Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.103909
Revised: January 14, 2025
Accepted: February 6, 2025
Published online: February 26, 2025
Processing time: 83 Days and 7.5 Hours
Unheralded cardiac arrest among previously healthy young people without antecedent illness, months or years after coronavirus disease 2019 (COVID-19) vaccination, highlights the urgent need for risk stratification. The most likely underlying pathophysiology is subclinical myopericarditis and reentrant ventri
Core Tip: This study reviews evidence linking mRNA vaccines to cardiac pathology and proposes a comprehensive risk stratification approach involving patient history, antibody testing, and cardiac diagnostics. By identifying high-risk individuals through measurable endpoints like spike protein exposure and cardiac biomarkers, this approach seeks to guide clinicians in addressing the risks of myocarditis, arrhythmias, and cardiac arrest post-vaccination. Implementing this framework in primary care settings may improve cardiovascular outcomes and reduce preventable deaths.
- Citation: McCullough PA, Hulscher N. Risk stratification for future cardiac arrest after COVID-19 vaccination. World J Cardiol 2025; 17(2): 103909
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/103909.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.103909
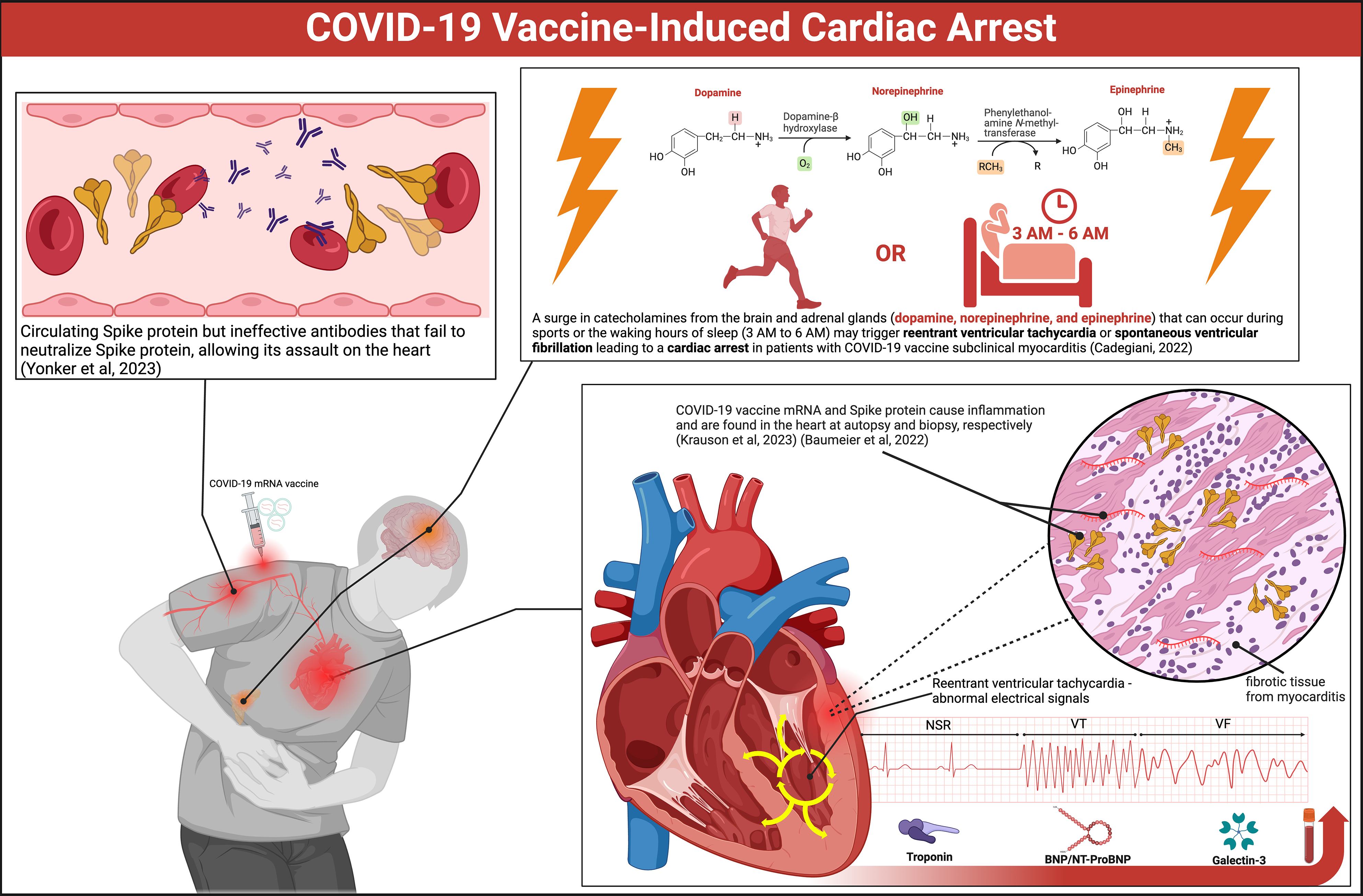
We continue to observe coronavirus disease 2019 (COVID-19) vaccinated persons suffer cardiac arrests since the inception of the mass vaccination campaign in late 2020[1-5]. Figure 1 illustrates the likely mechanisms. Both Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) mRNA have been found in human heart muscle at autopsy[6]. Spike protein has been stained in endomyocardial biopsy samples of young men suffering from COVID-19 vaccine-induced myocarditis[7]. Victims have been found to have circulating Spike protein but ineffective antibodies, likely IgG4 subclass, that fail to neutralize Spike protein and allow its assault on the heart[8]. Positron emission tomography data have revealed a shift from free fatty acid metabolism to glucose metabolism in the hearts of the majority of individuals who have received a COVID-19 vaccine[9]. The positron emission tomography pattern resembles global ischemia. This could be due to vaccine Spike protein hemagglutination in myocardial capillaries or cellular changes in mitochondrial respiration and substrate metabolism[10]. Small patches of dysfunctional, inflamed, or scarred myocardium are sufficient to serve as a nidus for re-entrant ventricular tachycardia that can degrade to ventricular fibrillation and lead to cardiac arrest[11]. A surge in catecholamines (epinephrine, norepinephrine, and dopamine) that can occur during sports or the waking hours of sleep (3 AM to 6 AM) may trigger reentrant ventricular tachycardia or spontaneous ventricular fibrillation leading to a cardiac arrest in patients with COVID-19 vaccine subclinical myocarditis[12].
Elevated numbers of sudden deaths among athletes after COVID-19 vaccination have raised concerns[13]. Alessandria et al[14] amplified these concerns, demonstrating higher all-cause death risks in COVID-19 vaccinated individuals compared to the unvaccinated. Participants that received 2 doses lost 37% of life expectancy compared to the unvacci
The United States Food and Drug Administration's Center for Biologics Evaluation and Research has established a follow-up period of 5 to 15 years for novel genetic products to monitor for any long-term adverse effects that may emerge in the exposed population[18]. With the passage of time, we have learned much from the evaluation of many vaccine-injured victims who have symptoms months to years after injection[19-21]. Brogna et al[22] found vaccine-generated stabilized prefusion Spike protein in subjects up to 6 months after COVID-19 mRNA vaccination. Cardiac abnorma
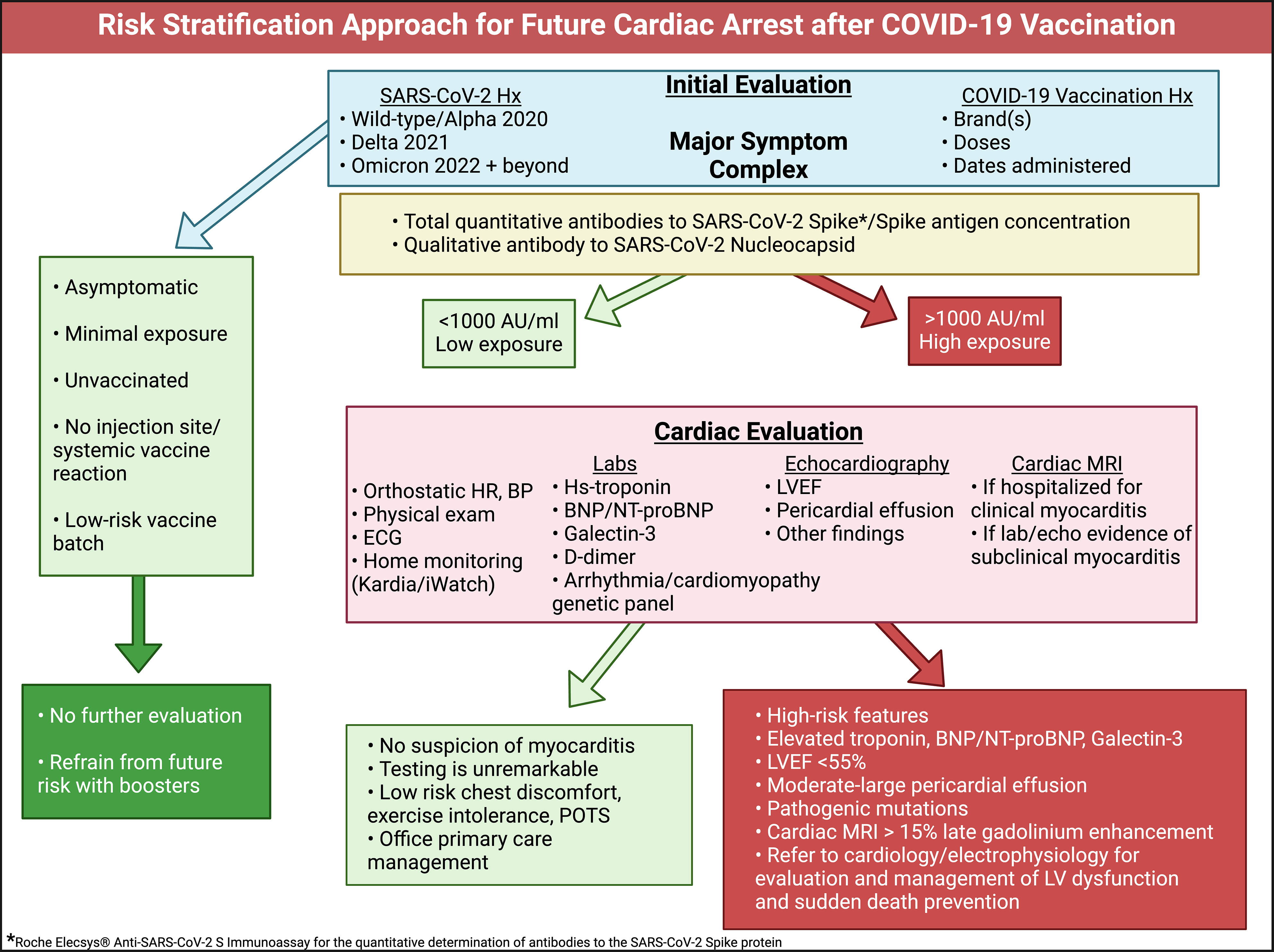
When patients are seen in clinical practice for the initial evaluation of cardiovascular symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or vaccination, a proposed approach is outlined in Figure 2. COVID-19 vaccine-induced myocarditis is caused principally by the Spike protein[7,8,16,24]. Measures of cytokine activation and inflammation are secondary to Spike protein resident in the myocardium and circulating in blood[7,8].
A detailed SARS-CoV-2 infection and vaccine administration history is essential to the evaluation. Serious COVID-19 requiring hospitalization and mechanical ventilation should be noted. Each SARS-CoV-2 infection and COVID-19 vaccine dose can be considered an ‘exposure’. It can be reasonably assumed that the greater the number of exposures, the more likely it is to have large quantities of Spike protein in the body, where natural proteases and lysosomes seem unable to clear it[25].
An extended range total antibody against the Spike protein is a useful proxy for prior Spike exposure. For example, the Roche Diagnostics Elecsys Anti-SARS-CoV-2 Spike assay measures antibodies against the receptor binding domain[26]. Roche Elecsys Anti-SARS-CoV-2 assay has a normal value of < 0.8 U/mL, and in clinical practice, 0-1000 is low risk, and > 1000 indicates higher risk with either multiple infections or COVID-19 vaccine administrations. We have observed that it is not uncommon to find patients with > 25000 U/mL, remaining unmeasurably high even years after vaccination. Antibody concentrations can take at least 12 months to taper off. Thus, a Spike antigen test measurable in whole blood, plasma, or serum is greatly needed to provide a real-time estimate of Spike toxicity.
If (1) the number of Spike protein exposures was low; (2) there were nonserious infections; (3) Spike antibody levels are below 1000 U/mL; (4) minimal or no injection site or initial systemic reaction to vaccination[27,28]; and (5) if the vaccine was obtained from a low-risk batch[29,30] (with zero hospitalizations and deaths found in a VAERS batch analysis[31]), then additional clinical investigation may not be warranted. If there are higher risk features as indicated in the Figure 2, then it is prudent to perform more formal cardiac testing and risk stratification.
It is reasonable to obtain an electrocardiogram and blood testing for high-sensitivity troponin, BNP/NT-ProBNP, galectin-3, and D-dimer. Elevated troponin may indicate ongoing myocarditis[32]. BNP/NT-proBNP are reliable indicators of cardiac pressure/volume overload and predict heart failure[33]. Galectin-3 is a chronic inflammation/fibrosis marker and when elevated predicts future heart failure[34]. D-dimer is a proxy for micro-blood clotting[35] and in our experience < 0.2 is low risk, 0.2-0.5 is moderate, and > 0.5 is high risk for thrombotic events. Patel et al[36] demon
Point-of-care echocardiography or formal cardiac ultrasound and Doppler can be used to evaluate cardiac structure, function, and presence of pericardial fluid[38]. This package of diagnostics, when results are completely normal, suggests a low risk for future heart failure and cardiac death[39]. Conversely, multiple abnormalities found in electrocardiogram, blood tests, and echocardiography can prompt the use of cardiac magnetic resonance imaging (MRI) with contrast[40]. The MRI is confirmatory for left ventricular function and importantly can detect areas of inflammation/scar with late gadolinium enhancement (LGE)[41]. In other types of cardiomyopathy, LGE ≥ 15% of the left ventricle indicates high risk for cardiac arrest[42].
Small patches of myocardial inflammation, edema, or fibrosis may not be detectable by axial slices on cardiac MRI or autopsy[43,44]. Thus, the heart may appear normal on post-mortem examination and the final report may indicate death due to “natural causes” in a healthy patient with no antecedent disease. We believe these cases likely represent previously silent subclinical myocarditis and cardiac fibrosis which serves as the substrate for re-entrant ventricular tachycardia that degenerates to ventricular fibrillation and asystole in patients that do not receive prompt defibrillation.
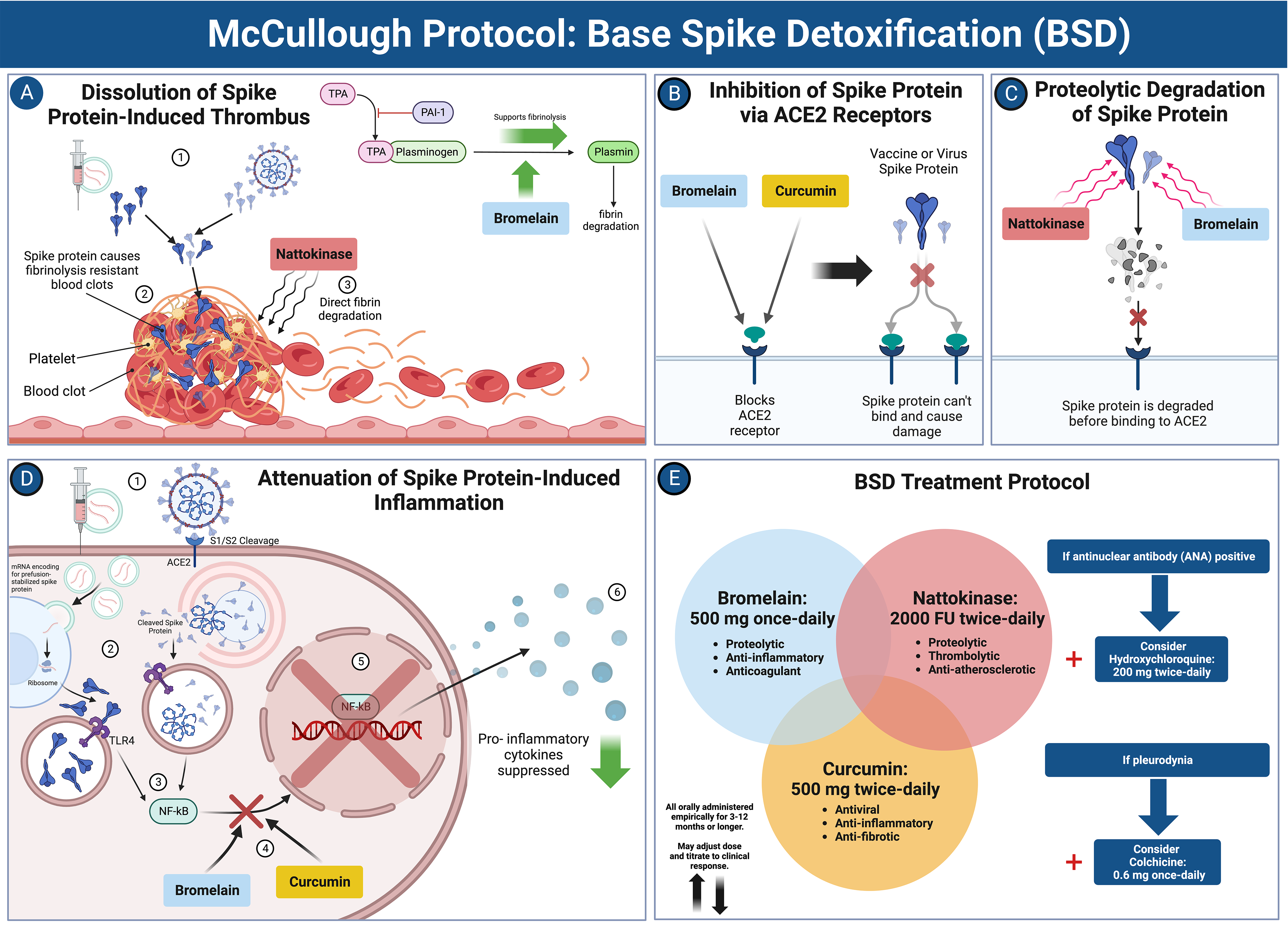
When risk stratification indicates low-risk, primary care office management is suggested with McCullough Protocol: Base Spike Detoxification and adjunctive medications depending on the syndrome (Figure 3)[25]. For subclinical myopericarditis, oral colchicine 0.6 mg twice a day (BID) or once a day is indicated for at least one year[45]. For COVID-19 vaccine-induced postural orthostatic tachycardia syndrome, use of colchicine and nadolol 20-40 mg BID can be helpful[46]. In patients who screen as high-risk, Base Spike Detoxification[25] is also indicated. For those at very high risk for cardiomyopathy and or ventricular arrhythmias, formal cardiology consultation is suggested with the main goals of preventing heart failure and sudden death. Additionally, some patients may require ICD implantation if there are symptomatic arrhythmias, LGE > 15%, or genetic predictors such as pathogenic SCN5A mutations[37,47].
While this framework is designed for the general population, it is important to recognize that specific subgroups, such as older adults and individuals with pre-existing cardiovascular conditions, may require additional considerations. Older adults, for example, may benefit from more frequent monitoring and tailored preventive strategies to mitigate heightened risks associated with age-related cardiovascular changes. Similarly, patients with a history of heart disease should undergo customized evaluations and interventions to address their unique vulnerabilities. Incorporating these subgroup-specific approaches will further enhance the practical utility of the proposed framework across diverse patient populations. While the proposed risk stratification framework is already supported by substantial indirect evidence and sound clinical principles, it is important to note that real-world implementation data are currently lacking. This represents an opportunity to validate its effectiveness through future retrospective analyses and prospective clinical studies, which will not only strengthen its utility but also refine its practical application. By bridging this gap, the framework has the potential to become an indispensable tool for improving patient outcomes and guiding evidence-based care.
In summary, we have proposed a risk stratification approach that addresses the clinical concern of future cardiac arrest following COVID-19 vaccination. The numerous studies highlighting serious cardiovascular safety concerns related to COVID-19 vaccines have raised public and physician awareness. It is prudent for each primary care physician to have a pre-established approach when addressing this issue in their practice.
| 1. | Hulscher N, Cook MJ, Stricker RB, McCullough PA. Excess Cardiopulmonary Arrest and Mortality after COVID-19 Vaccination in King County, Washington. J Emerg Med OA. 2024;2:1-11. |
| 2. | Li YE, Wang S, Reiter RJ, Ren J. Clinical cardiovascular emergencies and the cellular basis of COVID-19 vaccination: from dream to reality? Int J Infect Dis. 2022;124:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (5)] |
| 3. | Sun CLF, Jaffe E, Levi R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Sci Rep. 2022;12:6978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 4. | Sadiq W, Waleed MS, Suen P, Chalhoub MN. Cardiopulmonary Arrest After COVID-19 Vaccination: A Case Report. Cureus. 2022;14:e21141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (4)] |
| 5. | Maruyama T, Uesako H. Lessons Learnt from Case Series of Out-of-hospital Cardiac Arrest and Unexpected Death after COVID-19 Vaccination. Intern Med. 2023;62:3267-3275. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (2)] |
| 6. | Krauson AJ, Casimero FVC, Siddiquee Z, Stone JR. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines. 2023;8:141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 7. | Baumeier C, Aleshcheva G, Harms D, Gross U, Hamm C, Assmus B, Westenfeld R, Kelm M, Rammos S, Wenzel P, Münzel T, Elsässer A, Gailani M, Perings C, Bourakkadi A, Flesch M, Kempf T, Bauersachs J, Escher F, Schultheiss HP. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, Davis JP, Loiselle M, Novak T, Senussi Y, Cheng CA, Burgess E, Edlow AG, Chou J, Dionne A, Balaguru D, Lahoud-Rahme M, Arditi M, Julg B, Randolph AG, Alter G, Fasano A, Walt DR. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 9. | Nakahara T, Iwabuchi Y, Miyazawa R, Tonda K, Shiga T, Strauss HW, Antoniades C, Narula J, Jinzaki M. Assessment of Myocardial (18)F-FDG Uptake at PET/CT in Asymptomatic SARS-CoV-2-vaccinated and Nonvaccinated Patients. Radiology. 2023;308:e230743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Scheim DE, Vottero P, Santin AD, Hirsh AG. Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Vähätalo JH, Huikuri HV, Holmström LTA, Kenttä TV, Haukilahti MAE, Pakanen L, Kaikkonen KS, Tikkanen J, Perkiömäki JS, Myerburg RJ, Junttila MJ. Association of Silent Myocardial Infarction and Sudden Cardiac Death. JAMA Cardiol. 2019;4:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Cadegiani FA. Catecholamines Are the Key Trigger of COVID-19 mRNA Vaccine-Induced Myocarditis: A Compelling Hypothesis Supported by Epidemiological, Anatomopathological, Molecular, and Physiological Findings. Cureus. 2022;14:e27883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Polykretis P, McCullough PA. Rational harm-benefit assessments by age group are required for continued COVID-19 vaccination. Scand J Immunol. 2023;98:e13242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (3)] |
| 14. | Alessandria M, Malatesta GM, Berrino F, Donzelli A. A Critical Analysis of All-Cause Deaths during COVID-19 Vaccination in an Italian Province. Microorganisms. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (5)] |
| 15. | Faksova K, Walsh D, Jiang Y, Griffin J, Phillips A, Gentile A, Kwong JC, Macartney K, Naus M, Grange Z, Escolano S, Sepulveda G, Shetty A, Pillsbury A, Sullivan C, Naveed Z, Janjua NZ, Giglio N, Perälä J, Nasreen S, Gidding H, Hovi P, Vo T, Cui F, Deng L, Cullen L, Artama M, Lu H, Clothier HJ, Batty K, Paynter J, Petousis-Harris H, Buttery J, Black S, Hviid A. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine. 2024;42:2200-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 46.5] [Reference Citation Analysis (1)] |
| 16. | Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 17. | Rose J, Hulscher N, McCullough PA. Determinants of COVID-19 vaccine-induced myocarditis. Ther Adv Drug Saf. 2024;15:20420986241226566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Eisenman D, Swindle S. Food and Drug Administration Guidance on Design of Clinical Trials for Gene Therapy Products with Potential for Genome Integration or Genome Editing and Associated Long-Term Follow-Up of Research Subjects. Appl Biosaf. 2022;27:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Krumholz HM, Wu Y, Sawano M, Shah R, Zhou T, Arun AS, Khosla P, Kaleem S, Vashist A, Bhattacharjee B, Ding Q, Lu Y, Caraballo C, Warner F, Huang C, Herrin J, Putrino D, Hertz D, Dressen B, Iwasaki A. Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences After Covid-19 Immunization. medRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 20. | Shrestha Y, Venkataraman R. The prevalence of post-COVID-19 vaccination syndrome and quality of life among COVID-19-vaccinated individuals. Vacunas. 2023;25:7-18. [DOI] [Full Text] |
| 21. | Said KB, Al-Otaibi A, Aljaloud L, Al-Anazi B, Alsolami A, Alreshidi FS; On Behalf Of The Ha'il Com Research Unit Group. The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 22. | Brogna C, Cristoni S, Marino G, Montano L, Viduto V, Fabrowski M, Lettieri G, Piscopo M. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteomics Clin Appl. 2023;17:e2300048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Yu CK, Tsao S, Ng CW, Chua GT, Chan KL, Shi J, Chan YY, Ip P, Kwan MY, Cheung YF. Cardiovascular Assessment up to One Year After COVID-19 Vaccine-Associated Myocarditis. Circulation. 2023;148:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (2)] |
| 24. | Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J. 'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Hulscher N, Procter BC, Wynn C, McCullough PA. Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination. Cureus. 2023;15:e49204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 26. | Riester E, Findeisen P, Hegel JK, Kabesch M, Ambrosch A, Rank CM, Pessl F, Laengin T, Niederhauser C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J Virol Methods. 2021;297:114271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 27. | Komici K, Verderosa S, D'Amico F, Guerra G. Self-reported side effects following COVID-19 vaccination in athletes: A retrospective study. Hum Vaccin Immunother. 2023;19:2234788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Schwab C, Domke LM, Hartmann L, Stenzinger A, Longerich T, Schirmacher P. Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin Res Cardiol. 2023;112:431-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Schmeling M, Manniche V, Hansen PR. Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine. Eur J Clin Invest. 2023;53:e13998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 30. | Fürst T, Šourek P, Krátká Z, Janošek J. Batch-dependent safety of COVID-19 vaccines in the Czech Republic and comparison with data from Denmark. Eur J Clin Invest. 2024;54:e14271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Hulscher N, Mccullough PA. Delayed Fatal Pulmonary Hemorrhage Following COVID-19 Vaccination: Case Report, Batch Analysis, And Proposed Autopsy Checklist. 2024 Preprint. [DOI] [Full Text] |
| 32. | Manno EC, Amodio D, Cotugno N, Rossetti C, Giancotta C, Santilli V, Zangari P, Rotulo GA, Villani A, Giglioni E, Turchetta A, Cafiero G, Franceschini A, Chinali M, Porzio O, Secinaro A, Palma P. Higher Troponin Levels on Admission are associated With Persistent Cardiac Magnetic Resonance Lesions in Children Developing Myocarditis After mRNA-Based COVID-19 Vaccination. Pediatr Infect Dis J. 2023;42:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Silver MA, Maisel A, Yancy CW, McCullough PA, Burnett JC Jr, Francis GS, Mehra MR, Peacock WF 4th, Fonarow G, Gibler WB, Morrow DA, Hollander J; BNP Consensus Panel. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail. 2004;10:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | McCullough PA, Olobatoke A, Vanhecke TE. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479:537-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 36. | Patel F, Le Roux J, Sawry S, Kieser R, Dhar M, Gill K, Lazarus E, Nana A, Garrett N, Moore PL, Sigal A, Gray G, Rees HV, Jacobson BF, Fairlie L. Clot Twist – D-dimer analysis of healthy adults receiving heterologous or homologous booster COVID-19 vaccine after a single prime dose of Ad26.COV2.S in a phase II randomised open-label trial, BaSiS. 2024 Preprint. [DOI] [Full Text] |
| 37. | Ittiwut C, Mahasirimongkol S, Srisont S, Ittiwut R, Chockjamsai M, Durongkadech P, Sawaengdee W, Khunphon A, Larpadisorn K, Wattanapokayakit S, Wetchaphanphesat S, Arunotong S, Srimahachota S, Pittayawonganon C, Thammawijaya P, Sutdan D, Doungngern P, Khongphatthanayothin A, Kerr SJ, Shotelersuk V. Genetic basis of sudden death after COVID-19 vaccination in Thailand. Heart Rhythm. 2022;19:1874-1879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Maheshwari S, Dagor H. Evolving the Scope of Cardiac Point-of-Care Ultrasound in the Current Era. Cureus. 2024;16:e53985. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Money DB, Mehio M, Scoma C, Gupta S. Cardiac Point-of-Care Ultrasound (P.O.C.U.S.) Utilization for Hospitalists in the Assessment of Patients with Cardiac Complaints: An Educational Overview. J Community Hosp Intern Med Perspect. 2023;13:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 40. | Rajiah PS, François CJ, Leiner T. Cardiac MRI: State of the Art. Radiology. 2023;307:e223008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 41. | Sen G, Scully P, Gordon P, Sado D. Advances in the diagnosis of myocarditis in idiopathic inflammatory myopathies: an overview of diagnostic tests. Rheumatology (Oxford). 2024;63:1825-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 42. | Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 791] [Article Influence: 65.9] [Reference Citation Analysis (1)] |
| 43. | Karur GR, Aneja A, Stojanovska J, Hanneman K, Latchamsetty R, Kersting D, Rajiah PS. Imaging of Cardiac Fibrosis: An Update, From the AJR Special Series on Imaging of Fibrosis. AJR Am J Roentgenol. 2024;222:e2329870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Zhu L, Wang Y, Zhao S, Lu M. Detection of myocardial fibrosis: Where we stand. Front Cardiovasc Med. 2022;9:926378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (3)] |
| 45. | Valore L, Junker T, Heilmann E, Zuern CS, Streif M, Drexler B, Arranto C, Halter JP, Berger CT. Case report: mRNA-1273 COVID-19 vaccine-associated myopericarditis: Successful treatment and re-exposure with colchicine. Front Cardiovasc Med. 2023;10:1135848. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (2)] |
| 46. | Sanada Y, Azuma J, Hirano Y, Hasegawa Y, Yamamoto T. Overlapping Myocarditis and Postural Orthostatic Tachycardia Syndrome After COVID-19 Messenger RNA Vaccination: A Case Report. Cureus. 2022;14:e31006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 47. | Tran HH, Sherpa ML, Shrestha N, Ravi N, Choday S, Shantha Kumar V, Kc A, Parisapogu A, Ojinna BT, Mohammed L. Safety and Efficacy of an Implantable Cardioverter Defibrillator (ICD) in the Detection and Prevention of Cardiac Arrhythmia - A Systematic Review. Cureus. 2023;15:e48471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/