Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.106072
Revised: March 23, 2025
Accepted: April 7, 2025
Published online: April 26, 2025
Processing time: 65 Days and 11.4 Hours
Safety and efficacy of intravascular ultrasound (IVUS) guidance in percutaneous coronary intervention (PCI) has been consistently shown in recent trials. Ho
To investigate the outcomes of patients with STEMI undergoing IVUS-guided PCI and correlate derived IVUS measurements with clinical, procedural, imaging and follow-up outcomes of interest.
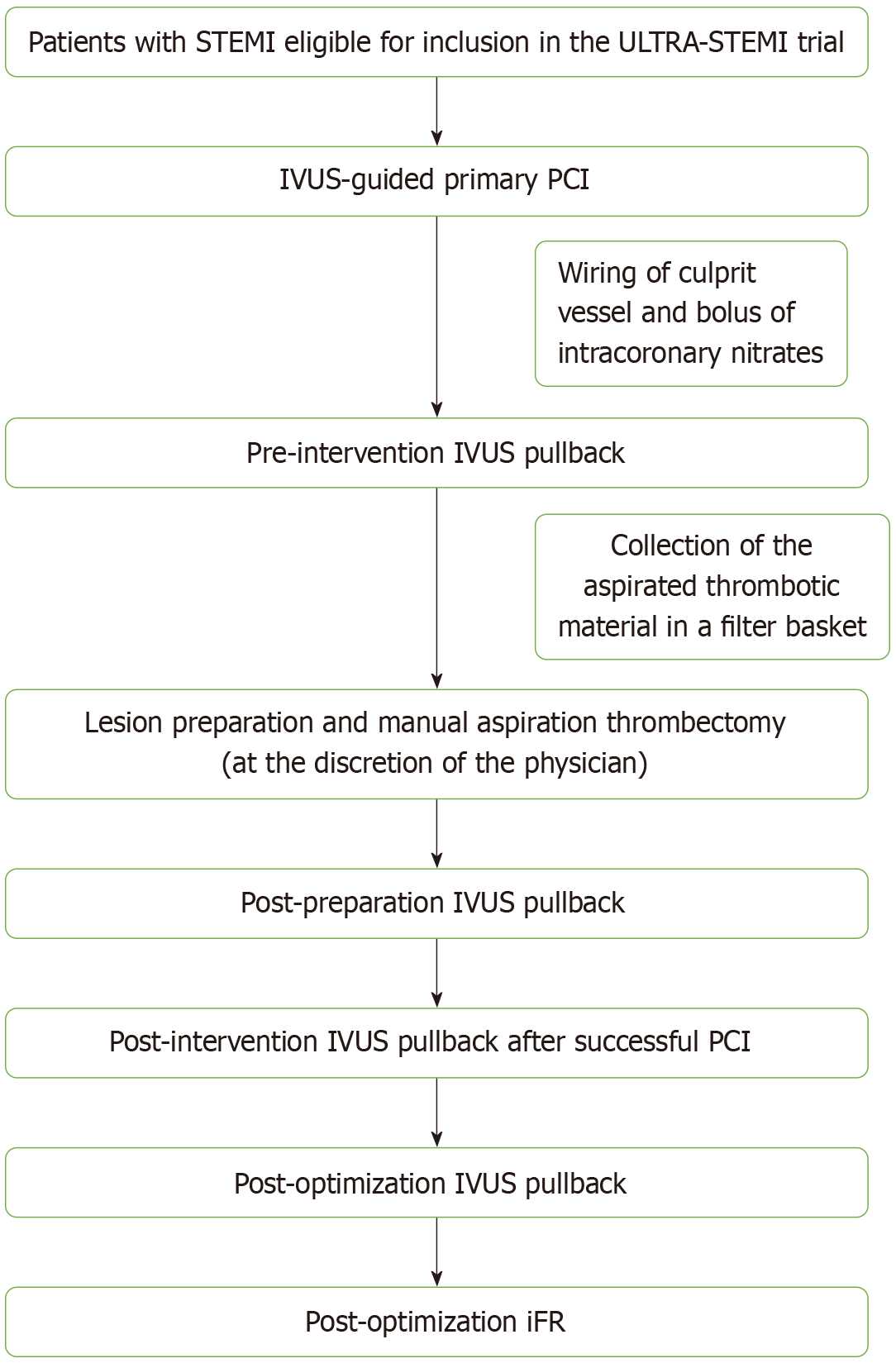
Study participants will undergo primary PCI as per standardized procedures. IVUS pullbacks will be performed pre-intervention, post-lesion preparation, post-intervention and post-optimization using a 20 MHz digital IVUS (Eagle Eye Platinum, Philips). Manual thrombus aspiration will be performed in cases of high thrombus burden. The aspirated thrombi will be scanned with micro-computed tomography to extract volumetric measurements of the aspirated thrombotic burden. Moreover, angiographic, peri-procedural and 3-year follow-up data will be gathered. Co-primary endpoints will be cardiovascular mortality and target vessel failure, defined as the composite of: Cardiovascular mortality, target vessel myocardial infarction and/or clinically driven target vessel revascularization.
The results of the study are expected by the third quarter of 2029.
The ULTRA-STEMI trial will add to the existing literature the clinical, angio
Core Tip: Intravascular ultrasound (IVUS) guidance in percutaneous coronary inter
- Citation: Karagiannidis E, Papazoglou AS, Samaras A, Nasoufidou A, Zormpas G, Tagarakis G, Theodoropoulos KC, Papadakis M, Tzikas A, Fragakis N, Kassimis G. Intravascular ULTRA sound-guided percutaneous coronary intervention in patients with STEMI: Rationale and design of the ULTRA-STEMI trial. World J Cardiol 2025; 17(4): 106072
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/106072.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.106072
Intracoronary imaging techniques, including intravascular ultrasound (IVUS) and optical coherence tomography, can overcome some limitations of coronary angio
Recent meta-analyses of randomized trials on the debate of IVUS-guidance vs CA-guidance of PCI procedures have demonstrated that IVUS-guided PCI improves peri-procedural and follow-up outcomes in a broad spectrum of patients in the era of drug-eluting stents leading to reduction in mortality rates and major adverse cardiovascular events (MACE)[5,6]. The studied populations were initially comprised mostly of pa
The use of IVUS in primary PCI of patients with acute myocardial infarction (AMI) has been first described in 2003[7]; however, patients with STEMI are still underrepresented in randomized trials[8,9]. Nevertheless, the real-world uti
However, IVUS-guided studies providing long-term outcome data in STEMI, evaluating and quantifying the precise effects of concomitant thrombus aspiration procedures, and comparing the utility of IVUS guidance in STEMI vs NSTEMI populations remain scarce. Particularly, research gaps exist in thrombus characterization, lesion morphology differences, and the impact of IVUS on stent optimization in STEMI. Hence, the prospective ULTRA-STEMI trial aims to be a pro
The study protocol is reported according to the SPIRIT 2013 checklist (Supplementary Table 1)[15]. ULTRA-STEMI (ClinicalTrials.gov Identifier: NCT05974930) is an investigator-initiated, prospective, single-arm, non-interventional cohort trial involving patients with STEMI, who undergo primary PCI within 12 hours of symptoms onset.
The trial will be conducted according to the principles set by the declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice[16]. The study protocol has been approved by the Scientific Committee and by the Directory Board of the Hippokration General Hospital of Thessaloniki (protocol code: 46/2024). Each subject will provide written informed consent before participating in the study.
A total of 80 patients presenting with STEMI to the Hippokration General Hospital of Thessaloniki and undergoing primary PCI will be enrolled in the study. Detailed eligibility criteria are described in Table 1.
| Inclusion criteria | Exclusion criteria |
| Patients with symptoms of myocardial ischemia for at least 30 minutes | Patients who have received thrombolytic therapy for index STEMI event |
| ECG changes indicating STEMI | Known intolerance to heparin, aspirin or P2Y12 inhibitor therapy (clopidogrel, prasugrel, or ticagrelor) |
| Minimum reference vessel diameter > 2.5 mm | Cardiogenic shock |
| Graft vessel as the culprit vessel | |
| Written informed consent | Presentation ≥ 12 hours after symptom onset |
Intravascular imaging for PCI guidance will be performed with the Eagle Eye Platinum digital IVUS (20 mHz) system (Philips, CA, United States). Two different thrombus aspiration systems will be used: The 6F EXPORT AP aspiration catheter (Medtronic) οr the 6F Thrombuster II catheter (Kaneka). All devices carry a Conformité Européenne mark and are routinely used in clinical practice.
CA and PCI: Every study participant will receive the standard of care treatment according to the current guidelines[17]. The pharmacological treatment of each patient prior to PCI will be according to standard practices [unfractionated heparin (100 IU/kg) and a loading dose of aspirin (325 mg) and either ticagrelor (180 mg) or prasugrel (60 mg) or clopidogrel (600 mg)][18].
Thrombus aspiration: Thrombus aspiration will be performed, in cases of definite thrombus burden [thrombolysis in myocardial infarction (TIMI) thrombus grade ≥ 3, as assessed via modified TIMI classification], as previously described[19,20]. Briefly, after crossing the lesion with a wire, the thrombus aspiration catheter will be advanced proximal to the lesion. Manual suction will begin before the catheter crosses the lesion. The thrombus aspiration catheter will be passed through the thrombotic occlusion many times, so that at least 40 mL of blood and material are aspirated. In case blood backflow stops suddenly during the procedure, the device will be removed to check for the presence of thrombus obstructing the lumen. The solid material aspirate will be captured in a filter basket provided by the manufacturer of the thrombus aspiration catheter.
IVUS: Four IVUS pull-backs will be performed (Figure 1): Pre-intervention, after lesion preparation, post-intervention, and post-optimization. IVUS image acquisition will follow established guidelines. All of the recorded variables for each study participant are summarized in Table 2. More specific, the primary IVUS parameters to be collected include vessel size, plaque burden, minimal lumen area, stent expansion, edge dissection, malapposition, and thrombus characteristics. Stent optimization will be defined as achieving minimal stent area > 5.5 mm², stent expansion > 80%, absence of significant edge dissection, and minimal residual disease at stent edges. These definitions align with prior IVUS-guided PCI studies[9].
| Data | Pre-intervention | Post-intervention | Post-optimization |
| IVUS data | Vessel, lumen and plaque cross-sectional area; Minimal lumen area; Plaque area and burden; Calcium severity; Supposed thrombus presence; Thrombus morphology (acute, subacute, organized) | Stent expansion; Plaque burden at stent edges; Edge dissection; Haematoma; Malapposition; Thrombus protrusion through stent struts; Percentage area stenosis | Minimum stent area; Stent underexpansion; Stent malapposition; Stent deformation; Stent edge dissection; Residual disease at stent edge |
| Pre-PCI | Post-PCI | ||
| Echocardiographic data | LVEF | LVEF | |
| Clinical data | Medical history; electrocardiographic and laboratory data; IIb/IIIa inhibitors/heparin administration | Electrocardiographic and laboratory data; Stent length and diameter; Amount of contrast drug administered; PCI and CA duration; Total effective radiation dose; Total fluoroscopy time; Number and type of stents; Number of catheters used; ST-segment resolution | |
| Micro-CT data | Thrombus volume | ||
| n.a. | Thrombus density | ||
| Angiographic data | Pre-procedural TIMI flow; Thrombus burden classification; Culprit vessel; Number of diseased vessels; SYNTAX score | Angiographically evident residual thrombus burden; Myocardial no-reflow phenomenon; Post-procedural TIMI flow; Distal embolization | |
| Follow-up data | n.a. | Mortality (+cause); CV-hospitalization; MI/revascularization(+vessel); Thromboembolic or bleeding event; Peri- and post-procedural complications | |
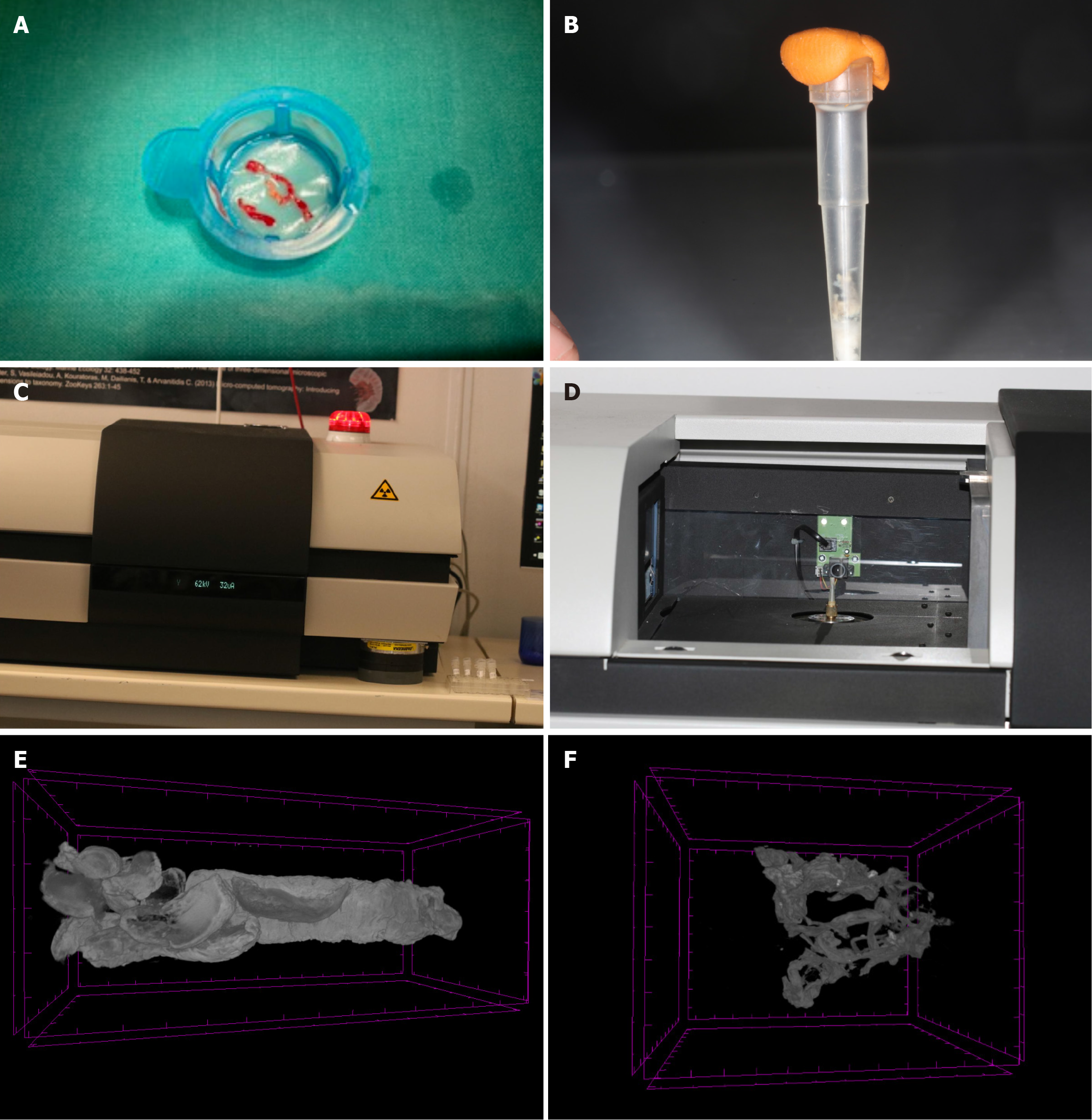
The aspirated thrombotic samples will be sent to the Biodiversity Laboratory of the Institute of Marine Biology, Biotechnology and Aquaculture (Herakleion, Crete). The samples will be first washed, and then subjected to dehydration procedures to be stored in alcohol solution 70% (Metscher protocol)[21]. Sample staining will be performed with a contrast-strengthening factor (phosphotungstic acid) to achieve best image quality as previously described[22]. The aspirated thrombi will be scanned with the micro-CT Sky-Scan 1172 (Bruker, Kontich, Belgium).
The scanning procedure will lead to the creation of a series of projection images in the form of image stacks. These will be then reconstructed using the NRecon (Bruker, Kontich, Belgium) software. Finally, 3D models of thrombi will be analyzed using the Amira (VSG, Burlington United States) and CTAnalyser (CTAn, Bruker, Kontich, Belgium) software to quantify the thrombotic volume (in mm3) and density (in Hounsefield Units). A micro-CT image of aspirated thrombotic material is illustrated in Figure 2, as generated during our previous QUEST-STEMI trial[14]. In cases of inadequate aspirated thrombotic material or when thrombus aspiration is not performed, no micro-CT analysis will be conducted. These patients will be included in overall clinical analyses but excluded from thrombus-specific correlations.
The study primarily aims to assess the rates of occurrence of target vessel failure (TVF) and cardiovascular death 3 years after IVUS-guided primary PCI in patients with STEMI. TVF is defined as the composite of: Cardiovascular death, target vessel myocardial infarction, clinically driven target vessel revascularization or target lesion revascularization according to the standardized end point definitions for coronary intervention trials[23]. At a second level, correlation of IVUS measurements with clinical, peri-procedural, angiographic, and micro-tomographic parameters will be performed. Detailed primary and secondary endpoints of the ULTRA-STEMI trial are described in Table 3.
| ULTRA-STEMI endpoints | |
| Clinical/procedural | Primary endpoints |
| Target vessel failure (36 months): Composite of cardiovascular death, target vessel myocardial infarction, clinically driven target vessel revascularization | |
| Cardiovascular death (36 months) | |
| Secondary endpoints | |
| MACE: Composite of cardiovascular mortality, any myocardial infarction and repeat revascularization | |
| Individual components of TVF and MACE | |
| Periprocedural data: Stent length and diameter, number and type of stents used, amount of contrast drug administered, total effective radiation dose, procedural duration | |
| In-hospital (post-PCI) adverse events: Composite of cardiac tamponade, need for CABG, shock, in-hospital mortality, acute kidney failure, bleeding, stroke | |
| IVUS | Post-PCI IVUS measurements: Stent underexpansion, malapposition, edge dissections, high plaque burden at stent edges, residual focal lesions, stent deformation, tissue protrusion through the stent struts |
| Angiographic | Angiographic outcomes: Pre-procedural and post-procedural TIMI flow, thrombus burden classification, culprit vessel, number of diseased vessels, angio-graphically evident residual thrombus, no-reflow phenomenon, post-procedural myocardial blush grade, distal embolization, SYNTAX score |
| Micro-CT | Volume and density of the aspirated thrombi |
Patients’ follow-up will be obtained using standardized telephone calls at 30 days, 1 and 3 years after the procedure and in-person follow-up visits at 6 and 18 months. Hospital and national healthcare system records will be also reviewed whenever possible to verify the occurrence of any adverse events and strengthen the reliability of our follow-up data. Angiographic and follow-up outcomes will be documented independently by two experienced interventional cardiologists based on pre-specified definitions.
With approximately 100 patients undergoing primary PCI in our hospital every year, the sample size of 80 patients is a feasible target enabling the completion of the enrolment phase within 12-18 months. A TVF percentage of approximately 15% at 36 months of follow-up in AMI patients[24] led us consider that a sample size of 80 patients may allow us to provide a TVF percentage in the range of 10% to 20% with reasonable precision. While this study is not powered for rare clinical endpoints, it aims to provide exploratory data on IVUS-guided PCI outcomes and thrombus burden quantification.
Normally distributed continuous variables will be presented as mean ± SD, whereas non-normally distributed variables will be presented as median with interquartile range. The Shapiro-Wilk test will be used to assess the normality of variable distributions. Categorical variables will be displayed as counts with percentages. For the comparison of continuous measurements (e.g., pre- and post-intervention IVUS measurements), the parametric student’s t-test will be used, if normally distributed, and the non-parametric Wilcoxon-Mann-Whitney test will be used, if non-normally distributed. Categorical variables will be compared using the χ2 test. Bonferroni correction will be used for the adjustment of multiple comparisons. The correlation of continuous IVUS measurements with micro-CT derived thrombotic measurements will be also investigated using the Spearman’s correlations. Any missing data in baseline characteristics and procedural outcomes will be handled using multiple imputation techniques when appropriate. Sensitivity analyses may also be performed excluding patients lost to follow-up.
Additionally, logistic or linear regression analyses will be performed to examine the association of IVUS measurements with binary or continuous procedural outcomes of interest, respectively. Cox regression analyses will be also conducted to evaluate the prognostic value of every recorded variable on the follow-up outcomes of interest. Kaplan-Meier curves will be plotted to display the cumulative incidence over time. At study completion, depending on the number of adverse events, we will assess the feasibility of performing propensity score matching or Cox regression analyses to adjust for patient characteristics (e.g., SYNTAX score, GRACE score, age, diabetes mellitus, renal function) and evaluate prognostic differences in specific population subsets (e.g., patients with standard modifiable risk factors vs. those without, patients aged ≥ 65 years vs < 65 years). If event rates are insufficient for multivariable adjustment, alternative approaches such as Kaplan-Meier analysis with log-rank testing may be considered. All statistical tests will be performed using the SPSS (version 27) and R 4.2.2 software; a two-tailed P value of less than 0.05 will be the statistical significance threshold for our study.
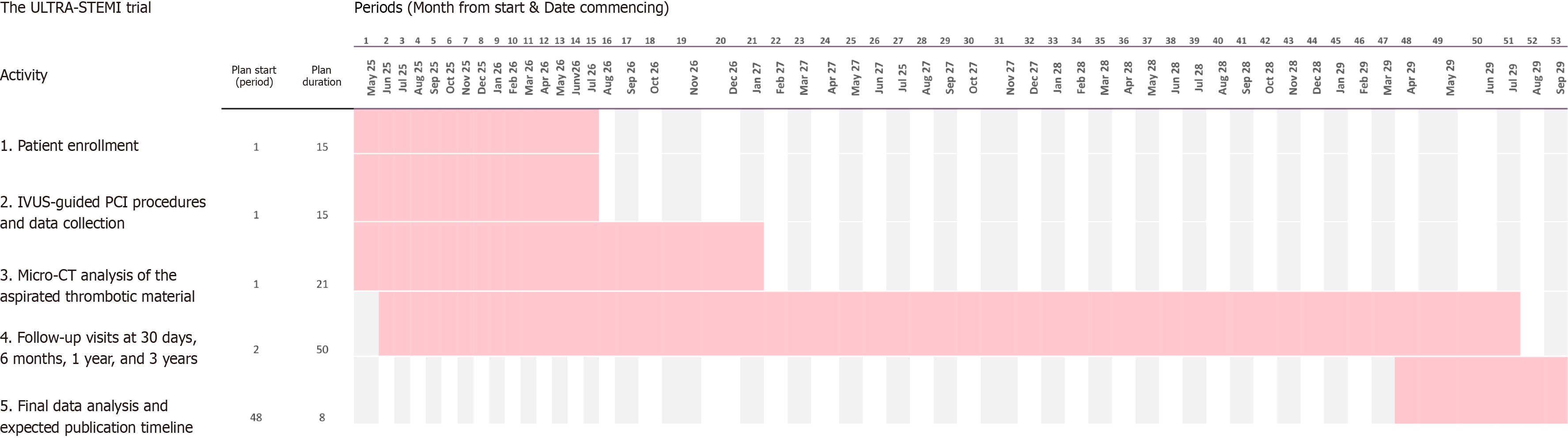
The first patient will be included in the study on 1st of May, 2025. Completion of enrollment is anticipated in the second quarter of 2026 and complete 3-year follow-up in the second quarter of 2029 (Figure 3).
The results of the study are expected by the third quarter of 2029.
ULTRA-STEMI is a prospective single-center registry designed to examine the value of IVUS-guided PCI in patients with STEMI. The aims of this study are: (1) To add further prospective data to the emerging body of evidence on the short- and long-term rates of adverse events after IVUS-guided primary PCI in STEMI; (2) To add prospective data on the procedural safety in IVUS-guided primary PCI of patients with STEMI; (3) To investigate for the first time the real-world outcomes of IVUS-guided PCI in STEMI using the Eagle Eye Platinum digital IVUS catheter; and (4) To quantify, if possible, the thrombotic material aspirated during IVUS-guided PCI in STEMI and perform correlations of specific IVUS measurements with the thrombotic volume and density.
With respect to the use of IVUS in the challenging setting of STEMI, we can observe that IVUS-guided PCI in AMI has gradually increased[8]. According to the multicenter KARMI-NIH registry, the percentage of IVUS usage elevated from 15% in 2011 to 26% in 2015, a tendency observed in both STEMI and non-STEMI subgroups[10]. The same investigators demonstrated that centers with higher usage of IVUS-guided PCI demonstrated a greater reduction in 3-year target lesion failure[10]. They also report that IVUS-guided PCI led to larger stent diameter, longer stent length and often multiple stent implantation in AMI[10]. The IVUS-ACS trial, a multicenter randomized study comparing IVUS-guided vs CA-guided PCI in ACS patients, showed lower rates of TVF and all-cause mortality with IVUS[12]. Unlike KAMIR-NIH and IVUS-ACS, ULTRA-STEMI is a prospective single-center trial focusing exclusively on STEMI with standardized imaging and procedural protocols, integrating objective micro-CT-based thrombus quantification, a feature not assessed in those trials. Although ULTRA-STEMI is a single-arm study, its findings will be placed in context with historical data from large registries and randomized trials, such as KAMIR-NIH and IVUS-ACS, to assess whether IVUS guidance leads to similar or improved long-term outcomes in STEMI patients.
IVUS seems to enable optimal stent selection because it helps to define stent landing zones and accurately assess vessel size, which is often affected by coronary spasm and high thrombus burden in patients with AMI[10]. Moreover, IVUS can provide an accurate evaluation of the acute stent result, quantifying stent expansion and identifying tissue protrusion, persisting thrombus and stent edge problems[10]. Hence, IVUS-guide PCI is encouraged to be considered in STEMI, where circumstances and operator experience allow, to enhance long-term patient outcomes[12,13].
The results of ULTRA-STEMI could have significant implications for both clinical guidelines and future trials. Our study aims to add upon the first prospective observational study designed to present dedicated procedural, imaging, safety, and outcome data on the use of IVUS in patients with STEMI, the SPECTRUM study[9]. If IVUS-derived parameters correlate with the aspirated thrombotic material, procedural and long-term outcomes, this study may further support routine IVUS use in primary PCI for thrombus-rich STEMI lesions. Additionally, micro-CT thrombus quantification may serve as a valuable tool for refining thrombus aspiration techniques, potentially influencing device selection and procedural strategies. Future randomized trials could expand on ULTRA-STEMI by integrating IVUS-based thrombus burden assessment into STEMI risk stratification models, further refining personalized PCI strategies for improved patient outcomes.
We estimate that it might be important to recognize whether IVUS-guided PCI has similar outcomes in different countries and healthcare systems using potentially different IVUS catheters. In the ULTRA-STEMI trial, the Eagle Eye Platinum digital IVUS catheter is projected to be used due to its user-friendly design, rapid image acquisition, and suitability for the acute STEMI setting. This catheter provides a fast and efficient approach for IVUS imaging as it does not require a motor drive, moving parts, or a pullback device, which simplifies procedural workflow. Unlike some high-resolution IVUS systems, the Eagle Eye Platinum catheter does not require pre-procedural flushing or transducer priming, minimizing procedural delays-a critical advantage in primary PCI for STEMI, where time-sensitive inter
Furthermore, the catheter is compatible with SyncVision co-registration, which establishes a three-way association between angiographic landmarks, longitudinal IVUS display, and tomographic IVUS frames, enhancing real-time PCI planning and optimization. The Eagle Eye Platinum catheter operates at 20 MHz, offering a balance between penetration depth and resolution, making it well-suited for assessing vessel dimensions, plaque burden, thrombus presence, and stent optimization. While higher-frequency IVUS catheters (e.g., 45-60 MHz) provide superior spatial resolution, they have reduced penetration depth, which may limit the assessment of deep-seated plaques and large thrombotic occlusions. Given the acute nature of STEMI, where procedural efficiency is crucial, the Eagle Eye Platinum IVUS catheter represents a clinically practical and widely adopted option that aligns well with the study’s objectives of evaluating IVUS-guided PCI outcomes in STEMI. The results of the ULTRA-STEMI trial will provide further insights on potential peri-procedural complications (such as increased radiation dose, PCI duration, creatinine elevation) associated with IVUS usage.
A preliminary analysis of the SPECTRUM study demonstrated that pre-intervention IVUS in 200 patients with STEMI allowed identification of culprit lesion plaque characteristics and thrombus load, thereby, it was helpful for the guidance of primary PCI[25]. This study also created an IVUS-derived thrombus score based on the total thrombus length, occlusive thrombus length and maximum thrombus angle[25]. This score can effectively differentiate between low (0-1 points) and high (2-3 points) thrombus burden. Our study cannot validate this IVUS-derived thrombus score because of the use of a different IVUS catheter of lower resolution; however, aims similarly to quantify the precise volume of the underlying thrombus burden by providing micro-CT measurements of the extracted thrombus. Our previous research demonstrated that micro-CT can be used for accurate characterization of the extracted thrombotic material, and that current thrombectomy devices might fail to deal adequately with large thrombotic material[14,22]. Perhaps, IVUS usage might also improve the aspiration procedure outcomes.
Admittedly, there are certain limitations to this study. Most importantly, the single-center, non-randomized character of the study and the enrollment of a small sample of Greek patients with STEMI may limit the generalizability of our findings. For instance, different institutional practices and country-specific patient population demographics and baseline characteristics might be better reflected in multi-center trial settings. Additionally, the existence of selection bias and other confounding factors (e.g., operator experience and different patients’ characteristics including age, gender, diabetes status, and SYNTAX score) cannot be eliminated even if efforts will be made to adjust for potential confounding factors. Furthermore, the inclusion of a relatively small population may not allow to conduct subgroup analyses or detect rare adverse events (such as in-hospital mortality, acute kidney failure, bleeding, cardiac tamponade or stroke). To address this limitation, we aim to collectively assess those in-hospital (post-PCI) adverse events as a composite “secondary” endpoint, which should be assessed in greater detail in larger IVUS-guided studies with STEMI populations undergoing primary PCI.
However, it is important to acknowledge that previous IVUS studies included a great diversity of patients, whereas our prospective study aims to enroll patients with STEMI and assess the aforementioned IVUS-related outcomes. Our study may be underpowered for clinical endpoints; however, its aim is not to answer whether someone would benefit from IVUS-guided PCI over angio-guided, but to provide an exploratory and descriptive patient registry adding further prospective data for the usage of a different IVUS catheter in STEMI.
In conclusion, this prospective study aims to shed light on the clinical, angiographic, micro-CT and follow-up outcomes of IVUS-guided PCI in 80 patients presenting with STEMI. These outcomes will be assessed through the synergy of different imaging modalities and will add to the existing literature.
| 1. | Burzotta F, Trani C. Intracoronary Imaging. Circ Cardiovasc Interv. 2018;11:e007461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, Tian N, Lin S, Lu Q, Wu X, Li Q, Liu Z, Chen Y, Qian X, Wang J, Chai D, Chen C, Li X, Gogas BD, Pan T, Shan S, Ye F, Chen SL. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 489] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 3. | Lee YJ, Zhang JJ, Mintz GS, Hong SJ, Ahn CM, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Kan J, Pan T, Gao X, Ge Z, Chen SL, Hong MK. Impact of Intravascular Ultrasound-Guided Optimal Stent Expansion on 3-Year Hard Clinical Outcomes. Circ Cardiovasc Interv. 2021;14:e011124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Mitsis A, Eftychiou C, Kadoglou NPE, Theodoropoulos KC, Karagiannidis E, Nasoufidou A, Ziakas A, Tzikas S, Kassimis G. Innovations in Intracoronary Imaging: Present Clinical Practices and Future Outlooks. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Buccheri S, Franchina G, Romano S, Puglisi S, Venuti G, D'Arrigo P, Francaviglia B, Scalia M, Condorelli A, Barbanti M, Capranzano P, Tamburino C, Capodanno D. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention With Stent Implantation: A Systematic Review and Bayesian Network Meta-Analysis of 31 Studies and 17,882 Patients. JACC Cardiovasc Interv. 2017;10:2488-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 6. | Darmoch F, Alraies MC, Al-Khadra Y, Moussa Pacha H, Pinto DS, Osborn EA. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2020;9:e013678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 7. | Kotani J, Mintz GS, Pregowski J, Kalinczuk L, Pichard AD, Satler LF, Suddath WO, Waksman R, Weissman NJ. Volumetric intravascular ultrasound evidence that distal embolization during acute infarct intervention contributes to inadequate myocardial perfusion grade. Am J Cardiol. 2003;92:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Karamasis GV, Varlamos C, Benetou DR, Kalogeropoulos AS, Keeble TR, Tsigkas G, Xenogiannis I. The Usefulness of Intracoronary Imaging in Patients with ST-Segment Elevation Myocardial Infarction. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Groenland FTW, Mahmoud KD, Neleman T, Ziedses des Plantes AC, Scoccia A, Ligthart J, Witberg KT, Nuis RJ, den Dekker WK, Wilschut JM, Diletti R, Zijlstra F, Kardys I, Cummins P, Van Mieghem NM, Daemen J. Tissue characterisation and primary percutaneous coronary intervention guidance using intravascular ultrasound: rationale and design of the SPECTRUM study. Open Heart. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Kim Y, Bae S, Johnson TW, Son NH, Sim DS, Hong YJ, Kim SW, Cho DK, Kim JS, Kim BK, Choi D, Hong MK, Jang Y, Jeong MH; KAMIR‐NIH (Korea Acute Myocardial Infarction Registry‐National Institutes of Health) Investigators [Link]. Role of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention in Optimizing Outcomes in Acute Myocardial Infarction. J Am Heart Assoc. 2022;11:e023481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Ya'qoub L, Gad M, Saad AM, Elgendy IY, Mahmoud AN. National trends of utilization and readmission rates with intravascular ultrasound use for ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2021;98:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Li X, Ge Z, Kan J, Anjum M, Xie P, Chen X, Khan HS, Guo X, Saghir T, Chen J, Gill BUA, Guo N, Sheiban I, Raza A, Wei Y, Chen F, Mintz GS, Zhang JJ, Stone GW, Chen SL; IVUS-ACS Investigators. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet. 2024;403:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 13. | Groenland FTW, Neleman T, Kakar H, Scoccia A, Ziedses des Plantes AC, Clephas PRD, Chatterjee S, Zhu M, den Dekker WK, Diletti R, Zijlstra F, Mahmoud KD, Van Mieghem NM, Daemen J. Intravascular ultrasound-guided versus coronary angiography-guided percutaneous coronary intervention in patients with acute myocardial infarction: A systematic review and meta-analysis. Int J Cardiol. 2022;353:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Karagiannidis E, Papazoglou AS, Sofidis G, Chatzinikolaou E, Keklikoglou K, Panteris E, Kartas A, Stalikas N, Zegkos T, Girtovitis F, Moysidis DV, Stefanopoulos L, Koupidis K, Hadjimiltiades S, Giannakoulas G, Arvanitidis C, Michaelson JS, Karvounis H, Sianos G. Micro-CT-Based Quantification of Extracted Thrombus Burden Characteristics and Association With Angiographic Outcomes in Patients With ST-Elevation Myocardial Infarction: The QUEST-STEMI Study. Front Cardiovasc Med. 2021;8:646064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, Doré CJ, Parulekar WR, Summerskill WS, Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 4989] [Article Influence: 383.8] [Reference Citation Analysis (0)] |
| 16. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 20192] [Article Influence: 1553.2] [Reference Citation Analysis (9)] |
| 17. | Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan GA, Dweck MR, Galbraith M, Gilard M, Hinterbuchner L, Jankowska EA, Jüni P, Kimura T, Kunadian V, Leosdottir M, Lorusso R, Pedretti RFE, Rigopoulos AG, Rubini Gimenez M, Thiele H, Vranckx P, Wassmann S, Wenger NK, Ibanez B; ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 2800] [Article Influence: 933.3] [Reference Citation Analysis (0)] |
| 18. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 4795] [Article Influence: 799.2] [Reference Citation Analysis (0)] |
| 19. | Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Kåregren A, Nilsson J, Robertson L, Sandhall L, Sjögren I, Ostlund O, Harnek J, James SK; TASTE Trial. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 848] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 20. | Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B-14B. [PubMed] |
| 21. | Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009;9:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 745] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 22. | Papazoglou AS, Karagiannidis E, Moysidis DV, Sofidis G, Bompoti A, Stalikas N, Panteris E, Arvanitidis C, Herrmann MD, Michaelson JS, Sianos G. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hellenic J Cardiol. 2021;62:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW; Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 620] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 24. | Choi IJ, Lim S, Choo EH, Hwang BH, Kim CJ, Park MW, Lee JM, Park CS, Kim HY, Yoo KD, Jeon DS, Youn HJ, Chung WS, Kim MC, Jeong MH, Ahn Y, Chang K. Impact of Intravascular Ultrasound on Long-Term Clinical Outcomes in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv. 2021;14:2431-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Groenland FTW, Ziedses des Plantes AC, Neleman T, Scoccia A, Ligthart JMR, Witberg KT, Mahmoud KD, Nuis RJ, den Dekker WK, Wilschut JM, Diletti R, Zijlstra F, Van Mieghem NM, Daemen J. Culprit lesion plaque characterization and thrombus grading by high-definition intravascular ultrasound in patients with ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv. 2023;102:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/