Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.491
Revised: July 30, 2024
Accepted: August 8, 2024
Published online: August 26, 2024
Processing time: 40 Days and 9.6 Hours
Antiphospholipid syndrome (APS) is a chronic autoimmune disease characterized by venous or arterial thrombosis, pregnancy morbidity and a variety of other autoimmune and inflammatory complications. Here, we report a case of APS associated with multiple coronary thromboses.
The patient, a 28-year-old male, suffered from recurrent coronary thromboses over a period of 31 months. Despite undergoing interventional coronary proce
APS can manifest as ACS. Screening for rheumatologic and immunological con
Core Tip: Antiphospholipid syndrome (APS) is primarily identified by its thrombotic phenomena. Thus, healthcare professionals should be highly vigilant for the assorted clinical symptoms that can stem from thromboembolic events, which have the potential to involve several organ systems. When encountering young individuals with frequent angina attacks who do not possess conventional risk factors, it is imperative not to pinpoint the cause solely on cardiac issues. The integration of percutaneous coronary intervention and specific treatment targeting the etiology of APS is essential. The need to preserve a heightened awareness of the spectrum of clinical signs linked to thromboembolic complications affecting diverse organ systems is required.
- Citation: Liu XC, Wang W, Wang LY. Antiphospholipid syndrome presenting as recurrent coronary thrombosis: A case report. World J Cardiol 2024; 16(8): 491-495
- URL: https://www.wjgnet.com/1949-8462/full/v16/i8/491.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i8.491
Antiphospholipid syndrome (APS) is a thromboinflammatory disease that complicates up to one third of cases of systemic lupus erythematosus, which may result in more organ damage over time. APS is most prominently characterized by thrombotic manifestations, such as common deep vein thrombosis, cerebral artery thrombosis, and so on[1]. Studies have suggested that the presence of lupus anticoagulant is more strongly associated with an increased risk of thromboembolic episodes than the detection of positive anti-cardiolipin antibodies[2]. Primary APS can also occur in the absence of other systemic autoimmune disorders. Acute coronary syndrome (ACS) refers to a spectrum of coronary artery pathologies, including unstable angina, non-ST segment elevation myocardial infarction and ST-segment elevation myocardial infarction, and its common manifestations include chest pain[3]. In clinical settings, patients with APS often present with a predominant thrombotic phenotype, which complicates the distinction between their symptoms and those associated with ACS, leading to diagnostic challenges.
The patient, a 28-year-old male, was admitted to the hospital with a history of paroxysmal chest tightness spanning approximately 31 months.
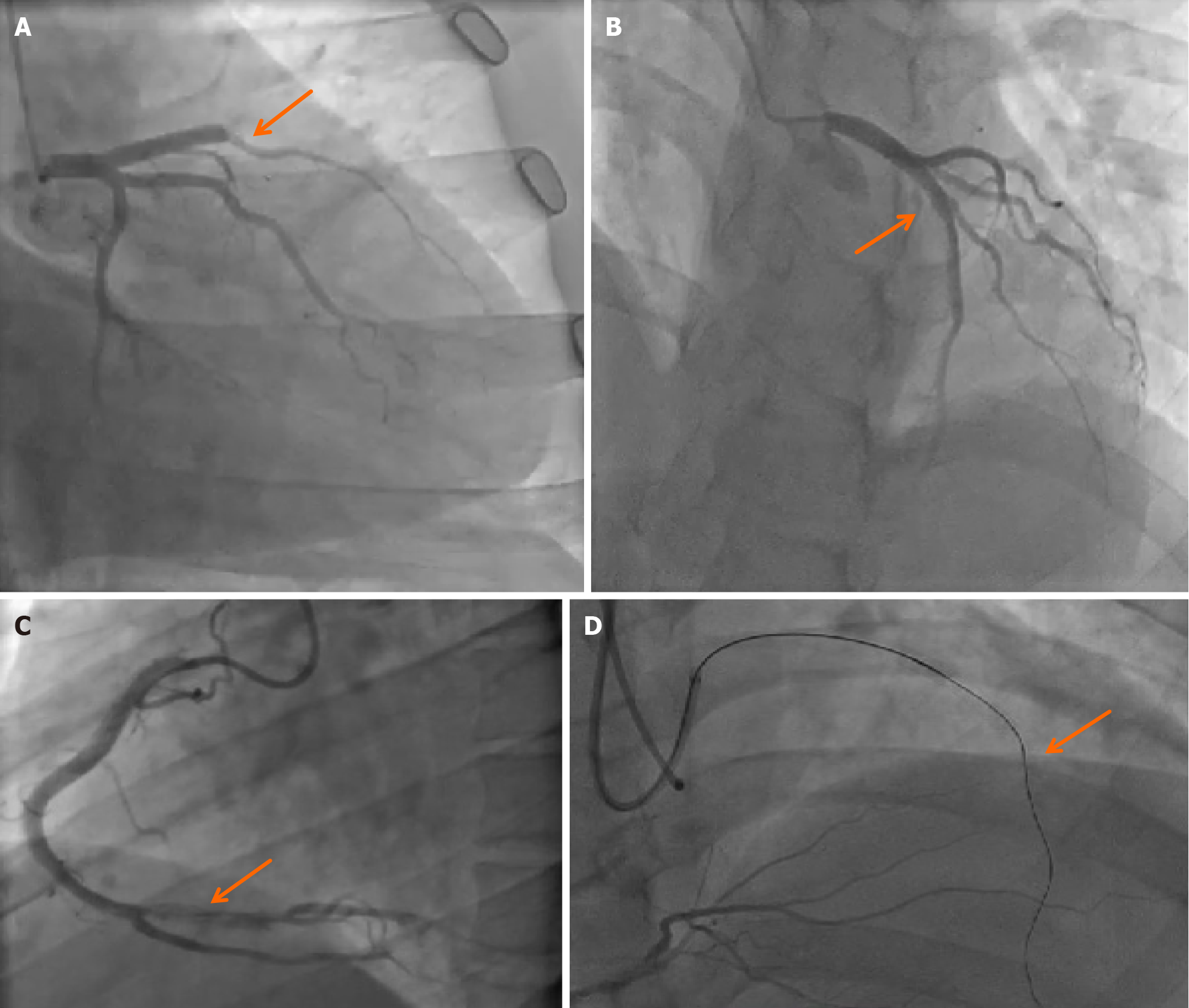
Thirty-one months ago, the patient experienced a sudden onset of chest discomfort, accompanied by difficulty breathing and diaphoresis, which was subsequently diagnosed as "acute ST segment elevation myocardial infarction" affecting both the anterior and inferior walls following electrocardiogram and cardiac biomarker assessments at a local hospital. Coronary angiography revealed occlusion of the proximal left anterior descending (LAD) coronary artery and complete occlusion at the posterior opening of the right coronary artery (Figure 1A). Interventional treatment included stent implantation in the LAD and percutaneous transluminal coronary angioplasty in the right coronary artery. After discharge, the patient maintained a regimen of antiplatelet and anticoagulant medications. A follow-up examination two weeks later showed thrombosis in the LAD, which was again relieved by balloon dilation (Figure 1B). Initial screening for lupus anticoagulants was positive, with an increased ratio of lupus anticoagulant initial screening to confirmation (LA1/LA2) of 1.39, indicating a low level of lupus anticoagulants, but no specific treatment was initiated. Two years ago, the patient presented to our hospital with recurrent chest tightness. Coronary angiography revealed thrombus formation within the LAD stent. Balloon dilation of the LAD and laser treatment of the right coronary artery were performed (Figure 1C). The initial lupus anticoagulant screening was again positive, with a slightly increased LA1/LA2 ratio of 1.23. In addition to the ongoing antiplatelet and anticoagulant therapy, hydroxychloroquine 100 mg twice daily was added to the treatment regimen. Six months ago, the patient experienced another episode of chest tightness, prompting a repeat coronary angiography, which showed occlusion of the LAD stent (Figure 1D), necessitating another balloon dilation treatment. Based on the APS diagnostic criteria and the patient's clinical presentation, a diagnosis of APS was confirmed.
The patient's medical history was remarkable by the recurrence of thrombotic episodes. Five years previously, the patient experienced an acute episode of dyspnea, and underwent pulmonary computed tomography angiography, which diagnosed bilateral pulmonary artery embolism. Concurrently, venous thrombosis was identified in the right superficial femoral, popliteal, anterior tibial, and posterior tibial veins. Approximately four and a half years ago, the patient subsequently developed additional thrombosis in the right popliteal and intermuscular veins of the lower leg.
The patient had a 10-year history of smoking, with an average consumption of 10 cigarettes daily, and occasionally indulges in alcohol. He did not have a family history of cardiovascular disease.
The patient’s body temperature was 36.5 °C, pulse rate was 69 beats/min, respiratory rate was 18 breaths/min and blood pressure was 98/69 mmHg. The patient was alert and oriented. Auscultation of the lungs revealed clear breath sounds with the absence of dry or wet rales. The heart rate was 69 beats/min and maintained a regular rhythm; no appreciable murmurs were detected in the valve areas. Abdominal examination was unremarkable, with no tenderness or rebound pain, and the liver and spleen were non-palpable. No edema was noted in the lower extremities. The admission electrocardiogram showed the presence of Q waves in leads V1 through V5.
Thirty-one months ago, initial screening for lupus anticoagulants was positive, with an increased LA1/LA2 ratio of 1.39, indicating a low level of lupus anticoagulants. Two years ago, the initial lupus anticoagulant screening was again positive, with a slightly increased LA1/LA2 ratio of 1.23. Six months ago, the LA1/LA2 ratio improved to 1.31.
Thirty-one months ago, coronary angiography revealed occlusion of the proximal LAD coronary artery and complete occlusion at the posterior opening of the right coronary artery. A follow-up examination two weeks later showed thrombosis in the LAD. Two years ago, coronary angiography revealed thrombus formation within the LAD stent. Six months ago, repeat coronary angiography showed occlusion of the LAD stent, and echocardiography revealed a thrombus at the left ventricular apex.
The diagnoses were APS, coronary artery thrombosis, coronary atherosclerotic heart disease, old extensive anterior and inferior wall myocardial infarction, and heart function Class II (NYHA).
In addressing the coronary artery occlusion, the patient underwent balloon angioplasty as a therapeutic intervention. Concurrent with the diagnosis of APS, the patient's treatment regimen included hydroxychloroquine, warfarin for anticoagulation purposes, indobufen as an antiplatelet agent, and rosuvastatin to reduce blood lipid levels and promote plaque stabilization.
The patient has experienced a marked reduction in chest pain symptoms compared to his previous condition. Accurate adjustment of the medication regimen led to the absence of further ACS episodes throughout the subsequent 20-month follow-up. While the patient may occasionally suffer from a sensation of chest constriction, there have been no additional reports of distressing symptoms.
Clinical diagnostic criteria for APS are: One or more episodes of thrombosis are present in arteries, veins, or small blood vessels of any organ or tissue (excluding superficial venous thrombosis as a diagnostic marker). Objective evidence, such as imaging or histopathological findings, is required. In cases where histopathology confirms thrombosis, the vessel walls at the site of the thrombus must exhibit no signs of vascular inflammation. Laboratory diagnostic criteria in terms of lupus anticoagulant level in plasma are: The level must be tested at a minimum interval of 12 weeks between tests, and the result should be positive on at least two separate occasions. For a diagnosis of APS, the positive antiphospholipid antibody test result should not be less than 12 weeks before or more than 5 years after the onset of clinical symptoms[4,5]. In the present case, based on the patient's clinical presentation and auxiliary diagnostic examinations, which align with the aforementioned criteria, the diagnosis of APS is established.
The distinctive aspect of this case is the patient's profile: A young male without conventional risk factors for coronary heart disease, such as hypertension, diabetes, hyperlipidemia, advanced age, or a family history of genetic disorders. Despite this, the patient has experienced repeated thrombotic events across multiple vascular beds, including the deep veins of the lower limbs, coronary arteries, pulmonary arteries, and the left ventricle. The clinical presentation of cardiovascular diseases alone fails to adequately explain the patient's symptoms. Furthermore, the patient's history of multiple emboli is not confined to the lower limb veins but is more significantly manifested in the coronary arteries. The negative results for anticardiolipin antibodies and anti-β2-glycoprotein I antibodies, coupled with the patient's cardiac symptoms, add to the diagnostic complexity and challenge. This case underscores the importance of looking beyond superficial symptoms and considering a broader range of diseases, including those related to the rheumatic and immune systems, in the diagnostic process. The significance of this case lies in its potential to inform a diagnostic and therapeutic approach for clinicians faced with similar presentations, enabling earlier diagnosis and treatment and preventing the progression of complications and adverse outcomes. Considering the proneness of APS patients to thrombosis and a hypercoagulable state, it is imperative to investigate optimal personalized treatment strategies for APS patients with ACS.
The clinical features of APS are predominantly marked by thrombotic events. It is crucial to maintain a high index of suspicion for the varied clinical manifestations that can result from thromboembolic complications affecting multiple organ systems. In young patients who lack traditional risk factors for coronary atherosclerosis and who suffer from recurrent episodes of angina, it is imperative not to limit the diagnostic focus solely to cardiac pathologies. Instead, the possibility of APS should also be actively considered in the differential diagnosis. In light of the heightened risk of thrombosis and the hypercoagulable state inherent in patients with APS, there is an urgent call for additional research and advancement to delineate the most effective personalized percutaneous coronary intervention treatment protocols for APS patients who are diagnosed with ACS.
| 1. | Knight JS, Branch DW, Ortel TL. Antiphospholipid syndrome: advances in diagnosis, pathogenesis, and management. BMJ. 2023;380:e069717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 136] [Reference Citation Analysis (0)] |
| 2. | Knight JS, Erkan D. Rethinking antiphospholipid syndrome to guide future management and research. Nat Rev Rheumatol. 2024;20:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 3. | Atwood J. Management of Acute Coronary Syndrome. Emerg Med Clin North Am. 2022;40:693-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 4. | Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4924] [Cited by in RCA: 4855] [Article Influence: 242.8] [Reference Citation Analysis (7)] |
| 5. | Barbhaiya M, Zuily S, Ahmadzadeh Y, Amigo MC, Avcin T, Bertolaccini ML, Branch DW, de Jesus G, Devreese KMJ, Frances C, Garcia D, Guillemin F, Levine SR, Levy RA, Lockshin MD, Ortel TL, Seshan SV, Tektonidou M, Wahl D, Willis R, Naden R, Costenbader K, Erkan D; New APS Classification Criteria Collaborators. Development of a New International Antiphospholipid Syndrome Classification Criteria Phase I/II Report: Generation and Reduction of Candidate Criteria. Arthritis Care Res (Hoboken). 2021;73:1490-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/