Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.484
Revised: July 4, 2024
Accepted: July 19, 2024
Published online: August 26, 2024
Processing time: 118 Days and 10.6 Hours
With the development of percutaneous coronary intervention (PCI), the number of interventional procedures without implantation, such as bioresorbable stents (BRS) and drug-coated balloons, has increased annually. Metal drug-eluting stent unloading is one of the most common clinical complications. Comparatively, BRS detachment is more concealed and harmful, but has yet to be reported in clinical research. In this study, we report a case of BRS unloading and successful rescue.
This is a case of a 59-year-old male with the following medical history: “Type 2 diabetes mellitus” for 2 years, maintained with metformin extended-release tablets, 1 g PO BID; “hypertension” for 20 years, with long-term use of metoprolol sustained-release tablets, 47.5 mg PO QD; “hyperlipidemia” for 20 years, without regular medication. He was admitted to the emergency department of our hospital due to intermittent chest pain lasting 18 hours, on February 20, 2022 at 15: 35. Electrocardiogram results showed sinus rhythm, ST-segment elevation in leads I and avL, and poor R-wave progression in leads V1–3. High-sensitivity troponin I level was 4.59 ng/mL, indicating an acute high lateral wall myocardial infarction. The patient’s family requested treatment with BRS, without implanta
We describe a case of a 59-year-old male experienced BRS unloading and successful rescue. By analyzing images, the causes of BRS unloading and the treatment plan are discussed to provide insights for BRS release operations. We discuss preventive measures for BRS unloading.
Core Tip: Percutaneous coronary intervention plays a vital role in the treatment of coronary artery diseases. Recently, considerable attention has been given to bioresorbable stents (BRS), which can relieve coronary artery occlusions and reduce vascular stenosis. BRS can be absorbed or degraded, which helps restore vascular endothelial function and normalize systolic and diastolic functions. However, reports of clinical cases of BRS-unloading have been limited. Here, we present a case of BRS unloading and successful rescue, providing a practical treatment plan for such clinical scenarios.
- Citation: Sun T, Zhang MX, Zeng Y, Ruan LH, Zhang Y, Yang CL, Qin Z, Wang J, Zhu HM, Long Y. Unloading and successful treatment with bioresorbable stents during percutaneous coronary intervention: A case report. World J Cardiol 2024; 16(8): 484-490
- URL: https://www.wjgnet.com/1949-8462/full/v16/i8/484.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i8.484
Percutaneous coronary intervention (PCI) plays a vital role in the treatment of coronary artery diseases. Recently, considerable attention has been given to bioresorbable stents (BRS); these can relieve coronary artery occlusion, reduce vascular stenosis, be absorbed or degraded, restore vascular endothelial function, and normalize systolic and diastolic functions. However, only a few publications have discussed clinical cases of BRS unloading.
The patient was a 59-year-old male admitted to the emergency department of our hospital at 15: 35 on February 20, 2022, due to intermittent chest pain for 18 hours.
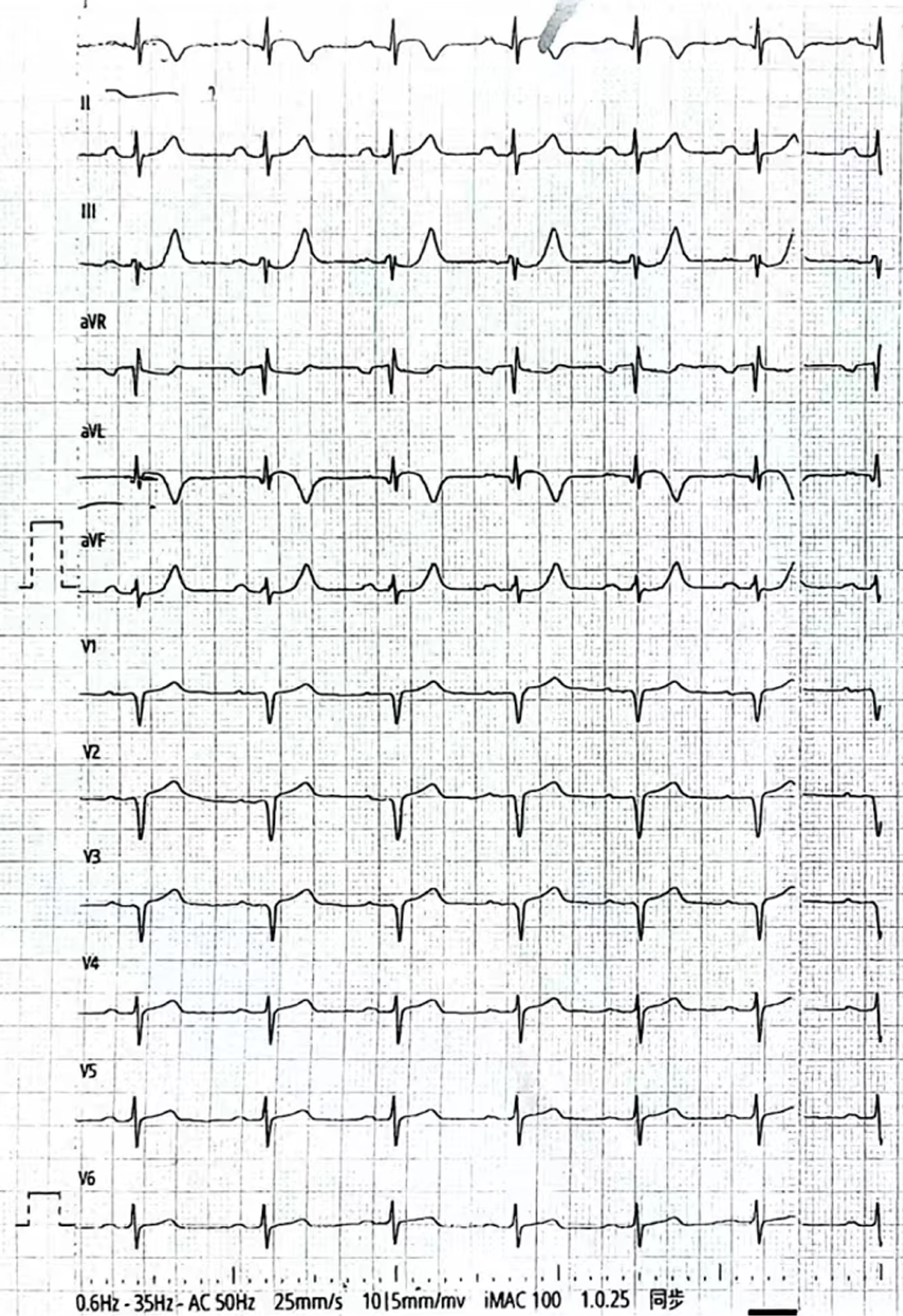
The patient was a 59-year-old male admitted to the emergency department of our hospital at 15: 35 on February 20, 2022, due to intermittent chest pain for 18 hours. Electrocardiogram (ECG) results showed sinus rhythm, ST-segment elevation in leads I and avL, and poor R-wave progression in leads V1–3. High-sensitivity troponin I (hs-TnI) level was at 4.59 ng/mL, indicating an acute high lateral wall myocardial infarction. The patient refused emergency PCI treatment as the chest pain had been relieved; therefore, he was transferred to the Department of Cardiology.
He had the following medical history: “Type 2 diabetes mellitus” for 2 years, maintained with metformin extended-release tablets, 1 g PO BID; “hypertension” for 20 years, with long-term use of metoprolol sustained-release tablets, 47.5 mg PO QD; “hyperlipidemia” for 20 years, without regular medication.
He denied a history of smoking and had no family history of related disease.
Admission examination: Temperature: 36.5 °C, Pulse: 75 beats/min, Respiration: 18 breaths/min, Blood Pressure: 138/74 mmHg (1 mmHg = 0.133 kPa), clear breath sounds in both lungs, no dry or wet rales, normal heart boundaries, heart rate 75 beats/min, regular rhythm, and no pathological murmur.
The ECG results showed sinus rhythm, ST-segment elevation in leads I and avL, and poor R-wave progression in leads V1–3 (Figure 1). hs-TnI level was at 4.59 ng/mL. N-terminal pro-brain natriuretic peptide levels were normal upon admission.
Echocardiography revealed abnormal left ventricular wall motion, mild mitral regurgitation, tricuspid and aortic valves, and a left ventricular ejection fraction of 62%.
(1) Acute high lateral myocardial infarction of coronary heart disease, cardiac function class I (Killip classification); (2) Hypertension grade 3, very high risk; and (3) Type 2 diabetes mellitus.
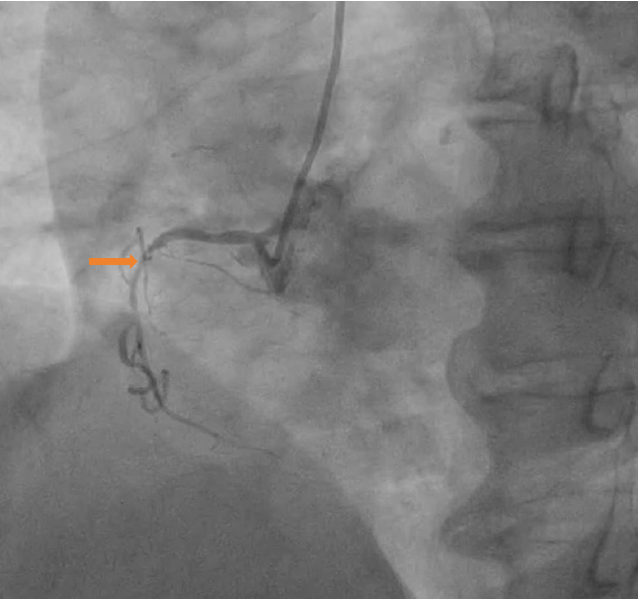
Upon admission, the patient was administered aspirin and clopidogrel for dual antiplatelet therapy, heparin for anticoagulation, and atorvastatin for lipid reduction. Coronary angiography was performed on the second day, revealing a dominant left coronary artery. Other findings were as follows: Non-stenotic left main artery, 85% stenosis in the proximal and middle segments of the anterior descending artery, 50% stenosis in the middle and distal segments of the circumflex artery, and 75% stenosis in the proximal segment of the right coronary artery (Figures 2 and 3). The patient’s family requested treatment with BRS without implantation. Because there was no stock in the catheter room, interventional treatment of the left anterior descending artery (LAD) was postponed until the fourth day. The 6F SPB 3.5 guiding catheter was sent along the sheath to connect the left coronary ostia, while the Rinato and Sion Blue guidewires were sent through the catheter to the LAD and distal segment of the first diagonal branch.
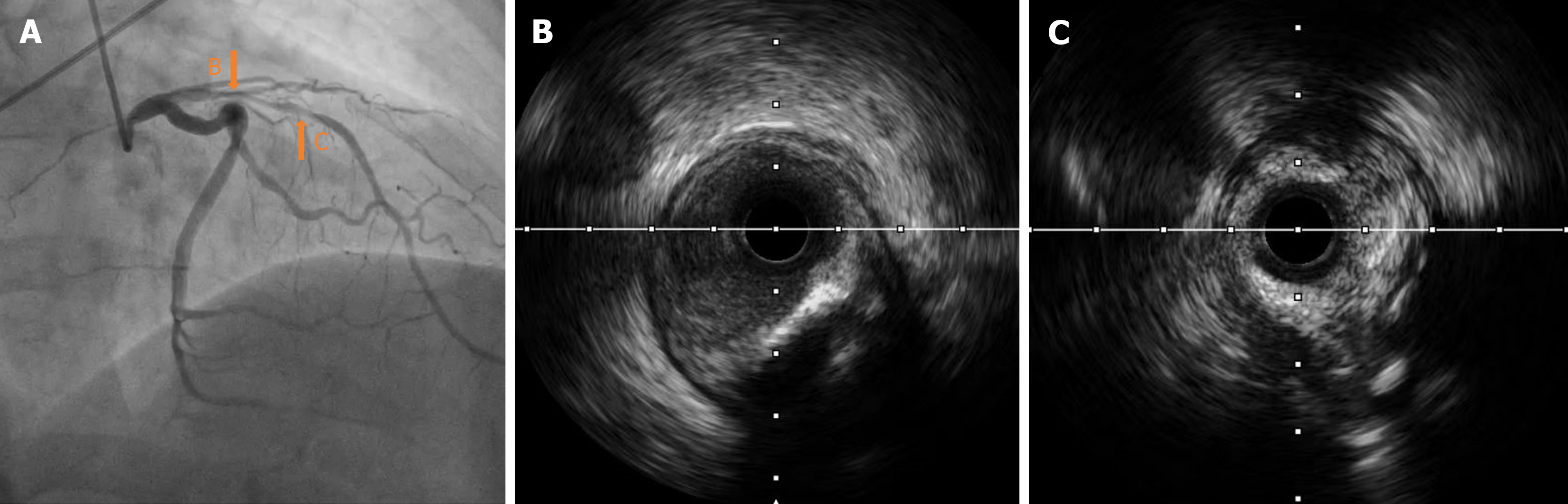
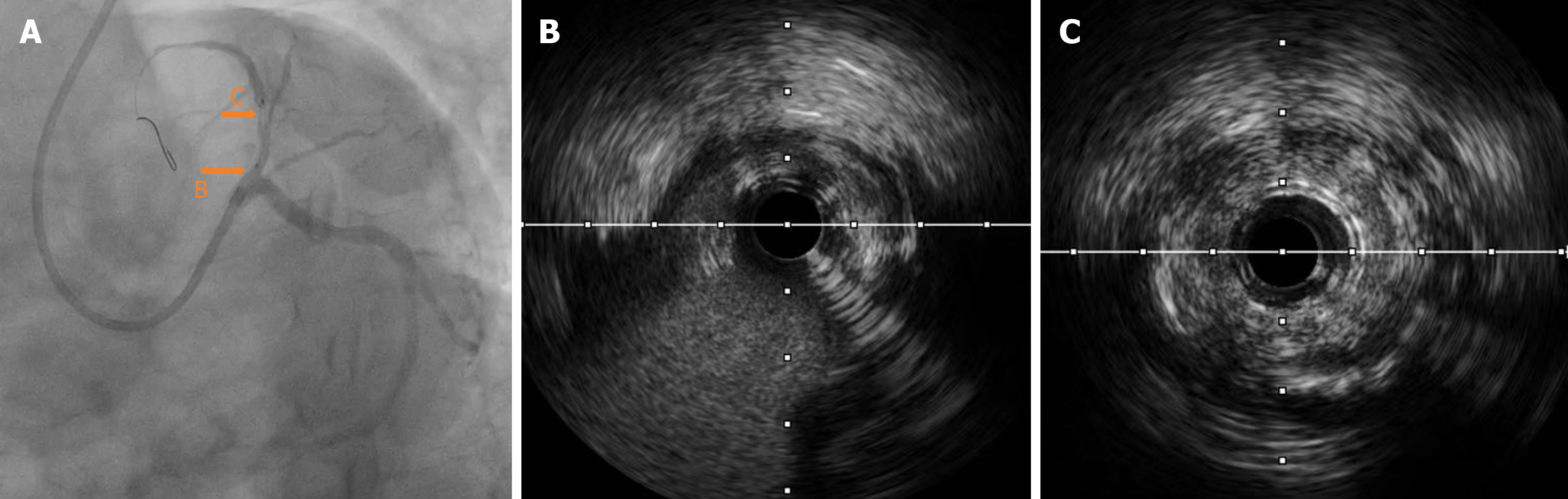
Intravascular ultrasound (IVUS) examination showed that the middle segment of the LAD was the myocardial bridge; diffused low-to-moderate echogenic plaques were observed in the lumen from the middle segment, close to the myocardial bridge to the ostium of the LAD. The minimum lumen area of the proximal segment was 1.85 mm², with mild local calcification (Figure 3). The 2.0 mm × 20 mm semi-compliant, 2.75 mm × 12 mm non-compliant, and 2.75 mm × 10 mm cutting balloons were used multiple times for pre-expansion at 6-10 atm at the lesion in the proximal and middle segments of the LAD; angiography showed a satisfactory expansion effect (Figure 4A). Subsequently, the first BRS, measuring 3.0 mm × 18 mm, was implanted at 12 atm in the proximal middle segment of the LAD, avoiding the myocardial bridge. A second BRS, measuring 3.5 mm × 18 mm, was also delivered along the Rinato guidewire to the lesion of the proximal part of the LAD.
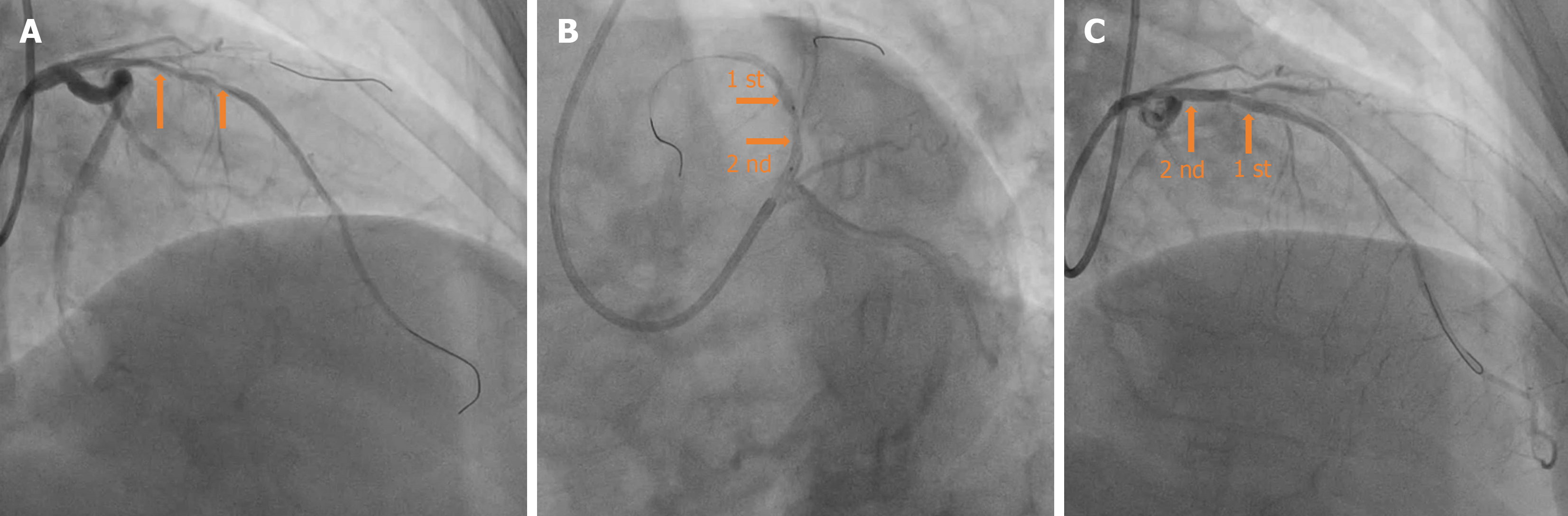
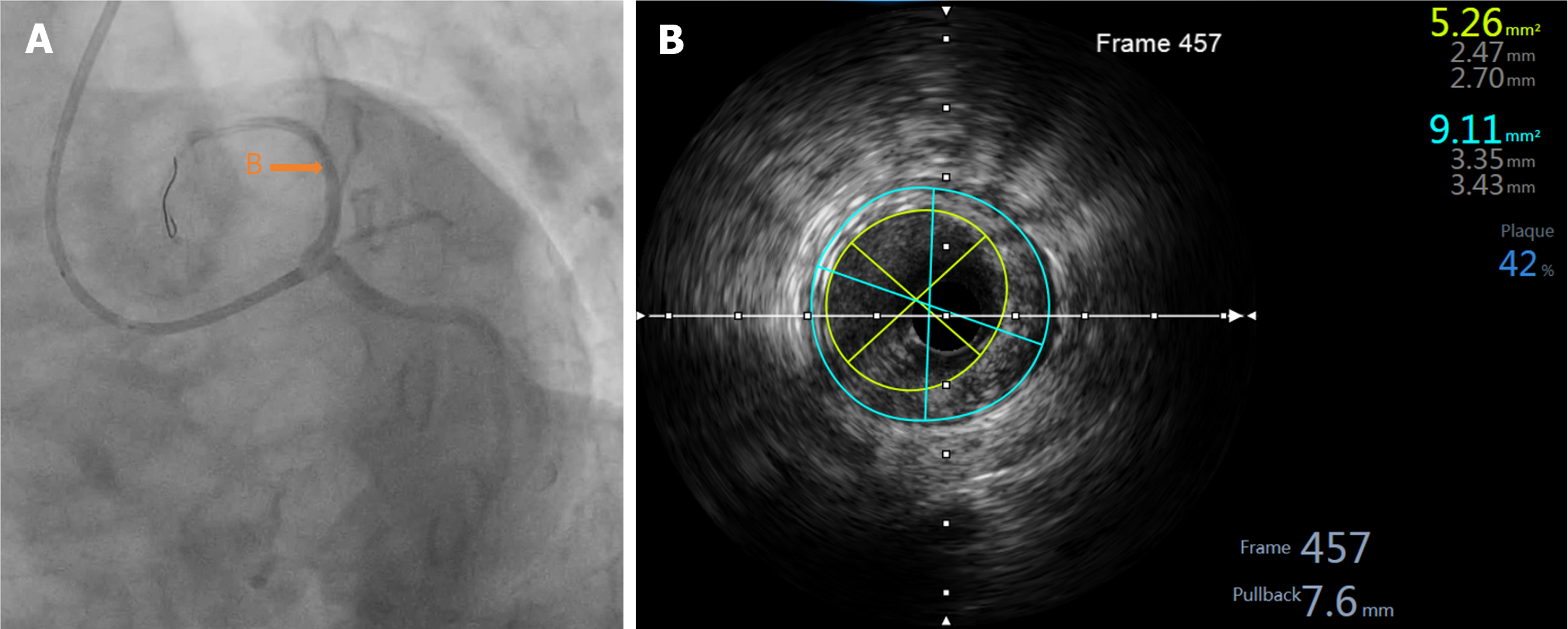
During the positioning process, the patient suddenly coughed. Consequently, the guiding catheter was inserted deeply into the proximal segment of the LAD with the BRS. The positions of the guiding catheter and BRS were then promptly adjusted. The appearance of “smoke” indicated a significant worsening of the lesions in the proximal segment and ostium of the LAD compared to the previous state (Figures 4B and 5). Considering the potential risk of local hematoma or dissection, we immediately withdrew the BRS and sent it to IVUS for detection; the results indicated that the BRS was unloaded. The distal end of the second BRS was positioned close to the afflux of the first diagonal branch. The proximal end intruded approximately 2 mm into the left main stem (Figure 5). The 2.0 mm × 20 mm semi-compliant balloons; and the 2.75 mm × 12 mm, 3.0 mm × 12 mm, and 3.5 mm × 12 mm non-compliant balloons, were sent along the Rinato guidewire into the second BRS and expanded at 6-12 atm for immediate in-situ release. IVUS revealed poor adherence at the proximal end of the BRS. Subsequently, a 4.0 mm × 12 mm non-compliant balloon was delivered into the BRS at the end of the left main stem and ostium of the LAD, which was then post-dilated at 14 atm. IVUS detection indicated that the structure of the BRS was complete and adherent. There was an approximately 2 mm gap between the two BRS at the LAD near the afflux of the first diagonal branch, without dissection or hematoma formation. The minimum lumen area was 5.26 mm² (Figure 6); angiography confirmed completely patent blood vessels with thrombolysis in TIMI grade 3 distal blood flow (Figure 4C).
Immediate IVUS evaluation was satisfactory. The patient was followed up for 2 years, having no episodes of angina pectoris and with generally good condition.
Emergency or elective PCI can relieve coronary artery occlusion or stenosis, and is one of the most effective methods for treating coronary heart diseases. BRS is a significant advancement in stent technology; it can achieve complete degradation of stents, restoration of blood vessels elasticity, and promotion of the normal blood vessel structure and function recovery[1,2]. While DES unloading is a known iatrogenic complication of PCI, few clinical cases of BRS detachment have been reported. Common causes of DES unloading include[3]: Pathological factors such as moderate to severe calcification, severe angulation, or distortion; device-related factors include using a smaller guiding catheter, misalignment of the guiding catheter and blood vessel axes, scratch on the extended catheter, and loose kneading of the stent and balloon; technical operation factors such as inadequate pretreatment.
In this case, the lesion had mild local calcification; the effect of angiographic evaluation after balloon pretreatment was acceptable. Furthermore, BRS unloading may be due to an unstable intraoperative guiding catheter, thus resulting in BRS scratching and reduced scaffold balloon adhesion; therefore, the BRS was completely separated from its stent balloon during PCI. Prevention of stent unloading is far more important than treatment; comprehensive preoperative lesion evaluation, formulation of a PCI strategy, selection of an appropriate guiding catheter, and good coaxial and gentle operations are indispensable. Because the lateral beam of BRS is thicker than that of DES, adequate pretreatment should be performed before BRS implantation. PSP operation[4] guarantees successful implantation and reduces poor BRS expansion.
In addition, precautions should be taken to prevent BRS unloading. Once the BRS is in place, operators should strive for a single release to prevent repetitive insertion and removal. In this case, the BRS is made of polylactic acid; its stress-relaxation properties have obvious temperature sensitivity[5]. The body temperature affects the adhesion performance between the scaffold balloon and stent during delivery. Therefore, it needs to be released as soon as possible. The resistance increases and the sense of loss occurs when DES is unloaded; however, BRS unloading differs from DES unloading in terms of hand-feeling. BRS features a thicker lateral wall that is susceptible to scratching. When unloading occurs, the operator may not feel it in his hands; X-ray fluoroscopy and angiography are not easy to find in time. Therefore, caution is warranted during PCI. During BRS localization, the possibility of stent unloading should be considered when coronary angiography reveals imaging changes, especially when the original coronary artery lesion seems to be aggravated. This is because the thickness of the unloaded BRS side wall overlaps with that of the original lesion, which leads to a change in contrast agent filling. Therefore, the BRS should not be withdrawn or immediately released for evaluation.
This study has limitations. There were differences between the treatments with unloaded BRS and DES. The use of a stent to crush an unloaded BRS into the vessel wall is inappropriate. First, it is difficult to crush thick biodegradable materials onto vessel walls to ensure adhesion. Second, the mural cavity formed after material degradation can easily lead to an increased risk of thrombosis. Because the BRS material is not radiopaque, X-rays can hardly detect it, making it very difficult to remove the unloaded BRS from the body. In this case, BRS unloading was found at the lesion site on IVUS examination, and was rescued by local release. Immediate IVUS evaluation was satisfactory. The outpatient was followed up for 2 years, having no episodes of angina pectoris and with generally good condition.
We report a case of BRS unloading and successful rescue, and provide a practical treatment plan for clinical BRS unloading. In the future, we plan to conduct research to completely prevent its occurrence.
| 1. | Gao LJ, Chen JL. [Advances in clinical application of biodegradable stents]. Zhongguo Xunhuan Zazhi. 2017;03:215-216. |
| 2. | Feng GK, Jiang XJ. [Research progress and future direction of bioabsorbable intravascular stents by polylactic acid organism]. Zhongguo Xunzheng Xinxueguan Yixue Zazhi. 2020;11:1393-1395+1402. [DOI] [Full Text] |
| 3. | Chinese Medical Association Cardiovascular Physicians Branch Guidelines and Consensus Working Committee Young and Middle aged Coronary Experts Salon. [Working Committee of Guidelines and Consensus of Chinese Society of Cardiovascular Physicians]. Zhognhua Xinxueguanbing Zazhi (Wangluoban). 2019;2: 1-10. [DOI] [Full Text] |
| 4. | Chinese Society of Cardiology of Chinese Medical Association. [Chinese expert consensus on the clinical application of coronary bioresorbable scaffold]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Chen C, Chen TN, Wang XP. [Stress Relaxation Properties of Biodegradable Polymer PLGA]. Gaofenzi Cailiao Kexue Yu Gogncheng. 2012;28:60-62. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/