Published online Apr 26, 2025. doi: 10.4330/wjc.v17.i4.104717
Revised: March 7, 2025
Accepted: April 1, 2025
Published online: April 26, 2025
Processing time: 112 Days and 20.2 Hours
Heart failure (HF) is a growing public health concern, with an increasing inci
Core Tip: Recent research hints at an alarming rise in heart failure cases among younger populations. The same is believed to stem from a complex interaction between various risk factors, including metabolic syndrome, environmental pollutants, unfavorable genetics, and lifestyle behaviors including substance abuse. This review examines these evolving determinants, discussing each in detail and exploring the key therapeutic strategies to disrupt this rapidly rising public health problem.
- Citation: Parizad R, Batta A, Hatwal J, Taban-sadeghi M, Mohan B. Emerging risk factors for heart failure in younger populations: A growing public health concern. World J Cardiol 2025; 17(4): 104717
- URL: https://www.wjgnet.com/1949-8462/full/v17/i4/104717.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i4.104717
Heart failure (HF) is one of the most significant causes of morbidity and mortality worldwide, and it predominantly affects the older people. Recent studies have revealed an alarming rise in HF cases among younger populations[1]. The prevalence and causes of HF vary significantly between developed and developing countries. Approximately 1%-3% of all HF cases occur in individuals under 40 years of age. Notably, between 1999 and 2019, hospitalizations due to HF among young people aged 18-44 years increased by 23% in the United States[2].
In developed countries, the rising prevalence of HF among younger populations is associated with lifestyle factors, such as increasing rates of obesity, hypertension (HTN), and diabetes. Despite improvements in medical care and management of cardiovascular diseases (CVD), the burden of HF continues to rise, especially among younger adults. Hospital admissions for HF in youth have markedly increased in the last two decades, highlighting the urgent need for early detection and intervention[2,3].
HF is a growing public health concern in developing countries, with prevalence rates increasing due to demographic changes, urbanization, and the rising burden of non-communicable diseases (NCDs)[4,5]. In many developing countries, HF affects younger populations compared to developed countries, often due to untreated or poorly managed risk factors such as HTN, diabetes, and rheumatic heart disease (RHD)[6].
In Sub-Saharan Africa, the prevalence of HF is estimated to be between 1%-2%, with RHD and hypertensive heart disease being the primary causes. Patients in this region tend to be younger and often present with more advanced stages of the disease compared to those in developed countries[7]. Similarly, in South Asian countries such as India and Pakistan, HF is predominantly caused by ischemic heart disease, HTN, and diabetes. Patients in these regions frequently experience a higher prevalence of comorbid conditions and worse health outcomes compared to Western populations[8]. Infectious diseases, such as Chagas disease, human immunodeficiency virus (HIV), and tuberculosis contribute significantly to HF in developing countries. Chagas disease, caused by the parasite Trypanosoma cruzi, is endemic in Latin America and leads to chronic cardiomyopathy and HF[9]. HIV-associated cardiomyopathy is another important cause of HF in sub-Saharan Africa, where HIV prevalence is high[10].
Many developing countries face significant challenges in providing timely and effective healthcare for HF patients. Limited access to diagnostic tools, essential medications, and specialized care contributes to poor outcomes[11].
Despite the rapid identification of new associations and risk factors, the burden of HF among younger individuals continues to rise. This trend underscores the urgent need for further investigation in this area. The increasing prevalence of HF in younger adults is particularly concerning due to its severe complications and long-term consequences, including frequent hospitalizations, reduced quality of life, and elevated healthcare costs[12].
Most epidemiological studies on HF have focused on its increased prevalence in recent decades, often without distinguishing between older and younger people. Most of these studies have involved cohorts over 40 years of age, resulting in a limited understanding of HF in younger age groups.
The primary objective of this review is to explore the interplay of various risk factors, including emerging ones associated with the rising incidence of HF in younger generations focusing on identifying key contributors such as behavioral, environmental, genetic, and socio-economic influences. Additionally, it emphasizes the importance of age-specific screening, early diagnosis, and individualized treatment strategies tailored to the unique characteristics of HF in youth. Furthermore, it aims to raise awareness among healthcare professionals and the broader community about the growing incidence of HF in younger populations. Ultimately, this work seeks to inform strategies for reducing the burden of HF through targeted prevention, early intervention, and comprehensive management approaches.
A systematic approach to literature selection was implemented to enhance the reproducibility and reliability of this review. The following steps were undertaken.
The primary databases consulted were PubMed, Scopus, and Web of Science, covering studies published between 2000 and 2024.
A combination of keywords and Medical Subject Headings (MeSH) terms was used, including "heart failure," "younger populations," "metabolic syndrome," "substance abuse," and "COVID-19 cardiovascular effects". Boolean operators (AND, OR) were utilized to refine the search strategy.
Studies were included if they met the following criteria: (1) Focused on individuals under 40 years old; (2) Examined risk factors for HF; and (3) Provided original data or systematic reviews relevant to the topic.
Non-English articles, conference abstracts, and studies lacking a clear methodology were systematically excluded.
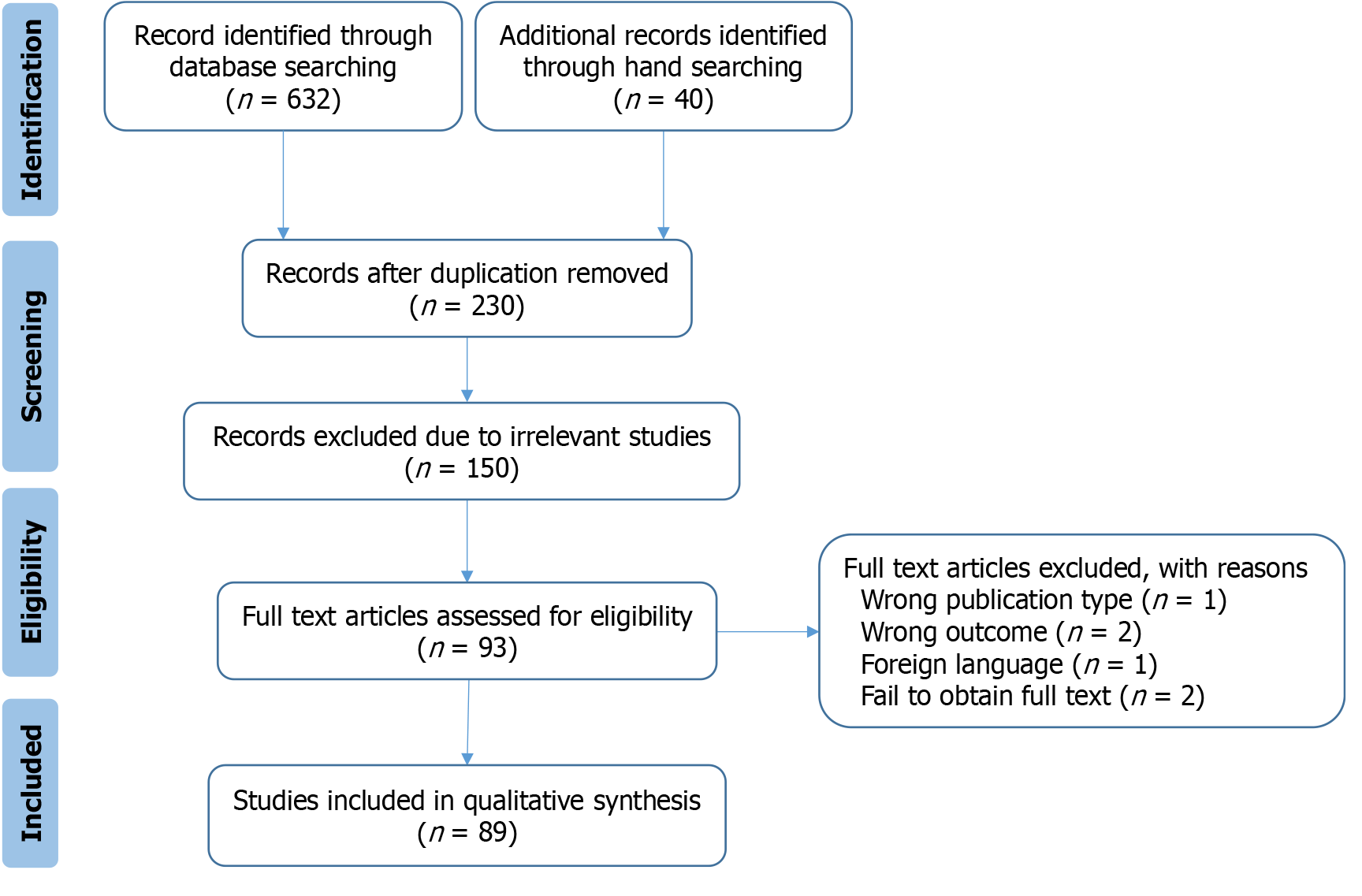
The initial search yielded 632 articles. After screening for relevance and applying inclusion and exclusion criteria, 89 studies were included in this review. A PRISMA flow diagram summarizing the selection process is provided in Figure 1.
The increasing prevalence of metabolic syndrome (MetS) and obesity is a significant factor contributing to HF among younger individuals. Obesity leads to structural and functional alterations in the heart, including left ventricular hypertrophy (LVH) and diastolic dysfunction, both of which are early indicators of HF with preserved ejection fraction (HFpEF)[13].
MetS, influenced by excessive consumption of high-calorie foods, physical inactivity, HTN, hyperglycemia, dyslipidemia, and central obesity, is being diagnosed more frequently in adolescents and young adults, particularly in high-income countries[14]. This growing trend substantially raises the likelihood of HF in this demographic. Additionally, MetS is strongly associated with an elevated risk of type 2 diabetes (T2D), CVD, non-alcoholic fatty liver disease, chronic kidney disease, and various cancers[15]. These comorbidities not only contribute to HFpEF but also play a critical role in the development of coronary artery disease (CAD), a key precursor to HF with reduced ejection fraction (HFrEF).
Recent studies have highlighted a concerning rise in T2D prevalence among younger populations, which significantly contributes to the development of HF. This increase is attributed to factors such as childhood obesity, unhealthy dietary habits, and sedentary lifestyles[16-18]. For instance, data from the Global Burden of Disease Study indicate that the incidence of T2D in adolescents and young adults has risen by approximately 30% over the past decade, particularly in high-income countries where MetS is more[7]. Early-onset T2D is strongly linked to accelerated cardiovascular complications, including LVH and diastolic dysfunction, both of which are precursors to HF[19]. These findings emphasize the urgent need for targeted interventions aimed at reducing obesity and HTN, and addressing the rising burden of diabetes in younger age groups.
A sedentary lifestyle and unhealthy eating habits among young individuals are growing public health concerns. Physical inactivity and reduced functional capacity are strongly associated with adverse cardiovascular outcomes, independent of other risk factors[20].
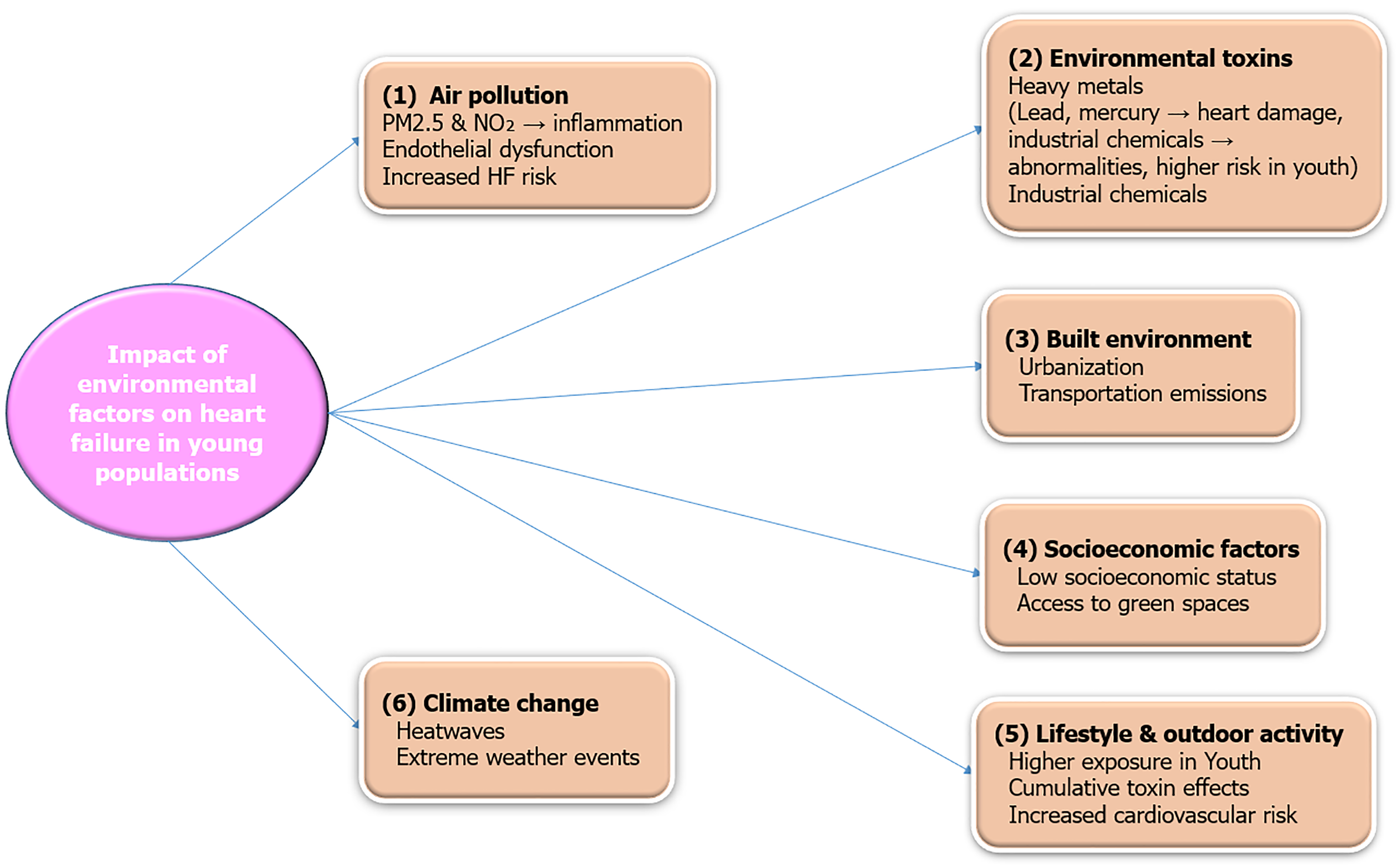
Environmental factors are often overlooked in clinical practice but, are increasingly recognized as an important risk factor for various CVDs. Air pollution and exposure to environmental toxins are emerging as critical contributors to HF, especially among young people, given their increased exposure. Oxides of nitrogen (NO2) and particulate matter smaller (PM2.5) than 2.5 microns are particularly concerning as they can easily reach the lungs and bloodstream. Long-term exposure to these pollutants causes inflammation and oxidative stress, affects endothelial function, and ultimately is closely linked to HF pathophysiology[21,22]. Young individuals living in polluted environments are at a higher risk of developing early-onset HTN and MetS, both of which increase their likelihood of HF in later years. For instance, one study revealed that young age groups exposed to higher PM2.5 concentrations had elevated biomarkers associated with various CVDs, including C-reactive protein, one of the most clinically relevant biomarkers linked to CVD and HF[23,24].
Built environment: Urban planning and access to green spaces are increasingly recognized as critical components of cardiovascular health. Studies have shown that individuals living in areas with limited recreational spaces and high pollution levels are at a higher risk of developing HF[25]. The lack of walkable neighborhoods and exposure to urban heat islands further exacerbate cardiovascular risks by promoting sedentary lifestyles and increasing thermal stress[2]. Additionally, residential proximity to major roadways and industrial zones has been associated with elevated levels of air pollutants, contributing to systemic inflammation and endothelial dysfunction, key precursors to HF[3].
Socioeconomic factors: Socioeconomic disparities significantly influence the prevalence of HF in younger populations. Low socioeconomic status is associated with limited access to healthcare, poor nutrition, and higher exposure to environmental pollutants[4]. These factors contribute to the development of MetS, obesity, and other precursors of HF. Financial stress and job insecurity can lead to chronic psychosocial stress, which is an independent risk factor for HF[5]. Furthermore, individuals from lower socioeconomic backgrounds often face barriers to early diagnosis and treatment, resulting in delayed interventions and worse outcomes[6].
Climate changes: Climate change has emerged as a significant contributor to cardiovascular morbidity, particularly among younger individuals who are more exposed to extreme weather conditions. Heatwaves increase the risk of dehydration, electrolyte imbalances, and acute cardiovascular events, while cold spells elevate blood pressure and myocardial oxygen demand[7]. Climate-induced displacement and migration also often result in overcrowded living conditions, worsening exposure to air pollution and infectious diseases predisposing individuals to HF[8]. Rising global temperatures have also been linked to increased hospitalizations for HF, particularly in vulnerable populations such as those with pre-existing cardiovascular conditions[9].
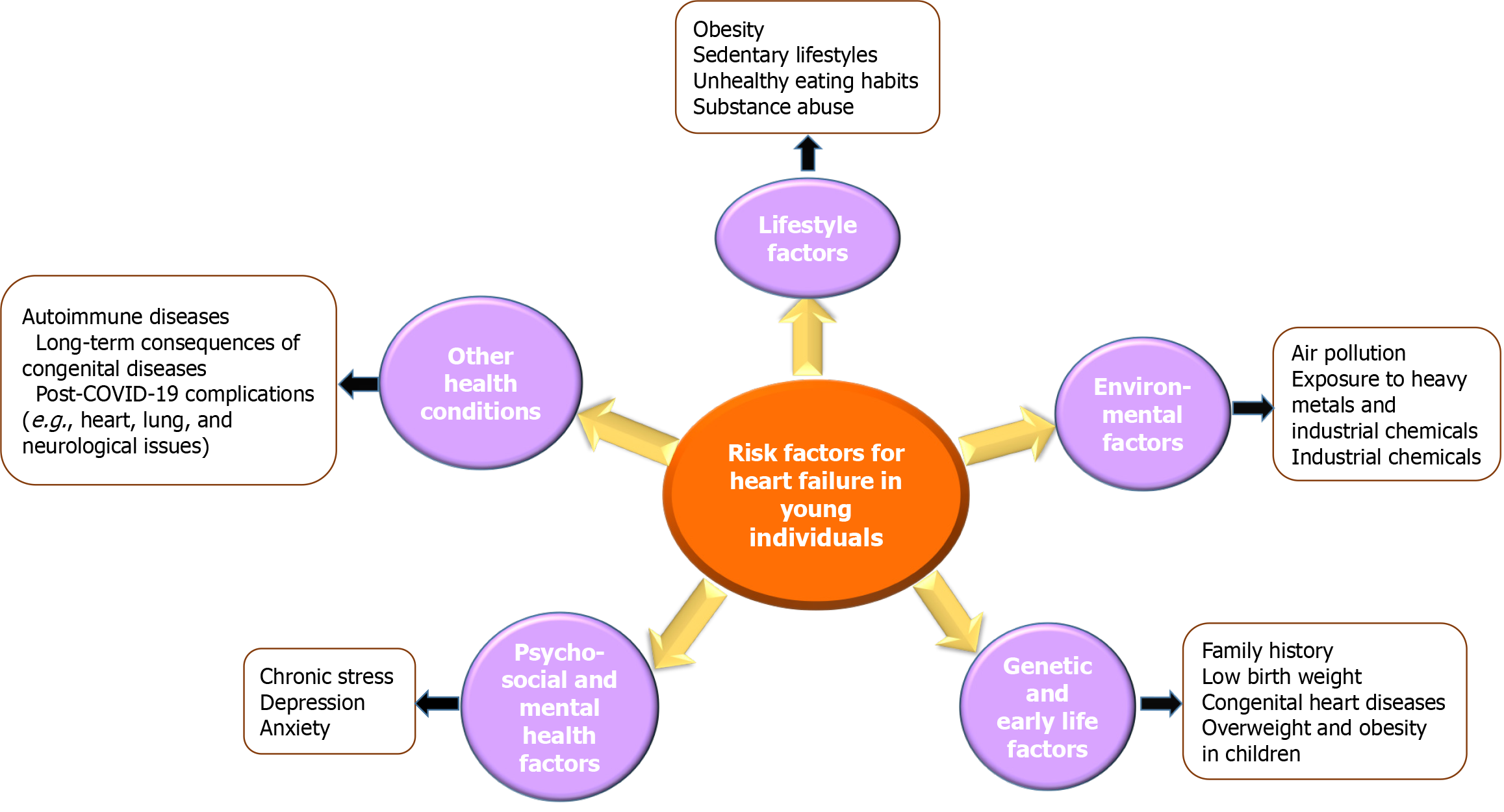
HF is becoming increasingly prevalent among younger populations, with environmental factors playing a significant role in its development. Prolonged exposure to air pollutants, particularly fine PM2.5 and NO2, has been linked to systemic inflammation, oxidative stress, and endothelial dysfunction, all of which contribute to CVD and HF. Moreover, exposure to heavy metals such as lead and mercury has been associated with structural changes in the myocardium, increasing the risk of early-onset HF. These environmental risk factors emphasize the need for preventive strategies and public health interventions to reduce exposure and mitigate their long-term impact (Figure 2).
Low birth weight (LBW) is increasingly recognized as a critical early-life risk factor for CVDs, including HF, particularly among younger populations. LBW is often associated with adverse perinatal circumstances such as maternal undernutrition, stress, and inadequate prenatal care, all of which can impair the growth and development of cardiovascular tissues. These factors contribute to structural and functional abnormalities in the heart, predisposing individuals to conditions like HTN and LVH, both of which are significant precursors to HF[26].
Furthermore, exposure to environmental pollutants during pregnancy has also been shown to exacerbate these effects, underscoring the multifactorial origins of LBW-related cardiovascular risks[27].
Recent studies have highlighted the significant impact of maternal nutritional deficiencies during pregnancy on fetal cardiac development, leading to long-term cardiovascular risks, including HF in adulthood. Maternal nutrition plays a crucial role in determining the risk of NCDs in offspring, such as heart disease, T2Ds, cancer, and chronic obstructive pulmonary diseases[28,29].
Research indicates that exposure to air pollution during pregnancy is associated with an increased risk of LBW, which in turn elevates the risk of CVD in adulthood. A study published in Environmental Health Perspectives found that exposure to air pollution, even at low levels, may increase the risk of LBW, particularly for certain segments of the population[30].
Childhood obesity is strongly associated with early markers of cardiovascular risk, including pre-HTN, pre-insulin resistance, and dyslipidemia, which contribute to adverse cardiac remodeling such as LVH and diastolic dysfunction[16].
Congenital heart diseases (CHDs) represent another major contributor to HF risk among young individuals.
Technological advancements in neonatal cardiac surgery have significantly improved survival rates for children born with CHD, enabling many to reach adulthood[26]. However, these individuals remain at an elevated risk of developing HF later in life due to a combination of factors, including residual anatomical defects, chronic morphological changes, and long-term complications associated with their condition[31]. For instance, even after successful surgical repair, patients often experience distorted cardiac geometry, conduction abnormalities, and prolonged QRS durations, all of which increase susceptibility to arrhythmias and HF[32,33]. Scarring of heart tissue following repeated surgical interventions can predispose individuals to both atrial and ventricular arrhythmias (VTs), further exacerbating their cardiovascular risk. Research indicates that myocardial scarring, resulting from surgical procedures, can lead to arrhythmogenic cardiomyopathy, characterized by the loss of ventricular myocardium and subsequent fibrous or fibro-fatty scar tissue replacement. This scarring predisposes individuals to potentially lethal VTs and impairs systolic ventricular function[34].
Moreover, specific types of CHDs, such as single ventricle physiology, present unique challenges. Despite advances in surgical techniques, these partially treatable defects often result in chronic hemodynamic stress, leading to progressive ventricular dysfunction and eventual HF[35]. Longitudinal studies have demonstrated that young adults with repaired tetralogy of fallot (TOF) or transposition of the great arteries (TGA) are particularly susceptible to adverse outcomes, including right ventricular (RV) failure and systemic complications. For instance, a longitudinal study by Kochav et al[36] evaluated changes in systemic right ventricular remodeling in adult patients with transposition of the great vessels using cardiovascular magnetic resonance imaging. The study highlighted that the systemic right ventricle in these patients is prone to structural and functional alterations, which may lead to significant long-term complications. Beyond the physiological burden, psychosocial factors also play a critical role. The fear of repeated medical interventions and the psychological impact of transitioning from pediatric to adult care can contribute to chronic stress, which has been linked to worsening cardiovascular health[37]. This underscores the need for comprehensive, multidisciplinary care that these patients' physical and emotional well-being[38].
Technological advancements in neonatal surgery have enabled more youngsters with CHD to survive into adulthood. However, this progress comes with increased risks of residual cardiac abnormalities and complications, such as arrhythmias, valvular dysfunction, and progressive ventricular failure[39]. According to Liu et al[26], patients with repaired CHDs, such as TOF or TGA, remain at elevated risk for developing HF due to chronic hemodynamic stress and myocardial scarring. Moreover, Fontan procedure survivors face unique challenges, including circulatory inefficiency and liver disease, further predisposing them to HF. The interplay between genetic predispositions and environmental factors, such as air pollution and socioeconomic disparities, also amplifies HF risks in this population[40].
Substance abuse has been involved in causing HF in young adults in recent years. Various illicit substances and excessive use of legal substances have been demonstrated to have cardiotoxic effects and can contribute to HF. For instance, cocaine and amphetamines have been associated with cardiomyopathy and sudden cardiac death (SCD). Cannabis use has been associated with cardiovascular risks, including tachycardia, HTN, and myocardial infarction (MI). A systematic review by Arenas et al[41] found that cocaine use significantly increases the risk of HF and SCD.
The purposes for which cocaine is used may be associated with vasoconstriction of the coronary arteries, myocardial ischemia, and arrhythmias, while its direct cardiotoxicity can result in permanent heart damage. Additionally, the cardiovascular risks of cocaine may be dose-dependent and influenced by co-existing factors such as polysubstance use or pre-existing CVD. For example, a study by Gagnon et al[42] found that occasional cocaine users had a lower risk of HF compared to chronic users.
Additionally, the role of cocaine in causing HF may be overstated in some populations, as other substances (e.g., alcohol, opioids) often co-occur and contribute to cardiovascular damage[43].
While cocaine is a well-established risk factor for CVD, the extent of its contribution to HF may vary depending on usage patterns and co-existing risk factors.
A study by Patel et al[44] found that cannabis use was associated with a 2–3 times higher risk of MI in young adults. Synthetic cannabinoids, in particular, have been linked to severe cardiovascular complications, including HF[45]. Nevertheless, other studies suggest that the cardiovascular risks of cannabis may be overstated. A systematic review by Sebastian et al[46] found limited evidence linking cannabis use to HF, with most studies being observational and prone to confounding. Some researchers argue that the cardiovascular effects of cannabis may depend on the method of consumption (e.g., smoking vs edibles) and the presence of co-existing risk factors[47,48].
In contrast, the chronic use of substances containing amphetamine is associated with sympathetic stimulation, leading to hypertensive cardiomyopathy, arrhythmias, and hypertrophy of the heart, all of which render the patient a candidate for developing HF[49].
Except for stimulants, opioids have gained significant attention in the last ten years due to their association with the opioid epidemic and have also been related to cardiovascular complications. Chronic opioid use results in bradycardia, hypotension, and myocardial injury. In addition, opioid-induced hypoxia may trigger ischemia, thereby predisposing individuals to myocardial injury and, over time, eventually leading to HF[50]. Additionally, long-term alcohol consumption and smoking are well-established risk factors for dilated cardiomyopathy (DCM)[51]. The rising use of alcohol and drugs among young people further exacerbates this risk[52].
Other abused drugs, such as amphetamines and synthetic cannabinoids, also carry a risk of severe cardiac complications[53]. Methamphetamine exposure induces oxidative stress, mitochondrial damage, and endothelial dysfunction, all of which predispose individuals to the development of DCM. Synthetic cannabinoids have less literature available, but associations have been found with tachyarrhythmia, MI, and even HF in selected cases[54,55]. While alcohol and tobacco are legal, these represent essential yet already-established risk factors in the causation of DCM[56]. Chronic alcohol consumption leads to a long-term decline in myocardial contractility, eventually resulting in a form of HF known as alcoholic cardiomyopathy. Chronic smoking accelerates atherosclerosis and HTN and, in combination with increased oxidative stress, contributes to the risk of HF in smokers[57].
The adverse effects of substance abuse are exacerbated by the increasing incidence of polysubstance use, particularly the combination of drugs with psychoactive properties. For instance, the concurrent use of alcohol and cocaine significantly impacts the cardiovascular system, while opioid and benzodiazepine co-ingestion frequently occurs in overdose cases.
Substance abuse has emerged as a significant contributor to HF among young populations, with distinct patterns of use and cardiotoxic effects observed in this demographic. Cocaine, for instance, is particularly prevalent among young adults and has been strongly associated with cardiomyopathy, arrhythmias, and SCD. Recent evidence highlights that chronic cocaine use in younger individuals leads to oxidative stress, endothelial dysfunction, and myocardial remodeling, all of which predispose them to HF at an earlier age compared to non-users[42]. Notably, the dose-dependent nature of cocaine's cardiotoxicity is exacerbated in young populations due to higher rates of binge use and polysubstance combinations, such as concurrent alcohol consumption, which further amplifies cardiovascular risks[43].
In addition to cocaine, synthetic cannabinoids have gained attention as a growing threat to cardiovascular health in young adults. These substances are linked to severe complications, including tachyarrhythmias, MI, and acute HF, even in otherwise healthy individuals[45]. A study demonstrated that young users of synthetic cannabinoids exhibited elevated biomarkers of cardiac injury, such as troponin levels, underscoring their potential to cause irreversible myocardial damage[44]. Furthermore, the psychosocial stressors often accompanying substance abuse, such as academic pressure or socioeconomic instability, disproportionately affect younger populations and exacerbate the progression to HF. Addressing these risks through targeted public health interventions and educational programs is critical to mitigating the rising burden of substance-induced HF in this vulnerable group[58]. This combination suppresses respiratory and cardiovascular function, potentially resulting in SCD or HF[59].
The impact of various substances of abuse on cardiovascular health is illustrated in Table 1.
| Substance | Mechanism of action | Cardiovascular effects | Association with HF | Ref. |
| Cocaine | Blocks dopamine, norepinephrine, and serotonin reuptake | Vasoconstriction, tachycardia, arrhythmias, and hypotension | Associated with cardiomyopathy, MI, arrhythmias, and increased HF risk | [30,46] |
| Amphetamines | Stimulates release of norepinephrine and dopamine | Tachycardia, hypertension, cardiomyopathy | Linked to hypertensive heart disease and increased HF risk | [47,48] |
| Alcohol | CNS depressant altering cardiac function | Chronic use leads to alcoholic cardiomyopathy, hypertension, arrhythmias | High association with dilated cardiomyopathy leading to HF | [30,49] |
| Opioids | Binds to opioid receptors, decreases pain, induces euphoria | Bradycardia, hypotension, respiratory depression | Chronic use may lead to myocardial injury and increase HF risk | [48,50] |
| Nicotine | Increases heart rate and blood pressure | Endothelial dysfunction, tachyarrhythmias, hypertension | Increases HF risk through oxidative stress and inflammation | [49,51] |
| Marijuana | Activates cannabinoid receptors | Tachycardia, hypotension, changes in vascular tone | Limited evidence; potential role in MI and arrhythmias | [50,52] |
| Methamphetamine | Elevates dopamine release | Cardiovascular strain, hypertension | High risk of cardiomyopathy and HF due to structural remodeling | [47,52] |
| Synthetic cannabinoids | Potent synthetic THC, stronger than natural cannabinoids | Tachyarrhythmias, MI, blood pressure changes | Severe cardiovascular complications including HF | [50,52] |
| Ecstasy (MDMA) | Increases serotonin, norepinephrine, and dopamine levels | Hypertension, tachycardia, hyperthermia | Acute HF risks during dehydration and hyperthermia episodes | [51,52] |
| Benzodiazepines | CNS inhibition causing sedation | Hypotension, respiratory depression | Limited association with HF; can exacerbate existing conditions | [48,51] |
The rising prevalence of substance abuse among young individuals highlights the urgent need for targeted public health interventions and educational programs to mitigate these risks.
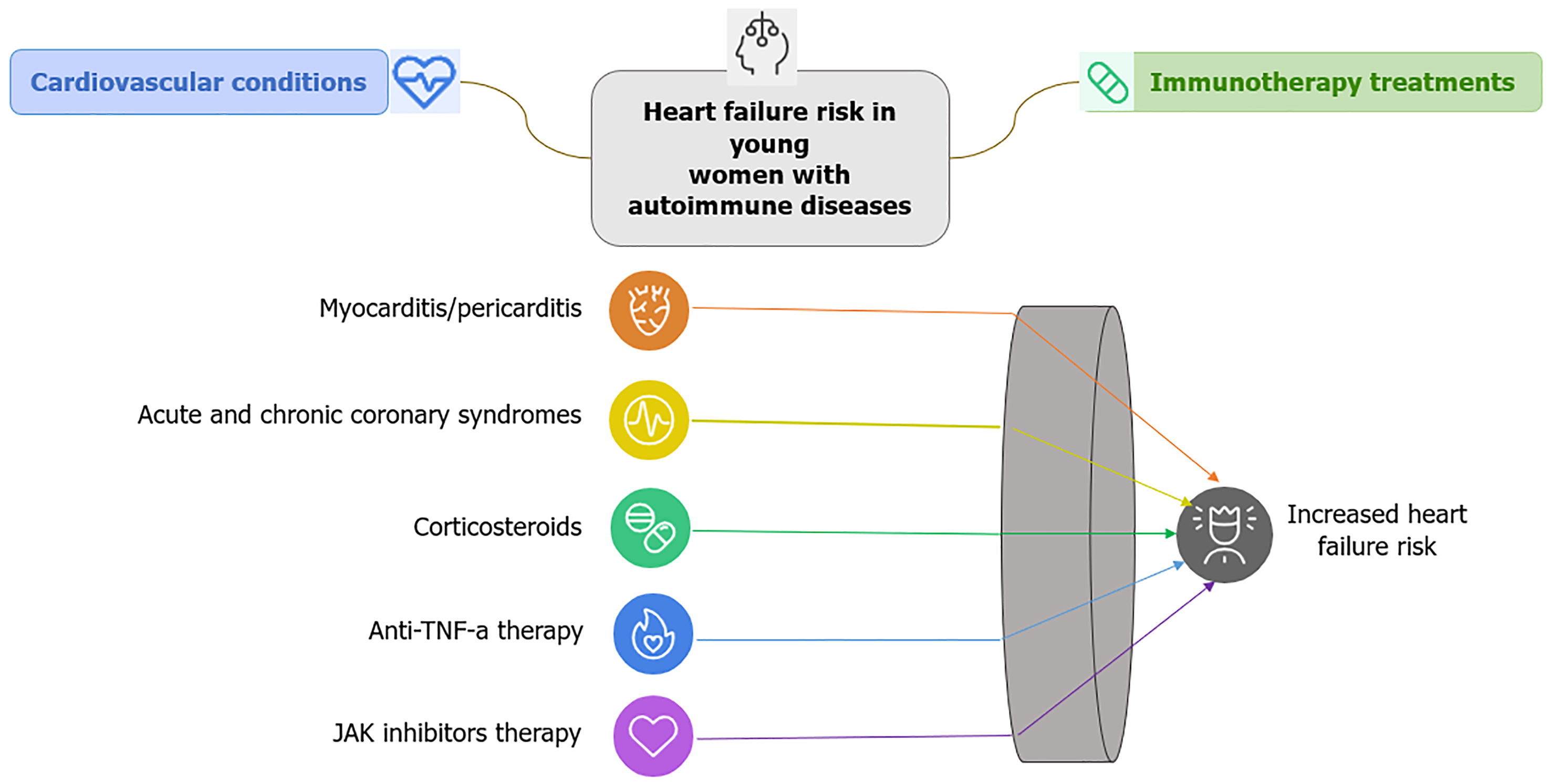
Autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), disproportionately affect young women and are significant contributors to HF risk. These conditions can lead to myocarditis and pericarditis, inflammatory diseases of the heart that heighten the risk of HF[60,61]. Additionally, autoimmune disorders are strongly linked to CAD and acute coronary syndrome (ACS), which are associated with a higher risk of complications and worse prognosis compared to the general population experiencing the same episode. Individuals with autoimmune diseases are more likely to have a reduced ejection fraction and a greater propensity to develop HF following an ACS[62]. Furthermore, the use of corticosteroids for treating autoimmune diseases has deleterious cardiovascular effects, further increasing this risk[61].
The primary treatments for autoimmune diseases include immunotherapy medications such as corticosteroids, tumor necrosis factor-alpha (TNF-α) inhibitors, and Janus kinase (JAK) inhibitors, all of which have serious cardiovascular side effects. The adverse cardiovascular effects of corticosteroids include alterations in lipid profiles, dyslipidemia, HTN, and hyperglycemia, all of which are recognized risk factors for CVD[63]. Prolonged corticosteroid therapy leads to arterial wall stiffening and atherosclerosis, consequently increasing the risk of MI and stroke[63].
Anti-TNF-α therapies, commonly used to manage autoimmune diseases such as RA and psoriasis, have been shown to influence patients' lipid profiles. A meta-analysis study (2023) indicated that these treatments might temporarily elevate high-density lipoprotein levels in psoriasis patients, with significant increases observed within the first three months of therapy. However, changes in triglyceride (TG) levels varied over time, showing significant increases between three to six months of treatment. These findings underscore the importance of regular lipid monitoring in patients undergoing anti-TNF-α therapy[64]. Another study highlighted that while anti-TNF-α treatments were associated with significant increases in total cholesterol and TG levels, the addition of statins helped reduce low-density lipoprotein levels. This suggests that combining statin therapy with anti-TNF-α treatments may mitigate potential atherogenic risks associated with lipid profile alterations[65]. While anti-TNF-α therapies effectively reduce inflammation in autoimmune conditions, their impact on lipid profiles necessitates careful management to address potential cardiovascular risks.
However, some studies suggest that these drugs may confer endothelial protection[66]. JAK inhibitors, the most recent class of immunomodulatory drugs, specifically target inflammatory pathways. Nevertheless, they have been associated with an increased risk of adverse cardiovascular events, including thromboembolic complications such as deep vein thrombosis and pulmonary embolism. Additionally, specific JAK inhibitors may exacerbate conventional CVD risk factors, such as HTN and dyslipidemia[67,68].
The cardiovascular side effects of these immunotherapies underscore the importance of cardiovascular screening to effectively manage CVD risk factors in patients undergoing long-term immunosuppressive treatment[69,70].
The complex interplay between autoimmune diseases, immunotherapy treatments, and cardiovascular complications is shown in Figure 3, which highlights the increased risk of HF in young women due to conditions such as myocarditis, pericarditis, and CAD, as well as the adverse effects of therapies like corticosteroids, anti-TNF-α inhibitors, and JAK inhibitors.
Genetics plays a crucial role in the prevalence of HF among younger individuals. Familial cardiomyopathies, such as hypertrophic cardiomyopathy (HCM) and arrhythmogenic RV dysplasia (ARVD), significantly contribute to the early onset of HF in genetically predisposed individuals[71]. Advances in genetic testing have facilitated the identification of at-risk individuals; however, its clinical application in younger populations remains limited[26].
Historically, genetics was regarded primarily as a predisposing factor for HF in young groups age. Conditions such as HCM and ARVD exemplify familial cardiomyopathies that lead to early-onset HF in patients with specific gene mutations, reinforcing the role of genetic factors[72]. Recent epidemiological evidence suggests that the incidence of genetic cardiomyopathies, particularly HCM, is higher than previously estimated, affecting approximately 0.2% of the global population. These conditions tend to present with more severe symptoms in younger individuals due to structural and functional cardiac abnormalities, increasing the risk of SCD and early HF onset compared to other HF types[73].
Recent studies have shown that mutations in the MY, MYBPC3, PKP2, DSP, TTN, and LMNA play a significant role in causing structural and functional myocardial abnormalities, which increase the risk of early-onset HF and SCD. HCM is one of the most common genetic causes of HF, especially in younger individuals. It is primarily caused by gene mutations that encode sarcomeric proteins, such as MYH7 and MYBPC3[74,75].
These mutations lead to abnormal thickening of the left ventricular (LV) wall, diastolic dysfunction, and an increased risk of arrhythmias and SCD[76].
Mutations in the MYBPC3 gene are also common, accounting for 20%-30% of HCM cases. These mutations typically result in a later onset of symptoms but still carry a significant risk of HF and arrhythmias[73].
Genetic testing for MYH7 and MYBPC3 mutations is recommended for individuals with a family history of HCM or unexplained cardiac hypertrophy. Early identification of these mutations allows targeted interventions, such as implantable cardioverter-defibrillators (ICDs), to prevent SCD[77].
ARVD is a genetic cardiomyopathy characterized by fibrofatty replacement of the RV myocardium, leading to arrhythmias and HF. It is commonly caused by mutations in desmosomal genes, such as PKP2 and DSP, which are crucial for maintaining the structural integrity of cardiac tissue. These mutations disrupt the normal functioning of desmosomes, resulting in structural instability and increased susceptibility to arrhythmias and progressive HF[78].
PKP2 mutations are the most common cause of ARVD, accounting for approximately 40%-50% of cases. These mutations disrupt cell-to-cell adhesion, resulting in myocardial damage and arrhythmias[79].
DSP mutations, while less common, are associated with a more severe phenotype, including biventricular involvement and a higher risk of HF[80].
Genetic testing for PKP2 and DSP mutations is recommended for individuals with a family history of ARVD or unexplained arrhythmias. Early diagnosis allows for the initiation of antiarrhythmic therapy and ICD implantation to prevent SCD[81].
DCM is characterized by LV dilation and systolic dysfunction, often leading to HF. It can be caused by mutations in genes encoding cytoskeletal, sarcomeric, and nuclear envelope proteins, such as TTN and LMNA. Mutations in the TTN gene, most notably truncating variants, are the most common genetic cause of DCM, accounting for approximately 20%-25% of cases. These mutations disrupt the structural integrity of the sarcomere, leading to progressive ventricular dilation and HF[82].
Mutations in the LMNA gene are associated with a more severe phenotype, including early-onset HF, conduction system disease, and a high risk of SCD[83].
Genetic testing for TTN and LMNA mutations is recommended for individuals with a family history of DCM or unexplained HF. Early identification of these mutations allows for the initiation of guidelines, directed medical therapy and consideration of ICD implantation[84].
Other genetic cardiomyopathies, such as LV non-compaction (LVNC) and restrictive cardiomyopathy (RCM), are also associated with an increased risk of HF. These conditions are often caused by mutations in genes such as MYH7, TNNT2, and TNNI3[84].
TNNT2 and TNNI3 mutations: Mutations in TNNT2 and TNNI3 are associated with RCM and LVNC, leading to diastolic dysfunction and HF. Although rare, these mutations carry a high risk of adverse outcomes[85].
Genetic testing for TNNT2 and TNNI3 mutations should be considered in individuals with unexplained HF or a family history of cardiomyopathy. Early diagnosis allows for tailored management strategies, including heart transplantation in severe cases[86].
Advancements in genetic testing methods have significantly improved over time. Next-generation sequencing, for instance, has facilitated the identification of pathogenic mutations underlying HCM, arrhythmogenic ARVD, and other inherited cardiomyopathies, thereby enabling earlier interventions[87].
However, the use of genetic testing in these patient populations remains limited due to factors such as cost, accessibility, and challenges with insurance reimbursement. Furthermore, interpreting genomic variants is complex, as many mutations exhibit low penetrance, meaning not all carriers will develop HF. These challenges hinder effective patient education and clinical management[88].
The increasing prevalence of genetic cardiomyopathies has been accompanied by advancements in diagnostic techniques, including cardiac magnetic resonance imaging (MRI) and genetic testing. These methods have enhanced understanding of disease progression, clinical heterogeneity, and genetic-morphological correlations[73,89]. Conse
Recent advancements in pediatric cardiology and surgical interventions have significantly improved survival rates for children born with CHD, enabling many affected individuals to reach adulthood[90]. However, these patients remain at lifelong risk of developing HF due to residual cardiac abnormalities and the long-term consequences of their initial corrective surgeries.
Specific forms of CHD are mainly associated with a high risk of HF. For instance, patients with TOF, even after surgical repair, may experience complications such as pulmonary regurgitation and subsequent RV dilatation, which can eventually lead to HF in adulthood[91,92]. Likewise, systemic RV failure is frequently observed in patients with TGA who have undergone Mustard or Senning procedures. In such cases, the RV is subjected unphysiologically designed systemic pressures[93].
Unrepaired lesions also pose significant risks for HF. Large ventricular septal defects (VSDs) and uncorrected single ventricle physiology are particularly notable. Patients with unoperated VSDs often develop pulmonary HTN and Eisenmenger syndrome, leading to severe HF due to increased pulmonary vascular resistance and RV strain. Even in cases where CHDs have been repaired, residual defects, arrhythmias, or ventricular dysfunction may predispose patients to HF[94,95]. Besides, patients with single ventricle physiology who have undergone the Fontan procedure face unique long-term complications, such as Fontan-associated liver disease and circulatory inefficiency, both of which often result in HF as they transition into adulthood[96].
The distinction between repaired and unrepaired CHD is crucial, as both pathways can lead to HF through different pathophysiological mechanisms. Repaired congenital defects may result in HF due to long-term surgical sequelae, including scarring, valvular insufficiency, and arrhythmias, all of which impose chronic stress on the heart. Conversely, unrepaired disabilities may cause HF through chronic volume overload, increased pulmonary pressures, and progressive RV failure. These risks emphasize the importance of lifelong cardiac monitoring and individualized management strategies tailored to each adult with CHD. Such approaches aim to optimize long-term outcomes[97].
Psychosocial stressors, particularly depression, significantly contribute to CVD and HF pathogenesis. An 18-year longitudinal study demonstrated that depression is an independent risk factor for incident coronary heart disease in women, mediated by chronic inflammation and autonomic nervous system dysregulation[98,99].
In other words, the rising prevalence of mental illnesses among high school students has become a growing public health concern, contributing to the increasing burden of HF. Currently, approximately one in five adolescents worldwide experience an anxiety disorder, depression, or substance use disorder, and most of these individuals do not receive treatment[100]. For example, the rate of major depressive episodes among children and adolescents aged 12 to 17 years in the United States increased from 13.3% in 2017 to 15.7% in 2021, mirroring global trends[101]. Research indicates that the coronavirus disease 2019 (COVID-19) pandemic exacerbated this issue, with anxiety and depression among adolescents increasing by 25% during this period[102,103].
Several contributing factors, including low socioeconomic status, financial and academic pressures, and associated lifestyle choices such as smoking, alcohol and drug use, poor nutrition, and physical inactivity, are recognized risk factors for CVD. Depression and other mental health disorders are not only independent predictors of HF but also contribute to its development through pathways such as increased pro-inflammatory cytokines, elevated cortisol levels, and autonomic dysfunction[104,105]. Furthermore, adults with a history of childhood mental health disorders face significantly higher risks of premature CVD, including HF. These risks can persist for decades, with mental health disorders serving as long-term predictors of cardiovascular outcomes[106].
Given the interplay between mental health and HF, a multisectoral approach to prevention is essential. Integrating mental health assessments and care with the management of CVD risk factors among youth could help mitigate this emerging public health issue. This combined strategy offers a promising pathway to reducing HF complications and promoting better long-term health outcomes[107,108].
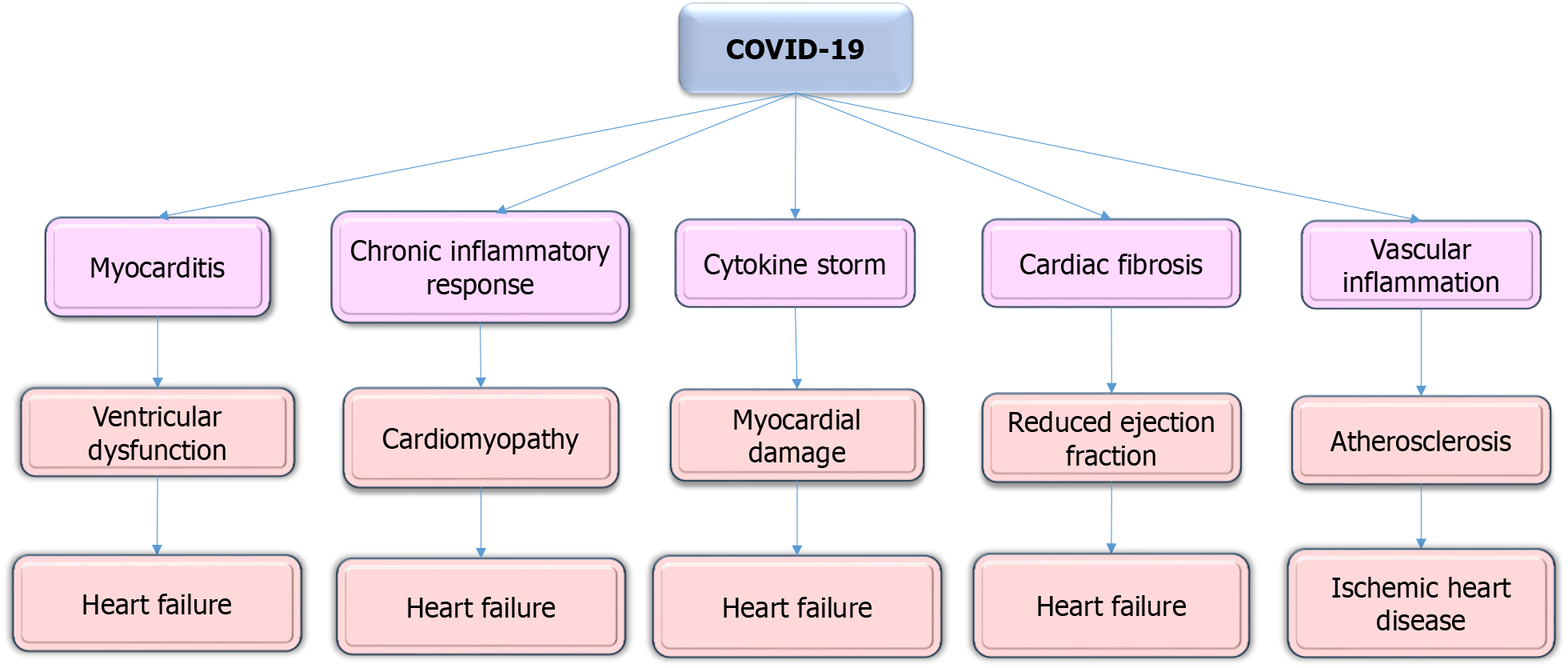
Emerging evidence thus demonstrates that COVID-19 is associated with significant long-term cardiovascular risk, particularly in young and otherwise healthy populations. Myocarditis has been reported at a rate of 11 per 100000 young adults following COVID-19, a rate notably higher than that observed after other viral infections[109]. This condition, characterized by persistent inflammation, can lead to ventricular dysfunction and increase the risk of HF[110].
Several studies have reported that COVID-19 can cause myocarditis, even in young and otherwise healthy individuals. The chronic inflammatory response triggered by the virus may predispose these individuals to HF in the future[111,112]. For example, a study by Puntmann et al[113] found that 78% of recovered COVID-19 patients exhibited cardiac involvement, including myocarditis, as detected by cardiac MRI.
A large population-based study by Tuvali et al[109] found that the incidence of myocarditis after COVID-19 was only 11 per 100000 individuals, which is lower than previously reported. Additionally, some researchers argue that the long-term cardiovascular risks of COVID-19 may be confounded by pre-existing conditions or the severity of the acute infection. For example, a study by Alvarez-Garcia et al[114] found that prior HF, rather than COVID-19 itself, was the strongest predictor of adverse cardiovascular outcomes post-infection.
The virus can also induce a cytokine storm, directly causing myocardial damage, fibrosis, and vascular inflammation factors that contribute to an elevated risk of cardiomyopathy and HF, even in individuals without prior CVD[115].
According to a study, post-recovery COVID-19 has been associated with a 45% increased risk of developing new-onset HF, particularly among those who required hospitalization during the acute phase of the infection[116]. Additionally, indicates that 10%-40% of COVID-19 survivors experience long COVID syndrome, often presenting with cardiovascular symptoms such as chest pain and fatigue, further contributing to the long-term cardiovascular burden in younger populations[117].
Despite this, other studies suggest that the risk of HF post-COVID-19 may be overstated. Trimaille et al[118] found that most young patients with COVID-19-related cardiac symptoms recovered fully without developing HF. Some researchers argue that the observed increase in HF cases may be due to heightened surveillance and diagnostic testing rather than a direct effect of the virus[110]. While COVID-19 can lead to HF in some patients, the overall risk may be lower than initially feared, especially in young and healthy individuals. More longitudinal studies are needed to assess the actual burden of COVID-19-related HF.
Given this evidence, it is clear that managing the long-term cardiovascular effects of COVID-19, will remain a critical public health priority. Future research should prioritize the development of cardiac monitoring and management strategies for COVID-19 survivors, emphasizing on early detection and intervention to prevent the potential progression to HF. Moreover, public health measures should shift toward systematic screening for cardiovascular sequelae, including among younger COVID-19 survivors, as they may face an increased risk of HF over an extended period following infection[119].
The long-term cardiovascular effects of COVID-19 on HF in young individuals have become a significant area of concern. Studies indicate that myocarditis, a condition characterized by persistent inflammation, occurs at a rate of 11 per 100000 young adults following COVID-19 infection, which is notably higher than the incidence observed after other viral infections[109].
A study conducted by Xie et al[120] revealed that survivors of COVID-19 had a significantly higher risk of developing HF compared to non-infected controls, with an increased risk of up to 45% among those hospitalized during the acute phase of the infection. Additionally, 10%-40% of survivors experience prolonged symptoms like chest pain and fatigue, increasing the cardiovascular burden[117].
Despite these findings, some studies suggest that the overall risk of HF post-COVID-19 may be lower than initially feared, especially in healthy young individuals. Trimaille et al[118] reported that most young patients with mild or moderate COVID-19-related cardiac symptoms fully recovered without progressing to HF. However, it remains crucial to monitor this population closely due to potential delayed complications.
The data illustrated the need for ongoing research and targeted interventions to address the long-term cardiovascular consequences of COVID-19 in younger individuals. Regular cardiac monitoring and early detection strategies are essential for mitigating the progression to HF in this demographic (Figure 4).
The interplay between MetS and substance abuse reveals a compounded risk for HF in younger populations. Studies have demonstrated that MetS, characterized by obesity, HTN, and insulin resistance, predisposes individuals to cardiovascular dysfunction, including LVH and diastolic dysfunction. When combined with substance abuse such as chronic alcohol consumption or cocaine use, the cardiovascular strain intensifies due to overlapping mechanisms, including oxidative stress, endothelial dysfunction, and systemic inflammation[101,121]. This synergistic effect suggests that youth with coexisting MetS and substance use disorders may have an accelerated trajectory toward HF.
Many studies on the cardiovascular sequelae of COVID-19 rely on retrospective designs, which may introduce selection bias. In addition, the long-term cardiovascular outcomes in younger populations remain underexplored, and further prospective cohort studies are needed[122,123]. A recent retrospective cohort study revealed that COVID-19 survivors face elevated long-term risks of cardiovascular, cerebrovascular, and thrombotic complications; however, the retrospective design may introduce selection bias (e.g., overrepresentation of hospitalized), underscoring the need for prospective studies to assess these outcomes in younger populations[124].
While the cardiotoxic effects of these substances are well-established, many of the existing studies are based on small sample sizes or animal models, which may limit the generalizability of the findings to human populations[125].
Furthermore, few studies have explicitly examined how risk factors such as MetS and substance abuse interact[13,42,43,126]. This gap highlights the need for research that integrates these overlapping pathways, particularly in young individuals at high risk for HF.
In summary, HF in younger populations is influenced by a complex interplay of various risk factors, including MetS, substance abuse, autoimmune diseases, genetic predispositions, environmental exposures, and the long-term cardiovascular effects of COVID-19. These factors often interact synergistically, exacerbating the risk of HF in this demographic. Figure 5 provides a comprehensive overview of these diverse risk factors, highlighting their cumulative impact on the rising incidence of HF among young individuals (Figure 5).
Gender differences in HF risk factors have gained increasing attention in recent years, as emerging evidence suggests that men and women may experience distinct pathways and risk profiles leading to HF. While the section on autoimmune diseases highlights the disproportionate impact of conditions such as SLE and RA on young women, other risk factors also exhibit significant gender disparities that warrant further exploration.
MetS and obesity: MetS and obesity are major contributors to HF, but their impact varies by gender. Women with MetS are more likely to develop HF with HFpEF, whereas men are more prone to HF with HFrEF[126,127]. This difference is partly attributed to hormonal influences, as estrogen has protective effects on vascular health. However, these benefits diminish after menopause, increasing women's susceptibility to HFpEF[128]. Additionally, women tend to accumulate more visceral fat, which is strongly associated with insulin resistance and diastolic dysfunction, further predisposing them to HFpEF[129].
Substance abuse: Substance abuse, specifically alcohol and illicit drugs, affects men and women differently. Men are more likely to engage in heavy alcohol consumption and illicit drug use, which are strongly linked to DCM and HFrEF[130]. However, the gender gap in substance abuse is closing, and healthcare practitioners may not always investigate substance abuse history in detail, which can limit the recognition of its role in the pathogenesis of cardiomyopathy and HF. In contrast, women are more susceptible to the cardiotoxic effects of substances Such as cocaine, even at lower doses, due to differences in body composition and metabolism[131]. Furthermore, women with substance use disorders often face additional psychosocial stressors, such as domestic violence and caregiving responsibilities, which can exacerbate HF risk[132].
Psychosocial stressors and mental health: Psychosocial stressors, including chronic stress, depression, and anxiety, disproportionately affect women and are independent risk factors for HF. Women are twice as likely as men to experience depression, which is associated with worse HF outcomes due to poor adherence to treatment and unhealthy lifestyle behaviors[133]. Chronic stress, often linked to caregiving roles, can lead to endothelial dysfunction and inflammation, further increasing HF risk in women[8].
Environmental factors: Exposure to environmental pollutants, such as PM2.5 and NO2, affects men and women differently. Women may be more vulnerable to the cardiovascular effects of air pollution due to hormonal fluctuations and differences in lung physiology[37]. For example, long-term exposure to PM2.5 has been related to a higher incidence of HFpEF in women than men[134].
Genetic predisposition: Genetic cardiomyopathies, such as HCM and arrhythmogenic ARVD, also exhibit gender differences. Men with HCM are more likely to experience SCD and severe hypertrophy, while women often present with more subtle symptoms but a higher risk of HF progression[135,136]. Furthermore, mutations in genes such as TTN and LMNA may have varying penetrance and clinical manifestations based on gender, necessitating gender-specific management strategies[137].
COVID-19 and cardiovascular risks: The long-term cardiovascular effects of COVID-19 also show gender disparities. Women are more likely to experience post-COVID-19 myocarditis and persistent cardiovascular symptoms, such as chest pain and fatigue, which can contribute to HF[138,139]. These differences may be due to variations in immune responses and hormonal influences, which modulate the inflammatory cascade triggered by the virus[140].
Gender differences in HF risk factors are significant and multifaceted, encompassing metabolic, behavioral, psychosocial, environmental, and genetic dimensions[141]. Addressing these disparities requires a gender-specific approach to prevention, diagnosis, and treatment. Future research should focus on elucidating the underlying mechanisms of these differences and developing targeted interventions to improve outcomes for both men and women at risk of HF.
Gender differences play a crucial role in the prevalence and progression of HF, with distinct risk factors influencing disease outcomes in men and women. Understanding these gender-specific variations is essential for developing targeted prevention and treatment strategies to improve cardiovascular health outcomes.
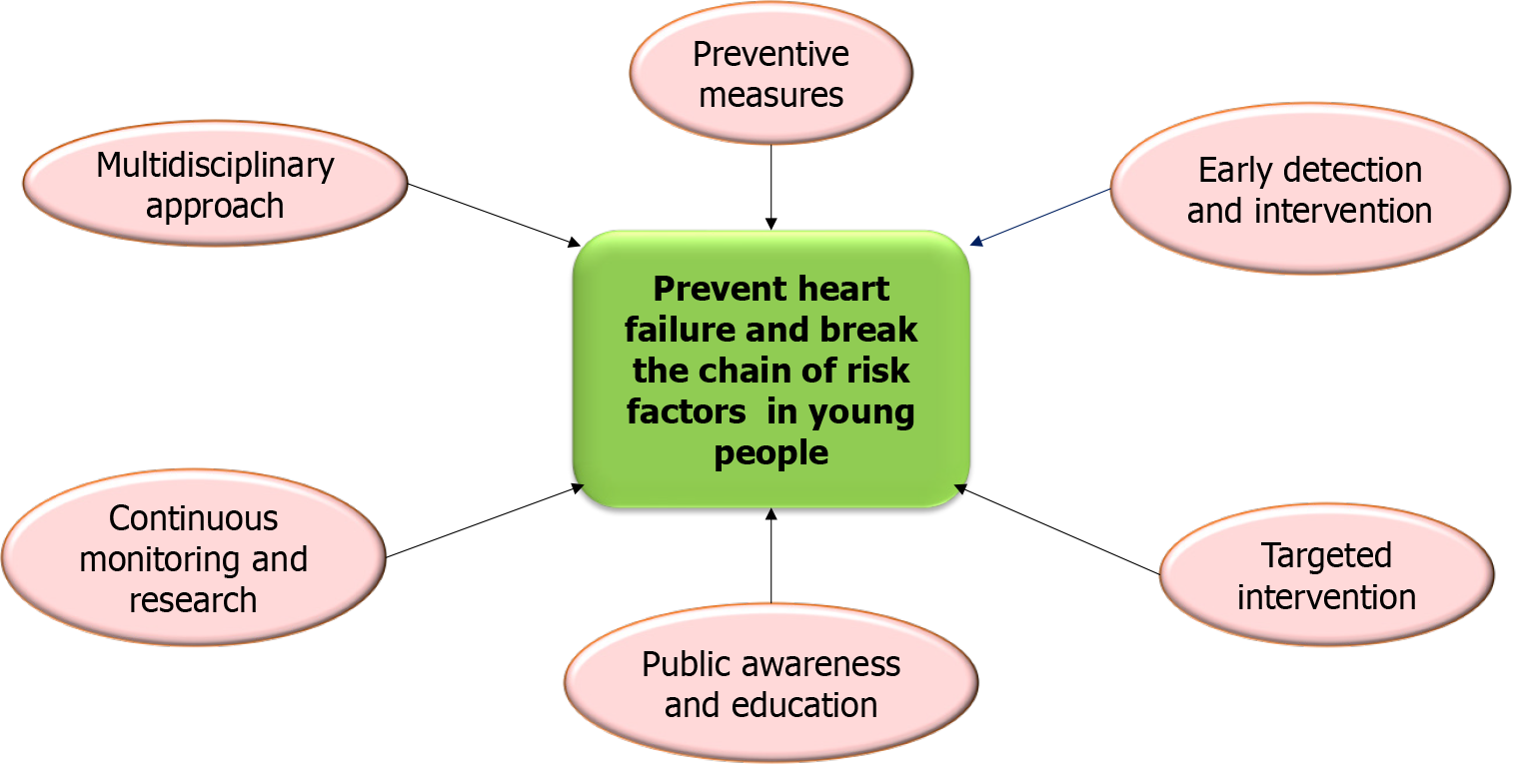
The rising prevalence of HF in younger populations is a significant public health concern driven by a complex interplay of emerging risk factors. These include MetS, environmental exposures, genetic predispositions, lifestyle behaviors, substance abuse, autoimmune diseases, and the long-term cardiovascular effects of COVID-19. Addressing these challenges requires a multidisciplinary approach incorporating lifestyle interventions, public health policies, and early screening programs.
Studies have emphasized the role of lifestyle modifications, including physical activity and dietary changes, in reducing the incidence of MetS and obesity, which are significant contributors to HF[142]. Moreover, environmental factors such as air pollution and exposure to heavy metals have been linked to adverse cardiovascular outcomes, emphasizing the need for stricter environmental regulations and public health initiatives[143] Furthermore, the COVID-19 pandemic has introduced new challenges, with emerging evidence suggesting that severe acute respiratory syndrome coronavirus 2 infection may exacerbate cardiovascular risks, notably in young individuals with pre-existing conditions[144]. Effectively mitigating these multifaceted risk factors through targeted interventions, public health initiatives, and interdisciplinary collaboration is essential preventing of HF and improving cardiovascular outcomes in young populations.
Social mobilization plays an essential role in encouraging heart care from childhood onward by reducing exposure to conditions like MetS, which can lead to LVH and CAD.
Implementing school-based and community programs to encourage regular physical activity is essential. Programs such as the Coordinated Approach to Child Health (CATCH) have proven effective in increasing physical activity levels and improving dietary habits among children and adolescents[145]. Encouraging diets rich in fruits, vegetables, and whole grains while reducing the consumption of processed foods, salt, and unhealthy fats is crucial. For instance, the Dietary Approaches to Stop HTN (DASH) diet, has been demonstrated to lower blood pressure and enhance cardiovascular health[140]. Also, introducing taxes on sugary drinks and providing subsidies for healthier food options can make nutritious choices more accessible. For example, Mexico’s tax on sugar-sweetened beverages significantly declined their consumption[146]. These measures can play a vital role in improving public health outcomes.
Environmental modifications are equally important, as poor air quality and exposure to heavy metal toxicity are known to increase the risk of CVD in young populations[147]. Strengthening air quality standards and enforcing regulations to reduce emissions of PM2.5 and NO2. are essential steps toward improving public health. For example, the Clean Air Act in the United States, has illustrated significant success in reducing air pollution and its associated health risks[148,149].
Promoting the development of green spaces in urban areas can help minimize exposure to harmful air pollutants. Research has consistently shown that urban green spaces are associated with better cardiovascular health outcomes[150,151]. Likewise, public awareness campaigns play a vital role in educating communities about the dangers of air pollution and encouraging the adoption of protective measures, such as using air purifiers in homes and schools, particularly in regions with high pollution levels. Effective communication strategies, including air quality advisories and targeted awareness campaigns, can help improve environmental health literacy and promote public understanding of the health impacts of air pollution. Participatory sensing activities and citizen science approaches can also enhance public engagement and interest in addressing air pollution issues. While public awareness campaigns can help protect against the decline of public concern and motivate individuals to take protective actions, they may not directly influence public protective behaviors in the absence of other supporting measures[151,152].
School-based substance abuse prevention programs, such as Project Adolescent Lifestyle Education and Risk Training and LifeSkills Training, have indicated effectiveness in reducing substance abuse among adolescents by providing valuable tools and education to help young people make healthier choices[153].
Increasing access to evidence-based treatment programs for substance abuse, including medication-assisted treatment (MAT) for opioid addiction, is essential. MAT integrates medications with counseling and behavioral therapies, offering a comprehensive approach to effectively manage and treat opioid use disorders[154].
Public health campaigns play a significant role in raising awareness about the cardiovascular risks associated with substance abuse. For instance, programs aimed at reducing smoking have successfully decreased smoking rates among young adults through targeted media campaigns that emphasize the dangers of tobacco use[155,156].
Early screening programs for autoimmune diseases, such as SLE and RA, are essential to prevent cardiovascular complications. The Lupus Foundation of America highlights the importance of routine cardiovascular risk assessments in patients with SLE to identify and manage potential issues at an early stage[157,158].
Cardiovascular risk assessment tools, such as the QRISK3 calculator, can help identify and manage cardiovascular risks more effectively in patients with autoimmune diseases. These tools provide valuable insights into an individual’s risk profile, enabling healthcare providers to tailor interventions accordingly[159].
Raising patient awareness regarding the cardiovascular risks tied to autoimmune diseases is crucial. Educating them on how adopting healthier habits, like stopping smoking and maintaining a healthy weight, is essential to lower their risk of cardiovascular issues. Such education empowers patients to take proactive steps toward improving their long-term health outcomes[160].
Early diagnosis and treatment are indispensable for preventing HF. Regular check-ups, including annual physical exams and cardiovascular screenings, should be emphasized, even for young individuals without evident CVD risk factors[147,161,162]. Identifying risk factors early allows for timely intervention, potentially preventing the progression to HF. Individualized care plans that consider genetic predispositions and social determinants of health can significantly enhance patient outcomes[163]. Promoting genetic screening for familial cardiomyopathies, such as HCM and ARVD, is critical for individuals with a family history of HF. For ARVD, Muller et al[164] emphasize that cascade genetic testing identifies 70% of at-risk relatives before symptom onset, enabling interventions like implantable cardioverter-defibrillator (ICD) placement to prevent sudden cardiac death. Their study highlights that early genetic screening reduces diagnostic delays by an average of 4 years compared to symptom-based diagnosis.
For HCM, Dellefave-Castillo et al[165] demonstrate that multi-gene panel testing identifies pathogenic variants in 15%-30% of cases, with mutations in MYBPC3 and MYH7 accounting for the majority of familial cases. This aligns with American Heart Association (AHA) guidelines, which recommend genetic testing for first-degree relatives to enable early risk stratification and management. Importantly, Dellefave-Castillo et al[165] also show that combined testing for cardiomyopathy and arrhythmia genes increases diagnostic yield by 40% compared to single-gene approaches, ensuring comprehensive identification of at-risk individuals across both HCM and ARVD. Integrating these strategies reduces morbidity and mortality in high-risk populations.
The application of precision medicine can significantly improve treatment strategies by customizing therapies based on individual genetic variations. Programs such as the National Institutes of Health’s (NIH) All of Us Research Programactively gathers comprehensive genetic data to advance personalized medicine and enhance patient outcomes[166,167].
Increasing public awareness about the importance of genetic testing and its role in preventing HF is equally vital. Educating communities about the benefits of genetic screening can encourage more individuals to seek testing, leading to earlier detection and better management of cardiovascular conditions[168-170].
Integrating mental health services into primary care settings is essential for addressing chronic stress, depression, and anxiety, significant contributors to cardiovascular risks. The Collaborative Care Model has proven effective in improving mental health outcomes and, in turn, reducing the likelihood of cardiovascular complications[171].
Incorporating mental health initiatives within school settings, such as Mind Matters and FRIENDS, is essential for mitigating anxiety and depression among adolescents. By equipping young individuals with the necessary coping strategies and support systems, these programs contribute to their long-term psychological well-being[172].
Encouraging the adoption of workplace wellness programs that integrate stress management and mental health support is a crucial initiative. The World Health Organization’s Mental Health Gap Action Programme is vital in addressing mental health challenges globally[173].
Young individuals recovering from COVID-19, including those with ongoing symptoms, should undergo regular cardiac monitoring to enable early detection and effective management of potential cardiovascular complications. According to the American College of Cardiology, cardiac MRI is suggested for patients suspected of myocarditis following a COVID-19 infection, as this method supports accurate diagnosis and timely intervention[113].
Launching public health campaigns to raise awareness about the long-term cardiovascular risks involved in COVID-19 is essential. These campaigns should emphasize the importance of early intervention and regular follow-ups to mitigate potential health issues and improve outcomes for affected individuals.
Investing in research to better understand the long-term cardiovascular effects of COVID-19 is equally important. Initiatives like the NIH RECOVER Initiative are actively examining the prolonged impacts of COVID-19, emphasizing on cardiovascular complications, to develop targeted interventions and enhance patient care[174].
Effective prevention also depends on interprofessional collaboration among cardiologists, primary care physicians, and other specialists focused on young adults[175]. Additionally, ongoing research the socioeconomic and environmental predictors of HF and implementing new policies addressing these factors, is essential for long-term prevention.
Raising awareness and promoting education are critical components in mitigating the risk of HF among younger individuals. Public health campaigns targeting schools, workplaces, and communities can play a pivotal role in educating young people about the importance of maintaining a healthy lifestyle, recognizing early warning signs of HF, and understanding the impact of modifiable risk factors such as obesity, substance abuse, and poor dietary habits[108]. For instance, school-based programs like the CATCH have adolescents' physical activity levels and nutritional choices[145]. Furthermore, integrating mental health education into existing frameworks can help address psychosocial stressors, which are increasingly recognized as significant contributors to CVD[133]. Additionally, educational initiatives to inform the public about the long-term cardiovascular effects of environmental pollutants and substance use disorders can empower individuals to make healthier decisions[143]. By fostering a culture of preventive care through targeted education and awareness campaigns, it is possible to reduce the burden of HF in younger populations.
To effectively mitigate the rising burden of HF in younger populations, it is essential to adopt a comprehensive and multidisciplinary approach targeting the complex network of risk factors. By advancing early intervention strategies and encouraging collaboration across healthcare disciplines, significant progress can be made in reducing HF prevalence and enhancing cardiovascular health outcomes[108]. Addressing these challenges requires integrating lifestyle modifications, public health policies, and early screening programs into routine care. Healthcare providers must remain vigilant in identifying cardiovascular risks, particularly among young patients with family histories of heart disease or additional risk factors such as obesity, substance abuse, or autoimmune disorders (Figure 6)[109].
Public health initiatives promoting physical activity, healthy diets, and substance abuse prevention are crucial for reducing the long-term cardiovascular burden, including complications associated with conditions like COVID-19[110]. Programs to educate young populations about the dangers of unhealthy behaviors and environmental exposures can play a pivotal role in fostering healthier lifestyles[119]. Furthermore, implementing school-based interventions, community awareness campaigns, and evidence-based treatments for substance use disorders will strengthen preventive efforts[153]. These coordinated actionscan break the chain of risk factors contributing to HF and improve long-term health outcomes in younger generations.
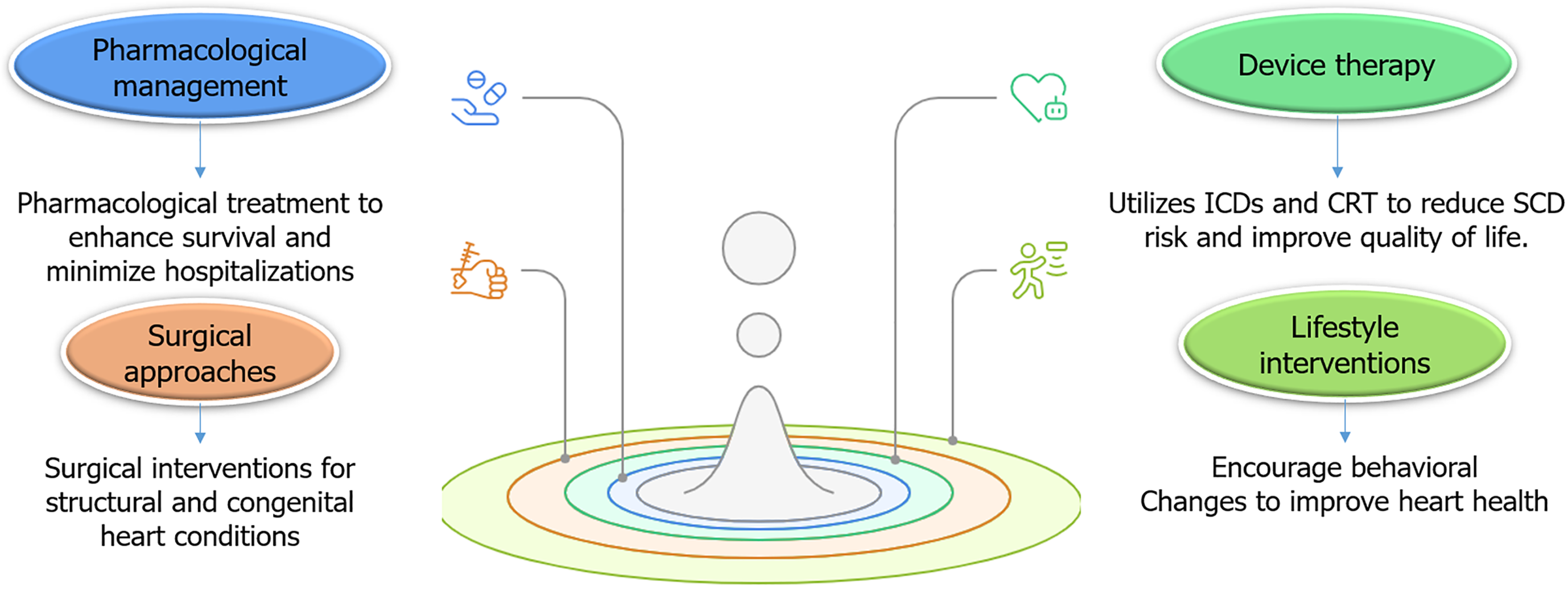
While prevention is imperative, effective treatment strategies are equally crucial for managing HF in younger populations. Given the unique risk factors and etiologies of HF in this demographic, treatment approaches must be tailored to meet the specific needs of younger patients. Recent evidence and guidelines suggested key treatment strategies.
Pharmacological treatment remains the cornerstone of HF management, even for younger patients. However, the selection of medications may vary depending on the underlying cause of HF, such as genetic cardiomyopathies, substance abuse, or autoimmune conditions, for patients with HF and HFrEF, guideline-directed medical therapy is recommended. This includes angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter-2 (SGLT2) inhibitors. These medications have demonstrated significant benefits in improving survival rates and reducing hospitalizations in younger patients, similar to their effects in older populations[176,177].
Recent research has emphasized the advantages SGLT2 inhibitors, such as dapagliflozin and empagliflozin, in lowering the risk of HF-related hospitalizations and improving outcomes in younger individuals, including those without diabetes[178,179]. These drugs are particularly noteworthy due to their cardioprotective properties and ability to address metabolic risk factors. In younger patients with genetic cardiomyopathies, such as HCM or ARVD, antiarrhythmic medications like amiodarone or beta-blockers are frequently prescribed to reduce the risk of SCD[180].
Device-based interventions are essential in the management of HF in younger patients, especially those with genetic or congenital heart conditions. ICDs are often recommended for younger individuals with HF and HFrEF who are at high risk of SCD. This is especially relevant for patients with genetic cardiomyopathies or a history of VT. The use of device-based therapies, including ICDs, is crucial for the management of cardiac rhythm abnormalities and congestive heart failure in patients with congenital heart disease and inherited cardiac conditions[181]. Early implantation of ICDs in high-risk patients has been shown to significantly lower mortality rates[182]. Moreover, cardiac resynchronization therapy (CRT) is a valuable treatment option for younger patients with HFrEF who exhibit electrical dyssynchrony, such as a wide QRS complex. CRT effectively improved symptoms, reduced hospitalizations, and enhanced overall quality of life in younger populations[183].
For younger patients with structural heart disease or CHD, surgical or interventional procedures may be necessary. In cases of valvular heart diseases, surgical repair or replacement is often required. For example, patients with RHD may benefit from mitral valve repair or replacement to prevent the progression to HF[184]. In addition, patients with unrepaired or residual CHD may require surgical or catheter-based interventions to correct structural abnormalities. For instance, patients with TOF may need pulmonary valve replacement to address pulmonary regurgitation and prevent RV failure[97].
Lifestyle and behavioral interventions play a crucial role in managing HF in young adults, especially with MetS or a history of substance abuse. Adopting a heart-healthy diet, such as the DASH diet, and engaging in regular physical activity, can significantly improve cardiovascular health and alleviate HF symptoms[185,186]. Structured exercise training programs, including aerobic and resistance training, have been demonstrated to enhance functional capacity and quality of life in younger patients with HF[187,188]. Furthermore, for individuals with HF related to substance abuse, such as cocaine or alcohol, cessation programs are essential. Behavioral interventions and medications like naltrexone for alcohol use disorder can significantly reduce substance consumption and lead to improved HF outcomes[189].
Diet and exercise: A heart-healthy diet, such as the DASH diet, combined with regular physical activity, can significantly improve cardiovascular health and reduce HF symptoms. Exercise training programs, including aerobic and resistance training, have enhanced functional capacity and quality of life in younger HF patients. Goyal et al[190] validates the DASH diet’s role in reducing heart failure incidence, aligning with the manuscript’s recommendation for dietary interventions.
While this study focuses on adults, its findings reinforce the broader applicability of heart-healthy diets (e.g., DASH) to younger populations with metabolic syndrome. The exercise training benefits cited in the text are supported by Volterrani et al[187] and Myers et al[188], ensuring a comprehensive evidence base for lifestyle strategies in HF management.
Substance abuse cessation: In patients with HF linked to substance abuse-particularly alcohol-cessation programs are a cornerstone of therapy. Chronic alcohol use not only exacerbates HF through direct myocardial toxicity and oxidative stress but also contributes to comorbidities like alcohol-associated cirrhosis, which further impairs cardiac function via systemic inflammation and hemodynamic instability[189]. Behavioral interventions, combined with pharmacotherapy such as naltrexone, have emerged as critical strategies to reduce alcohol dependence. A landmark Indian cohort study demonstrated that naltrexone significantly diminishes alcohol cravings and improves hepatic outcomes in cirrhotic patients, indirectly alleviating cardiac workload and reducing HF decompensation risks[189]. This dual benefit-targeting both addiction and its hepatic complications-highlights naltrexone’s role in mitigating the bidirectional relationship between liver dysfunction and cardiovascular decline. Furthermore, by antagonizing opioid receptors, naltrexone may attenuate neurohormonal activation and inflammation, key drivers of adverse cardiac remodeling in this population[189].
Given the high prevalence of mental health disorders among younger patients with HF and other chronic cardiac conditions, integrating psychosocial support into treatment plans is critical. A randomized controlled trial by Holdgaard et al[191] demonstrated that cognitive behavioral therapy (CBT) significantly reduces psychological distress in younger patients with cardiac disease, including those with chronic conditions such as HF. By addressing maladaptive thought patterns and promoting coping strategies, CBT not only alleviates anxiety and depression but also improves adherence to medical therapies-a key factor in optimizing HF outcomes. This aligns with growing evidence that psychological interventions should be a standard component of multidisciplinary care for younger HF patients, particularly given the bidirectional relationship between mental health and cardiovascular prognosis. Focusing on mental health concerns improves emotional well-being and enhances adherence to treatment and overall clinical outcomes. Moreover, a multidisciplinary care approach is essential for managing the complex needs of younger HF patients. This team-based model, which includes cardiologists, primary care physicians, mental health professionals, and social workers, ensures comprehensive care that addresses this population's physical and mental health challenges both the physical and mental health challenges faced by this population[38]. Such an integrated strategy is vital for optimizing long-term health and quality of life in younger individuals with HF.
For younger patients with end-stage HF, advanced therapies such as heart transplantation or mechanical circulatory support (MCS) may be considered.
Heart transplantation: Heart transplantation remains the gold standard for managing end-stage HF in younger individuals. Advances in immunosuppressive therapies have significantly improved long-term survival rates, with younger patients often achieving better outcomes than older recipients[192]. For patient’s ineligible for heart transplantation, MCS provides a life-saving alternative. LVADs, such as the Heart Mate 3, have shown promising results in real-world settings. A multicenter European study by Numan et al[193] reported 80% survival at 1 year and 70% at 2 years post-Heart Mate 3 implantation, with significant improvements in functional capacity and quality of life. Notably, younger patients (< 60 years) experienced fewer device-related complications (e.g., pump thrombosis, stroke) compared to older cohorts, reinforcing the role of continuous-flow LVADs as a durable therapy for select populations.
These advanced therapies represent critical options for extending and improving the lives of younger patients with severe HF (Figure 7).
The treatment of HF in younger populations requires a multifaceted approach tailored to address the unique risk factors and underlying causes specific to this demographic. An integrated approach, including pharmacological treatments, device-based therapies, surgical interventions, lifestyle changes, and psychosocial support is fundamental for effectively managing of HF and enhancing patient outcomes.
Early diagnosis is crucial for initiating timely interventions, while personalized treatment plans ensure that care aligns with individual patient needs. Additionally, a multidisciplinary care approach involving collaboration among cardiologists, primary care providers, mental health professionals, and other specialists. By integrating these strategies, healthcare providers can optimize long-term health and quality of life for this vulnerable population.
The increasing incidence of HF in younger populations represents a significant public health challenge that demands immediate attention. Emerging risk factors, such as MetS, substance use, autoimmune diseases, genetic predisposition, and the long-term cardiovascular effects of COVID-19, underscore the urgent need for a comprehensive approach to both the prevention and management of HF in this vulnerable group.
Future research should focus on understanding the mechanisms driving these risk factors and developing targeted interventions to curb the rising rates of HF in younger individuals. By proactively addressing these factors, significant strides can be made in reducing the burden of HF and improving long-term health outcomes for younger populations. Early intervention, personalized care, and a comprehensive strategy combining medical, surgical, lifestyle, and psychosocial approaches are crucial in mitigating the impact of HF.
These efforts not only have the potential to improve survival rates but also to enhance the quality of life for younger patients, allowing them to lead healthier and more fulfilling lives. This proactive approach emphasizes the importance of prevention, timely treatment, and holistic care in managing HF in this demographic.
We would like to thank the Clinical Research Development Unit of Shahid Madani Educational, Research and Treatment Center, Tabriz University of Medical Sciences, Tabriz, Iran, for their assistance in this research.
| 1. | Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153-e639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2995] [Cited by in RCA: 3638] [Article Influence: 909.5] [Reference Citation Analysis (0)] |
| 2. | Jain V, Minhas AMK, Morris AA, Greene SJ, Pandey A, Khan SS, Fonarow GC, Mentz RJ, Butler J, Khan MS. Demographic and Regional Trends of Heart Failure-Related Mortality in Young Adults in the US, 1999-2019. JAMA Cardiol. 2022;7:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Lecoeur E, Domeng O, Fayol A, Jannot AS, Hulot JS. Epidemiology of heart failure in young adults: a French nationwide cohort study. Eur Heart J. 2023;44:383-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, Brauer M, Kutty VR, Gupta R, Wielgosz A, AlHabib KF, Dans A, Lopez-Jaramillo P, Avezum A, Lanas F, Oguz A, Kruger IM, Diaz R, Yusoff K, Mony P, Chifamba J, Yeates K, Kelishadi R, Yusufali A, Khatib R, Rahman O, Zatonska K, Iqbal R, Wei L, Bo H, Rosengren A, Kaur M, Mohan V, Lear SA, Teo KK, Leong D, O'Donnell M, McKee M, Dagenais G. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 1324] [Article Influence: 220.7] [Reference Citation Analysis (0)] |
| 5. | Khan MS, Shahid I, Bennis A, Rakisheva A, Metra M, Butler J. Global epidemiology of heart failure. Nat Rev Cardiol. 2024;21:717-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 230] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 6. | Feng J, Zhang Y, Zhang J. Epidemiology and Burden of Heart Failure in Asia. JACC Asia. 2024;4:249-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 7. | Yang C, Jia Y, Zhang C, Jin Z, Ma Y, Bi X, Tian A. Global, regional, and national burdens of heart failure in adolescents and young adults aged 10-24 years from 1990 to 2021: an analysis of data from the Global Burden of Disease Study 2021. EClinicalMedicine. 2025;79:102998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Srinivasan N, Gullapalli N, Shah KS. Highlighting the South Asian Heart Failure Epidemic. Card Fail Rev. 2024;10:e07. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Wagle A, Goerlich E, Post WS, Woldu B, Wu KC, Hays AG. HIV and Global Cardiovascular Health. Curr Cardiol Rep. 2022;24:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Lumngwena EN, Mokaila D, Aremu O, Katoto PD, Blackburn J, Zilla P, Wiysonge CS, Ntusi N. Prevalence and Impact of HIV Infections in Patients with Rheumatic Heart Disease: A Systematic Review and Meta-Analysis. Glob Heart. 2023;18:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Agarwal A, Husain MJ, Datta B, Kishore SP, Huffman MD. Access to Heart Failure Medicines in Low- and Middle-Income Countries: An Analysis of Essential Medicines Lists, Availability, Price, and Affordability. Circ Heart Fail. 2022;15:e008971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 12. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5792] [Article Influence: 965.3] [Reference Citation Analysis (2)] |
| 13. | Alebna PL, Mehta A, Yehya A, daSilva-deAbreu A, Lavie CJ, Carbone S. Update on obesity, the obesity paradox, and obesity management in heart failure. Prog Cardiovasc Dis. 2024;82:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 14. | Ahima RS. Overview of Metabolic Syndrome. Metab Syndr. 2023;. [DOI] [Full Text] |
| 15. | Shiferaw WS, Akalu TY, Gedefaw M, Anthony D, Kassie AM, Misganaw Kebede W, Mulugeta H, Dessie G, Aynalem YA. Metabolic syndrome among type 2 diabetic patients in Sub-Saharan African countries: A systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Chung ST, Krenek A, Magge SN. Childhood Obesity and Cardiovascular Disease Risk. Curr Atheroscler Rep. 2023;25:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 17. | Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Toor PP, Banfield L, Thabane L, Chaudhary V, Samaan MC. Global Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023;6:e231887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 18. | Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2021;12:344-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (12)] |
| 19. | Ceriello A, Catrinoiu D, Chandramouli C, Cosentino F, Dombrowsky AC, Itzhak B, Lalic NM, Prattichizzo F, Schnell O, Seferović PM, Valensi P, Standl E; D&CVD EASD Study Group. Heart failure in type 2 diabetes: current perspectives on screening, diagnosis and management. Cardiovasc Diabetol. 2021;20:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Stamatakis E, Rezende LFM, Rey-lópez JP. Sedentary Behaviour and Cardiovascular Disease. Springer Ser Epidemiol Public Health. 2023;. [DOI] [Full Text] |
| 21. | Bravo MA, Fang F, Hancock DB, Johnson EO, Harris KM. Long-term air pollution exposure and markers of cardiometabolic health in the National Longitudinal Study of Adolescent to Adult Health (Add Health). Environ Int. 2023;177:107987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17:656-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 23. | Hayes RB, Lim C, Zhang Y, Cromar K, Shao Y, Reynolds HR, Silverman DT, Jones RR, Park Y, Jerrett M, Ahn J, Thurston GD. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int J Epidemiol. 2020;49:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 24. | Ning J, Zhang Y, Hu H, Hu W, Li L, Pang Y, Ma S, Niu Y, Zhang R. Association between ambient particulate matter exposure and metabolic syndrome risk: A systematic review and meta-analysis. Sci Total Environ. 2021;782:146855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | . Correction to: Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147:e622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 91] [Reference Citation Analysis (1)] |
| 26. | Liu A, Diller GP, Moons P, Daniels CJ, Jenkins KJ, Marelli A. Changing epidemiology of congenital heart disease: effect on outcomes and quality of care in adults. Nat Rev Cardiol. 2023;20:126-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 27. | Hao H, Yoo SR, Strickland MJ, Darrow LA, D'Souza RR, Warren JL, Moss S, Wang H, Zhang H, Chang HH. Effects of air pollution on adverse birth outcomes and pregnancy complications in the U.S. state of Kansas (2000-2015). Sci Rep. 2023;13:21476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Kankowski L, Ardissino M, McCracken C, Lewandowski AJ, Leeson P, Neubauer S, Harvey NC, Petersen SE, Raisi-Estabragh Z. The Impact of Maternal Obesity on Offspring Cardiovascular Health: A Systematic Literature Review. Front Endocrinol (Lausanne). 2022;13:868441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Qu Y, Liu X, Lin S, Bloom MS, Wang X, Li X, Wang H, Han F, Liu JE, Pan W, Zhang W, Zou X, Zhuang J, Li J, Chen J. Maternal Serum Folate During Pregnancy and Congenital Heart Disease in Offspring. JAMA Netw Open. 2024;7:e2438747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Fu Z, Liu Q, Liang J, Huang T, Liang G, Zhou Y, Gu A. Association of ambient air pollution exposure with low birth weight. Environ Res. 2022;215:114164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 1375] [Article Influence: 275.0] [Reference Citation Analysis (0)] |
| 32. | Paul T, Krause U, Sanatani S, Etheridge SP. Advancing the science of management of arrhythmic disease in children and adult congenital heart disease patients within the last 25 years. Europace. 2023;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 33. | Krieger EV, Zeppenfeld K, DeWitt ES, Duarte VE, Egbe AC, Haeffele C, Lin KY, Robinson MR, Sillman C, Upadhyay S; American Heart Association Adults With Congenital Heart Disease Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Council on Clinical Cardiology. Arrhythmias in Repaired Tetralogy of Fallot: A Scientific Statement From the American Heart Association. Circ Arrhythm Electrophysiol. 2022;15:e000084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 34. | Corrado D, Zorzi A, Cipriani A, Bauce B, Bariani R, Brunetti G, Graziano F, De Lazzari M, Mattesi G, Migliore F, Pilichou K, Rigato I, Rizzo S, Thiene G, Perazzolo Marra M, Basso C. Scarring/arrhythmogenic cardiomyopathy. Eur Heart J Suppl. 2023;25:C144-C154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 35. | Corno AF, Findley TO, Salazar JD. Narrative review of single ventricle: where are we after 40 years? Transl Pediatr. 2023;12:221-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 36. | Kochav J, DiLorenzo MP, Lewis MJ, Groenink M, van den Boogaard M, Mulder B, Rosenbaum M. Longitudinal changes in systemic right ventricular remodeling in adult patients with transposition of the great vessels as assessed by cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2024;26:101107. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Ebong IA, Quesada O, Fonkoue IT, Mattina D, Sullivan S, Oliveira GMM, Spikes T, Sharma J, Commodore Y, Ogunniyi MO, Aggarwal NR, Vaccarino V; American College of Cardiology Cardiovascular Disease in Women Committee. The Role of Psychosocial Stress on Cardiovascular Disease in Women: JACC State-of-the-Art Review. J Am Coll Cardiol. 2024;84:298-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 38. | Carmin CN, Ownby RL, Fontanella C, Steelesmith D, Binkley PF. Impact of Mental Health Treatment on Outcomes in Patients With Heart Failure and Ischemic Heart Disease. J Am Heart Assoc. 2024;13:e031117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Ma K, He Q, Dou Z, Hou X, Li X, Zhao J, Rao C, Feng Z, Sun K, Chen X, He Y, Zhang H, Li S. Current treatment outcomes of congenital heart disease and future perspectives. Lancet Child Adolesc Health. 2023;7:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Gagliardi F, Baldini E, Lori E, Cardarelli S, Pironi D, Lauro A, Tripodi D, Palumbo P, D'Armiento E, Cavallaro G, Polistena A, D'Orazi V, Sibio S, Fallahi P, Antonelli A, D'Andrea V, Ulisse S, Sorrenti S. Insights on the Association between Thyroid Diseases and Colorectal Cancer. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Arenas DJ, Beltran S, Zhou S, Goldberg LR. Cocaine, cardiomyopathy, and heart failure: a systematic review and meta-analysis. Sci Rep. 2020;10:19795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 42. | Gagnon LR, Sadasivan C, Perera K, Oudit GY. Cardiac Complications of Common Drugs of Abuse: Pharmacology, Toxicology, and Management. Can J Cardiol. 2022;38:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Minhas AMK, Kewcharoen J, Hall ME, Warraich HJ, Greene SJ, Shapiro MD, Michos ED, Sauer AJ, Abramov D. Temporal Trends in Substance Use and Cardiovascular Disease-Related Mortality in the United States. J Am Heart Assoc. 2024;13:e030969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 44. | Patel B, Lapsiwala B, Vanani S, Patel S, Suresh A, Jariwala P, Rajani A, Desai R, Krishnamoorthy G. Abstract 4146725: Young Adults with Migraine and Established Cardiovascular Disease Risk: Studying the Impact of Cannabis Use Disorder on Major Adverse Cardiac and Cerebrovascular Events in a Nationwide Study. Circulation. 2024;150. [DOI] [Full Text] |
| 45. | Roque-Bravo R, Silva RS, Malheiro RF, Carmo H, Carvalho F, da Silva DD, Silva JP. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu Rev Pharmacol Toxicol. 2023;63:187-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 46. | Sebastian SA, Shah Y, Krishnamoorthy G. Abstract P152: Association of Cannabis Use with Atherosclerotic Cardiovascular Diseases Outcomes: Insights from a Meta-Analysis of a Multinational Cohort of 1.9 Million Individuals. Hypertension. 2024;81. [DOI] [Full Text] |
| 47. | Theerasuwipakorn N, Prechawat S, Chokesuwattanaskul R, Siranart N, Marsukjai A, Thumtecho S, Rungpradubvong V. Cannabis and adverse cardiovascular events: A systematic review and meta-analysis of observational studies. Toxicol Rep. 2023;10:537-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Jeffers AM, Glantz S, Byers AL, Keyhani S. Association of Cannabis Use With Cardiovascular Outcomes Among US Adults. J Am Heart Assoc. 2024;13:e030178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 49. | Paneta M, Waring WS. Literature review of the evidence regarding intravenous lipid administration in drug-induced cardiotoxicity. Expert Rev Clin Pharmacol. 2019;12:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Larsson SC, Wallin A, Wolk A. Alcohol consumption and risk of heart failure: Meta-analysis of 13 prospective studies. Clin Nutr. 2018;37:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Pitman A, Stevenson F, King M, Osborn D. Self-Reported Patterns of Use of Alcohol and Drugs After Suicide Bereavement and Other Sudden Losses: A Mixed Methods Study of 1,854 Young Bereaved Adults in the UK. Front Psychol. 2020;11:1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Nawi AM, Ismail R, Ibrahim F, Hassan MR, Manaf MRA, Amit N, Ibrahim N, Shafurdin NS. Risk and protective factors of drug abuse among adolescents: a systematic review. BMC Public Health. 2021;21:2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 53. | Conklin DJ, Schick S, Blaha MJ, Carll A, DeFilippis A, Ganz P, Hall ME, Hamburg N, O'Toole T, Reynolds L, Srivastava S, Bhatnagar A. Cardiovascular injury induced by tobacco products: assessment of risk factors and biomarkers of harm. A Tobacco Centers of Regulatory Science compilation. Am J Physiol Heart Circ Physiol. 2019;316:H801-H827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 54. | Shabani M, Jamali Z, Bayrami D, Salimi A. Vanillic acid alleviates methamphetamine-induced mitochondrial toxicity in cardiac mitochondria via antioxidant activity and inhibition of MPT Pore opening: an in-vitro study. BMC Pharmacol Toxicol. 2023;24:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 55. | Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Fernandes A, Manivannan A, Schou M, Fosbøl E, Køber L, Gustafsson F, Gislason GH, Torp-Pedersen C, Andersson C. Clinical Trajectories and Long-Term Outcomes of Alcoholic Versus Other Forms of Dilated Cardiomyopathy. Heart Lung Circ. 2024;33:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Ferreira A, Ferreira V, Antunes MM, Lousinha A, Pereira-da-Silva T, Antunes D, Cunha PS, Oliveira M, Ferreira RC, Rosa SA. Dilated Cardiomyopathy: A Comprehensive Approach to Diagnosis and Risk Stratification. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 58. | Fuster D, Zuluaga P, Muga R. Substance use disorder: Epidemiology, medical consequences and treatment. Med Clin (Barc). 2024;162:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 59. | Danpanichkul P, Wattanachayakul P, Duangsonk K, Ongsupankul S, Sripusanapan A, Uawithya E, Benjanuwattra J, Trongtorsak A, Nathisuwan S, Navaravong L. The burden of alcohol-related cardiovascular complications in young and middle-aged adults: rising burden of atrial fibrillation and hypertensive heart disease. Acta Cardiol. 2024;79:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Schmidt T, Mankad R. Assessment of Cardiac Risk in Women with Autoimmune Disease. Curr Cardiol Rep. 2022;24:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Mehta PK, Levit RD, Wood MJ, Aggarwal N, O'Donoghue ML, Lim SS, Lindley K, Gaignard S, Quesada O, Vatsa N, Leon A, Volgman AS, Malas W, Pepine CJ; American College of Cardiology Cardiovascular Disease in Women Committee. Chronic rheumatologic disorders and cardiovascular disease risk in women. Am Heart J Plus. 2023;27:100267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Palomäki A, Kerola AM, Malmberg M, Rautava P, Kytö V. Patients with rheumatoid arthritis have impaired long-term outcomes after myocardial infarction: a nationwide case-control registry study. Rheumatology (Oxford). 2021;60:5205-5215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Porsch F, Binder CJ. Autoimmune diseases and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2024;21:780-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 64. | Su L, Xu C, Huang H, Zhang P, Wang J, Ouyang X, Yang X, Ye J. Effects of tumor necrosis factor-alpha inhibitors on lipid profiles in patients with psoriasis: a systematic review and meta-analysis. Front Immunol. 2024;15:1354593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 65. | Macáková K, Tekeľová M, Mlynáriková V, Šebeková K, Vlková B, Celec P, Šteňová E. Metabolic Effects of Anti-TNF-α Treatment in Rheumatoid Arthritis. Diseases. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 66. | Conrad N, Verbeke G, Molenberghs G, Goetschalckx L, Callender T, Cambridge G, Mason JC, Rahimi K, McMurray JJV, Verbakel JY. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. 2022;400:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 67. | Atzeni F, Popa CD, Nucera V, Nurmohamed MT. Safety of JAK inhibitors: focus on cardiovascular and thromboembolic events. Expert Rev Clin Immunol. 2022;18:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Yang V, Kragstrup TW, McMaster C, Reid P, Singh N, Haysen SR, Robinson PC, Liew DFL. Managing Cardiovascular and Cancer Risk Associated with JAK Inhibitors. Drug Saf. 2023;46:1049-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Mirabel M, Eslami A, Thibault C, Oudard S, Mousseaux E, Wahbi K, Fabre E, Terrier B, Marijon E, Villefaillot A, Fayol A, Dragon-Durey MA, Le Louet AL, Bruno RM, Soulat G, Hulot JS. Adverse myocardial and vascular side effects of immune checkpoint inhibitors: a prospective multimodal cardiovascular assessment. Clin Res Cardiol. 2024;113:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Opałka B, Żołnierczuk M, Grabowska M. Immunosuppressive Agents-Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 71. | Palmieri G, D'Ambrosio MF, Correale M, Brunetti ND, Santacroce R, Iacoviello M, Margaglione M. The Role of Genetics in the Management of Heart Failure Patients. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Yamada T, Nomura S. Recent Findings Related to Cardiomyopathy and Genetics. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Maron BJ, Rowin EJ, Maron MS. Global Burden of Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018;6:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 74. | Höller V, Seebacher H, Zach D, Schwegel N, Ablasser K, Kolesnik E, Gollmer J, Waltl G, Rainer PP, Verheyen S, Zirlik A, Verheyen N. Myocardial Deformation Analysis in MYBPC3 and MYH7 Related Sarcomeric Hypertrophic Cardiomyopathy-The Graz Hypertrophic Cardiomyopathy Registry. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:3022-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 76. | Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res. 2017;121:749-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 1016] [Article Influence: 112.9] [Reference Citation Analysis (1)] |
| 77. | Hershberger RE, Givertz MM, Ho CY, Judge DP, Kantor PF, McBride KL, Morales A, Taylor MRG, Vatta M, Ware SM; ACMG Professional Practice and Guidelines Committee. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018;20:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 78. | Pérez-Hernández M, van Opbergen CJM, Bagwan N, Vissing CR, Marrón-Liñares GM, Zhang M, Torres Vega E, Sorrentino A, Drici L, Sulek K, Zhai R, Hansen FB, Christensen AH, Boesgaard S, Gustafsson F, Rossing K, Small EM, Davies MJ, Rothenberg E, Sato PY, Cerrone M, Jensen THL, Qvortrup K, Bundgaard H, Delmar M, Lundby A. Loss of Nuclear Envelope Integrity and Increased Oxidant Production Cause DNA Damage in Adult Hearts Deficient in PKP2: A Molecular Substrate of ARVC. Circulation. 2022;146:851-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 79. | Bosman LP, Te Riele ASJM. Arrhythmogenic right ventricular cardiomyopathy: a focused update on diagnosis and risk stratification. Heart. 2022;108:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 80. | Di Lorenzo F, Marchionni E, Ferradini V, Latini A, Pezzoli L, Martino A, Romeo F, Iorio A, Bianchi S, Iascone M, Calò L, Novelli G, Mango R, Sangiuolo F. DSP-Related Cardiomyopathy as a Distinct Clinical Entity? Emerging Evidence from an Italian Cohort. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 81. | Cianci V, Forzese E, Sapienza D, Cianci A, Ieni A, Germanà A, Guerrera MC, Omero F, Speranza D, Cracò A, Asmundo A, Gualniera P, Mondello C. Arrhythmogenic Right Ventricular Cardiomyopathy Post-Mortem Assessment: A Systematic Review. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Mukhopadhyay A, Devi B, Baidya AT, Yadav ML, Kumar R, Mohapatra B. Implication of TITINV ariations in Dilated Cardiomyopathy: Integrating Whole Exome Sequencing With Molecular Dynamics Simulation Study. 2024 Preprint. Available from: bioRxiv:622829. [DOI] [Full Text] |
| 83. | Forleo C, Carella MC, Basile P, Carulli E, Dadamo ML, Amati F, Loizzi F, Sorrentino S, Dentamaro I, Dicorato MM, Ricci S, Bagnulo R, Iacoviello M, Santobuono VE, Caiati C, Pepe M, Desaphy JF, Ciccone MM, Resta N, Guaricci AI. Missense and Non-Missense Lamin A/C Gene Mutations Are Similarly Associated with Major Arrhythmic Cardiac Events: A 20-Year Single-Centre Experience. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Dziewięcka E, Winiarczyk M, Wiśniowska-Śmiałek S, Karabinowska-Małocha A, Robak J, Kaciczak M, Baranowski F, Rubiś P. Comparison of Clinical Course and Outcomes between Dilated and Hypokinetic Non-Dilated Cardiomyopathy. Cardiology. 2023;148:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 85. | Bagnall RD, Singer ES, Wacker J, Nowak N, Ingles J, King I, Macciocca I, Crowe J, Ronan A, Weintraub RG, Semsarian C. Genetic Basis of Childhood Cardiomyopathy. Circ Genom Precis Med. 2022;15:e003686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:e159-e240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 490] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 87. | Hespe S, Gray B, Puranik R, Peters S, Sweeting J, Ingles J. The role of genetic testing in management and prognosis of individuals with inherited cardiomyopathies. Trends Cardiovasc Med. 2025;35:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 88. | Murdock DR, Venner E, Muzny DM, Metcalf GA, Murugan M, Hadley TD, Chander V, de Vries PS, Jia X, Hussain A, Agha AM, Sabo A, Li S, Meng Q, Hu J, Tian X, Cohen M, Yi V, Kovar CL, Gingras MC, Korchina V, Howard C, Riconda DL, Pereira S, Smith HS, Huda ZA, Buentello A, Marino PR, Leiber L, Balasubramanyam A, Amos CI, Civitello AB, Chelu MG, Maag R, McGuire AL, Boerwinkle E, Wehrens XHT, Ballantyne CM, Gibbs RA. Genetic testing in ambulatory cardiology clinics reveals high rate of findings with clinical management implications. Genet Med. 2021;23:2404-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Bourfiss M, van Vugt M, Alasiri AI, Ruijsink B, van Setten J, Schmidt AF, Dooijes D, Puyol-Antón E, Velthuis BK, van Tintelen JP, Te Riele ASJM, Baas AF, Asselbergs FW. Prevalence and Disease Expression of Pathogenic and Likely Pathogenic Variants Associated With Inherited Cardiomyopathies in the General Population. Circ Genom Precis Med. 2022;15:e003704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 90. | Bouma BJ, Mulder BJ. Changing Landscape of Congenital Heart Disease. Circ Res. 2017;120:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 91. | Wang T, Chen L, Yang T, Huang P, Wang L, Zhao L, Zhang S, Ye Z, Chen L, Zheng Z, Qin J. Congenital Heart Disease and Risk of Cardiovascular Disease: A Meta-Analysis of Cohort Studies. J Am Heart Assoc. 2019;8:e012030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 92. | Hinton RB, Ware SM. Heart Failure in Pediatric Patients With Congenital Heart Disease. Circ Res. 2017;120:978-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 93. | Surkova E, Segura T, Dimopoulos K, Bispo D, Flick C, West C, Babu-Narayan SV, Senior R, Gatzoulis MA, Li W. Systolic dysfunction of the subpulmonary left ventricle is associated with the severity of heart failure in patients with a systemic right ventricle. Int J Cardiol. 2021;324:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Arvanitaki A, Giannakoulas G, Baumgartner H, Lammers AE. Eisenmenger syndrome: diagnosis, prognosis and clinical management. Heart. 2020;106:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Liu YC, Chen YW, Chen IC, Wu YH, Lo SH, Hsu JS, Hsu JH, Wu BN, Cheng YF, Dai ZK. Long-Term Study on Therapeutic Strategy for Treatment of Eisenmenger Syndrome Patients: A Case Series Study. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Surkova E, Segura T, Dimopoulos K, Flick C, West C, Senior R, Gatzoulis M, Li W. P1292 Prevalence and mechanisms of mitral regurgitation and its association with advanced heart failure in patients with a systemic right ventricle. Eur Heart J Cardiovasc Imaging. 2020;21. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 97. | Egidy Assenza G, Krieger EV, Baumgartner H, Cupido B, Dimopoulos K, Louis C, Lubert AM, Stout KK, Valente AM, Zeppenfeld K, Opotowsky AR. AHA/ACC vs ESC Guidelines for Management of Adults With Congenital Heart Disease: JACC Guideline Comparison. J Am Coll Cardiol. 2021;78:1904-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 98. | Eisenberg-Guyot J, Presskreischer R, Pamplin JR 2nd. Psychiatric Epidemiology During the COVID-19 Pandemic. Curr Epidemiol Rep. 2024;11:120-130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 99. | O'Neil A, Fisher AJ, Kibbey KJ, Jacka FN, Kotowicz MA, Williams LJ, Stuart AL, Berk M, Lewandowski PA, Taylor CB, Pasco JA. Depression is a risk factor for incident coronary heart disease in women: An 18-year longitudinal study. J Affect Disord. 2016;196:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 100. | Iranpour S, Sabour S, Koohi F, Saadati HM. The trend and pattern of depression prevalence in the U.S.: Data from National Health and Nutrition Examination Survey (NHANES) 2005 to 2016. J Affect Disord. 2022;298:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 101. | Kieling C, Buchweitz C, Caye A, Silvani J, Ameis SH, Brunoni AR, Cost KT, Courtney DB, Georgiades K, Merikangas KR, Henderson JL, Polanczyk GV, Rohde LA, Salum GA, Szatmari P. Worldwide Prevalence and Disability From Mental Disorders Across Childhood and Adolescence: Evidence From the Global Burden of Disease Study. JAMA Psychiatry. 2024;81:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 300] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 102. | Marques de Miranda D, da Silva Athanasio B, Sena Oliveira AC, Simoes-E-Silva AC. How is COVID-19 pandemic impacting mental health of children and adolescents? Int J Disaster Risk Reduct. 2020;51:101845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 103. | Hawes MT, Szenczy AK, Klein DN, Hajcak G, Nelson BD. Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol Med. 2022;52:3222-3230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 415] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 104. | Kivimäki M, Batty GD, Pentti J, Shipley MJ, Sipilä PN, Nyberg ST, Suominen SB, Oksanen T, Stenholm S, Virtanen M, Marmot MG, Singh-Manoux A, Brunner EJ, Lindbohm JV, Ferrie JE, Vahtera J. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5:e140-e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 476] [Article Influence: 79.3] [Reference Citation Analysis (1)] |
| 105. | Psaltopoulou T, Hatzis G, Papageorgiou N, Androulakis E, Briasoulis A, Tousoulis D. Socioeconomic status and risk factors for cardiovascular disease: Impact of dietary mediators. Hellenic J Cardiol. 2017;58:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 106. | Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 677] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 107. | McGorry PD, Mei C, Chanen A, Hodges C, Alvarez-Jimenez M, Killackey E. Designing and scaling up integrated youth mental health care. World Psychiatry. 2022;21:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 275] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 108. | Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis JPA. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20:200-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 109. | Tuvali O, Tshori S, Derazne E, Hannuna RR, Afek A, Haberman D, Sella G, George J. The Incidence of Myocarditis and Pericarditis in Post COVID-19 Unvaccinated Patients-A Large Population-Based Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Standl E, Schnell O. Heart failure outcomes and Covid-19. Diabetes Res Clin Pract. 2021;175:108794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Paraskevaidis I, Farmakis D, Papingiotis G, Tsougos E. Inflammation and Heart Failure: Searching for the Enemy-Reaching the Entelechy. J Cardiovasc Dev Dis. 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 112. | Adeghate EA, Eid N, Singh J. Mechanisms of COVID-19-induced heart failure: a short review. Heart Fail Rev. 2021;26:363-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 113. | Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1240] [Cited by in RCA: 1511] [Article Influence: 251.8] [Reference Citation Analysis (3)] |
| 114. | Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, Brunjes D, Glicksberg BS, Levin MA, Nadkarni G, Fayad Z, Fuster V, Mancini D, Lala A. Prognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19. J Am Coll Cardiol. 2020;76:2334-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 115. | Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 1078] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 116. | Salah HM, Fudim M, O'Neil ST, Manna A, Chute CG, Caughey MC. Post-recovery COVID-19 and incident heart failure in the National COVID Cohort Collaborative (N3C) study. Nat Commun. 2022;13:4117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 117. | Guo B, Zhao C, He MZ, Senter C, Zhou Z, Peng J, Li S, Fitzpatrick AL, Lindström S, Stebbins RC, Noppert GA, Li C. Long-term cardiac symptoms following COVID-19: a systematic review and meta-analysis. medRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 118. | Trimaille A, Ribeyrolles S, Fauvel C, Chaumont C, Weizman O, Pommier T, Cellier J, Geneste L, Panagides V, Marsou W, Deney A, Attou S, Delmotte T, Chemaly P, Karsenty C, Giordano G, Gautier A, Guilleminot P, Sagnard A, Pastier J, Duceau B, Sutter W, Waldmann V, Pezel T, Mika D, Cohen A, Bonnet G; The Critical Covid-France Investigators. Cardiovascular Characteristics and Outcomes of Young Patients with COVID-19. J Cardiovasc Dev Dis. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Wan EYF, Mathur S, Zhang R, Yan VKC, Lai FTT, Chui CSL, Li X, Wong CKH, Chan EWY, Yiu KH, Wong ICK. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: a prospective cohort in UK Biobank. Cardiovasc Res. 2023;119:1718-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 120. | Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1452] [Cited by in RCA: 1306] [Article Influence: 326.5] [Reference Citation Analysis (0)] |
| 121. | O'Keefe EL, Dhore-Patil A, Lavie CJ. Early-Onset Cardiovascular Disease From Cocaine, Amphetamines, Alcohol, and Marijuana. Can J Cardiol. 2022 Sep;38(9):1342-1351. doi: 10.1016/j.cjca.2022.06.027 Epub 2022 Jul 14. PMID: 35840019.. |
| 122. | Mukkawar RV, Reddy H, Rathod N, Kumar S, Acharya S. The Long-Term Cardiovascular Impact of COVID-19: Pathophysiology, Clinical Manifestations, and Management. Cureus. 2024;16:e66554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 123. | Zhang B, Thacker D, Zhou T, Zhang D, Lei Y, Chen J, Chrischilles E, Christakis DA, Fernandez S, Garg V, Kim S, Mosa ASM, Sills MR, Taylor BW, Williams DA, Wu Q, Forrest CB, Chen Y. Post-Acute Cardiovascular Outcomes of COVID-19 in Children and Adolescents: An EHR Cohort Study from the RECOVER Project. medRxiv. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 124. | Lim JT, Liang En W, Tay AT, Pang D, Chiew CJ, Ong B, Lye DCB, Tan KB. Long-term Cardiovascular, Cerebrovascular, and Other Thrombotic Complications in COVID-19 Survivors: A Retrospective Cohort Study. Clin Infect Dis. 2024;78:70-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 125. | Reddy PKV, Ng TMH, Oh EE, Moady G, Elkayam U. Clinical Characteristics and Management of Methamphetamine-Associated Cardiomyopathy: State-of-the-Art Review. J Am Heart Assoc. 2020;9:e016704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 126. | Kim TE, Kim H, Sung J, Kim DK, Lee MS, Han SW, Kim HJ, Kim SH, Ryu KH. The association between metabolic syndrome and heart failure in middle-aged male and female: Korean population-based study of 2 million individuals. Epidemiol Health. 2022;44:e2022078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 360] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 128. | Tibrewala A, Yancy CW. Heart Failure with Preserved Ejection Fraction in Women. Heart Fail Clin. 2019;15:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 129. | Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2023;118:3434-3450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 288] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 130. | Grubb AF, Greene SJ, Fudim M, Dewald T, Mentz RJ. Drugs of Abuse and Heart Failure. J Card Fail. 2021;27:1260-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 131. | Ukah UV, Potter BJ, Paradis G, Low N, Ayoub A, Auger N. Cocaine and the Long-Term Risk of Cardiovascular Disease in Women. Am J Med. 2022;135:993-1000.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 132. | Mehr JB, Bennett ER, Price JL, de Souza NL, Buckman JF, Wilde EA, Tate DF, Marshall AD, Dams-O'Connor K, Esopenko C. Intimate partner violence, substance use, and health comorbidities among women: A narrative review. Front Psychol. 2022;13:1028375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 133. | Rashid S, Qureshi AG, Noor TA, Yaseen K, Sheikh MAA, Malik M, Malik J. Anxiety and Depression in Heart Failure: An Updated Review. Curr Probl Cardiol. 2023;48:101987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 134. | Cui F, Zheng L, Zhang J, Tang L, Ma Y, Li D, Wang J, Xing M, Xie J, Yang J, Tian Y. Long-term exposure to fine particulate matter constituents, genetic susceptibility, and incident heart failure among 411 807 adults. Eur J Heart Fail. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 135. | Zhao H, Tan Z, Liu M, Yu P, Ma J, Li X, Wang J, Zhao Y, Zhu W, Liu X. Is There a Sex Difference in the Prognosis of Hypertrophic Cardiomyopathy? A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2023;12:e026270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 136. | Butters A, Lakdawala NK, Ingles J. Sex Differences in Hypertrophic Cardiomyopathy: Interaction With Genetics and Environment. Curr Heart Fail Rep. 2021;18:264-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 137. | Lamounier Júnior A, Ferrari F, Max R, Ritt LEF, Stein R. Importance of Genetic Testing in Dilated Cardiomyopathy: Applications and Challenges in Clinical Practice. Arq Bras Cardiol. 2019;113:274-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Vardar U, Shaka H, Kumi D, Gajjar R, Bess O, Kanemo P, Shaka A, Baskaran N. Gender disparities, causes and predictors of immediate and short-term cardiovascular readmissions following COVID-19-related hospitalisations in the USA. BMJ Open. 2023;13:e073959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 139. | Mangion K, Morrow AJ, Sykes R, Kamdar A, Bagot C, Bruce G, Connelly P, Delles C, Gibson VB, Gillespie L, Barrientos PH, Lennie V, Roditi G, Sattar N, Stobo D, Allwood-Spiers S, McConnachie A, Berry C; CISCO-19 investigators. Post-COVID-19 illness and associations with sex and gender. BMC Cardiovasc Disord. 2023;23:389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 140. | Shoaibinobarian N, Danehchin L, Mozafarinia M, Hekmatdoost A, Eghtesad S, Masoudi S, Mohammadi Z, Mard A, Paridar Y, Abolnezhadian F, Malihi R, Rahimi Z, Cheraghian B, Mir-Nasseri MM, Shayesteh AA, Poustchi H. The Association between DASH Diet Adherence and Cardiovascular Risk Factors. Int J Prev Med. 2023;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 141. | Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J. 2019;40:3859-3868c. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 142. | Alkhulaifi HSM, Alshammari NN, Hakami AHO, Alqarnii MSM, Alshehri KM, Alshammary HH, Somily TI, Mahnashi AB, Albalawi SES, Al Otabei MHS, Alshammari AHO, Alqahtani ARM. Impact of Lifestyle Interventions on Heart Disease Prevention-Hypertension and Atherosclerotic Cardiovascular Disease. Egypt J Chem. 2024;67:881-909. [DOI] [Full Text] |
| 143. | Scimeca M, Palumbo V, Giacobbi E, Servadei F, Casciardi S, Cornella E, Cerbara F, Rotondaro G, Seghetti C, Scioli MP, Montanaro M, Barillà F, Sisto R, Melino G, Mauriello A, Bonfiglio R. Impact of the environmental pollution on cardiovascular diseases: From epidemiological to molecular evidence. Heliyon. 2024;10:e38047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 144. | Kemerley A, Gupta A, Thirunavukkarasu M, Maloney M, Burgwardt S, Maulik N. COVID-19 Associated Cardiovascular Disease-Risks, Prevention and Management: Heart at Risk Due to COVID-19. Curr Issues Mol Biol. 2024;46:1904-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 145. | Dauenhauer B, Stoepker P. Physical Education and Physical Activity Within a Whole School, Whole Community, Whole Child Approach. J Phys Educ Recreat Dance. 2022;93:12-19. [DOI] [Full Text] |
| 146. | Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 471] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 147. | US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW Jr, Kubik M, Landefeld S, Ogedegbe G, Pbert L, Silverstein M, Simon MA, Tseng CW, Wong JB. Behavioral Counseling Interventions to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults With Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;324:2069-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 148. | Buonocore JJ, Fisher J, Prull D, Willis MD, Arunachalam S, Perera F, Kinney P, Sousa B, Levy JI. Federal Policy Platforms and Public Health: Reinforcing the Benefits of Air Pollution Control Devices at Power Plants in the United States. Am J Public Health. 2025;115:30-33. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 149. | Henning RJ. Particulate Matter Air Pollution is a Significant Risk Factor for Cardiovascular Disease. Curr Probl Cardiol. 2024;49:102094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 150. | Nguyen PY, Astell-Burt T, Rahimi-Ardabili H, Feng X. Green Space Quality and Health: A Systematic Review. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 151. | Astell-Burt T, Feng X. Urban green space, tree canopy and prevention of cardiometabolic diseases: a multilevel longitudinal study of 46 786 Australians. Int J Epidemiol. 2020;49:926-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 152. | Ramírez AS, Ramondt S, Van Bogart K, Perez-Zuniga R. Public Awareness of Air Pollution and Health Threats: Challenges and Opportunities for Communication Strategies To Improve Environmental Health Literacy. J Health Commun. 2019;24:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 153. | Haug S, Paz Castro R, Wenger A, Schaub MP. A Mobile Phone-Based Life-Skills Training Program for Substance Use Prevention Among Adolescents: Cluster-Randomized Controlled Trial. JMIR Mhealth Uhealth. 2021;9:e26951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 154. | Hyatt JM, Lobmaier PP. Medication assisted treatment (MAT) in criminal justice settings as a double-edged sword: balancing novel addiction treatments and voluntary participation. Health Justice. 2020;8:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 155. | Kaminsky LA, German C, Imboden M, Ozemek C, Peterman JE, Brubaker PH. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog Cardiovasc Dis. 2022;70:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 156. | Hacker KA, Briss PA, Richardson L, Wright J, Petersen R. COVID-19 and Chronic Disease: The Impact Now and in the Future. Prev Chronic Dis. 2021;18:E62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 157. | Drosos GC, Vedder D, Houben E, Boekel L, Atzeni F, Badreh S, Boumpas DT, Brodin N, Bruce IN, González-Gay MÁ, Jacobsen S, Kerekes G, Marchiori F, Mukhtyar C, Ramos-Casals M, Sattar N, Schreiber K, Sciascia S, Svenungsson E, Szekanecz Z, Tausche AK, Tyndall A, van Halm V, Voskuyl A, Macfarlane GJ, Ward MM, Nurmohamed MT, Tektonidou MG. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis. 2022;81:768-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 158. | Kostopoulou M, Nikolopoulos D, Parodis I, Bertsias G. Cardiovascular Disease in Systemic Lupus Erythematosus: Recent Data on Epidemiology, Risk Factors and Prevention. Curr Vasc Pharmacol. 2020;18:549-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 159. | Restivo V, Candiloro S, Daidone M, Norrito R, Cataldi M, Minutolo G, Caracci F, Fasano S, Ciccia F, Casuccio A, Tuttolomondo A. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: Symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun Rev. 2022;21:102925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 160. | Lai CH, Hsieh CY, Barnado A, Huang LC, Chen SC, Tsai LM, Shyr Y, Li CY. Outcomes of acute cardiovascular events in rheumatoid arthritis and systemic lupus erythematosus: a population-based study. Rheumatology (Oxford). 2020;59:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 161. | Bushey E, Wu Y, Wright A, Pescatello L. The Influence of Physical Activity and Diet Mobile Apps on Cardiovascular Disease Risk Factors: Meta-Review. J Med Internet Res. 2024;26:e51321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 162. | Tromp J, Paniagua SMA, Lau ES, Allen NB, Blaha MJ, Gansevoort RT, Hillege HL, Lee DE, Levy D, Vasan RS, van der Harst P, van Gilst WH, Larson MG, Shah SJ, de Boer RA, Lam CSP, Ho JE. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 163. | Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, Mentz RJ, O'Brien E, Correa A, Suthahar N, de Boer RA, Wilkins JT, Lloyd-Jones DM. 10-Year Risk Equations for Incident Heart Failure in the General Population. J Am Coll Cardiol. 2019;73:2388-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 164. | Muller SA, Gasperetti A, Bosman LP, Schmidt AF, Baas AF, Amin AS, Houweling AC, Wilde AAM, Compagnucci P, Targetti M, Casella M, Calò L, Tondo C, van der Harst P, Asselbergs FW, van Tintelen JP, Oerlemans MIFJ, Te Riele ASJM. Individualized Family Screening for Arrhythmogenic Right Ventricular Cardiomyopathy. J Am Coll Cardiol. 2023;82:214-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 165. | Dellefave-Castillo LM, Cirino AL, Callis TE, Esplin ED, Garcia J, Hatchell KE, Johnson B, Morales A, Regalado E, Rojahn S, Vatta M, Nussbaum RL, McNally EM. Assessment of the Diagnostic Yield of Combined Cardiomyopathy and Arrhythmia Genetic Testing. JAMA Cardiol. 2022;7:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 166. | Ju D, Hui D, Hammond DA, Wonkam A, Tishkoff SA. Importance of Including Non-European Populations in Large Human Genetic Studies to Enhance Precision Medicine. Annu Rev Biomed Data Sci. 2022;5:321-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 167. | Vlasschaert C, Mack T, Heimlich JB, Niroula A, Uddin MM, Weinstock J, Sharber B, Silver AJ, Xu Y, Savona M, Gibson C, Lanktree MB, Rauh MJ, Ebert BL, Natarajan P, Jaiswal S, Bick AG. A practical approach to curate clonal hematopoiesis of indeterminate potential in human genetic data sets. Blood. 2023;141:2214-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 168. | Haga SB, Barry WT, Mills R, Ginsburg GS, Svetkey L, Sullivan J, Willard HF. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomarkers. 2013;17:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 169. | Geelen E, Van Hoyweghen I, Horstman K. Making genetics not so important: family work in dealing with familial hypertrophic cardiomyopathy. Soc Sci Med. 2011;72:1752-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 170. | Beattie JM, Castiello T, Jaarsma T. The Importance of Cultural Awareness in the Management of Heart Failure: A Narrative Review. Vasc Health Risk Manag. 2024;20:109-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 171. | Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, Dickens C, Coventry P. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 172. | Stallard P. School-based interventions for depression and anxiety in children and adolescents. Evid Based Ment Health. 2013;16:60-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 173. | mhGAP Intervention Guide for Mental, Neurological and Substance Use Disorders in Non-Specialized Health Settings: Mental Health Gap Action Programme (mhGAP): Version 2.0. Geneva: World Health Organization; 2016– . [PubMed] |
| 174. | Horwitz LI, Thaweethai T, Brosnahan SB, Cicek MS, Fitzgerald ML, Goldman JD, Hess R, Hodder SL, Jacoby VL, Jordan MR, Krishnan JA, Laiyemo AO, Metz TD, Nichols L, Patzer RE, Sekar A, Singer NG, Stiles LE, Taylor BS, Ahmed S, Algren HA, Anglin K, Aponte-Soto L, Ashktorab H, Bassett IV, Bedi B, Bhadelia N, Bime C, Bind MC, Black LJ, Blomkalns AL, Brim H, Castro M, Chan J, Charney AW, Chen BK, Chen LQ, Chen P, Chestek D, Chibnik LB, Chow DC, Chu HY, Clifton RG, Collins S, Costantine MM, Cribbs SK, Deeks SG, Dickinson JD, Donohue SE, Durstenfeld MS, Emery IF, Erlandson KM, Facelli JC, Farah-Abraham R, Finn AV, Fischer MS, Flaherman VJ, Fleurimont J, Fonseca V, Gallagher EJ, Gander JC, Gennaro ML, Gibson KS, Go M, Goodman SN, Granger JP, Greenway FL, Hafner JW, Han JE, Harkins MS, Hauser KSP, Heath JR, Hernandez CR, Ho O, Hoffman MK, Hoover SE, Horowitz CR, Hsu H, Hsue PY, Hughes BL, Jagannathan P, James JA, John J, Jolley S, Judd SE, Juskowich JJ, Kanjilal DG, Karlson EW, Katz SD, Kelly JD, Kelly SW, Kim AY, Kirwan JP, Knox KS, Kumar A, Lamendola-Essel MF, Lanca M, Lee-Lannotti JK, Lefebvre RC, Levy BD, Lin JY, Logarbo BP Jr, Logue JK, Longo MT, Luciano CA, Lutrick K, Malakooti SK, Mallett G, Maranga G, Marathe JG, Marconi VC, Marshall GD, Martin CF, Martin JN, May HT, McComsey GA, McDonald D, Mendez-Figueroa H, Miele L, Mittleman MA, Mohandas S, Mouchati C, Mullington JM, Nadkarni GN, Nahin ER, Neuman RB, Newman LT, Nguyen A, Nikolich JZ, Ofotokun I, Ogbogu PU, Palatnik A, Palomares KTS, Parimon T, Parry S, Parthasarathy S, Patterson TF, Pearman A, Peluso MJ, Pemu P, Pettker CM, Plunkett BA, Pogreba-Brown K, Poppas A, Porterfield JZ, Quigley JG, Quinn DK, Raissy H, Rebello CJ, Reddy UM, Reece R, Reeder HT, Rischard FP, Rosas JM, Rosen CJ, Rouphael NG, Rouse DJ, Ruff AM, Saint Jean C, Sandoval GJ, Santana JL, Schlater SM, Sciurba FC, Selvaggi C, Seshadri S, Sesso HD, Shah DP, Shemesh E, Sherif ZA, Shinnick DJ, Simhan HN, Singh U, Sowles A, Subbian V, Sun J, Suthar MS, Teunis LJ, Thorp JM Jr, Ticotsky A, Tita ATN, Tragus R, Tuttle KR, Urdaneta AE, Utz PJ, VanWagoner TM, Vasey A, Vernon SD, Vidal C, Walker T, Ward HD, Warren DE, Weeks RM, Weiner SJ, Weyer JC, Wheeler JL, Whiteheart SW, Wiley Z, Williams NJ, Wisnivesky JP, Wood JC, Yee LM, Young NM, Zisis SN, Foulkes AS. Researching COVID to Enhance Recovery (RECOVER) adult study protocol: Rationale, objectives, and design. PLoS One. 2023;18:e0286297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 175. | Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. 2019;364:l223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 176. | Writing Committee; Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:772-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 606] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 177. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 8547] [Article Influence: 1709.4] [Reference Citation Analysis (0)] |
| 178. | Greene SJ, Butler J, Kosiborod MN. Chapter 3: Clinical Trials of Sodium-Glucose Co-Transporter-2 Inhibitors for Treatment of Heart Failure. Am J Med. 2024;137:S25-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 179. | Zannad F, Ferreira JP, Pocock SJ, Zeller C, Anker SD, Butler J, Filippatos G, Hauske SJ, Brueckmann M, Pfarr E, Schnee J, Wanner C, Packer M. Cardiac and Kidney Benefits of Empagliflozin in Heart Failure Across the Spectrum of Kidney Function: Insights From EMPEROR-Reduced. Circulation. 2021;143:310-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 180. | Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O'Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJV. Efficacy of Dapagliflozin on Renal Function and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction: Results of DAPA-HF. Circulation. 2021;143:298-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 181. | Shah MJ, Silka MJ, Silva JNA, Balaji S, Beach CM, Benjamin MN, Berul CI, Cannon B, Cecchin F, Cohen MI, Dalal AS, Dechert BE, Foster A, Gebauer R, Gonzalez Corcia MC, Kannankeril PJ, Karpawich PP, Kim JJ, Krishna MR, Kubuš P, LaPage MJ, Mah DY, Malloy-Walton L, Miyazaki A, Motonaga KS, Niu MC, Olen M, Paul T, Rosenthal E, Saarel EV, Silvetti MS, Stephenson EA, Tan RB, Triedman J, Von Bergen NH, Wackel PL; Document Reviewers: Philip M. Chang, Fabrizio Drago, Anne M. Dubin, Susan P. Etheridge, Apichai Kongpatanayothin, Jose Manuel Moltedo, Ashish A. Nabar and George F. Van Hare. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Cardiol Young. 2021;31:1738-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 182. | Singh B, Hsieh YC, Liu YB, Lin KH, Joung B, Rodriguez DA, Chasnoits AR, Huang D, Zhang S, O'Brien JE, Lexcen DR, Cerkvenik J, Van Dorn B, Ching CK. Cardioverter-defibrillator reduces mortality risk in eligible ischemic and non-ischemic cardiomyopathy patients: Sub-analysis of the multi-center Improve SCA study. Indian Heart J. 2023;75:115-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 183. | Fastner C, Varma N, Rao I, Falk P, Remppis BA, Najarian K, Burkhoff D, Akin I, Kuschyk J. Cardiac contractility modulation in heart failure with reduced ejection fraction patients with QRS duration 120-149 ms: Reduction in heart failure hospitalizations and improvement in functional outcome. Heart Rhythm. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 184. | Writing Committee Members; Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:450-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 820] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 185. | Vacca A, Wang R, Nambiar N, Capone F, Farrelly C, Mostafa A, Sechi LA, Schiattarella GG. Lifestyle interventions in cardiometabolic HFpEF: dietary and exercise modalities. Heart Fail Rev. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 186. | Guo R, Li N, Yang R, Liao XY, Zhang Y, Zhu BF, Zhao Q, Chen L, Zhang YG, Lei Y. Effects of the Modified DASH Diet on Adults With Elevated Blood Pressure or Hypertension: A Systematic Review and Meta-Analysis. Front Nutr. 2021;8:725020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 187. | Volterrani M, Caminiti G, Perrone MA, Cerrito A, Franchini A, Manzi V, Iellamo F. Effects of Concurrent, Within-Session, Aerobic and Resistance Exercise Training on Functional Capacity and Muscle Performance in Elderly Male Patients with Chronic Heart Failure. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 188. | Myers J, Kokkinos P, Arena R, LaMonte MJ. The impact of moving more, physical activity, and cardiorespiratory fitness: Why we should strive to measure and improve fitness. Prog Cardiovasc Dis. 2021;64:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 189. | Varshney M, Kaur A, Sarin SK, Shasthry SM, Arora V. Safety and Effectiveness of Naltrexone in the Management of Alcohol Use Disorder in Patients With Alcohol-associated Cirrhosis: First Clinical Observation From Indian Cohort. J Clin Exp Hepatol. 2025;15:102447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 190. | Goyal P, Balkan L, Ringel JB, Hummel SL, Sterling MR, Kim S, Arora P, Jackson EA, Brown TM, Shikany JM, Judd SE, Safford MM, Levitan EB. The Dietary Approaches to Stop Hypertension (DASH) Diet Pattern and Incident Heart Failure. J Card Fail. 2021;27:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 191. | Holdgaard A, Eckhardt-Hansen C, Lassen CF, Kjesbu IE, Dall CH, Michaelsen KL, Sibilitz KL, Prescott E, Rasmusen HK. Cognitive-behavioural therapy reduces psychological distress in younger patients with cardiac disease: a randomized trial. Eur Heart J. 2023;44:986-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 192. | Bounader K, Flécher E. End-stage heart failure: The future of heart transplant and artificial heart. Presse Med. 2024;53:104191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 193. | Numan L, Schramm R, Oerlemans MIFJ, van der Kaaij NP, Aarts E, Ramjankhan FZ, Oppelaar AM, Morshuis M, Guenther SPW, Zimpfer D, Riebandt J, Wiedemann D, Asselbergs FW, Van Laake LW. Survival after HeartMate 3 left ventricular assist device implantation: real-world data from Europe. ESC Heart Fail. 2023;10:2754-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/