Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.106593
Revised: March 14, 2025
Accepted: April 1, 2025
Published online: April 26, 2025
Processing time: 50 Days and 20.5 Hours
Integrating exhaled breath analysis into the diagnosis of cardiovascular diseases holds significant promise as a valuable tool for future clinical use, particularly for ischemic heart disease (IHD). However, current research on the volatilome (exhaled breath composition) in heart disease remains underexplored and lacks sufficient evidence to confirm its clinical validity. Key challenges hindering the application of breath analysis in diagnosing IHD include the scarcity of studies (only three published papers to date), substantial methodological bias in two of these studies, and the absence of standardized protocols for clinical imple
Core Tip: Exhaled breath analysis offers a non-invasive, cost-effective alternative to traditional ischemic heart disease diagnostics, with superior accuracy (84% vs 60%–70% for stress electrocardiography). To enhance reliability, standardized protocols for breath collection and the integration of machine learning are essential. Collaborative efforts among clinicians, chemists, and data scientists are key to unlocking its full clinical potential.
- Citation: Marzoog BA, Kopylov P. Volatilome and machine learning in ischemic heart disease: Current challenges and future perspectives. World J Cardiol 2025; 17(4): 106593
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/106593.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.106593
Ischemic heart disease (IHD) and its associated sequelae remain the leading cause of global mortality and morbidity, posing a critical public health challenge despite advances in diagnostic and therapeutic strategies[1-4]. While emerging technologies, such as machine learning (ML)-enhanced medical applications, hold promise for refining diagnostic accuracy and treatment efficacy, significant gaps persist. A key barrier to the effective management of IHD is the limited availability of highly sensitive, widely accessible diagnostic tools, particularly in resource-constrained settings.
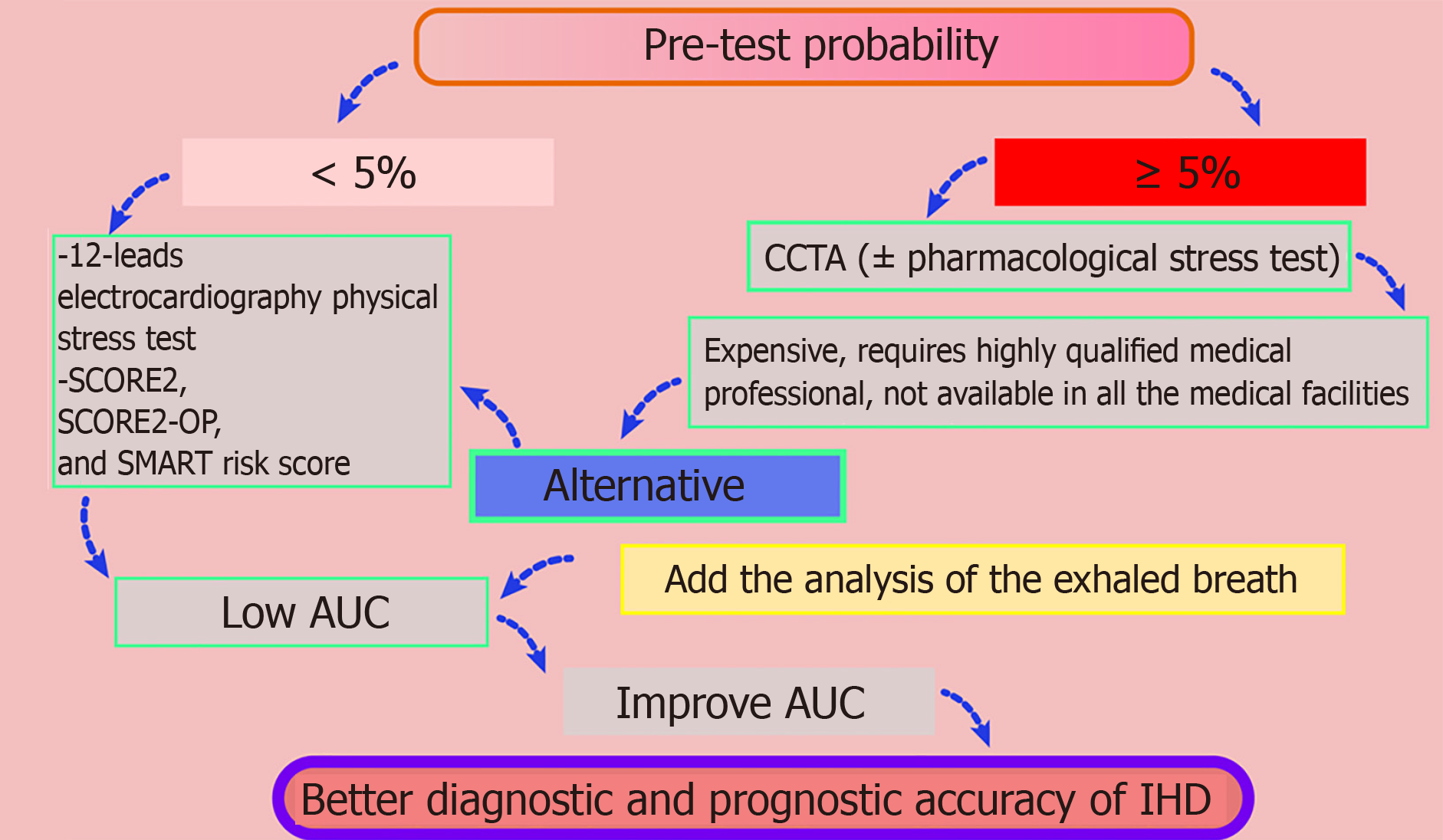
For the diagnosis of IHD, current guidelines from the European Society of Cardiology prioritize coronary computed tomography angiography and stress testing (e.g., pharmacological vasodilation with adenosine triphosphate) for moderate-to-high-risk patients[5]. However, these modalities face practical limitations: (1) They are costly, especially in poor countries; (2) They require specialized infrastructure; and (3) They depend on highly trained personnel to mitigate risks such as arrhythmias during vasodilator administration. 12-lead electrocardiography (ECG) with physical stress testing is a simple and cost-effective alternative but exhibits poor diagnostic accuracy, particularly in postmenopausal women, with high false-positive and false-negative rates.
To address these challenges, integrating exhaled breath analysis to identify disease-specific volatile organic compounds (VOCs) presents a novel, non-invasive strategy. This approach could complement existing methods by detecting metabolic biomarkers linked to ischemic pathophysiology, offering potential for an earlier and more precise diagnosis (Figure 1).
Current findings advocate advancing the use of exhaled breath analysis to diagnose IHD[6]. Although few studies have explored exhaled breath in this context, existing research demonstrates promising results, identifying VOCs linked to IHD, such as 2-pentanone or 3-methyl-2-butanone (C4H8O+), C2H7NO3H+, and ions at m/z 87.9337 and 144.9178[7-9]. Notably, a recent prospective study highlights an 84% diagnostic accuracy (area under the curve) for breath-based detection, outperforming traditional 12-lead ECG stress tests (60%–70%)[10,12]. The study included 80 participants, divided into two groups according to the results of the stress computed tomography with myocardial perfusion (CTP) imaging with vasodilation test with adenosine triphosphate[10]. The first group (n = 31) included patients with IHD, confirmed by positive post-stress myocardial perfusion defect on the CTP. The second group (n = 49) included participants without IHD, confirmed by the negative post-stress myocardial perfusion defect on the CTP[10]. Participants provided breath samples using PTR-TOF-MS-1000 at three time points: at rest, at peak physical stress, and 3 min after the second breath sample. ML methods were employed to analyze the obtained concentrations of the compounds based on the mass/charge ratio, using an internal validation method[10].
Current evidence suggests these VOCs primarily reflect myocardial membrane breakdown rather than atherosclerotic plaque metabolism[13]. The temporal pattern of VOC release (peaking during exercise-induced ischemia) supports direct cardiac origin over systemic inflammation[8]. However, controlled studies using isotope-labeled lipid tracers are needed to confirm the biochemical pathways[14].
Breathomics presents a compelling, cost-effective alternative to invasive diagnostics such as coronary computed tomography angiography and stress ECG, particularly in resource-limited settings, by offering a non-invasive, low-risk approach that eliminates radiation exposure and procedural complications while enhancing patient compliance through simplicity and comfort[15–17]. Its portability and minimal infrastructure requirements—operability with basic training and without the need for expensive imaging equipment or reliable electricity—reduce both upfront and operational costs, making it scalable for rural or remote areas. Rapid results and screening potential enable timely triage and early intervention, supported by synergies with mobile health technologies for cloud-based data analysis and telemedicine integration. While further validation of diagnostic accuracy and addressing of regulatory and cultural adoption barriers remain critical, breathomics prioritizes affordability, accessibility, and sustainability, addressing systemic gaps in healthcare delivery for underserved populations.
Challenges in clinical implementation persist, including the absence of standardized protocols—from patient preparation (diet, medication, exhalation technique) to analytical methods (mass spectrometry workflows, ML models)[13,18–20]. The following critical questions remain unresolved: (1) Should only the final exhaled breath (reflecting lower bronchial regions) be analyzed? and (2) Which ML algorithms (e.g., support vector machine, neural networks) are optimal? Additionally, offline methods risk sample degradation during storage, whereas real-time (online) mass spectrometry enhances accuracy by preserving biochemical integrity.
The integration of ML in breathomics, such as random forest (RF) algorithms, has demonstrated proven utility in analyzing high-dimensional breathomics data, such as VOC profiles, owing to their inherent advantages in handling noisy, multi-dimensional datasets[21-24]. RF operates by constructing an ensemble of decision trees, each trained on random subsets of features and data, which reduces overfitting—a critical concern in breathomics where datasets are often small but rich in features (e.g., thousands of VOCs)[25-28]. Its ability to rank feature importance (e.g., identifying key biomarkers such as aldehydes or ketones linked to diseases such as lung cancer or chronic obstructive pulmonary disease) enables interpretable insights, even with limited clinical samples[29–32]. For instance, RF has been successfully applied in studies distinguishing asthma subtypes or early-stage cancers by prioritizing robust VOC signatures amid confounding variables (e.g., environmental VOCs)[33,34]. Unlike “black-box” models (e.g., deep neural networks), the computational efficiency and tolerance to missing data make RF particularly suitable for resource-limited settings, where rapid, low-power analysis is essential[35-38]. However, its efficacy hinges on standardized data collection (to minimize batch effects) and validation across diverse cohorts to ensure generalizability—a gap that must be addressed through collaborative benchmarking initiatives[29,31,39].
The clinical translation of breathomics faces critical challenges that must be addressed. Key hurdles include methodological bias in biomarker identification due to variability in sample collection (e.g., breath sampling techniques, environmental contaminants) and analytical platforms (e.g., mass spectrometry vs. sensor-based devices), which can skew results and limit reproducibility[40–43]. A lack of standardization across studies—in protocols for breath capture, storage, and data processing—further complicates cross-validation and comparability of findings[44]. Additionally, the influence of confounding factors such as diet, medications, and comorbidities on VOC profiles raises concerns about diagnostic specificity, potentially leading to false positives or negatives[45]. While portable devices and artificial intelligence-driven analytics offer scalability, the limited validation against gold-standard diagnostics (e.g., coronary computed tomography angiography) in diverse populations undermines clinical confidence, and regulatory frameworks lag in adapting to these novel technologies[46]. Finally, the integration of breathomics into existing healthcare workflows requires addressing data interpretation complexities (e.g., distinguishing disease-specific biomarkers from noise) and ensuring equitable access to avoid exacerbating health disparities[47]. Overcoming these challenges demands collaborative efforts to establish standardized protocols, robust validation studies, and adaptive regulatory pathways to realize the potential of breathomics as a transformative diagnostic tool.
To address methodological variability and accelerate the clinical translation of breathomics, a standardized minimal breath collection checklist—inspired by the European Respiratory Society and American Thoracic Society guidelines—should be implemented[48-50]. Key elements of patient preparation include fasting for ≥ 6 h, avoiding exercise/smoking, and documenting current medications. The exhalation technique should involve the use of a nose clip (or nasal inhalation followed by oral exhalation), with a slow vital capacity exhalation at 200–300 mL/s, discarding dead-space air. Sample capture should involve the use of inert containers such as Tedlar® bags to collect mid-exhalation alveolar air at a standardized volume while controlling ambient VOCs and recording environmental metadata such as temperature and humidity. Post-collection protocols must prioritize immediate analysis or storage at −80 °C, include procedural blanks to detect contamination, and require rigorous documentation of patient demographics (e.g., body mass index, smoking status) and technical parameters (e.g., device type, operator ID). To ensure utility, this framework requires validation through multicenter trials assessing cross-platform reproducibility (e.g., gas chromatography–mass spectrometry vs e-noses vs PTR-TOF-MS-1000) and open-access protocol sharing via platforms such as European Multicenter Bronchiectasis Audit and Research Collaboration, coupled with regulatory collaboration to harmonize standards and address confounders, such as diet and comorbidities, that threaten diagnostic specificity.
A 2018 chronic obstructive pulmonary disease/asthma study using eNose technology demonstrated that breathomics could identify distinct inflammatory profiles independent of traditional diagnoses[51]. This shows that exhaled VOCs reflect systemic metabolic changes rather than just local lung pathology[52]. The same principle likely applies to cardiac conditions where systemic inflammation and oxidative stress drive pathology.
Research on 893 patients with chronic obstructive pulmonary disease revealed that breath analysis predicted the development of lung cancer within 2 years with 87% cross-validated accuracy (area under the receiver operating characteristic curve = 0.90)[53]. This proves that breath biomarkers can detect subclinical systemic metabolic shifts–a critical capability needed for early detection of IHD, where traditional tests often miss pre-symptomatic stages.
The pathophysiological mechanisms underlying IHD provide a foundation for hypothesizing the origins of detected VOCs, such as C4H8O+ and ions at m/z 87.9337 and 144.9178, with three proposed hypotheses[32,54–56]. The first posits that myocardial ischemia triggers mitochondrial lipid peroxidation, generating reactive breakdown products such as
The biomarker m/z 87.9337 (C4H8O+), likely reflecting VOCs such as butyraldehyde or fragmented ketone derivatives, may arise from oxidative stress and lipid peroxidation in IHD[57–59]. During ischemia-reperfusion injury, reactive oxygen species surge via mitochondrial dysfunction and enzymatic activation (e.g., xanthine oxidase), oxidizing polyunsaturated fatty acids in cell membranes to generate reactive aldehydes such as malondialdehyde or 4-hydroxynonenal, whose breakdown could yield smaller fragments such as C4H8O+. These aldehydes disrupt mitochondrial integrity, impair adenosine triphosphate production, and promote apoptosis while also modifying proteins/DNA, exacerbating cardiomyocyte dysfunction. Concurrently, lipid peroxidation products activate inflammatory pathways (e.g., nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 inflammasomes) and endothelial dysfunction by scavenging nitric oxide, worsening ischemia. Clinically, detecting such VOCs in breath or blood could offer real-time insights into oxidative damage, while therapies targeting aldehyde detoxification (e.g., aldehyde dehydrogenase 2 activators) or antioxidant strategies may mitigate injury, bridging metabolic dysregulation to IHD pathophysiology for improved diagnostics and treatment[60].
The integration of exhaled breath analysis into clinical practice holds significant promise for revolutionizing the diagnosis, prognosis, and personalized treatment of IHD. Encouragingly, clinical trials have demonstrated its potential, achieving 84% diagnostic accuracy—surpassing traditional 12-lead ECG stress tests—and identifying key biomarkers such as C4H8O+ and ions at m/z 87.9337 and 144.9178. While challenges such as protocol standardization, ML model variability, and pre-analytical variables (e.g., breath collection methods) remain, the scientific community has begun to address these barriers through collaborative efforts in order to establish robust guidelines. As these hurdles are systematically resolved, exhaled breath analysis is poised to transition from experimental validation to routine clinical use, offering a non-invasive and cost-effective tool for early detection and tailored therapeutic strategies. The future of breath-based diagnostics hinges on multidisciplinary collaboration, bridging gaps between clinicians, chemists, and data scientists to unlock its full potential in improving cardiovascular care.
| 1. | Genders TSS, Hunink MGM. Epidemiology of Coronary Artery Disease. In: Cademartiri F, Casolo G, Midiri M, editor. Clinical Applications of Cardiac CT. Milano: Springer Milan, 2012: 3–6 . [DOI] [Full Text] |
| 2. | Matskeplishvili S, Kontsevaya A. Cardiovascular Health, Disease, and Care in Russia. Circulation. 2021;144:586-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Barbarash OL, Karpov YA, Panov A V. , Akchurin RS, Alekyan BG, Alekhin MN, Aronov DM, Harutyunyan GK, Belenkov YN, Boytsov SA, Boldueva SA, Boschenko AA, Bubnova MG, Bulkina OS, Vasyuk YA, Galyavich AS, Glezer MG, Golubev EP, Golukhova EZ, Grinstein YI, Davidovich IM, Yezhov M V., Zavadovsky K V., Irtyuga OB, Karpov RS, Koziolova V V., Koziolova NA, Korennova OY, Kosmacheva ED, Koshelskaya OA, Kukharchuk V V., Lopatin YM, Merkulov E V., Mironov VM, Martsevich SY, Mirolyubova OA, Mikhin VP, Nedoshivin AO, Nikulina NN, Nikulina SY, Oleinikov VE, Panchenko EP, Perepech NB, Petrova MM, Protasov K V., Saidova MA, Samko AN, Sergienko I V., Sinitsyn VE, Skibitsky V V., Soboleva GN, Shalaev S V., Shaposhnik II, Shevchenko AO, Shiryaev AA, Shlyakhto E V., Chumakova GA, Yakushin SS. 2024 Clinical practice guidelines for Stable coronary artery disease. Russ J Cardiol. 2024;29:6110. [DOI] [Full Text] |
| 4. | Restrepo Tique M, Araque O, Sanchez-Echeverri LA. Technological Advances in the Diagnosis of Cardiovascular Disease: A Public Health Strategy. Int J Environ Res Public Health. 2024;21:1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4818] [Article Influence: 803.0] [Reference Citation Analysis (0)] |
| 6. | Bykova AA, Malinovskaya LK, Chomakhidze PS, Trushina OV, Shaltaeva YR, Belyakov VV, Golovin AV, Pershenkov VS, Syrkin AL, Betelin VB, Kopylov PY. [Exhaled Breath Analysis in Diagnostics of Cardiovascular Diseases]. Kardiologiia. 2019;59:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nardi Agmon I, Broza YY, Alaa G, Eisen A, Hamdan A, Kornowski R, Haick H. Detecting Coronary Artery Disease Using Exhaled Breath Analysis. Cardiology. 2022;147:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Marzoog BA, Chomakhidze P, Gognieva D, Parunova AY, Demchuk SN, Silantyev A, Kuznetsova N, Kostikova A, Podgalo D, Nagornov E, Gadzhiakhmedova A, Kopylov P. Updates in breathomics behavior in ischemic heart disease and heart failure, mass-spectrometry. World J Cardiol. 2025;17:102851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 9. | Lombardi M, Segreti A, Miglionico M, Pennazza G, Tocca L, Amendola L, Vergallo R, Di Sciascio G, Porto I, Grigioni F, Antonelli Incalzi R. Breath Analysis via Gas Chromatography-Mass Spectrometry (GC-MS) in Chronic Coronary Syndrome (CCS): A Proof-of-Concept Study. J Clin Med. 2024;13:5857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Marzoog BA, Chomakhidze P, Gognieva D, Gagarina NV, Silantyev A, Suvorov A, Fominykha E, Mustafina M, Natalya E, Gadzhiakhmedova A, Kopylov P. Machine Learning Model Discriminate Ischemic Heart Disease Using Breathome Analysis. Biomedicines. 2024;12:2814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O'Connor RE, Ross MA, Shaw LJ. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368-e454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 12. | Mancini GB, Gosselin G, Chow B, Kostuk W, Stone J, Yvorchuk KJ, Abramson BL, Cartier R, Huckell V, Tardif JC, Connelly K, Ducas J, Farkouh ME, Gupta M, Juneau M, O'Neill B, Raggi P, Teo K, Verma S, Zimmermann R; Canadian Cardiovascular Society. Canadian Cardiovascular Society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol. 2014;30:837-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Marzoog BA. Volatilome is Inflammasome- and Lipidome-dependent in Ischemic Heart Disease. Curr Cardiol Rev. 2024;20:e190724232038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Fenn D, Lilien TA, Hagens LA, Smit MR, Heijnen NFL, Tuip-de Boer AM, Neerincx AH, Golebski K, Bergmans DCJJ, Schnabel RM, Schultz MJ, Maitland-van der Zee AH, Brinkman P, Bos LDJ. Validation of volatile metabolites of pulmonary oxidative injury: a bench to bedside study. ERJ Open Res. 2023;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Christiansen A, Davidsen JR, Titlestad I, Vestbo J, Baumbach J. A systematic review of breath analysis and detection of volatile organic compounds in COPD. J Breath Res. 2016;10:034002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Fu XA, Li M, Knipp RJ, Nantz MH, Bousamra M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014;3:174-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Ibrahim W, Carr L, Cordell R, Wilde MJ, Salman D, Monks PS, Thomas P, Brightling CE, Siddiqui S, Greening NJ. Breathomics for the clinician: the use of volatile organic compounds in respiratory diseases. Thorax. 2021;76:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Marzoog BA. Volatilome: A Novel Tool for Risk Scoring in Ischemic Heart Disease. Curr Cardiol Rev. 2024;20:e080724231719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Marzoog B. Breathomics Detect the Cardiovascular Disease: Delusion or Dilution of the Metabolomic Signature. Curr Cardiol Rev. 2024;20:e020224226647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Marzoog BA, Chomakhidze P, Gognieva D, Silantyev A, Suvorov A, Fedorova AY, Kopylov P. Exhaled Breath Biomarkers Reflect the Inflammasome and Lipidome Changes in Ischemic Heart Disease: Using Machine Learning Models and Network Analysis. Preprints. 2025;. [DOI] [Full Text] |
| 21. | Smolinska A, Hauschild AC, Fijten RR, Dallinga JW, Baumbach J, van Schooten FJ. Current breathomics--a review on data pre-processing techniques and machine learning in metabolomics breath analysis. J Breath Res. 2014;8:027105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 22. | Mathur P, Srivastava S, Xu X, Mehta JL. Artificial Intelligence, Machine Learning, and Cardiovascular Disease. Clin Med Insights Cardiol. 2020;14:1179546820927404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Alabdaljabar MS, Hasan B, Noseworthy PA, Maalouf JF, Ammash NM, Hashmi SK. Machine Learning in Cardiology: A Potential Real-World Solution in Low- and Middle-Income Countries. J Multidiscip Healthc. 2023;16:285-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Ben Ali W, Pesaranghader A, Avram R, Overtchouk P, Perrin N, Laffite S, Cartier R, Ibrahim R, Modine T, Hussin JG. Implementing Machine Learning in Interventional Cardiology: The Benefits Are Worth the Trouble. Front Cardiovasc Med. 2021;8:711401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Sandeep B, Liu X, Huang X, Wang X, Mao L, Xiao Z. Feasibility of artificial intelligence its current status, clinical applications, and future direction in cardiovascular disease. Curr Probl Cardiol. 2024;49:102349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Quer G, Arnaout R, Henne M, Arnaout R. Machine Learning and the Future of Cardiovascular Care: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:300-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 27. | Pickering JW. Machine learning for decision-making in cardiology: a narrative review to aid navigating the new landscape. Rev Esp Cardiol (Engl Ed). 2023;76:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Upton R, Mumith A, Beqiri A, Parker A, Hawkes W, Gao S, Porumb M, Sarwar R, Marques P, Markham D, Kenworthy J, O'Driscoll JM, Hassanali N, Groves K, Dockerill C, Woodward W, Alsharqi M, McCourt A, Wilkes EH, Heitner SB, Yadava M, Stojanovski D, Lamata P, Woodward G, Leeson P. Automated Echocardiographic Detection of Severe Coronary Artery Disease Using Artificial Intelligence. JACC Cardiovasc Imaging. 2022;15:715-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Sardar P, Abbott JD, Kundu A, Aronow HD, Granada JF, Giri J. Impact of Artificial Intelligence on Interventional Cardiology: From Decision-Making Aid to Advanced Interventional Procedure Assistance. JACC Cardiovasc Interv. 2019;12:1293-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 30. | Geltser BI, Tsivanyuk MM, Shakhgeldyan KI, Rublev VY. Machine learning as a tool for diagnostic and prognostic research in coronary artery disease. Russ J Cardiol. 2020;25:3999. [DOI] [Full Text] |
| 31. | Glass C, Lafata KJ, Jeck W, Horstmeyer R, Cooke C, Everitt J, Glass M, Dov D, Seidman MA. The Role of Machine Learning in Cardiovascular Pathology. Can J Cardiol. 2022;38:234-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Han C, Song Y, Lim HS, Tae Y, Jang JH, Lee BT, Lee Y, Bae W, Yoon D. Automated Detection of Acute Myocardial Infarction Using Asynchronous Electrocardiogram Signals-Preview of Implementing Artificial Intelligence With Multichannel Electrocardiographs Obtained From Smartwatches: Retrospective Study. J Med Internet Res. 2021;23:e31129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Rufo JC, Madureira J, Fernandes EO, Moreira A. Volatile organic compounds in asthma diagnosis: a systematic review and meta-analysis. Allergy. 2016;71:175-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Suzukawa M, Ohta K, Sugimoto M, Ohshima N, Kobayashi N, Tashimo H, Tanimoto Y, Itano J, Kimura G, Takata S, Nakano T, Yamashita T, Ikegame S, Hyodo K, Abe M, Chibana K, Kamide Y, Sasaki K, Hashimoto H. Identification of exhaled volatile organic compounds that characterize asthma phenotypes: A J-VOCSA study. Allergol Int. 2024;73:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, Marwick TH. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1317-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 433] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 36. | Stamate E, Piraianu AI, Ciobotaru OR, Crassas R, Duca O, Fulga A, Grigore I, Vintila V, Fulga I, Ciobotaru OC. Revolutionizing Cardiology through Artificial Intelligence-Big Data from Proactive Prevention to Precise Diagnostics and Cutting-Edge Treatment-A Comprehensive Review of the Past 5 Years. Diagnostics (Basel). 2024;14:1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, Min JK, Tang WHW, Halperin JL, Narayan SM. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J. 2019;40:2058-2073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (2)] |
| 38. | Martin-Isla C, Campello VM, Izquierdo C, Raisi-Estabragh Z, Baeßler B, Petersen SE, Lekadir K. Image-Based Cardiac Diagnosis With Machine Learning: A Review. Front Cardiovasc Med. 2020;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Kuznetsova N, Gubina A, Sagirova Z, Dhif I, Gognieva D, Melnichuk A, Orlov O, Syrkina E, Sedov V, Chomakhidze P, Saner H, Kopylov P. Left Ventricular Diastolic Dysfunction Screening by a Smartphone-Case Based on Single Lead ECG. Clin Med Insights Cardiol. 2022;16:11795468221120088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Chen T, Liu T, Li T, Zhao H, Chen Q. Exhaled breath analysis in disease detection. Clin Chim Acta. 2021;515:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Müller-Wirtz LM, Kiefer D, Ruffing S, Brausch T, Hüppe T, Sessler DI, Volk T, Fink T, Kreuer S, Maurer F. Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients. Molecules. 2021;26:3089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Spanĕl P, Smith D. Selected ion flow tube mass spectrometry for on-line trace gas analysis in biology and medicine. Eur J Mass Spectrom (Chichester). 2007;13:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Martinez-Lozano Sinues P, Zenobi R, Kohler M. Analysis of the exhalome: a diagnostic tool of the future. Chest. 2013;144:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Khoubnasabjafari M, Mogaddam MRA, Rahimpour E, Soleymani J, Saei AA, Jouyban A. Breathomics: Review of Sample Collection and Analysis, Data Modeling and Clinical Applications. Crit Rev Anal Chem. 2022;52:1461-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 45. | Taylor A, Blum S, Ball M, Birch O, Chou H, Greenwood J, Swann S, Pocock L, Allsworth M, Boyle B, Geillinger-Kaestle K. Development of a new breath collection method for analyzing volatile organic compounds from intubated mouse models. Biol Methods Protoc. 2024;9:bpae087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Lin GP, Vadhwana B, Belluomo I, Boshier PR, Španěl P, Hanna GB. Cross Platform Analysis of Volatile Organic Compounds Using Selected Ion Flow Tube and Proton-Transfer-Reaction Mass Spectrometry. J Am Soc Mass Spectrom. 2021;32:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Manlhiot C, van den Eynde J, Kutty S, Ross HJ. A Primer on the Present State and Future Prospects for Machine Learning and Artificial Intelligence Applications in Cardiology. Can J Cardiol. 2022;38:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Armoundas AA, Narayan SM, Arnett DK, Spector-Bagdady K, Bennett DA, Celi LA, Friedman PA, Gollob MH, Hall JL, Kwitek AE, Lett E, Menon BK, Sheehan KA, Al-Zaiti SS; American Heart Association Institute for Precision Cardiovascular Medicine; Council on Cardiovascular and Stroke Nursing; Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; Council on the Kidney in Cardiovascular Disease; and Stroke Council. Use of Artificial Intelligence in Improving Outcomes in Heart Disease: A Scientific Statement From the American Heart Association. Circulation. 2024;149:e1028-e1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 133] [Article Influence: 66.5] [Reference Citation Analysis (1)] |
| 49. | Ayalew BD, Rodoshi ZN, Patel VK, Alresheq A, Babu HM, Aurangzeb RF, Aurangzeb RI, Mdivnishvili M, Rehman A, Shehryar A, Hassan A. Nuclear Cardiology in the Era of Precision Medicine: Tailoring Treatment to the Individual Patient. Cureus. 2024;16:e58960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 50. | Visco V, Ferruzzi GJ, Nicastro F, Virtuoso N, Carrizzo A, Galasso G, Vecchione C, Ciccarelli M. Artificial Intelligence as a Business Partner in Cardiovascular Precision Medicine: An Emerging Approach for Disease Detection and Treatment Optimization. Curr Med Chem. 2021;28:6569-6590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Scarlata S, Finamore P, Meszaros M, Dragonieri S, Bikov A. The Role of Electronic Noses in Phenotyping Patients with Chronic Obstructive Pulmonary Disease. Biosensors (Basel). 2020;10:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | de Vries R, Dagelet YWF, Spoor P, Snoey E, Jak PMC, Brinkman P, Dijkers E, Bootsma SK, Elskamp F, de Jongh FHC, Haarman EG, In 't Veen JCCM, Maitland-van der Zee AH, Sterk PJ. Clinical and inflammatory phenotyping by breathomics in chronic airway diseases irrespective of the diagnostic label. Eur Respir J. 2018;51:1701817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 53. | de Vries R, Farzan N, Fabius T, De Jongh FHC, Jak PMC, Haarman EG, Snoey E, In 't Veen JCCM, Dagelet YWF, Maitland-Van Der Zee AH, Lucas A, Van Den Heuvel MM, Wolf-Lansdorf M, Muller M, Baas P, Sterk PJ. Prospective Detection of Early Lung Cancer in Patients With COPD in Regular Care by Electronic Nose Analysis of Exhaled Breath. Chest. 2023;164:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 54. | Atasoy H, Greenwood BN, McCullough JS. The Digitization of Patient Care: A Review of the Effects of Electronic Health Records on Health Care Quality and Utilization. Annu Rev Public Health. 2019;40:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Gognieva D, Mitina Y, Gamilov T, Pryamonosov R, Vasilevskii Y, Simakov S, Liang F, Ternovoy S, Serova N, Tebenkova E, Sinitsyn V, Pershina E, Abugov S, Mardanian G, Zakarian N, Kirakosian V, Betelin V, Shchekochikhin D, Syrkin A, Kopylov P. Noninvasive Assessment of the Fractional Flow Reserve with the CT FFRc 1D Method: Final Results of a Pilot Study. Glob Heart. 2021;16:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Siontis KC, Friedman PA. The Role of Artificial Intelligence in Arrhythmia Monitoring. Card Electrophysiol Clin. 2021;13:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Bresser LRF, de Goffau MC, Levin E, Nieuwdorp M. Gut Microbiota in Nutrition and Health with a Special Focus on Specific Bacterial Clusters. Cells. 2022;11:3091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 58. | Joloudari JH, Joloudari EH, Saadatfar H, GhasemiGol M, Razavi SM, Mosavi A, Nabipour N, Shamshirband S, Nadai L. Coronary Artery Disease Diagnosis; Ranking the Significant Features Using a Random Trees Model. Int J Environ Res Public Health. 2020;17:731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Emrich T, Halfmann M, Schoepf UJ, Kreitner KF. CMR for myocardial characterization in ischemic heart disease: state-of-the-art and future developments. Eur Radiol Exp. 2021;5:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 60. | Nycyk JA, Drury JA, Cooke RW. Breath pentane as a marker for lipid peroxidation and adverse outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79:F67-F69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/