Published online Nov 26, 2025. doi: 10.4330/wjc.v17.i11.109739
Revised: June 16, 2025
Accepted: September 16, 2025
Published online: November 26, 2025
Processing time: 185 Days and 11.8 Hours
Voltage substrate mapping is a promising tool for the treatment of atrial fibrillation (AF). It is helpful to detect atrial fibrosis, which includes areas with low bipolar voltage, heterogeneous conduction properties, and shortened effective refractory period. The voltage amplitude is typically defined as the maximal peak-to-peak level within a specified time window of interest. Contemporary elec
Core Tip: Voltage mapping is commonly used during substrate mapping to detect target areas for ablation in patients with atrial fibrillation (AF). Analysis of the substrate may take the form of detecting low voltage areas or the average voltage over an entire region. Often, areas which are targets for ablation are located where the voltage level is lower than normal, indicating disease and damage to the substrate. Furthermore, as AF progresses from paroxysmal to persistent type, the voltage level of the left atrium tends to diminish. This is often associated with atrial remodeling, in which structural and electrical changes occur during arrhythmia. Protocols for finding target areas using voltage mapping are described, and future research efforts to improve mapping and outcome are discussed.
- Citation: Ciaccio EJ, Hsia HH, Yarmohammadi H, Wan EY, Peters NS, Saluja D, Biviano AB. Atrial fibrillation substrate mapping with emphasis on voltage-based guidance. World J Cardiol 2025; 17(11): 109739
- URL: https://www.wjgnet.com/1949-8462/full/v17/i11/109739.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i11.109739
In this review, aspects of substrate voltage mapping will be described and discussed for the detection and localizing of ablation targets in patients with atrial fibrillation (AF).
Electroanatomic mapping provides information pertaining to local voltage abnormalities that may be used as surrogate markers for the presence of atrial fibrosis and other structural impediments to conduction of the electrical activation wavefront[1]. The implementation of voltage mapping and analysis during electrophysiologic (EP) study enables the in vivo assessment of scar, which can then be targeted with radiofrequency, pulsed field[2], or other ablation energy[3,4]. Low voltage is often a focus of ablation treatment in AF, but its relationship to onset, perpetuation, and termination of the arrhythmia requires further elucidation[5]. A cutoff value for left atrial low bipolar voltage mapping has been described as less than or equal to 0.5 mV, as measured during sinus rhythm[6]. Further, a specific voltage range of 0.2–0.45 mV during sinus rhythm has been reported to accurately delineate left atrial scar distribution[7]. The single optimal cutoff value for detection of low voltage when recording during AF rather than sinus rhythm is 0.2 mV[8]. Although a low voltage threshold or voltage range can thus be utilized to indicate presence of atrial fibrosis, the threshold voltage level defining fibrosis is not necessarily constant, and may diminish with increased atrial structural remodeling[9]. Voltage depression when recording during AF as opposed to sinus rhythm may be caused by functional refractoriness and rapid electrical activation[10]; rhythm type during data acquisition is therefore an important consideration. Although bipolar recordings are often utilized for analysis, unipolar recordings may better characterize left atrial fibrosis by capturing more comprehensive transmural features[11].
Regional voltage differences are also apparent in patients with atrial arrhythmias, which may reflect the presence of altered tissue thickness and variable electrical activation wavefront propagation. The most frequent location of sinus rhythm low-voltage areas (LVA) is at the anterior wall, the septum, and the posterior wall of the left atrium (LA)[12-14]. Posterior LVA is associated with a significant increase in persistent AF recurrence[15]. Substrate voltage levels also depend upon patient characteristics of age, sex, co-occurrence of dilated or hypertrophic cardiomyopathy, the type of AF, left atrial surface area and volume, sinoatrial node dysfunction, and glomerular filtration rate[16]. These parameters correlate with whether LVA are present, and they may be useful in selecting an appropriate strategy for AF ablation[17,18]. Furthermore, it should be noted that left atrial LVAs are associated with a history of stroke and subclinical silent cerebral ischæmia in patients with non-valvular AF[19]. Yet, most AF patients who suffer stroke have no evidence of arrhythmia in temporal proximity, implying that the stroke is mechanically based and operates via a generalized atrial cardiomyopathy, rather than originating from electrical substrate[20-22].
In terms of correlation, statistical significance, and prediction, left atrial voltage is related to the total sinus rhythm activation time throughout the chamber[23]. Moreover, the total sinus rhythm activation time is greater in patients with persistent AF as compared to those with paroxysmal AF[24]. Hence, conduction velocity is likely to be slower in persistent AF, and a slower conduction velocity is consonant with a diminished left atrial voltage. Patients with persistent AF have significantly lower left atrial voltage levels during AF as compared with the paroxysmal type, even after adjustment for differences in indexed left atrial volume[25]. Moreover, LVAs are larger in patients with persistent as compared with paroxysmal AF[16]. Although persistent AF can be terminated by catheter ablation without linear lesioning, some of these cases require straight-line lesions to prevent macro re-entrant atrial tachycardia (AT)[26,27]. Left atrial voltage is an independent predictor of arrhythmia recurrence, whether the LA is mapped during sinus rhythm or during AF[28]. For paroxysmal AF, the existence of left atrial voltage areas less than 0.4 mV in greater than 10% of the total LA surface area is predictive of arrhythmia recurrence following pulmonary vein ablation[29]. In both paroxysmal and persistent AF, the presence of left atrial LVA after pulmonary vein isolation (PVI) predicts AF recurrence[30,31]. Notably however, recurrence of AF after PVI, which is common, may be due to pulmonary vein reconnections, rather than the presence of the LVA themselves[32,33]. Furthermore, LVA ablation has no beneficial impact on 1-year rhythm outcomes[34]. In addition, preprocedural cardiac magnetic resonance imaging-particularly for substrate mapping-can be useful; however, identifying pulmonary vein reconnections remains challenging, even with cardiovascular magnetic resonance (CMR)-gadolinium-enhanced (GE) imaging[35].
AF triggers can be defined as electrical impulses leading to AF onset from a non-fibrillatory rhythm, and AF drivers can be characterized as electrical phenomena critical to the arrhythmia’s perpetuation[36-39]. Several harbingers of AF triggers and drivers exist. LVAs are linked to AF trigger sites[40]. Pulmonary vein triggers are associated with diminished voltage levels in the pulmonary vein antra, while superior vena cava triggers correlate with lower voltages in the right atrium[41]. Non-pulmonary vein triggers arise from left atrial degeneration, which thereby contribute to persistence in AF[42]. Even when there are no LVA, it has been suggested that left atrial electrophysiological function may be alterable to form AF substrate[43]. The delineation of electrogram morphology has also been proposed to identify AF triggers and drivers. Complex fractionated atrial electrograms (CFAEs) are defined as electrograms with a very short cycle length
Atrial fibrosis is a demonstrated substrate for AF[3]. The appearance of fibrosis enhances conduction heterogeneity throughout the LA, causing disturbances in the electrical activation wavefront, including delay, conduction block, wave break, and reentry in the atrial wall, all of which may assist in perpetuating arrhythmia[55,56]. Rotational electrical activity, commonly termed rotors, can be meandering or stationary, and they anchor at regions of slow conduction, at gradients in refractoriness, structural discontinuities, and low voltage fibrotic regions[39,57]. A strong correlation has been established between left atrial fibrosis detected via CMR–GE and low endocardial voltage measured during electroanatomic mapping[58]. In AF patients, late gadolinium enhancement (LGE) regions exhibit a pronounced and consistent reduction in unipolar and bipolar voltage compared with non-LGE regions[59]. Left atrial bipolar voltage level is associated with the image attenuation ratio derived from contrast-enhanced multidetector computed tomography[60]. Low atrial endocardial bipolar voltage, as measured during AF, is a commonly used surrogate marker for the presence of atrial fibrosis[61]. The extent of left atrial fibrosis predicts the success of AF ablation at up to five years of follow-up[62]. AF voltage values sampled over several seconds show a strong temporal reproducibility[63], suggesting the duplicability of this measurement. However, of those left atrial targets which result in successful termination, more than half are entirely remote from low voltage areas[64], and in patients with advanced fibrosis, AF ablation has a high failure rate[62]. Thus, AF voltage as a sole parameter has limited value in efficaciously guiding the catheter toward target areas.
Catheter ablation, particularly PVI, is an established first-line treatment for paroxysmal and persistent AF[65]. LVA in the LA serves to flag the presence of arrhythmogenic substrate and it is a strong predictor of AF recurrence after PVI in both paroxysmal and persistent AF[66,67]. The extent of LVA is an independent predictor of arrhythmia recurrence, even after substrate homogenization[68,69]. Thus, targeting for ablation sites with distinct activation within or bordering LVAs may yield better outcomes than PVI alone[69]. Multiple studies and meta-analyses have shown that substrate modification targeting LVAs, when added to PVI, results in improved arrhythmia-free survival in patients with persistent or long-standing persistent AF, without increasing procedural risk[70-73]. In one analysis, freedom from atrial arrhythmia at one year was achieved in nearly three-quarters of patients receiving LVA-guided ablation[71]. The SUPPRESS-AF Trial found that LVA-guided ablation decreased recurrence rates compared to PVI alone in persistent AF, supporting such individualized substrate-guided approaches[74]. Ablation of sites with distinct activation characteristics within or at LVA border zones, in addition to PVI, is more effective than a conventional PVI-only strategy for persistent AF[69]. A meta-analysis has shown that ablation of LVAs in addition to PVI reduces arrhythmia recurrence without significant increase in procedure duration, fluoroscopy time, or complication rate[75]. Individualized ablation with high-density left atrial mapping followed by LVA-guided ablation, when LVAs are present, significantly improves outcomes, although at the cost of longer EP procedure time[76]. Such voltage-guided ablation increases one-year AF/AT-free survival in patients when compared to stepwise ablation[3]. In fact, voltage-guided ablation is the only independent predictor of AF/AT-free survival. When AF patients were assigned to LVA isolation vs standard treatment, there was a significantly increased arrhythmia-free survival in the LVA-guided group at one year[77,78]. Patients with LVA have an equally favorable long-term ablation outcome as compared to those without[79]. In both paroxysmal and persistent AF patients who lack LVAs, solo PVI leads to successful outcome[66].
Despite much progress in substrate mapping, AF reappearance rates remain high, ranging to as much as 40% at one-year post-ablation[34]. In patients with paroxysmal AF, LVA ablation beyond PVI may not improve outcome, and the presence of LVAs strongly predicts AF recurrence[80]. Less successful outcomes occurring in persistent AF may result from a longer AF time duration since onset of arrhythmia, which is associated with more extensive substrate remodeling, as well as to the presence of atrial dilatation[81]. Some randomized trials do suggest that the benefit of LVA ablation beyond PVI is inconsistent. Adjunctive ablation strategies following PVI has not consistently improved outcomes in randomized controlled trials[82]. The size and distribution of LVA affect AF initiation, perpetuation, and termination[5]. Furthermore, LVA ablation may not always reduce AF recurrence nor increase AT incidence vs PVI alone[83], despite a connection between LVA determined by voltage mapping in AF and scar observed on CMR–GE imagery[84]. In patients with minimal LVA, it may therefore be sufficient to apply only PVI[66]. When there is extensive atrial remodeling, such as is indicated by the presence of fatty tissue, a predominance of low voltage zones, and/or electrogram fractionation, only then may a more aggressive substrate modification be required[85].
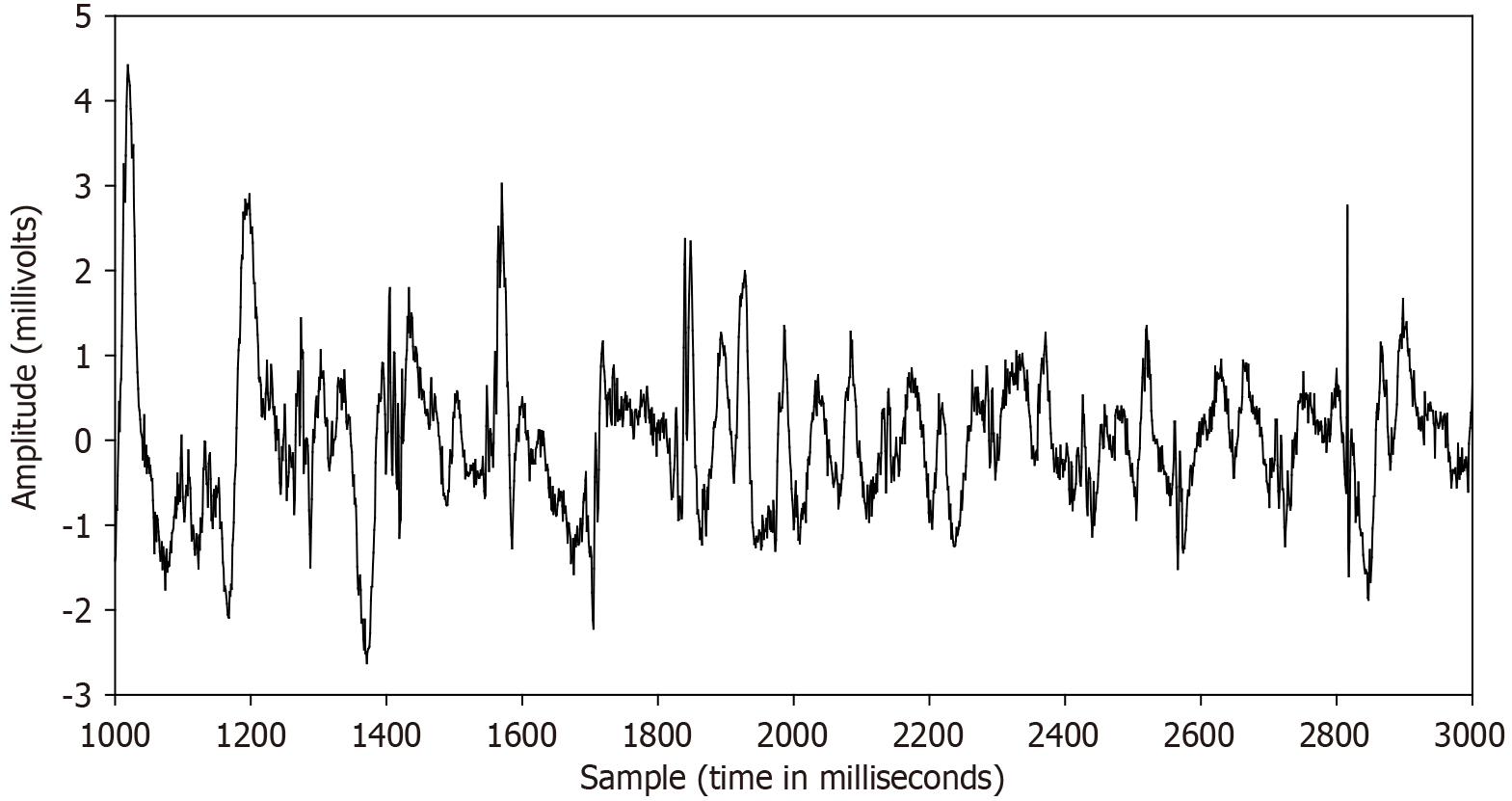
Difficulties in the use of LVA for substrate mapping may result in part from the variability of its characteristics, which can complicate the targeting of arrhythmogenic tissue for ablation[86]. Examples are shown in Figure 3 to distinguish and note the partial but by no means complete repetitiveness of atrial electrogram deflection and amplitude during AF, hence the possibility of variable efficacy in the use of the electrogram for substrate mapping. There is to some extent a linear voltage correlation between sinus rhythm and AF, suggesting a similar extent of left atrial fibrotic substrate may be identifiable during voltage mapping in any rhythm by adjusting the voltage cutoff[87]. When voltage mapping is done in AF, the LVAs are more evident and prominent than in other rhythms[69,87], with a tendency to reveal a larger low voltage extent[6], and indeed AF voltage is superior to sinus rhythm voltage for localizing CMR-GE regions[63].
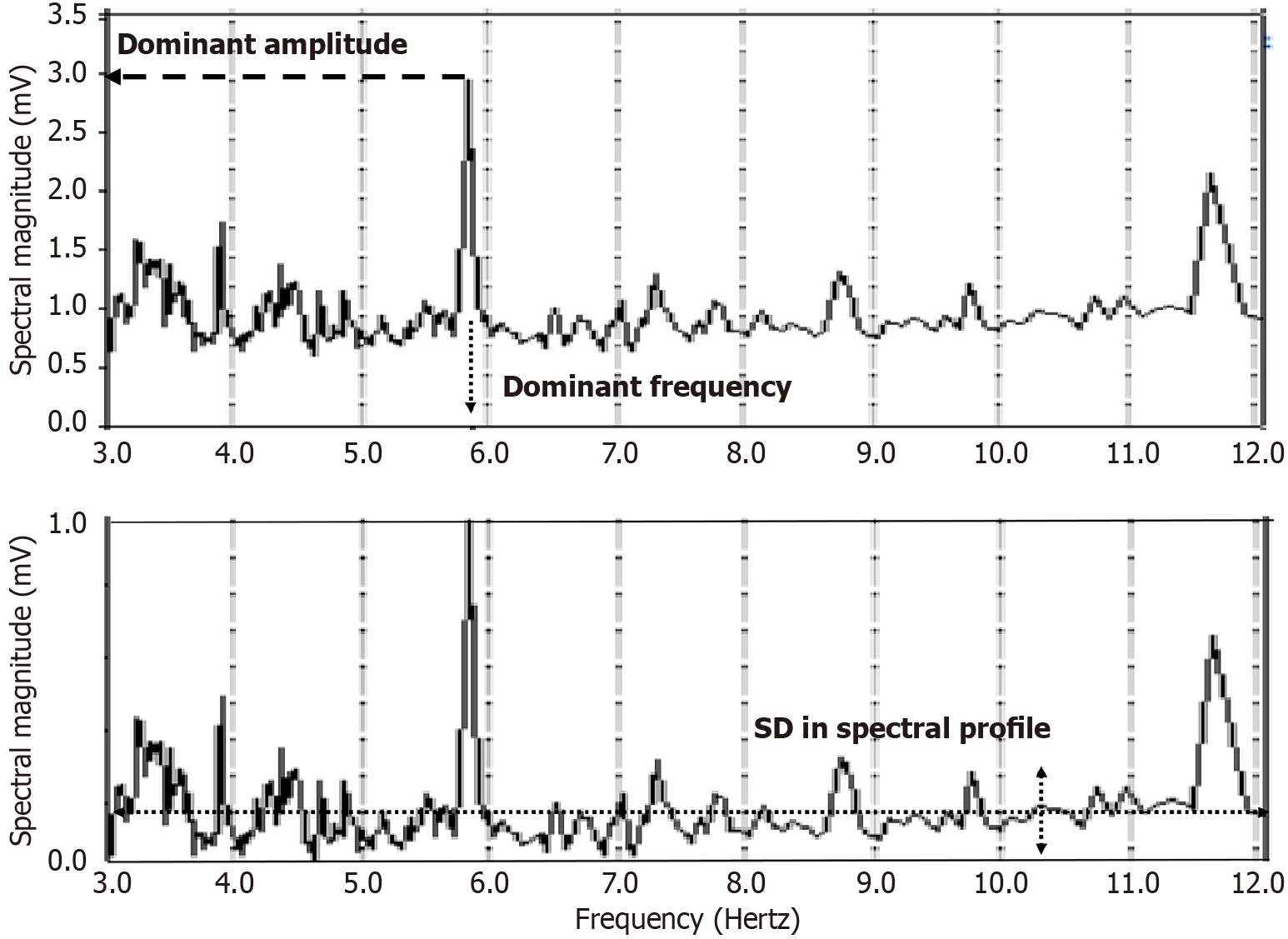
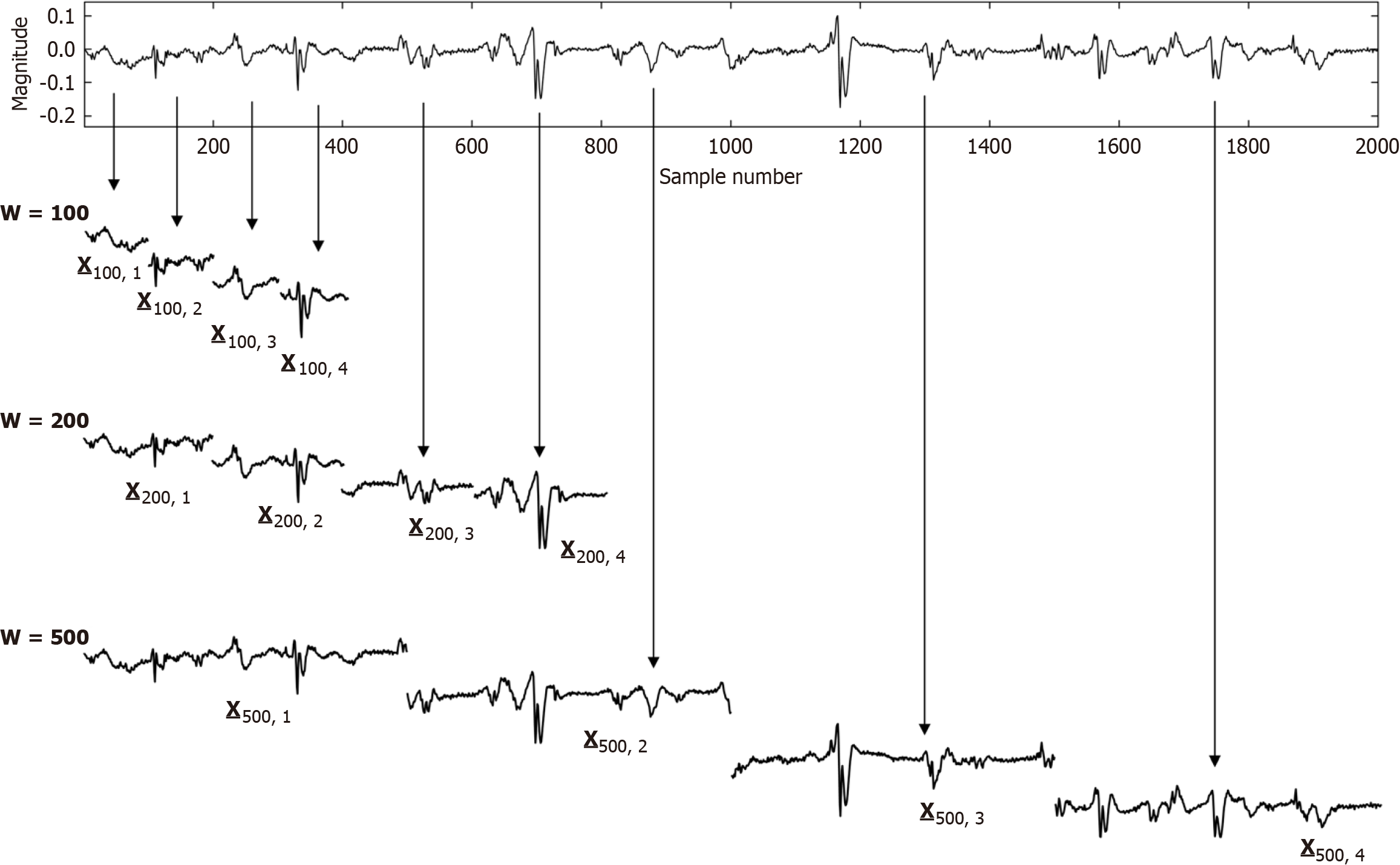
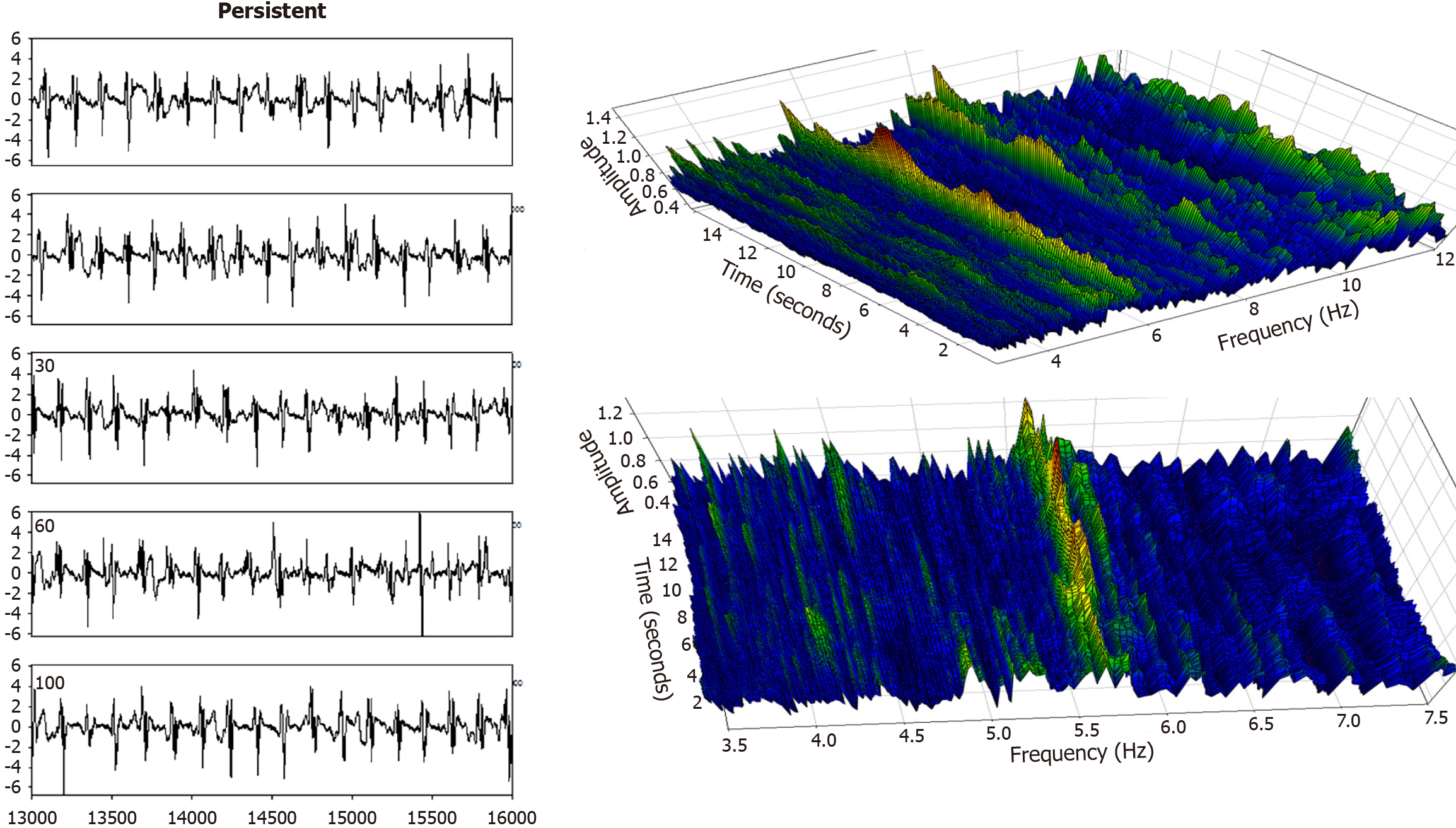
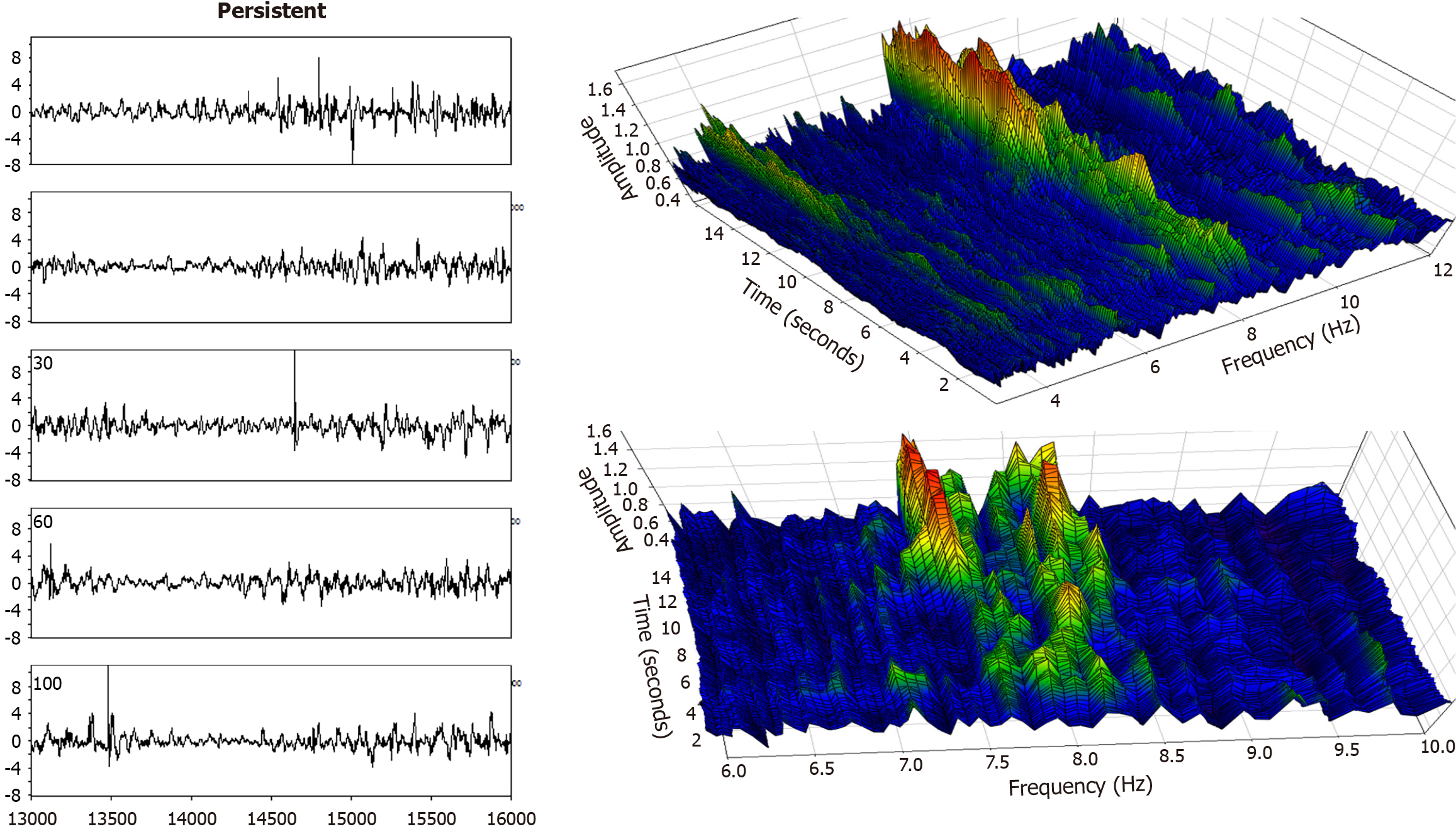
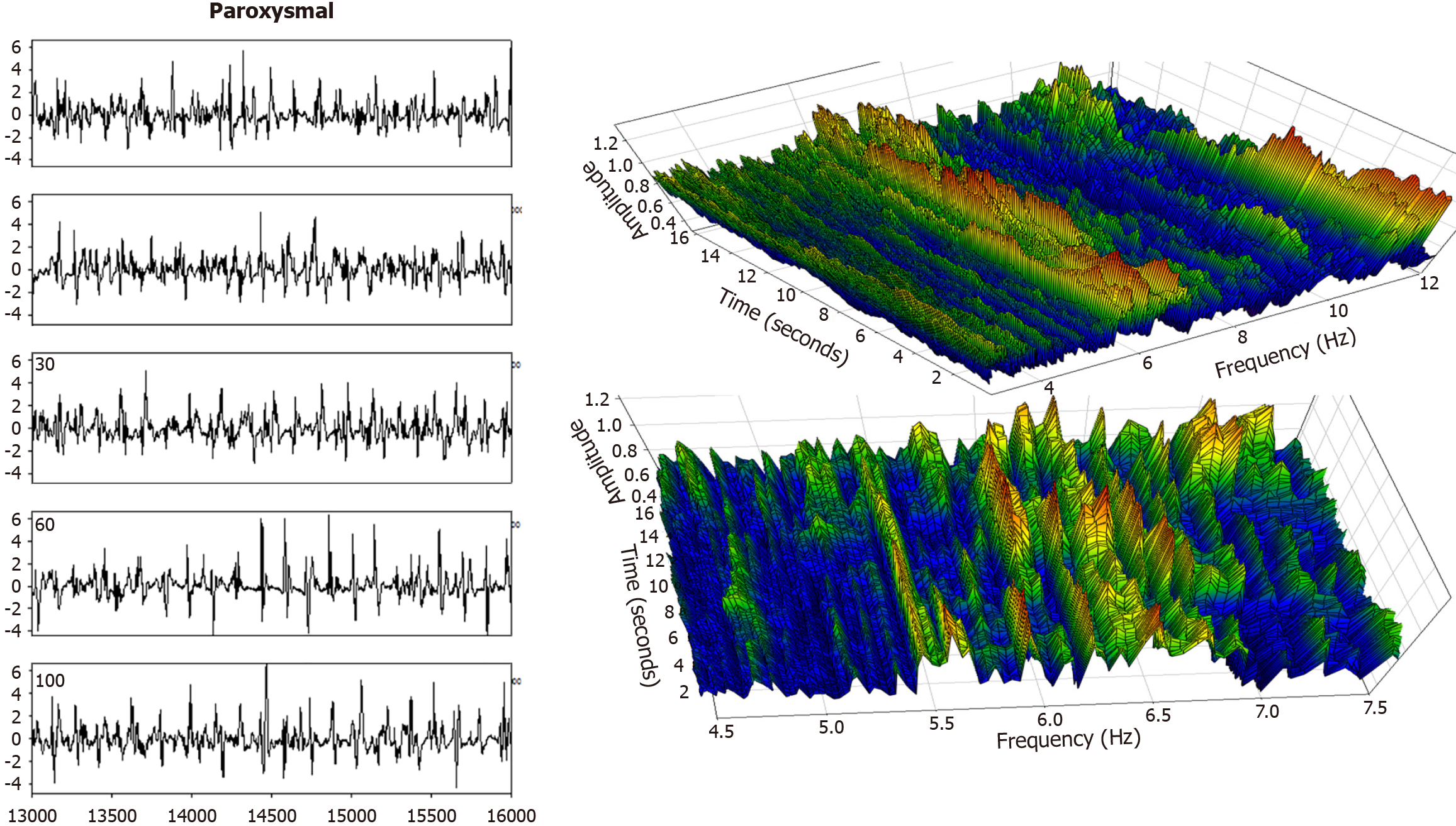
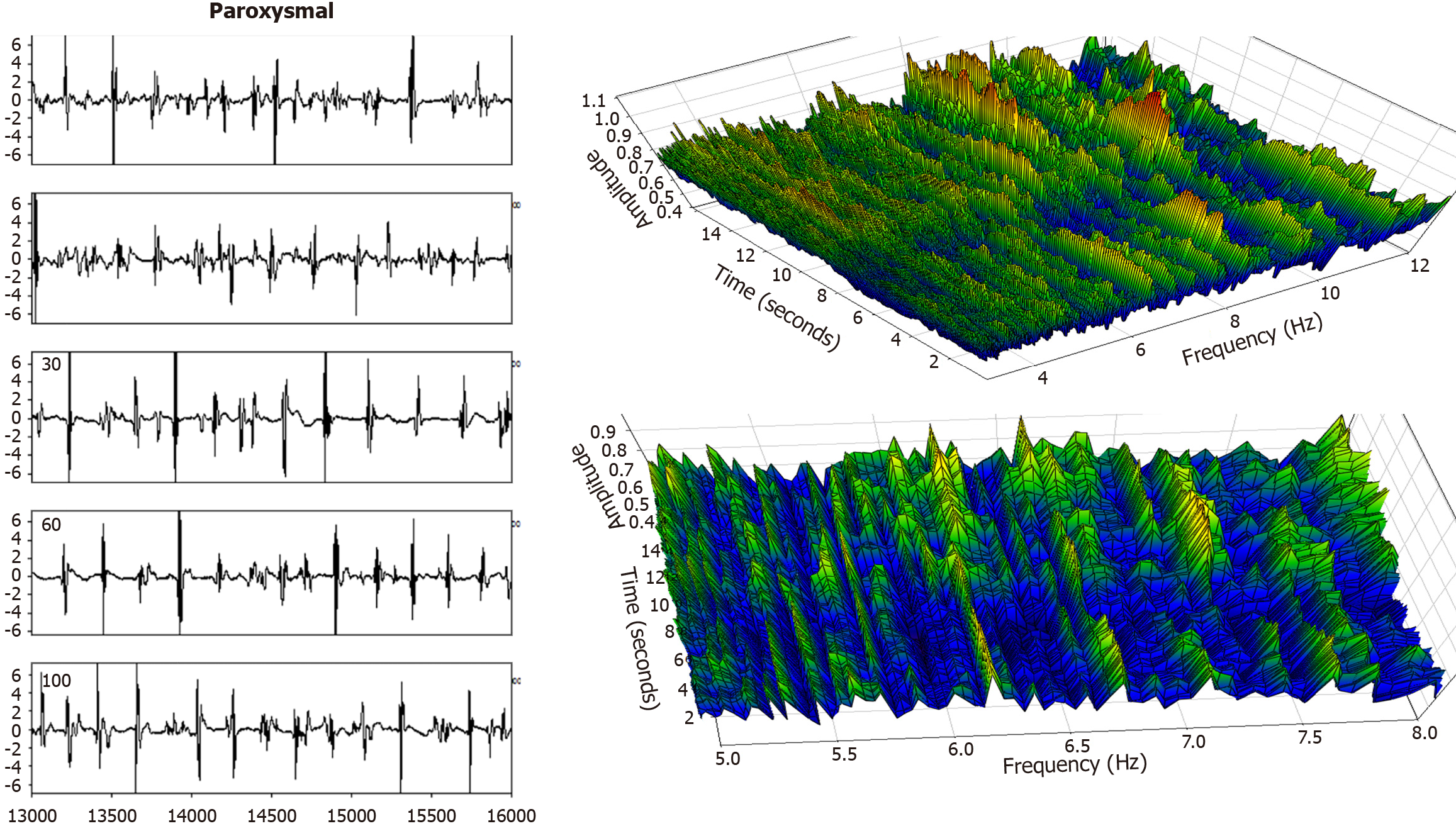
Structural remodeling includes atrial dilatation, fibrosis, and reduced myocardial voltage, and for AF patients, it begins near the pulmonary vein antra and progressively involves other left atrial regions[87-89]. The electroanatomic changes are reflected in reduced regional voltage, slowed conduction velocity, and an increased presence of fractionated electrograms and LVAs. These alterations are more pronounced in patients with persistent vs paroxysmal AF, suggesting that there is a progressive remodeling of substrate as the time since AF onset increases[90]. From the first to second substrate modification procedure, when needed, bipolar mean peak-to-peak voltage in the LA can decrease significantly, with marked increase in LVA surface area[91]. No similar significant changes are evident in the right atrial bipolar mean peak-to-peak voltage or in the LVA surface area during the second procedure, suggesting a predominant role of the LA, but not the right atrium, during disease progression. Electrogram morphology, in terms of the activation wavefront power (as estimated by spectral amplitude) and refractoriness (as estimated by DF) can be utilized to express the severity of disease progression. A schematic is illustrated in Figure 4. The electrogram (top graph) is segmented by windowing. At top, for a window length w = 100 sample points, the first four segments are shown, and similarly for window lengths w = 200 sample points and w = 500 sample points at middle and bottom, which are segmentations of differing window length applied to the original time series signal. For each window length, all segments in the series are then overlapped, and the ensemble mean power is computed and graphed for all segment lengths plotted on the ordinate axis, with the abscissa scale converted to frequency = 1/w. This method of frequency analysis, termed the New Spectral Estimator, can be helpful to use as a comparator to Fourier analysis, and it shows similar levels of significance in the difference in spectral parameters when comparing paroxysmal vs persistent AF[92]. Local bipolar voltage and its remodeled characteristics can in part be surmised from the spectral power, although it is also dependent on electrode size and interelectrode distance, tissue thickness/health, and activation wavefront orientation.
During remodeling, a longer AF time duration since onset is also associated with an increased left atrial scar burden, which can negatively impact the success of ablation procedures[93]. However, all patients with AF have lower regional voltages, increased proportions of LVAs, slower conduction velocities, and more complex electrograms as compared to control patients without AF[90]. Since these abnormalities are observed even in the absence of overt structural heart disease, they support the notion of primary electrical remodeling (i.e., electrical remodeling that occurs primarily in response to a functional effect, such as an altered electrical activation sequence)[94,95].
Although prompt termination of paroxysmal AF episodes may temporarily suppress arrhythmia symptoms, it does not halt disease progression, nor does it reverse the underlying progress of atrial remodeling[96]. LVA grade, which is reflective of remodeling, is an independent predictor of paroxysmal AF recurrence following PVI[97]. In a randomized comparison, AF recurred in half of patients who underwent PVI alone, compared to about a third of those who received PVI with additional substrate modification; hence targeting the underlying atrial substrate is appropriate for selected patients[82]. In AF ablation, scar formation as evident on CMR–GE is associated with AF recurrence[98]. However, CMR–GE is currently unsatisfactory in terms of its spatial resolution. Another hurdle as yet to be accomplished is that by integrating imaging with mapping data, a goal as yet unattained, patient stratification might more readily be implemented. Patients with persistent AF demonstrate more advanced remodeling compared to those with paroxysmal AF, including a lower median left atrial voltage, a greater percentage of bipolar voltage regions below a 0.2 mV threshold level, and a higher indexed LA volume (volume/body surface area)[25]. Of note, the shape of the left atrial chamber is predictive of AF ablation failure, independently from and more accurately as compared with atrial volume and AF persistence[99]. Hence, structural properties are of importance in determining arrhythmia persistence. Clinical trials suggest that earlier use of rhythm control, along with AF ablation, is associated with better outcome[100,101], which may indicate the possibility of dampening the progression of remodeling.
Systemic hypertension, especially when accompanied by left ventricular hypertrophy, is another property associated with atrial remodeling[102]. This hypertension is manifested as a global slowing of electrical conduction, regional conduction delay (notably at the crista terminalis), and increased susceptibility to AF induction[102]. While lower global left atrial conduction velocity and widespread voltage reduction are predictive of AF recurrence, the presence of discrete LVAs alone is not independently predictive[103], perhaps indicating the necessity of global property transformation in the remodeling process, to affect change in arrhythmia propensity. The relationship between voltage amplitude and wavefront activation slowing is complex, with some regions of low voltage exhibiting normal activation while other areas with normal voltage demonstrate significant activation slowing[86]. This supports the hypothesis that AF maintenance may also involve diffuse and more globalized substrate remodeling, rather than being dependent on localized LVAs alone. Furthermore, it is known that left atrial voltage reduction is a diffuse process related to the underlying fibrosis pattern, reinforcing the idea that atrial remodeling is not strictly limited to localized, well-defined low-voltage zones[104]. A persistent AF diagnosis, however, does not necessarily indicate the presence of voltage-defined fibrosis, nor does paroxysmal AF indicate a lack of fibrosis[105].
One of the known and hallmark features of electrical remodeling in AF is a reduction in the atrial effective refractory period (ERP), and an impaired ERP adaptation to increased rates, which contributes to local reentry and AF maintenance[106,107]. At the cellular level, AF leads to a marked shortening of action potential duration and impaired rate adaptation. These changes are largely attributed to reductions in the cellular-level calcium current and the transient outward current, thereby altering calcium and potassium handling in atrial myocytes[108,109], which confers remodeling at the microscopic-level.
Epicardial adipose tissue (EAT) remodeling also plays a crucial role in AF development and progression[110]. Patients with AF exhibit a higher volume of EAT and a lower image attenuation value[111]. The volume and attenuation of left atrial-EAT are associated with the presence of LVA within the myocardium. Myocardial bipolar voltage on electroanatomic mapping of the atrium is negatively associated with age and the presence of overlying EAT[112]. Electrogram fractionation in sinus rhythm is positively associated with age, male gender, diabetes, hypertension, and EAT. Electrogram widening is correlated to age, male gender, and EAT. Body mass index is directly associated with the presence of EAT in patients with AF. However, EAT is not a statistical mediator of the association between clinical variables and left atrial scar. Ultimately, lipomatous dysplasia, characterized by excessive fat accumulation in the heart which alters electrical current load and creates reentrant circuit corridors, may contribute to onset of both AF and ventricular tachycardia[113,114].
Voltage signals recorded for AF analysis are influenced by many factors-including wavefront direction, electrode configuration, tissue properties, activation rate, contact force, and filter settings[115]. There is only a partial correspondence between the voltages obtained in sinus rhythm vs AF-acquired signals, with weaker AF signal recordings being found in patients with persistent AF[87]. Although voltages acquired in both atria are generally lower during AF vs sinus rhythm, there is a greater voltage reduction observed at sites exhibiting the shortest AF cycle lengths (shortest frequencies)[1]. This may be due to the diminished coupling interval, disorganized depolarization, or partial depolarization of atrial tissue. Faster activation rates in general result in larger observed LVAs, particularly in paroxysmal AF[116]. Pacing from the coronary sinus at a rate 50 megaseconds faster than sinus rhythm reduces bipolar voltage and also increases the extent of LVA[117]. The voltage attenuation that occurs with faster pacing rate, which is termed Dynamic Voltage Mapping, demonstrates that left atrial voltage decreases at more than two-thirds of recording sites as coupling intervals shorten[116].
Voltage amplitude is highly influenced by the structural properties of the substrate. It is directly affected by atrial wall thickness, which varies regionally, even in normal atria[14]. Bipolar voltage is significantly higher on the epicardium (mean: 3.05 ± 1.31 mV) as compared to the endocardium (mean: 1.65 ± 0.75 mV)[116]. Increased atrial size and pressure are both associated with a lower atrial voltage[118]. Atrial wall stress and acute atrial dilatation have been linked to the formation of LVAs, particularly in the posterior wall[119]. Impaired mechanical function can result in lower voltage levels[6]. Aberrant atrial structure may cause complex and patient-variable three-dimensional bioelectrical transformations resulting in activation wavefront asynchrony, with observed endo-epi dissociation[120,121]. Hence, endocardial substrate mapping alone may not fully characterize the mechanisms of AF, and sole reliance on endocardial ablation may be insufficient in the treatment of AF.
The data acquisition system utilized to record the electrograms is a major source of variability between investigator teams. Bipolar electrode spacing determines the temporal offset in wavefront arrival, which influences both the signal morphology and the signal amplitude. Greater interelectrode spacing increases electrogram voltage until a plateau is asymptotically reached-typically beyond a 4-mm electrode spacing in healthy tissue-but this plateau is absent in diseased myocardium[122]. Increased bipolar electrode spacing also leads to greater far-field signal contribution. Larger electrodes record higher amplitude signals due to the larger tissue contact area, but these larger electrodes more likely include both normal and diseased myocardium. Adequate contact force is also necessary to ensure reliable voltage recordings. There is a weak correlation between contact force and voltage at low force levels, but no further voltage increase occurs beyond a certain threshold[52]. The filter settings during data acquisition additionally affect electrogram amplitude[123]. Increasing the high-pass filter frequency substantially reduces the amplitude of electrogram deflections. During clinical mapping, typical bipolar filters include high-pass corners between 1–30 Hz and low-pass corners between 300–500 Hz, with the high pass filter in this range significantly altering both electrogram shape and its amplitude.
Much attention has recently been given to the polarity of the signal recording. Unipolar and bipolar voltage maps are congruous in both sinus rhythm and AF[124], though it is bipolar electrode technology that is ubiquitously utilized for substrate voltage mapping. It is based on the principle that the differential of two separate electrode pole signals results in cancellation of the far-field component. The bipolar signal is formed when the local extracellular signal generated by the activation wavefront arrives at the positive pole of a bipolar electrode, which is added to form a positive deflection in that pole’s signal, and then arrives at the negative pole, which is subtracted by the differential transformer, causing a negative deflection, or vice versa. Thus, a biphasic electrogram shape is obtained when an activation wavefront sequentially propagates past the two poles of a bipolar electrode. If, however, the wavefront leading edge arrives at both poles simultaneously, the near-field as well as the far-field signal may be mostly cancelled, resulting in a low-voltage electrogram output. Hence, voltage level differs for parallel vs perpendicular orientated bipoles with respect to wavefront propagation direction, particularly in sinus rhythm when the orientation of the oncoming wavefront has a tendency to be more constant. Yet due to the randomness and time-variability of recordings made during AF, wavefront orientation then has a less clear effect on bipolar signal voltage[125]. Unipolar electrograms have no such oncoming wavefront orientation bias, but they do not remove atrial far-field activity[39].
Recently, omnipolar technology, which utilizes multiple electrodes to form a differentiated signal, has been introduced[126]. This arrangement can eliminate the wavefront orientation bias evident in bipolar electrode recordings, particularly during sinus rhythm. Omnipolar electrogram amplitudes match those of the largest bipolar electrogram, because the diminishing amplitude effect perpetuated by a perpendicular activation wavefront direction is removed. Yet, omnipolar and bipolar scar maps differ due to the effects of an altered voltage threshold selection, and variations in oncoming wavefront orientation. Although a potentially promising technology, published clinical AF data in which omnipolar mapping overcomes the directional limitation of bipolar voltage mapping is scarce[127]. Since omnipolar electrograms do provide consistent and independent voltage information in AF[125], they may eventually obviate the need for cardioversion to sinus rhythm to undertake voltage mapping.
Atrial fibrosis promotes arrhythmogenesis by supporting reentrant and focal mechanisms, and it contributes to the post-ablation transition from AF to more organized rhythms such as atrial flutter and AT[128]. Most post-ablation ATs originate in the LVAs, often coinciding with sites of prior ablation lesioning, and thereby underscore the role of LVAs as a source of conduction block in arrhythmia recurrence[129]. Bipolar voltages are significantly lower in AF than in sinus rhythm or atrial flutter due to the presence of rapid and disorganized activation, shortened cycle lengths, and potential functional refractoriness of the atrial substrate[10,107]. In the LA, voltages during sinus rhythm average around 3.0 ± 2.9 mV, while they drop substantially to approximately 0.9 ± 0.6 mV during AF[130]. Left atrial posterior and right atrial septal regions exhibit the most significant voltage reductions during AF[130]. In many persistent AF patients, LVAs identified in sinus rhythm correspond to those seen in AF, although the extent of LVAs is often larger in AF when using the same threshold voltage for detection[1]. At the posterior LA, AF voltage correlates with fibrotic tissue detected by CMR-GE better than sinus rhythm voltage, likely due to the functional unmasking of latent substrate under the conditions imposed by the arrhythmia[63].
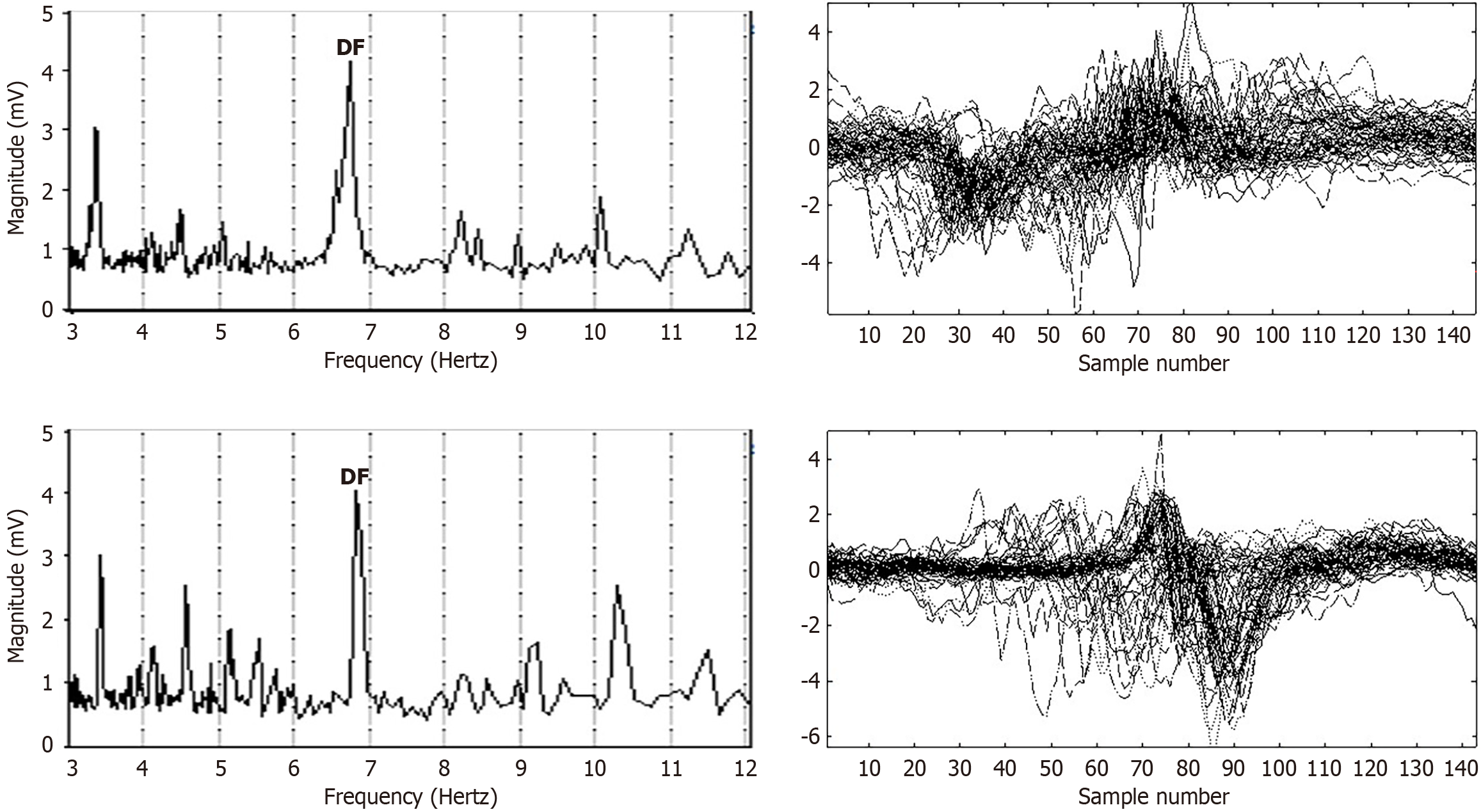
Electrogram fractionation is more prevalent during AF than in sinus rhythm, especially in areas of abnormal substrate. In persistent AF patients, fractionated electrograms occurred in 45% of sites during AF, as compared to 13% in sinus rhythm[30]. Sites exhibiting fractionation during AF were more likely to also show abnormalities during sinus rhythm, suggesting an underlying and fixed substrate at many locations with abnormality. During induced paroxysmal AF patients, shortening of the systolic interval, following either drift or acceleration of a source, results in intermittent fibrillatory conduction with formation of fractionated electrograms at the posterior left atrial wall[131]. Detailed examples of such fractionated AF electrograms are shown in Figures 5, 6, 7 and 8. In each Figure, to the left are the sequential time series traces. To the right are three-dimensional graphs of spectral estimation, showing frequency magnitude (Z-axis), and time vs frequency (X-axes and Y-axes). The upper panel portrays the global view of the entire spectrum of length 16 seconds and 2–12 Hz frequency, while the lower panel depicts a close-up or zoom-in of the frequency range, which varies in each of the Figures 5, 6, 7 and 8 to show detail. Frequency magnitude is color-coded, with low amplitudes blue and highest values red. The DF is often more regular and stable in persistent (Figures 5 and 6) as compared to paroxysmal AF (Figures 7 and 8). This is evident at the peak magnitudes (red, orange and yellow color) for persistent AF, both at anterior and posterior free wall sites (Figures 5 and 6, respectively) vs increased variability in paroxysmal AF (Figures 7 and 8). Thus, there is greater spatiotemporal dispersion in paroxysmal AF spectra. The spatiotemporal dispersion of the electrogram activation sequence reflects reentrant-like conduction, and is indicative of its contribution in initiating or maintaining AF[132].
The surface P-wave on a standard 12-lead electrocardiogram (ECG) offers valuable noninvasive insights into atrial conduction and remodeling. Numerous studies have linked P-wave characteristics, including amplitude, duration, axis, and dispersion, to AF onset, recurrence, PR interval, and atrial substrate abnormalities[133,134]. P-wave dispersion (Pd), defined as the difference between the maximum and minimum P-wave durations across 12 ECG leads, reflects the presence of heterogeneity in atrial conduction[135]. Increased Pd measured within 24 hours of an acute ischemic stroke may predict paroxysmal AF and identify patients at risk for recurrent stroke[136]. In stroke patients, Pd normalized to P-wave vector magnitude (Pd/Pvm) is the only ECG parameter independently associated with subsequent AF detection[137]. Lower P-wave voltage in lead 1 (PVL1) corresponds to increased AF recurrence risk following ablation[138]. Furthermore, a PVL1 magnitude less than 0.062 mV predicts the presence of left atrial LVAs overlying more than 35% of the atrial surface[139]. Reduced PVL1, and presence of interatrial block, are both associated with new-onset AF in patients with non-ST elevation myocardial infarction (MI), suggesting a shared pathophysiological mechanism which is linked to the presence of structural heart disease[138]. Prolonged P-wave duration is correlated with increased left atrial activation time, particularly in the presence of low-voltage substrates[140]. Left atrial low-voltage substrate significantly delays regional conduction[141], thus increasing P-wave duration. This contributes to arrhythmia recurrence after PVI, especially in persistent AF. Prolonged P-wave duration and reduced amplitude correspond to the extent of atrial scarring. These parameters may therefore serve as noninvasive markers of electroanatomic remodeling[139,140].
The P-wave duration-to-voltage ratio has emerged as a simple and cost-effective ECG metric. It shows promise in predicting new-onset AF, and may also provide mechanistic insight into atrial conduction abnormality[142]. The ratio integrates conduction time and atrial electrical amplitude, potentially serving as a surrogate for atrial remodeling severity[143]. A lower Pvm has been linked to the presence of extensive LVAs, and it may function as a complementary tool, alongside conventional ECG metrics, in left atrial substrate assessment[139]. An increased left atrial diameter observed on echocardiography is often associated with prolonged P-wave duration, and enhances AF risk stratification[144]. Thus, P-wave characteristics on the standard ECG, including dispersion, duration, voltage, and derived ratios, serve as important noninvasive tools for predicting AF risk, identifying atrial remodeling, and guiding patient selection for ablation[145]. These markers also hold relevance in stroke populations, post-MI patients, and those undergoing rhythm control strategies.
Voltage-guided substrate mapping is an important tool for the treatment of AF. Low voltage areas have been detected in a majority of patients with paroxysmal AF[97] and it is also useful for guiding linear ablation in patients with persistent AF[81]. This finding challenges the notion that substrate severity and the need for ablation are solely dependent on the time duration of the arrhythmia since onset. Rather, a median voltage of 0.99 mV in paroxysmal AF patients vs 0.41 mV in patients with persistent AF is useful to inform on AF type[25], thereby suggesting that substrate remodeling is important to AF progression, and in transition between AF types. Further study of remodeling would be useful to determine mechanistically salient aspects of disease progression, and those which are most important for substrate modification. Voltage-guided ablation using the established 0.5 mV cut-point during sinus rhythm for targeting low voltage regions improves clinical outcome, adding credibility to this metric as a useful tool. However, there is as yet no standard cut-point, and regions below the 0.5 mV cutoff in sinus rhythm typically exhibit lower thresholds during AF (approximately 0.2 mV) and atrial flutter (0.35 mV)[10]. Hence, there is a need to develop standards to target for ablation during substrate mapping, and to understand how these are affected by rhythm type. Although a simple peak-to-peak voltage measurement is normally used to determine level, the AF atrial electrogram has a rich morphology which might be considered for further appraisal (Figures 5, 6, 7 and 8). Many facets of atrial structure and of the data acquisition system influence voltage substrate mapping, and these should be considered, and if possible normalized, when comparing and contrasting the results from different studies. Ultimately, personalized ablation of LVA in persistent AF may provide further insight for substrate mapping and modification[146].
| 1. | Andrés Lahuerta A, Roberto C, Saiz FJ, Cano Ó, Martínez-Mateu L, Alonso P, Saurí A, Quesada A, Osca J. Atrial low voltage areas: A comparison between atrial fibrillation and sinus rhythm. Cardiol J. 2022;29:252-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Mergani M, Mannion J, Healy L, Ramsamy K, Egan S, Boles U. Pulsed field ablation using additional mapping and substrate modification to treat atrial fibrillation - one year outcome comparison. Eur Heart J. 2024;45:ehae666.373. [DOI] [Full Text] |
| 3. | Cutler MJ, Johnson J, Abozguia K, Rowan S, Lewis W, Costantini O, Natale A, Ziv O. Impact of Voltage Mapping to Guide Whether to Perform Ablation of the Posterior Wall in Patients With Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Verma A, Feld GK, Cox JL, Dewland TA, Babkin A, De Potter T, Raju N, Haissaguerre M. Combined pulsed field ablation with ultra-low temperature cryoablation: A preclinical experience. J Cardiovasc Electrophysiol. 2023;34:2124-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Liu Z, Xia Y, Guo C, Li X, Fang P, Yin X, Yang X. Low-Voltage Zones as the Atrial Fibrillation Substrates: Relationship With Initiation, Perpetuation, and Termination. Front Cardiovasc Med. 2021;8:705510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Liu W, Li S, Han B. It Is Necessary to Re-understand the Low-Voltage Area in Atrial Fibrillation Patients. Front Cardiovasc Med. 2022;9:919873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Kapa S, Desjardins B, Callans DJ, Marchlinski FE, Dixit S. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Kawaji T, Hojo S, Kushiyama A, Nakatsuma K, Kaneda K, Kato M, Yokomatsu T, Miki S. Optimal cutoff value of bipolar low-voltage in electroanatomic voltage mapping during atrial fibrillation rhythm. Pacing Clin Electrophysiol. 2019;42:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 849] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 10. | Rodríguez-Mañero M, Valderrábano M, Baluja A, Kreidieh O, Martínez-Sande JL, García-Seara J, Saenen J, Iglesias-Álvarez D, Bories W, Villamayor-Blanco LM, Pereira-Vázquez M, Lage R, Álvarez-Escudero J, Heidbuchel H, González-Juanatey JR, Sarkozy A. Validating Left Atrial Low Voltage Areas During Atrial Fibrillation and Atrial Flutter Using Multielectrode Automated Electroanatomic Mapping. JACC Clin Electrophysiol. 2018;4:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Qi X, Chen H, Yang G, Liu H, Wang Z, Jiang X, Cui C, Cai C, Ju W, Chen M. Unipolar Voltage for Better Characterizing Left Atrium Substrates: Comparing the Predictive Efficacy for Recurrence Post Atrial Fibrillation Ablation in a Post Hoc Analysis of STABLE-SR-III Trial. J Cardiovasc Electrophysiol. 2025;36:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Huo Y, Gaspar T, Pohl M, Sitzy J, Richter U, Neudeck S, Mayer J, Kronborg MB, Piorkowski C. Prevalence and predictors of low voltage zones in the left atrium in patients with atrial fibrillation. Europace. 2018;20:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Ding X, Li M, Chen H, Yang G, Zhang F, Ju W, Gu K, Li J, Chen M. Low-Voltage Area at the Anterior Wall of the Left Atrium Is Associated With Thromboembolism in Atrial Fibrillation Patients With a Low CHA(2)DS(2)-VA Score. Front Cardiovasc Med. 2022;9:869862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Marcus GM, Yang Y, Varosy PD, Ordovas K, Tseng ZH, Badhwar N, Lee BK, Lee RJ, Scheinman MM, Olgin JE. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm. 2007;4:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Chieng D, Sugumar H, Hunt A, Ling LH, Segan L, Al-Kaisey A, Hawson J, Prabhu S, Voskoboinik A, Wong G, Morton JB, Lee G, Ginks M, Sterns L, Sanders P, Kalman JM, Kistler PM. Impact of Posterior Left Atrial Voltage on Ablation Outcomes in Persistent Atrial Fibrillation: CAPLA Substudy. JACC Clin Electrophysiol. 2023;9:2291-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Ikoma T, Naruse Y, Kaneko Y, Sakakibara T, Narumi T, Sano M, Mogi S, Suwa K, Ohtani H, Saotome M, Urushida T, Maekawa Y. Pre-procedural predictors of left atrial low-voltage zones in patients undergoing catheter ablation of atrial fibrillation. PLoS One. 2022;17:e0266939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Nery PB, Al Dawood W, Nair GM, Redpath CJ, Sadek MM, Chen L, Green MS, Wells G, Birnie DH. Characterization of Low-Voltage Areas in Patients With Atrial Fibrillation: Insights From High-Density Intracardiac Mapping. Can J Cardiol. 2018;34:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Ammar-Busch S, Buiatti A, Tatzber A, Reents T, Bourier F, Semmler V, Telishevska M, Hessling G, Deisenhofer I. Predictors of low voltage areas in persistent atrial fibrillation: is it really a matter of time? J Interv Card Electrophysiol. 2020;57:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Müller P, Makimoto H, Dietrich JW, Fochler F, Nentwich K, Krug J, Duncker D, Blockhaus C, Kelm M, Fürnkranz A, Deneke T, Halbfass P. Association of left atrial low-voltage area and thromboembolic risk in patients with atrial fibrillation. Europace. 2018;20:f359-f365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, Ziegler PD; TRENDS Investigators. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 21. | Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm From Cardiac Electronic Implanted Devices. Circ Arrhythm Electrophysiol. 2015;8:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Matsuda Y, Kudo S, Masuda M, Uematsu H, Sugino A, Ooka H, Fujii S, Okamoto S, Ishihara T, Nanto K, Tsujimura T, Hata Y, Nakao S, Kusuda M, Ariyasu W, Mano T. Progression of Atrial Cardiomyopathy Predicts Subsequent Stroke: An Analysis of Left Atrial Low-Voltage Areas in Patients With Atrial Fibrillation Ablation. Circ Arrhythm Electrophysiol. 2025;18:e013550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Chang SL, Tai CT, Lin YJ, Wongcharoen W, Lo LW, Tuan TC, Udyavar AR, Chang SH, Tsao HM, Hsieh MH, Hu YF, Chen YJ, Chen SA. Biatrial substrate properties in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | van der Does WFB, Heida A, van der Does LJME, Bogers AJJC, de Groot NMS. Conduction Disorders during Sinus Rhythm in Relation to Atrial Fibrillation Persistence. J Clin Med. 2021;10:2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Fiala M, Wichterle D, Chovancík J, Bulková V, Wojnarová D, Nevralová R, Januska J. Left atrial voltage during atrial fibrillation in paroxysmal and persistent atrial fibrillation patients. Pacing Clin Electrophysiol. 2010;33:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Lockwood D, Nakagawa H, Peyton MD, Edgerton JR, Scherlag BJ, Sivaram CA, Po SS, Beckman KJ, Abedin M, Jackman WM. Linear left atrial lesions in minimally invasive surgical ablation of persistent atrial fibrillation: techniques for assessing conduction block across surgical lesions. Heart Rhythm. 2009;6:S50-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Knecht S, Hocini M, Wright M, Lellouche N, O'Neill MD, Matsuo S, Nault I, Chauhan VS, Makati KJ, Bevilacqua M, Lim KT, Sacher F, Deplagne A, Derval N, Bordachar P, Jaïs P, Clémenty J, Haïssaguerre M. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008;29:2359-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 28. | Begg GA, Karim R, Oesterlein T, Graham LN, Hogarth AJ, Page SP, Pepper CB, Rhode K, Lip GYH, Holden AV, Plein S, Tayebjee MH. Left atrial voltage, circulating biomarkers of fibrosis, and atrial fibrillation ablation. A prospective cohort study. PLoS One. 2018;13:e0189936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Vlachos K, Efremidis M, Letsas KP, Bazoukis G, Martin R, Kalafateli M, Lioni L, Georgopoulos S, Saplaouras A, Efremidis T, Liu T, Valkanas K, Karamichalakis N, Asvestas D, Sideris A. Low-voltage areas detected by high-density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Sunaga A, Tsujimura T, Matsuda Y, Ohashi T, Uematsu M. Comparison of Left Atrial Voltage between Sinus Rhythm and Atrial Fibrillation in Association with Electrogram Waveform. Pacing Clin Electrophysiol. 2017;40:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Zhang W, Wang Y, Wang H, Shao Y, Dong Q, Li S, Gu Y. Left Atrial Low Voltage Areas Predicts Recurrence of Atrial Fibrillation after Catheter Ablation: A Meta-Analysis. Heart Surg Forum. 2024;27:E058-E067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Cappato R, Negroni S, Pecora D, Bentivegna S, Lupo PP, Carolei A, Esposito C, Furlanello F, De Ambroggi L. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Nagashima K, Watanabe I, Okumura Y, Iso K, Takahashi K, Watanabe R, Arai M, Kurokawa S, Nakai T, Ohkubo K, Yoda S, Hirayama A. High-voltage zones within the pulmonary vein antra: Major determinants of acute pulmonary vein reconnections after atrial fibrillation ablation. J Interv Card Electrophysiol. 2017;49:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y, Okuno S, Hata Y, Mano T. Additional Low-Voltage-Area Ablation in Patients With Paroxysmal Atrial Fibrillation: Results of the Randomized Controlled VOLCANO Trial. J Am Heart Assoc. 2020;9:e015927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 35. | Spragg DD, Khurram I, Zimmerman SL, Yarmohammadi H, Barcelon B, Needleman M, Edwards D, Marine JE, Calkins H, Nazarian S. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012;9:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. II. The role of nonuniform recovery of excitability in the occurrence of unidirectional block, as studied with multiple microelectrodes. Circ Res. 1976;39:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 319] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5432] [Cited by in RCA: 5500] [Article Influence: 196.4] [Reference Citation Analysis (0)] |
| 38. | Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol. 1998;9:S2-12. [PubMed] |
| 39. | de Groot NMS, Kleber A, Narayan SM, Ciaccio EJ, Doessel O, Bernus O, Berenfeld O, Callans D, Fedorov V, Hummel J, Haissaguerre M, Natale A, Trayanova N, Spector P, Vigmond E, Anter E. Atrial fibrillation nomenclature, definitions, and mechanisms: Position paper from the international Working Group of the Signal Summit. Heart Rhythm. 2025;22:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Sim I, Bishop M, O'Neill M, Williams SE. Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol. 2019;56:213-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 41. | Huang SY, Lin YJ, Tsao HM, Chang SL, Lo LW, Hu HF, Suenari K, Lin YK, Huang JH, Chen IC, Ko WC, Ong ET, Chen SA. The biatrial substrate properties in different types of paroxysmal atrial fibrillation. Heart Rhythm. 2011;8:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Kawai S, Mukai Y, Inoue S, Yakabe D, Nagaoka K, Sakamoto K, Takase S, Chishaki A, Tsutsui H. Non-Pulmonary Vein Triggers of Atrial Fibrillation Are Likely to Arise from Low-Voltage Areas in the Left Atrium. Sci Rep. 2019;9:12271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Yamaji H, Higashiya S, Murakami T, Hina K, Kawamura H, Murakami M, Kamikawa S, Hirohata S, Kusachi S. Efficacy of an Adjunctive Electrophysiological Test-Guided Left Atrial Posterior Wall Isolation in Persistent Atrial Fibrillation Without a Left Atrial Low-Voltage Area. Circ Arrhythm Electrophysiol. 2020;13:e008191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Frederick M, Fortino J, Benloucif-Moore S, Jongnarangsin K, Pelosi F Jr, Bogun F, Morady F. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007;115:2606-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 45. | Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1497] [Cited by in RCA: 1492] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 46. | Roberts-Thomson KC, Kistler PM, Sanders P, Morton JB, Haqqani HM, Stevenson I, Vohra JK, Sparks PB, Kalman JM. Fractionated atrial electrograms during sinus rhythm: relationship to age, voltage, and conduction velocity. Heart Rhythm. 2009;6:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Biviano AB, Ciaccio EJ, Knotts R, Fleitman J, Lawrence J, Iyer V, Whang W, Garan H. Atrial electrogram discordance during baseline vs reinduced atrial fibrillation: Potential ramifications for ablation procedures. Heart Rhythm. 2015;12:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Saghy L, Callans DJ, Garcia F, Lin D, Marchlinski FE, Riley M, Dixit S, Tzou WS, Haqqani HM, Pap R, Kim S, Gerstenfeld EP. Is there a relationship between complex fractionated atrial electrograms recorded during atrial fibrillation and sinus rhythm fractionation? Heart Rhythm. 2012;9:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Kofune M, Okumura Y, Watanabe I, Nagashima K, Sonoda K, Mano H, Kogawa R, Sasaki N, Ohkubo K, Nakai T, Nikaido M, Hirayama A. Comparative distribution of complex fractionated atrial electrograms, high dominant frequency (HDF) sites during atrial fibrillation and HDF sites during sinus rhythm. J Interv Card Electrophysiol. 2013;36:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Sasaki N, Watanabe I, Okumura Y, Nagashima K, Kogawa R, Sonoda K, Iso K, Takahashi K, Arai M, Watanabe R, Kurokawa S, Ohkubo K, Nakai T, Hirayama A, Nikaido M. Complex fractionated atrial electrograms, high dominant frequency regions, and left atrial voltages during sinus rhythm and atrial fibrillation. J Arrhythm. 2017;33:185-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Sparks PB, Morton JB, Sanders P, Kalman JM. The relationship between complex fractionated electrograms and atrial low-voltage zones during atrial fibrillation and paced rhythm. Europace. 2011;13:1709-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Sasaki N, Okumura Y, Watanabe I, Sonoda K, Kogawa R, Takahashi K, Iso K, Nakahara S, Maruyama A, Takemura S, Hirayama A. Relations between contact force, bipolar voltage amplitude, and mapping point distance from the left atrial surfaces of 3D ultrasound- and merged 3D CT-derived images: Implication for atrial fibrillation mapping and ablation. Heart Rhythm. 2015;12:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Seitz J, Bars C, Théodore G, Beurtheret S, Lellouche N, Bremondy M, Ferracci A, Faure J, Penaranda G, Yamazaki M, Avula UM, Curel L, Siame S, Berenfeld O, Pisapia A, Kalifa J. AF Ablation Guided by Spatiotemporal Electrogram Dispersion Without Pulmonary Vein Isolation: A Wholly Patient-Tailored Approach. J Am Coll Cardiol. 2017;69:303-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 54. | Seitz J, Durdez TM, Albenque JP, Pisapia A, Gitenay E, Durand C, Monteau J, Moubarak G, Théodore G, Lepillier A, Zhao A, Bremondy M, Maluski A, Cauchemez B, Combes S, Guyomar Y, Heuls S, Thomas O, Penaranda G, Siame S, Appetiti A, Milpied P, Bars C, Kalifa J. Artificial intelligence software standardizes electrogram-based ablation outcome for persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33:2250-2260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 55. | Krul SP, Berger WR, Smit NW, van Amersfoorth SC, Driessen AH, van Boven WJ, Fiolet JW, van Ginneken AC, van der Wal AC, de Bakker JM, Coronel R, de Groot JR. Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 56. | Ma J, Chen Q, Ma S. Left atrial fibrosis in atrial fibrillation: Mechanisms, clinical evaluation and management. J Cell Mol Med. 2021;25:2764-2775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 57. | Vandersickel N, Watanabe M, Tao Q, Fostier J, Zeppenfeld K, Panfilov AV. Dynamical anchoring of distant arrhythmia sources by fibrotic regions via restructuring of the activation pattern. PLoS Comput Biol. 2018;14:e1006637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Zghaib T, Keramati A, Chrispin J, Huang D, Balouch MA, Ciuffo L, Berger RD, Marine JE, Ashikaga H, Calkins H, Nazarian S, Spragg DD. Multimodal Examination of Atrial Fibrillation Substrate: Correlation of Left Atrial Bipolar Voltage Using Multi-Electrode Fast Automated Mapping, Point-by-Point Mapping, and Magnetic Resonance Image Intensity Ratio. JACC Clin Electrophysiol. 2018;4:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Ali SY, Mohsen Y, Mao Y, Sakata K, Prakosa A, Yamamoto C, Kholmovski E, Sinha S, Marine J, Sommer PH, Sohns CH, Stoeckigt F, Spragg D, Trayanova N. Exploring LGE areas in the atria: Insights from electroanatomical voltage mapping in AF patients. Eur Heart J. 2024;45:ehae666.303. [DOI] [Full Text] |
| 60. | Ling Z, McManigle J, Zipunnikov V, Pashakhanloo F, Khurram IM, Zimmerman SL, Philips B, Marine JE, Spragg DD, Ashikaga H, Calkins H, Nazarian S. The association of left atrial low-voltage regions on electroanatomic mapping with low attenuation regions on cardiac computed tomography perfusion imaging in patients with atrial fibrillation. Heart Rhythm. 2015;12:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Wu Y, Qin X, Gao P, Liu Y, Fang Q, Deng H, Cheng K, Cheng Z, Yang D, Chen T. Relationship between the distribution of left atrial low-voltage zones and post-ablation atrial arrhythmia recurrence in patients with atrial fibrillation. Hellenic J Cardiol. 2022;66:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 62. | Chelu MG, King JB, Kholmovski EG, Ma J, Gal P, Marashly Q, AlJuaid MA, Kaur G, Silver MA, Johnson KA, Suksaranjit P, Wilson BD, Han FT, Elvan A, Marrouche NF. Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging and Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up Data. J Am Heart Assoc. 2018;7:e006313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 63. | Qureshi NA, Kim SJ, Cantwell CD, Afonso VX, Bai W, Ali RL, Shun-Shin MJ, Malcolme-Lawes LC, Luther V, Leong KMW, Lim E, Wright I, Nagy S, Hayat S, Ng FS, Wing MK, Linton NWF, Lefroy DC, Whinnett ZI, Davies DW, Kanagaratnam P, Peters NS, Lim PB. Voltage during atrial fibrillation is superior to voltage during sinus rhythm in localizing areas of delayed enhancement on magnetic resonance imaging: An assessment of the posterior left atrium in patients with persistent atrial fibrillation. Heart Rhythm. 2019;16:1357-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Schade A, Nentwich K, Costello-Boerrigter LC, Halbfass P, Mueller P, Roos M, Barth S, Krug J, Szoelloesi GA, Lapp H, Deneke T. Spatial Relationship of Focal Impulses, Rotors and Low Voltage Zones in Patients With Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J. 2017;38:20-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 66. | Yamaguchi T, Tsuchiya T, Nakahara S, Fukui A, Nagamoto Y, Murotani K, Eshima K, Takahashi N. Efficacy of Left Atrial Voltage-Based Catheter Ablation of Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 67. | Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, Hindricks G, Piorkowski C. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 434] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 68. | Yamaguchi T, Tsuchiya T, Fukui A, Kawano Y, Otsubo T, Takahashi Y, Hirota K, Murotani K, Eshima K, Takahashi N. Impact of the extent of low-voltage zone on outcomes after voltage-based catheter ablation for persistent atrial fibrillation. J Cardiol. 2018;72:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, Park CI, Denis A, Jaïs P, Hocini M, Potocnik C, Allgeier J, Hochholzer W, Herrera-Siklody C, Kim S, Omri YE, Neumann FJ, Weber R, Haïssaguerre M, Arentz T. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas With Selective Activation Characteristics. Circ Arrhythm Electrophysiol. 2016;9:e002962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 70. | Junarta J, Siddiqui MU, Riley JM, Dikdan SJ, Patel A, Frisch DR. Low-voltage area substrate modification for atrial fibrillation ablation: a systematic review and meta-analysis of clinical trials. Europace. 2022;24:1585-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Moustafa A, Karim S, Kahaly O, Elzanaty A, Meenakshisundaram C, Abi-Saleh B, Eltahawy E, Chacko P. Low voltage area guided substrate modification in nonparoxysmal atrial fibrillation: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2023;34:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 72. | Hwang J, Park HS, Han S, Lee CH, Kim IC, Cho YK, Yoon HJ, Chung JW, Kim H, Nam CW, Hur SH, Kim JY, Kim YS, Jang WS. Ablation of persistent atrial fibrillation based on high density voltage mapping and complex fractionated atrial electrograms: A randomized controlled trial. Medicine (Baltimore). 2021;100:e26702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Blandino A, Bianchi F, Grossi S, Biondi-Zoccai G, Conte MR, Gaido L, Gaita F, Scaglione M, Rametta F. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin Electrophysiol. 2017;40:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 74. | Sunaga A, Masuda M, Inoue K, Tanaka N, Watanabe T, Furukawa Y, Egami Y, Hirata A, Makino N, Minamiguchi H, Oka T, Minamisaka T, Takeda T, Yamada T, Kitamura T, Kida H, Oeun B, Sato T, Sotomi Y, Dohi T, Okada K, Suna S, Mizuno H, Nakatani D, Hikoso S, Sakata Y; OCVC-SUPPRESS-AF investigators. The efficacy and safety of left atrial low-voltage area guided ablation for recurrence prevention compared to pulmonary vein isolation alone in patients with persistent atrial fibrillation trial: Design and rationale. Clin Cardiol. 2021;44:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Valcher S, Villaschi A, Falasconi G, Chiarito M, Giunti F, Novelli L, Addeo L, Taormina A, Panico C, Francia P, Saglietto A, Del Monaco G, Latini AC, Carli S, Frittella S, Giaj Levra A, Antonelli G, Preda A, Guarracini F, Mazzone P, Berruezo A, Tritto M, Condorelli G, Penela D. Low-Voltage Area Ablation in Addition to Pulmonary Vein Isolation in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. J Clin Med. 2024;13:4541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Nery PB, Alqarawi W, Nair GM, Sadek MM, Redpath CJ, Golian M, Al Dawood W, Chen L, Hansom SP, Klein A, Wells GA, Birnie DH. Catheter Ablation of Low-Voltage Areas for Persistent Atrial Fibrillation: Procedural Outcomes Using High-Density Voltage Mapping. Can J Cardiol. 2020;36:1956-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer P, Dagres N, Richter S, Breithardt OA, Dinov B, Husser D, Eitel C, Gaspar T, Piorkowski C, Hindricks G. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2018;20:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 78. | Cutler MJ, Sattayaprasert P, Pivato E, Jabri A, AlMahameed ST, Ziv O. Low voltage-guided ablation of posterior wall improves 5-year arrhythmia-free survival in persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33:2475-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 79. | Yagishita A, Gimbel JR, DE Oliveira S, Manyam H, Sparano D, Cakulev I, Mackall J, Arruda M. Long-Term Outcome of Left Atrial Voltage-Guided Substrate Ablation During Atrial Fibrillation: A Novel Adjunctive Ablation Strategy. J Cardiovasc Electrophysiol. 2017;28:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 80. | Schreiber D, Rieger A, Moser F, Kottkamp H. Catheter ablation of atrial fibrillation with box isolation of fibrotic areas: Lessons on fibrosis distribution and extent, clinical characteristics, and their impact on long-term outcome. J Cardiovasc Electrophysiol. 2017;28:971-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 81. | Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011;8:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 82. | Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger FX, Martinek M, Pürerfellner H, Kirstein B, Richter U, Ulbrich S, Mayer J, Krahnefeld O, Agdirlioglu T, Zedda A, Piorkowski J, Piorkowski C. Low-Voltage Myocardium-Guided Ablation Trial of Persistent Atrial Fibrillation. NEJM Evid. 2022;1:EVIDoa2200141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 83. | Jia H, Wang W, Yu B. Efficacy and safety of low voltage area ablation for atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2023;66:1519-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Bijvoet GP, Nies HMJM, Holtackers RJ, Linz D, Adriaans BP, Nijveldt R, Wildberger JE, Vernooy K, Chaldoupi SM, Mihl C. Correlation between Cardiac MRI and Voltage Mapping in Evaluating Atrial Fibrosis: A Systematic Review. Radiol Cardiothorac Imaging. 2022;4:e220061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 85. | Szilágyi J, Sághy L. Atrial Remodeling in Atrial Fibrillation. Comorbidities and Markers of Disease Progression Predict Catheter Ablation Outcome. Curr Cardiol Rev. 2021;17:217-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 86. | Anter E, Neuzil P, Reddy VY, Petru J, Park KM, Sroubek J, Leshem E, Zimetbaum PJ, Buxton AE, Kleber AG, Shen C, Wit AL. Ablation of Reentry-Vulnerable Zones Determined by Left Ventricular Activation From Multiple Directions: A Novel Approach for Ventricular Tachycardia Ablation: A Multicenter Study (PHYSIO-VT). Circ Arrhythm Electrophysiol. 2020;13:e008625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 87. | Yagishita A, DE Oliveira S, Cakulev I, Gimbel JR, Sparano D, Manyam H, Manrique-Garcia A, Arredondo M, Mackall J, Arruda M. Correlation of Left Atrial Voltage Distribution Between Sinus Rhythm and Atrial Fibrillation: Identifying Structural Remodeling by 3-D Electroanatomic Mapping Irrespective of the Rhythm. J Cardiovasc Electrophysiol. 2016;27:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 88. | Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 870] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 89. | Mannion J, Hong K, Lennon SJ, Kenny A, Galvin J, O'Brien J, Jauvert G, Keelan E, Boles U. Comparing Left Atrial Low Voltage Areas in Sinus Rhythm and Atrial Fibrillation Using Novel Automated Voltage Analysis: A Pilot Study. Cardiol Res. 2023;14:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 90. | Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Sparks PB, Morton JB, Kalman JM. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J Cardiovasc Electrophysiol. 2012;23:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 91. | Lo LW, Tai CT, Lin YJ, Chang SL, Wongcharoen W, Chang SH, Hsieh MH, Tuan TC, Udyavar AR, Chen YJ, Tsao HM, Chen SA. Progressive remodeling of the atrial substrate--a novel finding from consecutive voltage mapping in patients with recurrence of atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol. 2007;18:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Ciaccio EJ, Biviano AB, Garan H. Comparison of spectral estimators for characterizing fractionated atrial electrograms. Biomed Eng Online. 2013;12:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 93. | Mannion J, Galvin J, Boles U. Left atrial scar identification and quantification in sinus rhythm and atrial fibrillation. J Arrhythm. 2020;36:967-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 94. | Jeyaraj D, Wilson LD, Zhong J, Flask C, Saffitz JE, Deschênes I, Yu X, Rosenbaum DS. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115:3145-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 95. | Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci. 2011;32:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts-Thomson KC, Wilson L, De Sciscio P, Young GD, Sanders P. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the "second factor". J Am Coll Cardiol. 2009;53:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 97. | Wang XH, Li Z, Mao JL, Zang MH, Pu J. Low voltage areas in paroxysmal atrial fibrillation: The prevalence, risk factors and impact on the effectiveness of catheter ablation. Int J Cardiol. 2018;269:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Parmar BR, Jarrett TR, Kholmovski EG, Hu N, Parker D, MacLeod RS, Marrouche NF, Ranjan R. Poor scar formation after ablation is associated with atrial fibrillation recurrence. J Interv Card Electrophysiol. 2015;44:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Jia S, Nivet H, Harrison J, Pennec X, Camaioni C, Jaïs P, Cochet H, Sermesant M. Left atrial shape is independent predictor of arrhythmia recurrence after catheter ablation for atrial fibrillation: A shape statistics study. Heart Rhythm O2. 2021;2:622-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Andrade JG, Champagne J, Deyell MW, Essebag V, Lauck S, Morillo C, Sapp J, Skanes A, Theoret-Patrick P, Wells GA, Verma A; EARLY-AF Study Investigators. A randomized clinical trial of early invasive intervention for atrial fibrillation (EARLY-AF) - methods and rationale. Am Heart J. 2018;206:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Ng GA, Schnabel RB, Suling A, Szumowski L, Themistoclakis S, Vardas P, van Gelder IC, Wegscheider K, Kirchhof P. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 102. | Medi C, Kalman JM, Spence SJ, Teh AW, Lee G, Bader I, Kaye DM, Kistler PM. Atrial electrical and structural changes associated with longstanding hypertension in humans: implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 103. | Kurata N, Masuda M, Kanda T, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Tsujimura T, Matsuda Y, Hata Y, Uematsu H, Mano T. Left Atrial Localized Low-Voltage Areas Indicate Whole Left Atrial Electrophysiological Degeneration in Atrial Fibrillation Patients. Circ J. 2022;86:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Yamaguchi T, Otsubo T, Takahashi Y, Nakashima K, Fukui A, Hirota K, Ishii Y, Shinzato K, Osako R, Tahara M, Kawano Y, Kawaguchi A, Aishima S, Takahashi N, Node K. Atrial Structural Remodeling in Patients With Atrial Fibrillation Is a Diffuse Fibrotic Process: Evidence From High-Density Voltage Mapping and Atrial Biopsy. J Am Heart Assoc. 2022;11:e024521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 105. | Kiedrowicz RM, Wielusinski M, Wojtarowicz A, Kazmierczak J. Predictors of the voltage derived left atrial fibrosis in patients with long-standing persistent atrial fibrillation. Cardiol J. 2022;29:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 107. | Spragg DD, Zghaib T. Veracity of Voltage Mapping During Atrial Fibrillation and Flutter: How Good Is Good Enough? JACC Clin Electrophysiol. 2018;4:1553-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 108. | Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1057] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 109. | Diness JG, Bentzen BH, Sørensen US, Grunnet M. Role of Calcium-activated Potassium Channels in Atrial Fibrillation Pathophysiology and Therapy. J Cardiovasc Pharmacol. 2015;66:441-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Conte M, Petraglia L, Cabaro S, Valerio V, Poggio P, Pilato E, Attena E, Russo V, Ferro A, Formisano P, Leosco D, Parisi V. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front Cardiovasc Med. 2022;9:932262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 111. | Shao Y, Chen L, Chen W, Sang C, Xu C, Zhang C. Left atrial epicardial adipose tissue is associated with low voltage zones in the left atrium in patients with non-valvular atrial fibrillation. Front Cardiovasc Med. 2022;9:924646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Zghaib T, Ipek EG, Zahid S, Balouch MA, Misra S, Ashikaga H, Berger RD, Marine JE, Spragg DD, Zimmerman SL, Zipunnikov V, Trayanova N, Calkins H, Nazarian S. Association of left atrial epicardial adipose tissue with electrogram bipolar voltage and fractionation: Electrophysiologic substrates for atrial fibrillation. Heart Rhythm. 2016;13:2333-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Burke AP, Litovsky S, Virmani R. Lipomatous hypertrophy of the atrial septum presenting as a right atrial mass. Am J Surg Pathol. 1996;20:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Xu L, Khoshknab M, Berger RD, Chrispin J, Dixit S, Santangeli P, Callans D, Marchlinski FE, Zimmerman SL, Han Y, Trayanova N, Desjardins B, Nazarian S. Lipomatous Metaplasia Enables Ventricular Tachycardia by Reducing Current Loss Within the Protected Corridor. JACC Clin Electrophysiol. 2022;8:1274-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 115. | de Bakker JM. Electrogram recording and analyzing techniques to optimize selection of target sites for ablation of cardiac arrhythmias. Pacing Clin Electrophysiol. 2019;42:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 116. | Lemery R, Birnie D, Tang AS, Green M, Gollob M, Hendry M, Lau E. Normal atrial activation and voltage during sinus rhythm in the human heart: an endocardial and epicardial mapping study in patients with a history of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 117. | Wong CX, Stiles MK, John B, Brooks AG, Lau DH, Dimitri H, Kuklik P, Shipp NJ, Sullivan T, Sanders P. Direction-dependent conduction in lone atrial fibrillation. Heart Rhythm. 2010;7:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 118. | Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, Choi JI, Hwang C, Kim YH. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three-dimensional computed tomographic images and voltage mapping. J Cardiovasc Electrophysiol. 2009;20:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 119. | Hayashida S, Nagashima K, Kurokawa S, Arai M, Watanabe R, Wakamatsu Y, Otsuka N, Yagyu S, Iso K, Okumura Y. Formation of low-voltage zones on the anterior left atrial wall due to mechanical compression by the ascending aorta. J Cardiovasc Electrophysiol. 2021;32:2275-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 120. | Parameswaran R, Kalman JM, Royse A, Goldblatt J, Larobina M, Watts T, Walters TE, Nalliah CJ, Wong G, Al-Kaisey A, Douglas Anderson R, Voskoboinik A, Sugumar H, Chieng D, Sanders P, Kistler PM, Gerstenfeld EP, Lee G. Endocardial-Epicardial Phase Mapping of Prolonged Persistent Atrial Fibrillation Recordings: High Prevalence of Dissociated Activation Patterns. Circ Arrhythm Electrophysiol. 2020;13:e008512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 121. | Tung R, Burris R, Salazar P, Aziz Z. Human Recordings of Left Atrial Epicardial-Endocardial Asynchrony During Persistent Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2022;15:e010605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 122. | Beheshti M, Magtibay K, Massé S, Porta-Sanchez A, Haldar S, Bhaskaran A, Nayyar S, Glover B, Deno DC, Vigmond EJ, Nanthakumar K. Determinants of atrial bipolar voltage: Inter electrode distance and wavefront angle. Comput Biol Med. 2018;102:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Shimizu A, Centurion OA. Electrophysiological properties of the human atrium in atrial fibrillation. Cardiovasc Res. 2002;54:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Nairn D, Lehrmann H, Müller-Edenborn B, Schuler S, Arentz T, Dössel O, Jadidi A, Loewe A. Comparison of Unipolar and Bipolar Voltage Mapping for Localization of Left Atrial Arrhythmogenic Substrate in Patients With Atrial Fibrillation. Front Physiol. 2020;11:575846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Haldar SK, Magtibay K, Porta-Sanchez A, Massé S, Mitsakakis N, Lai PFH, Azam MA, Asta J, Kusha M, Dorian P, Ha ACT, Chauhan V, Deno DC, Nanthakumar K. Resolving Bipolar Electrogram Voltages During Atrial Fibrillation Using Omnipolar Mapping. Circ Arrhythm Electrophysiol. 2017;10:e005018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 126. | Tung R, Josephson ME, Bradfield JS, Shivkumar K. Directional Influences of Ventricular Activation on Myocardial Scar Characterization: Voltage Mapping With Multiple Wavefronts During Ventricular Tachycardia Ablation. Circ Arrhythm Electrophysiol. 2016;9:e004155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 127. | Liang JJ, Tschabrunn CM, Marchlinski FE. Omnipolar Mapping: A Method to Improve the Fidelity of Voltage Mapping to Guide Substrate-Based Atrial Fibrillation Ablation? Circ Arrhythm Electrophysiol. 2017;10:e005700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Castrejón-Castrejón S, Ortega M, Pérez-Silva A, Doiny D, Estrada A, Filgueiras D, López-Sendón JL, Merino JL. Organized atrial tachycardias after atrial fibrillation ablation. Cardiol Res Pract. 2011;2011:957538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Kitamura T, Takigawa M, Derval N, Denis A, Martin R, Vlachos K, Nakatani Y, Frontera A, Cheniti G, Martin CA, Bourier F, Lam A, Duchateau J, Pambrun T, Sacher F, Cochet H, Hocini M, Haïssaguerre M, Jaïs P. Atrial tachycardia circuits include low voltage area from index atrial fibrillation ablation relationship between RF ablation lesion and AT. J Cardiovasc Electrophysiol. 2020;31:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 130. | Ndrepepa G, Schneider MA, Karch MR, Weber S, Schreieck J, Zrenner B, Schmitt C. Impact of atrial fibrillation on the voltage of bipolar signals acquired from the left and right atria. Pacing Clin Electrophysiol. 2003;26:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martínez-Alzamora N, González-Torrecilla E, Arenal A, Fernández-Avilés F, Berenfeld O. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 132. | Deisenhofer I, Albenque JP, Busch S, Gitenay E, Mountantonakis SE, Roux A, Horvilleur J, Bakouboula B, Oza S, Abbey S, Theodore G, Lepillier A, Guyomar Y, Bessiere F, Jan Smit J, Mohr Durdez T, Milpied P, Appetiti A, Guerrero D, De Potter T, De Chillou C, Goldbarg S, Verma A, Hummel JD; TAILORED-AF Investigators. Artificial intelligence for individualized treatment of persistent atrial fibrillation: a randomized controlled trial. Nat Med. 2025;31:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 133. | Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009;2:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 134. | Salah A, Zhou S, Liu Q, Yan H. P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arq Bras Cardiol. 2013;101:519-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Pérez-Riera AR, de Abreu LC, Barbosa-Barros R, Grindler J, Fernandes-Cardoso A, Baranchuk A. P-wave dispersion: an update. Indian Pacing Electrophysiol J. 2016;16:126-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 136. | Dogan U, Dogan EA, Tekinalp M, Tokgoz OS, Aribas A, Akilli H, Ozdemir K, Gok H, Yuruten B. P-wave dispersion for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Int J Med Sci. 2012;9:108-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 137. | Cortez D, Baturova M, Lindgren A, Carlson J, Shubik YV, Olsson B, Platonov PG. Atrial time and voltage dispersion are both needed to predict new-onset atrial fibrillation in ischemic stroke patients. BMC Cardiovasc Disord. 2017;17:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Alexander B, Haseeb S, van Rooy H, Tse G, Hopman W, Martinez-Selles M, de Luna AB, Çinier G, Baranchuk A. Reduced P-wave Voltage in Lead I is Associated with Development of Atrial Fibrillation in Patients with Coronary Artery Disease. J Atr Fibrillation. 2017;10:1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Nakatani Y, Sakamoto T, Yamaguchi Y, Tsujino Y, Kataoka N, Kinugawa K. P-wave vector magnitude predicts the left atrial low-voltage area in patients with paroxysmal atrial fibrillation. J Electrocardiol. 2020;59:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 140. | Schreiber T, Kähler N, Tscholl V, Nagel P, Blaschke F, Landmesser U, Attanasio P, Huemer M. Correlation of P-wave properties with the size of left atrial low voltage areas in patients with atrial fibrillation. J Electrocardiol. 2019;56:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 141. | Jadidi A, Müller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier J, Moreno-Weidmann Z, Trenk D, Neumann FJ, Lehrmann H, Arentz T. The Duration of the Amplified Sinus-P-Wave Identifies Presence of Left Atrial Low Voltage Substrate and Predicts Outcome After Pulmonary Vein Isolation in Patients With Persistent Atrial Fibrillation. JACC Clin Electrophysiol. 2018;4:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 142. | Karacop E, Enhos A, Bakhshaliyev N, Ozdemir R. P Wave Duration/P Wave Voltage Ratio Plays a Promising Role in the Prediction of Atrial Fibrillation: A New Player in the Game. Cardiol Res Pract. 2021;2021:8876704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 143. | Yano M, Egami Y, Kawanami S, Ukita K, Kawamura A, Yasumoto K, Tsuda M, Okamoto N, Matsunaga-Lee Y, Nishino M. Ratio of P-Wave Duration to P-Wave Amplitude and Left Atrial Remodeling: Insights from Electrophysiological Findings and Myocardial Injury After Cryoballoon Ablation. Am J Cardiol. 2024;212:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Miao Y, Xu M, Yang L, Zhang C, Liu H, Shao X. Investigating the association between P wave duration and atrial fibrillation recurrence after radiofrequency ablation in early persistent atrial fibrillation patients. Int J Cardiol. 2022;351:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Chen LY, Ribeiro ALP, Platonov PG, Cygankiewicz I, Soliman EZ, Gorenek B, Ikeda T, Vassilikos VP, Steinberg JS, Varma N, Bayés-de-Luna A, Baranchuk A. P Wave Parameters and Indices: A Critical Appraisal of Clinical Utility, Challenges, and Future Research-A Consensus Document Endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ Arrhythm Electrophysiol. 2022;15:e010435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 146. | Paul Nordin A, Jensen-Urstad M, Charitakis E, Åkerström F, Almroth H, Herczku C, Tapanainen J, Höglund N, Drca N. Individually designed ablation of low-voltage areas in persistent atrial fibrillation-a randomized controlled trial (IDEAL-AF): study design and rationale. Eur Heart J Open. 2025;5:oeaf037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/