Published online Feb 26, 2025. doi: 10.4330/wjc.v17.i2.102851
Revised: December 18, 2024
Accepted: January 23, 2025
Published online: February 26, 2025
Processing time: 117 Days and 18.9 Hours
Cardiovascular disease (CVD) and associated sequalae remain the leading cause of disability worldwide. Ischemic heart disease (IHD) and heart failure are the most common etiologies of morbidity and mortality worldwide. This is due to the poor diagnostic and management methods for heart failure and IHD. Early detection of related risk factors through modern strategies is underestimated and requires further research.
To interpret data from the published literature on volatile organic compounds (VOC), including all the methods used to analyze exhaled breath in patients with IHD and heart failure.
Searches for specific keywords were performed on Scopus and PubMed. A total of 20 studies were identified in breath analysis and IHD and heart failure. The study is registered in PROSPERO (Registration No. CRD42023470556).
Considering the articles found, more research is required to gain a full understanding of the role of VOCs in IHD and heart failure. However, the existing literature demonstrates that cardiac metabolic changes can be expressed in exhaled air. The number of papers found is extremely low, making interpretation extremely difficult.
Exhaled breath analysis can be a novel biomarker for the diagnosis and prevention of heart failure and IHD. Exhaled breath analysis can be used as a mirror to reflect the metabolic changes related to IHD and heart failure.
Core Tip: The breathome in heart disease diagnosis remains relatively unexplored in the medical field. Using of exhaled breath analysis to diagnose ischemic heart disease or heart failure is a promising tool for future diagnostic methods.
- Citation: Marzoog BA, Chomakhidze P, Gognieva D, Parunova AY, Demchuk SN, Silantyev A, Kuznetsova N, Kostikova A, Podgalo D, Nagornov E, Gadzhiakhmedova A, Kopylov P. Updates in breathomics behavior in ischemic heart disease and heart failure, mass-spectrometry. World J Cardiol 2025; 17(2): 102851
- URL: https://www.wjgnet.com/1949-8462/full/v17/i2/102851.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i2.102851
Exhaled breath analysis remains in the early stages of development and requires further elaboration in terms of its uses in clinical practice. Since the start of the medical examination, exhaled air has been suggested to hide the potential for use in different stages of the physical examination.
The use of exhaled air in clinical practice continues to be infrequent and has demonstrated mild alterations in different pathologies of different organs.
Cardiovascular disease (CVD) remains the pathology with the highest morbidity and mortality in our era. Currently used methods are not sufficient for early detection of CVD in individuals at risk of CVD development. Exhaled volatile organic compounds (VOCs) can be a mirror of heart homeostasis and, in particular, cardiomyocyte health at the subcellular and cellular level[1]. The use of exhaled VOCs in medical practice remains underestimated and requires further elaboration in terms of unifying and developing a single universal guideline. The heterogeneity of exhaled VOCs is partially due to variations in the methods and techniques used, as well as devices and protocols. To overcome these issues, a universal clinical recommendation must be developed.
The relationship between exhaled VOCs and CVD is rooted in the fact that these compounds can reflect underlying metabolic processes and pathological changes in the body.
VOCs are produced as a result of metabolic processes, including those associated with inflammation, oxidative stress, and tissue damage, which are common in CVD. For instance, certain VOCs may be indicative of lipid peroxidation, a process that occurs when free radicals attack lipids in cell membranes, leading to inflammation and atherosclerosis, a key factor in CVD. In addition, the presence of specific VOCs in exhaled breath can correlate with the severity of heart conditions, providing information on the state of the disease.
Several methods have been used to assess the components of VOC. One accurate method is the proton transfer reaction time of flight-mass spectrometry (PTR-TOF-MS). PTR-TOF-MS is a cutting-edge analytical technique that allows for the real-time detection and quantification of VOCs in exhaled breath. The advantages of PTR-TOF-MS include high sensitivity and specificity, real-time analysis, a wide detection range, and non-invasive nature.
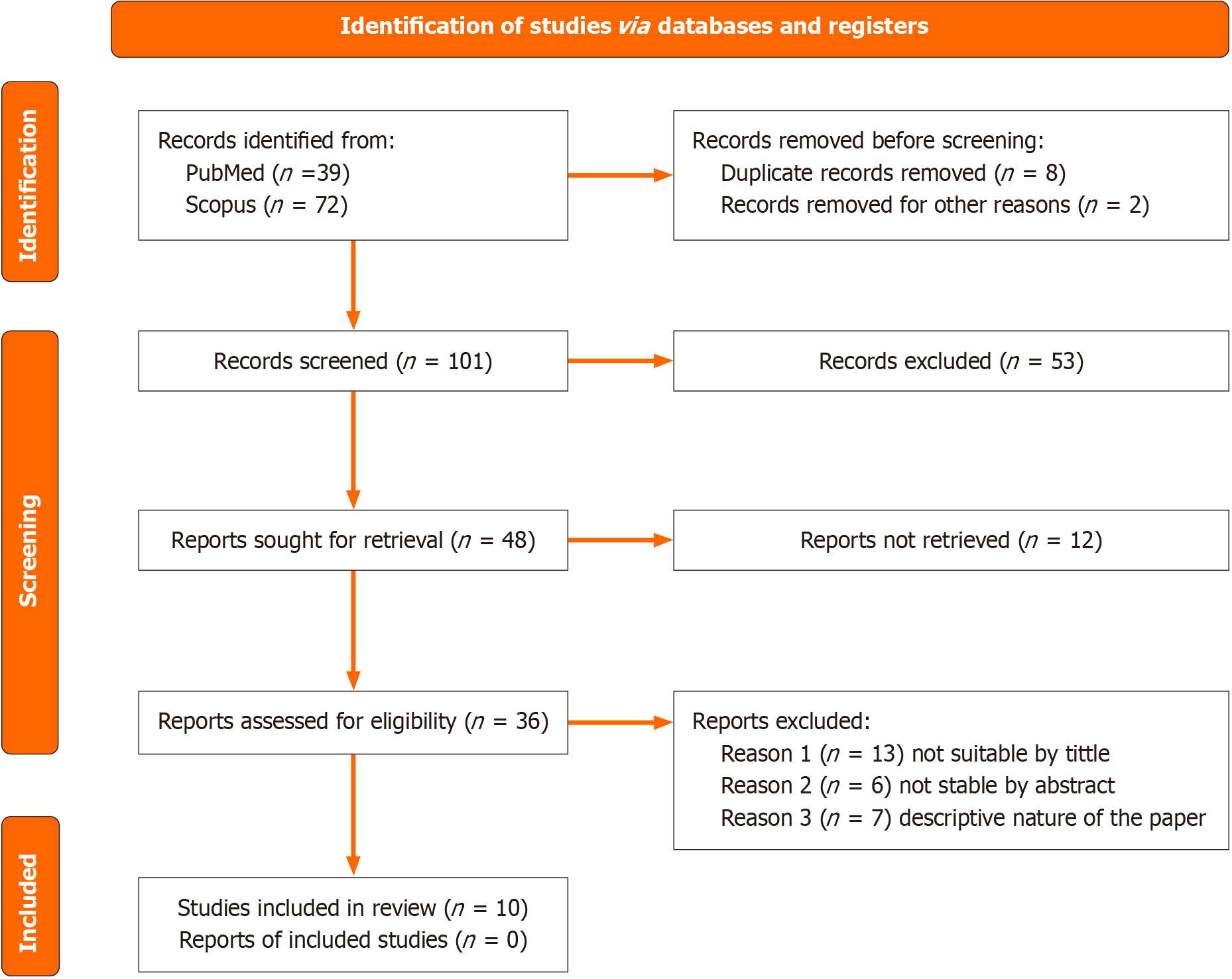
We searched Scopus and PubMed for the period 2000-2024 (submission date). The number of studies found in breath analysis and ischemic heart disease (IHD) and heart failure is 20. One of these studies was a letter to the editor and one was a case report, and a third was a meta-analysis (no full access to the article). An additional two articles were without full access (Supplementary Figure 1).
The study is registered in PROSPERO (registration number: CRD42023470556). The study follows the PRISMA guidelines for the last update 2020[2,3] (Figure 1).
Two independent authors separately searched Scopus and PubMed for the following keywords: ‘VOC AND ischemic AND heart AND disease’, ‘exhaled AND breath AND analysis AND ischemic AND heart AND disease’, ‘VOCs AND in AND heart AND failure’ and ‘exhaled AND breath AND analysis AND in AND heart AND failure’.
Inclusion criteria included patients with confirmed IHD or coronary artery disease, or IHD using the classical physical exertion test confirmed by electrocardiographic changes. Additionally, patients with heart failure classified according to the New York Heart Failure Classification were included. It is applicable to have hypertension with IHD/heart failure. In addition, diabetes mellitus was included in the selected studies due to the small number of studies.
The strength of the recommendations is determined by a critical evaluation of the evidence. The researchers consider the potential limitations of the studies, such as inaccuracies, incomplete data, or indirect evidence. In addition, factors such as publication bias were assessed. The Newcastle-Ottawa scale is a useful tool for evaluating the risk of bias in included studies[4]. All existing studies were case-control or non-cohort, nonrandomized studies. We excluded case reports, letter-to-editor, descriptive nature studies, articles without full access, articles that included patients with heart failure and diabetes mellitus, as well as asthma and chronic obstructive pulmonary disease and effects on the components of exhaled VOCs.
Unfortunately, existing studies have severe limitations and lack clear inclusion and exclusion criteria, and it was impossible to develop a meta-analysis or to perform statistical analysis. None of the papers used similar methods for analyzing exhaled breath analysis, and each paper utilized different materials and methods. Our search included studies in the following languages, ‘English’, ‘Russian’, ‘French’, ‘German’, ‘Persian’, ‘Arabic’. We searched the database for the period January 1, 2000- June 1, 2024. Included studies were controlled clinical trials and randomized clinical trials. The search results detected 39 PubMed and 72 Scopus records (Figure 2).
The researchers used a method developed by the Cochrane Collaboration to assess the reliability of the included studies (Table 1 and Table 2). This method, called “risk of bias”, looks for factors that could have skewed the study’s results[5,6]. These factors include random assignment, blinding, missing data, selective reporting, and others.
| Bias domain | Low risk | High risk | Unclear risk |
| Selection bias | Random sequence generation and allocation concealment both well described | Random sequence generation or allocation concealment not well described or inappropriate method used | Random sequence generation or allocation concealment not reported |
| Performance bias | Blinding of participants and personnel, and the outcome is not likely to be influenced by lack of blinding | Blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding | Blinding of participants and personnel not reported |
| Detection bias | Blinding of outcome assessment, and the outcome measurement is not likely to be influenced by lack of blinding | Blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding | Blinding of outcome assessment not reported |
| Attrition bias | No missing outcome data or reason for missing data unlikely to be related to true outcome | Missing outcome data likely to be related to true outcome | Incomplete outcome data reported, and the reason for missing data not described |
| Reporting bias | The study protocol is available, and all of the study's pre-specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre-specified way | One or more reported primary outcomes were not pre-specified (or, if they were, they were measured differently), or the reporting of primary outcomes is incomplete and not according to the protocol | No study protocol is available, and it is unclear whether the reported outcomes were pre-specified or not |
| Other bias | The study appears to be free of other sources of bias | The study has at least one important risk of bias | There is insufficient information to assess whether an important risk of bias exists |
| Ref. | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | Overall bias |
| Modak et al[27] | + | - | - | + | + | + | + |
| Tang et al[11] | - | - | - | - | - | - | - |
| Biagini et al[10] | + | - | + | + | - | + | + |
| Mollar et al[19] | + | - | - | - | + | + | + |
| Marcondes-Braga et al[17] | ! | - | ! | + | - | ! | ! |
| Bykova et al[12] | + | - | - | - | - | + | ± |
| Yokokawa et al[20] | + | - | - | - | + | + | + |
| Biagini et al[13] | - | - | - | - | + | + | ± |
| Marcondes-Braga et al[16] | + | + | - | + | - | ! | + |
| Yokokawa et al[18] | - | - | + | - | + | - | ± |
| Overall bias | + | - | ± | ± | ± | + | + |
To conduct a systematic review on breathomics behavior in IHD and the role of mass spectrometry, the available sources provide valuable information on the diagnostic potential of breath analysis for disease detection. Breath analysis, particularly the detection of VOC in exhaled air, holds promise for disease monitoring due to its noninvasive nature and reproducible results[7,8]. Various techniques such as gas chromatography mass spectrometry, selected ion flow tube mass spectrometry and ion mobility spectrometry are commonly used for breath analysis[8]. Recent advances in sensors and e-Noses show potential to become strong diagnostic tools through breath analysis, offering real-time results and portability for point-of-care applications[8]. Clinical trials are also underway to assess the diagnostic precision of breathomics in coronary artery disease using mass spectrometry methods such as PTR-TOF-MS-1000 (NCT06181799).
In the context of mental health disorders associated with heart disease, it is essential to expand traditional risk assessments to include mental health disorders and develop reliable screening instruments to identify vulnerable populations. Positive psychological well-being has been associated with cardiovascular health, emphasizing the importance of managing emotions and fostering positive relationships for general health. Understanding the interaction between mental health and heart disease is crucial for comprehensive health care, and efforts are being made to integrate mental health assessments into cardiovascular risk assessments.
Current studies on changes in VOC in IHD are descriptive in nature and did not use clear methods to confirm the presence or absence of IHD. Two studies showed that exhaled breath analysis in patients with coronary artery disease or acute coronary syndrome has been separated using exhaled breath analysis from a healthy control or one needing coronary artery revascularization[9,10]. However, the first study described the acute coronary syndrome to separate patients with coronary artery disease from random individuals without known coronary artery disease[10]. There are no well-established inclusion and exclusion criteria to confirm that these changes are due to acute coronary syndrome or other chronic diseases such as asthma, diabetes mellitus, or chronic obstructive pulmonary disease. Regarding the second study, the same concerns arise. There are no clear inclusion and exclusion criteria, and the article stated that exhaled VOCs have a sensitivity (78.3%) and specificity (68.4%) for detecting the need for coronary artery revascularization compared to coronary arteries that do not require revascularization. The study performed statistical analysis using machine learning model building and logistic regression analysis[7,9]. However, there is an ongoing clinical trial to explore the changes in exhaled VOCs in patients with IHD confirmed by computed stress myocardial perfusion imaging and stress ATP (NCT06181799). Exhaled ethane levels are likely related to IHD[7]. The study results showed a diagnostic accuracy of 84% in patients with IHD. These results are statistically significant, higher than the classically used method for the early detection of IHD, the physical stress test[11].
Changes in exhaled breath analysis have been well studied in patients with heart failure. Several studies have been published on disturbance in patients suffering from acute heart failure, chronic heart failure, and decompensated heart failure. However, the lack of standardization of the methods used makes it difficult to interpret the results. Studies have shown that elevated acetone and pentane levels are related to altered oxidation of fatty acids and oxidative stress[12]. Patients with heart failure experience an increase in the level of exhaled acetone and pentane in exhaled breath[12–16]. Furthermore, a single meta-analysis demonstrated that exhaled breath acetone levels are related to heart failure[17]. High levels of exhaled breath acetone associated with a poor prognosis in heart failure, including patients with heart failure with reduced ejection fraction patients[18–20]. Acute decompensated heart failure is associated with intestinal microbiota dysbiosis. The metabolites produced by the overgrowth of harmful bacteria in patients with heart failure are trimethylamine N-oxide, acetone, and butyrate[21,22].
Considering the studies discussed, changes in exhaled breath VOCs, are associated with one of the following hypotheses. The first hypothesis suggests that the change in concentrations of specific VOCs in exhaled breath of individuals with IHD is related to the peroxidation process, lipid, protein, and carbohydrate peroxidation in cardiomyocytes due to oxygen deficiency.
The second hypothesis proposes that changes in the concentrations of specific exhaled VOCs in individuals with IHD are related to changes in the gut microflora, where several studies demonstrated a strong correlation between dysbiosis and the existence of specific microflora that increase cardiovascular risk, including IHD. Furthermore, disturbance in the metabolism of the gut microbiome is a sword of two edges; first, gut microbiota metabolite disorder is a reason for IHD; second, the gut microbiota metabolite disorder is a result of IHD. However, in both cases, changes in VOC concentration in patients with IHD are an early biomarker of exhaled breath that can be used to assess the risk of developing IHD in the near future.
The role of endothelial dysfunction in the pathogenesis of IHD is well known[23-27]. The consequences of endothelial dysfunction include a reduction in endothelial nitric oxide production, which is responsible for the relaxation of vascular smooth muscle cells. Persistent spasm of smooth muscle cells results in hypertrophy and loss of the ability of the coronary vessels to regulate blood flow to the myocardium. Furthermore, pathological blood flow is associated with mechanical damage to the coronary arteries, which exposes the tunica intima to the risk of formation of atherosclerotic plaques. Therefore, endothelial nitric oxide levels are reduced in patients with IHD, and further nitric oxide is reduced in the analysis of exhaled breath. This is confirmed by the fact that physical activity is associated with an increase in the nitric oxide level in exhaled breath[27]. In this sense, patients with stable coronary artery disease at a functional stage that are taking nitroglycerine or analogs will have relatively normal or lower border values than normal values of endothelial nitric oxide levels in their exhaled breath.
In addition to the unclear inclusion and exclusion criteria, the methods that were used for the examination of the exhaled breath are extremely varied. This dramatically affects the results of exhaled VOC analysis. Additionally, using machine learning models for the analysis of the huge volume of mass/charge of the detected exhaled VOCs is an innovative method and requires a separate guideline and recommendation for use for the evaluation of the huge medical data. Basal levels of pentane are less useful than MDA as an index of lipid peroxidation in patients with coronary artery disease. However, pentane in exhaled breath appears to be a sensitive index of reperfusion-induced lipid peroxidation[2].
Regarding the level of exhaled acetone in patients with heart failure, several studies demonstrated a direct correlation between the level of exhaled acetone and the NTproBNP[12].
Exhaled breath analysis contains reflexes for a multitude of VOC that reflect the internal state of a physiological and pathological organism, including IHD. Breathomics behavior in IHD and heart failure should focus on the diagnostic potential of breath analysis, advances in mass spectrometry techniques, the role of VOCs in disease monitoring, and the integration into the cardiovascular risk assessments for a holistic approach to healthcare. Resolving the variability factors such as the fasting state, sampling type, and analytical method is important to produce a protocol for exhaled breath analysis in clinical practice.
| 1. | Dummer J, Storer M, Swanney M, McEwan M, Scott-Thomas A, Bhandari S, Chambers S, Dweik R, Epton M. Analysis of biogenic volatile organic compounds in human health and disease. Trends Anal Chem. 2011;30:960-967. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1310] [Cited by in RCA: 2109] [Article Influence: 421.8] [Reference Citation Analysis (0)] |
| 3. | Wells G, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Ottawa Hosp. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 4. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26303] [Article Influence: 1753.5] [Reference Citation Analysis (4)] |
| 5. | Lundh A, Gøtzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Marzoog B. Breathomics Detect the Cardiovascular Disease: Delusion or Dilution of the Metabolomic Signature. Curr Cardiol Rev. 2024;20:e020224226647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Kaloumenou M, Skotadis E, Lagopati N, Efstathopoulos E, Tsoukalas D. Breath Analysis: A Promising Tool for Disease Diagnosis-The Role of Sensors. Sensors (Basel). 2022;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Segreti A, Incalzi RA, Lombardi M, Miglionico M, Nusca A, Pennazza G, Santonico M, Grasso S, Grigioni F, Di Sciascio G. Characterization of inflammatory profile by breath analysis in chronic coronary syndromes. J Cardiovasc Med (Hagerstown). 2020;21:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Nardi Agmon I, Broza YY, Alaa G, Eisen A, Hamdan A, Kornowski R, Haick H. Detecting Coronary Artery Disease Using Exhaled Breath Analysis. Cardiology. 2022;147:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Biagini D, Pugliese NR, Vivaldi FM, Ghimenti S, Lenzi A, De Angelis F, Ripszam M, Bruderer T, Armenia S, Cappeli F, Taddei S, Masi S, Francesco FD, Lomonaco T. Breath analysis combined with cardiopulmonary exercise testing and echocardiography for monitoring heart failure patients: the AEOLUS protocol. J Breath Res. 2023;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Tang WHW, Tranchito L, Albert C, Gul ZG, Cikach FS Jr, Grove D, Wu Y, Dweik RA. Exhaled Breath Analysis Using Selected Ion Flow Tube Mass Spectrometry and Disease Severity in Heart Failure. Metabolites. 2023;13:1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Bykova AA, Malinovskaya LK, Trushina O V. , Chomakhidze PS, Shaltaeva YR, Proshlyakov AY, Serditenko E V., Syrkin AL, Betelin VB, Kopylov PY. Exhaled breath analysis in diagnosis of chronic heart failure with reduced left ventricular ejection fraction. Kardiol i Serdechno-Sosudistaya Khirurgiya. 2019;12:568-576. [DOI] [Full Text] |
| 13. | Biagini D, Lomonaco T, Ghimenti S, Bellagambi FG, Onor M, Scali MC, Barletta V, Marzilli M, Salvo P, Trivella MG, Fuoco R, Di Francesco F. Determination of volatile organic compounds in exhaled breath of heart failure patients by needle trap micro-extraction coupled with gas chromatography-tandem mass spectrometry. J Breath Res. 2017;11:047110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Yokokawa T, Sato T, Suzuki S, Oikawa M, Yoshihisa A, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Ishida T, Shimouchi A, Takeishi Y. Elevated exhaled acetone concentration in stage C heart failure patients with diabetes mellitus. BMC Cardiovasc Disord. 2017;17:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Gouzi F, Ayache D, Hédon C, Molinari N, Vicet A. Breath acetone concentration: too heterogeneous to constitute a diagnosis or prognosis biomarker in heart failure? A systematic review and meta-analysis. J Breath Res. 2021;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Marcondes-Braga FG, Batista GL, Gutz IG, Saldiva PH, Mangini S, Issa VS, Ayub-Ferreira SM, Bocchi EA, Pereira AC, Bacal F. Impact of Exhaled Breath Acetone in the Prognosis of Patients with Heart Failure with Reduced Ejection Fraction (HFrEF). One Year of Clinical Follow-up. PLoS One. 2016;11:e0168790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Marcondes-Braga FG, Gioli-Pereira L, Bernardez-Pereira S, Batista GL, Mangini S, Issa VS, Fernandes F, Bocchi EA, Ayub-Ferreira SM, Mansur AJ, Gutz IGR, Krieger JE, Pereira AC, Bacal F. Exhaled breath acetone for predicting cardiac and overall mortality in chronic heart failure patients. ESC Heart Fail. 2020;7:1744-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Yokokawa T, Sugano Y, Shimouchi A, Shibata A, Jinno N, Nagai T, Kanzaki H, Aiba T, Kusano K, Shirai M, Takeishi Y, Yasuda S, Ogawa H, Anzai T. Exhaled Acetone Concentration Is Related to Hemodynamic Severity in Patients With Non-Ischemic Chronic Heart Failure. Circ J. 2016;80:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Mollar A, Marrachelli VG, Núñez E, Monleon D, Bodí V, Sanchis J, Navarro D, Núñez J. Bacterial metabolites trimethylamine N-oxide and butyrate as surrogates of small intestinal bacterial overgrowth in patients with a recent decompensated heart failure. Sci Rep. 2021;11:6110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Yokokawa T, Sato T, Suzuki S, Oikawa M, Yoshihisa A, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Ishida T, Shimouchi A, Takeishi Y. Change of Exhaled Acetone Concentration Levels in Patients with Acute Decompensated Heart Failure. Int Heart J. 2018;59:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Marzoog BA. Autophagy Behavior in Endothelial Cell Regeneration. Curr Aging Sci. 2024;17:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (1)] |
| 22. | Marzoog BA. Endothelial Dysfunction under the Scope of Arterial Hypertension, Coronary Heart Disease, and Diabetes Mellitus using the Angioscan. Cardiovasc Hematol Agents Med Chem. 2024;22:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 23. | Bussotti M, Andreini D, Agostoni P. Exercise-induced changes in exhaled nitric oxide in heart failure. Eur J Heart Fail. 2004;6:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Mendis S, Sobotka PA, Leja FL, Euler DE. Breath pentane and plasma lipid peroxides in ischemic heart disease. Free Radic Biol Med. 1995;19:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Gladding PA, Cooper M, Young R, Loader S, Smith K, Zarate E, Green S, Villas Boas SG, Shepherd P, Kakadiya P, Thorstensen E, Keven C, Coe M, Jüllig M, Zhang E, Schlegel TT. Metabolomics and a Breath Sensor Identify Acetone as a Biomarker for Heart Failure. Biomolecules. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 26. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester, United Kingdom: John Wiley and Sons, 2019. |
| 27. | Modak AS. Regulatory issues on breath tests and updates of recent advances on [13C]-breath tests. J Breath Res. 2013;7:037103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/