Published online Jun 26, 2024. doi: 10.4330/wjc.v16.i6.355
Revised: May 7, 2024
Accepted: May 27, 2024
Published online: June 26, 2024
Processing time: 97 Days and 14.1 Hours
The utility of D-dimer (DD) as a biomarker for acute aortic dissection (AD) is recognized. Yet, its predictive value for in-hospital mortality remains uncertain and subject to conflicting evidence.
To conduct a meta-analysis of AD-related in-hospital mortality (ADIM) with elevated DD levels.
We searched PubMed, Scopus, Embase, and Google Scholar for AD and ADIM literature through May 2022. Heterogeneity was assessed using I2 statistics and effect size (hazard or odds ratio) analysis with random-effects models. Sample size, study type, and patients’ mean age were used for subgroup analysis. The significance threshold was P < 0.05.
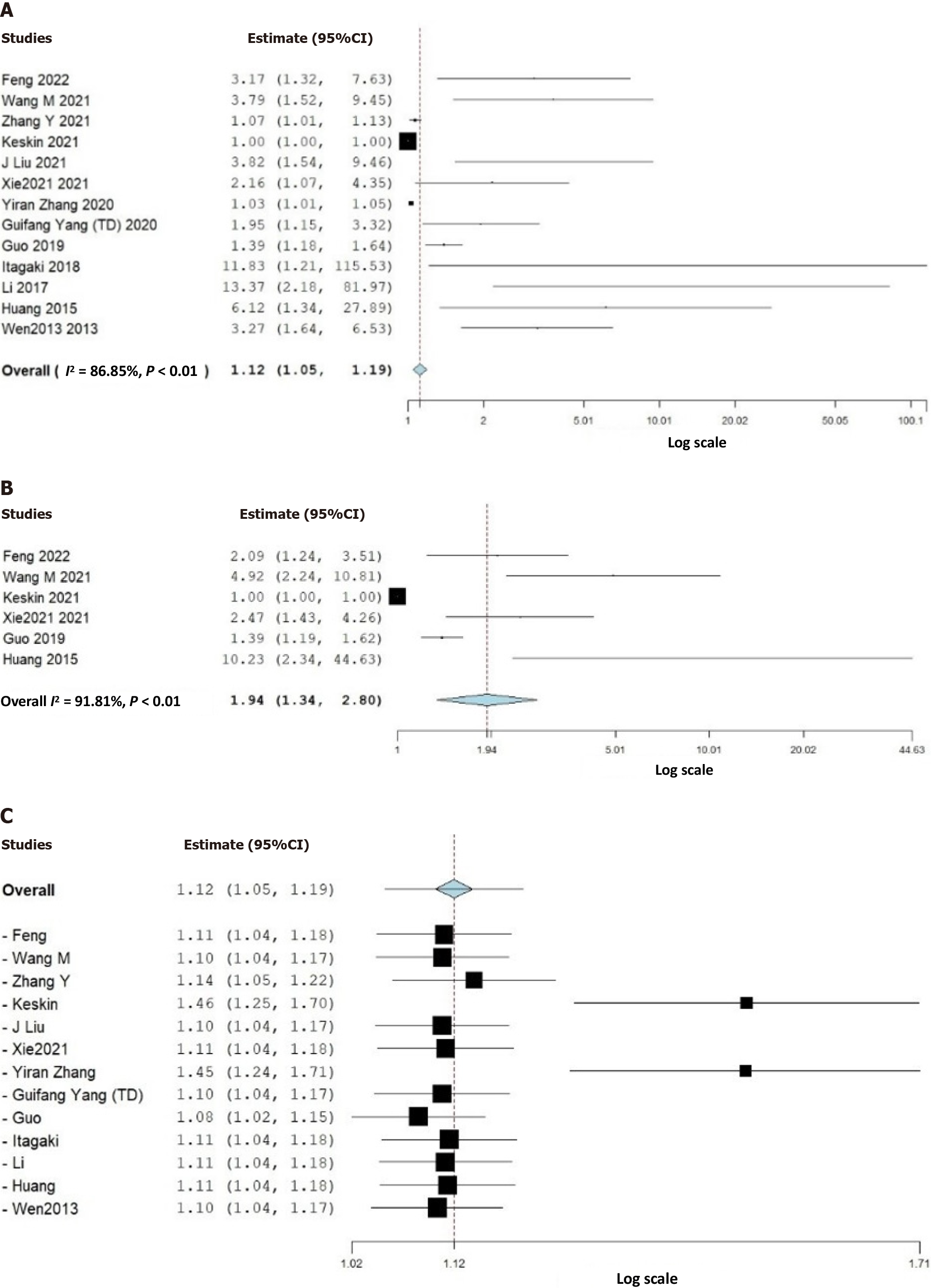
Thirteen studies (3628 patients) were included in our study. The pooled prevalence of ADIM was 20% (95%CI: 15%-25%). Despite comparable demographic characteristics and comorbidities, elevated DD values were associated with higher ADIM risk (unadjusted effect size: 1.94, 95%CI: 1.34-2.8; adjusted effect size: 1.12, 95%CI: 1.05-1.19, P < 0.01). Studies involving patients with a mean age of < 60 years exhibited an increased mortality risk (effect size: 1.43, 95%CI: 1.23-1.67, P < 0.01), whereas no significant difference was observed in studies with a mean age > 60 years. Prospective and larger sample size studies (n > 250) demonstrated a heightened likelihood of ADIM associated with elevated DD levels (effect size: 2.57, 95%CI: 1.30-5.08, P < 0.01 vs effect size: 1.05, 95%CI: 1.00-1.11, P = 0.05, respectively).
Our meta-analysis shows elevated DD increases in-hospital mortality risk in AD patients, highlighting the need for larger, prospective studies to improve risk prediction models.
Core Tip: This study illuminates the significant prognostic value of D-dimer (DD) levels in predicting in-hospital mortality among patients with aortic dissection (AD). By systematically reviewing and meta-analyzing 13 studies encompassing 3628 patients, we found a compelling association between elevated DD levels and increased risk of in-hospital mortality in AD patients. This relationship held strong across various subgroups, notably in larger sample sizes and prospective studies. Our findings suggest that incorporating DD into risk assessment models could greatly enhance the prediction of mortality risk, offering a crucial tool for early intervention and improved patient management in AD.
- Citation: Srikanth S, Abrishami S, Subramanian L, Mahadevaiah A, Vyas A, Jain A, Nathaniel S, Gnanaguruparan S, Desai R. Impact of D-dimer on in-hospital mortality following aortic dissection: A systematic review and meta-analysis. World J Cardiol 2024; 16(6): 355-362
- URL: https://www.wjgnet.com/1949-8462/full/v16/i6/355.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i6.355
Aortic dissection (AD) is a critical medical emergency characterized by the perforation of the aortic wall, resulting in significant morbidity and mortality. Multiple factors, including hypertension, connective tissue disorders, atherosclerosis, trauma, and genetic predisposition, contribute to the pathogenesis of AD, rendering the aortic wall susceptible to tearing. Survival in AD hinges on several factors, including the location and extent of the dissection, the presence of comor
AD has a mortality rate of 1% to 2% per hour after symptom onset if not treated promptly. Thus, early diagnosis plays a pivotal role in successful management and favorable outcomes post-AD[1]. Biomarkers may offer valuable insights into AD diagnosis and prognosis. D-dimer (DD) and other biomarkers have been investigated for their prognostic value in AD. DD is a fibrin breakdown product that is released into the circulation during fibrinolysis and holds promise as an AD biomarker. However, conflicting and limited evidence exists regarding its predictive value for in-hospital mortality in AD patients. While some studies have reported inconsistent findings, others have linked elevated DD levels and increased mortality in AD patients[2,3]. To address these discrepancies, we conducted a comprehensive systematic review and meta-analysis to evaluate the impact of DD on in-hospital mortality following AD. Our study aims to provide insights into the potential of DD as a biomarker for risk stratification and further highlight the importance of early diagnosis and timely intervention in managing AD.
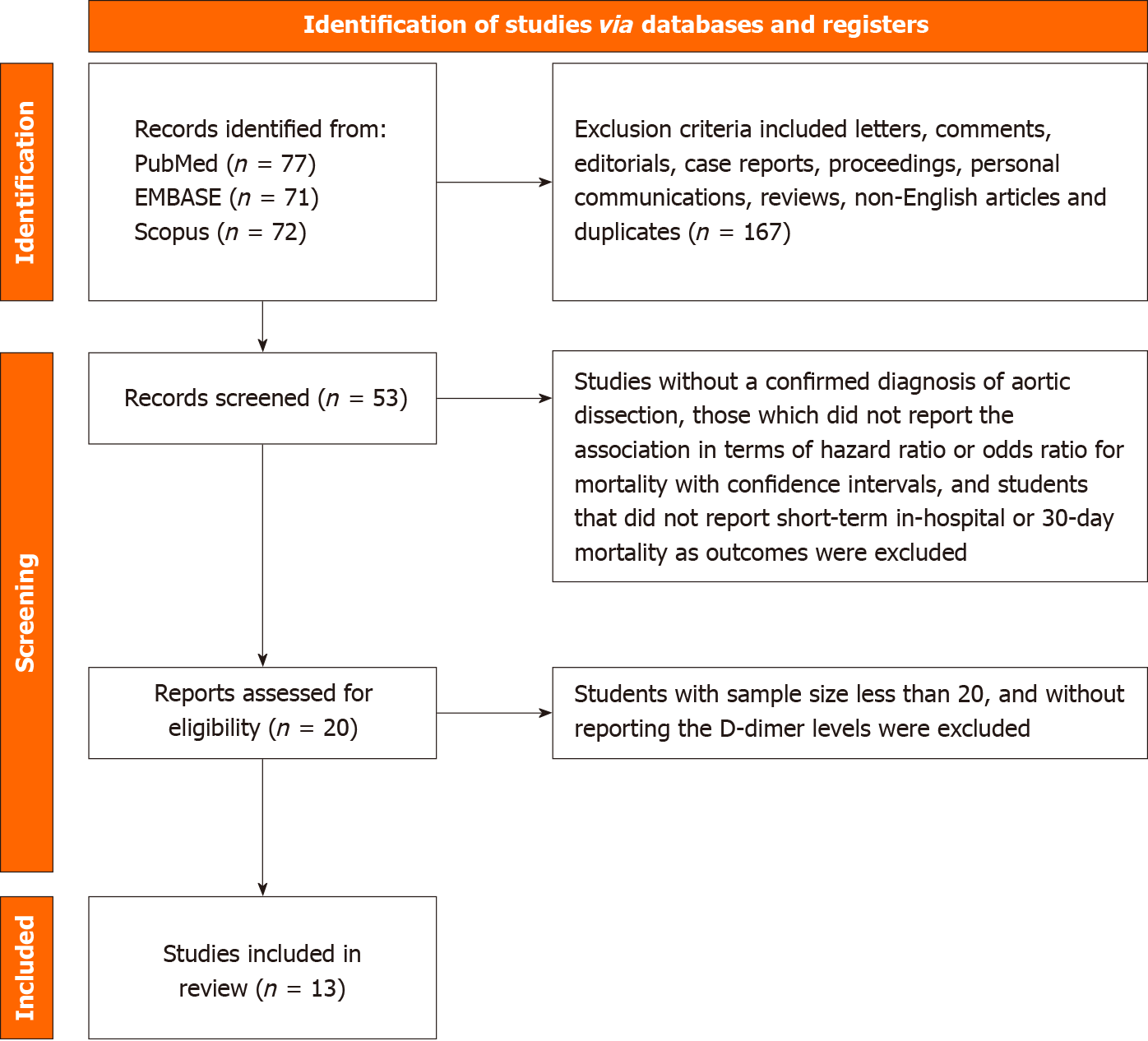
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used to establish a complete search strategy and selection criteria. PubMed/Medline, Scopus, Embase, Google Scholar, and Embase were systematically searched, with articles included until May 2022. Search terms “D-dimer,” “aortic dissection,” and “in-hospital mortality” were utilized to retrieve relevant literature. Additionally, further publications were identified through manual examination of the reference lists of relevant studies. The following were the study inclusion criteria: (1) Studies including patients aged 18 and greater and sample size greater than 20; (2) Studies with a confirmed diagnosis of AD; (3) DD levels were obtained; (4) The study design was two-armed, and the association was reported in terms of hazard or odds ratio for mortality with confidence intervals; and (5) In-hospital mortality reported as outcomes. Exclusion criteria included letters, comments, editorials, case reports, proceedings, personal communications, reviews, and non-English articles.
Two independent reviewers (Srikanth and Desai) screened identified articles based on title and abstract. Any discrepancies were resolved through consensus or consultation with a third reviewer (Subramanian). Subsequently, full-text publications from potentially relevant research were retrieved and evaluated for eligibility. Relevant data, including the first author’s name, year of publication, study design, number of participants in each treatment group, participants’ age and sex, type of AD, medical conditions other than AD, DD levels, and accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of DD levels for the diagnosis of acute AD were extracted from included studies.
The Newcastle-Ottawa Quality Assessment Scale with modification was utilized to evaluate the quality of the included studies. This scale ranks non-randomized studies based on patient selection, research group comparability, and outcome assessment. Two reviewers (Srikanth and Desai) independently assessed the quality, with any disagreements resolved through consensus or consultation with a third reviewer (Abrishami). Each study’s quality assessment score was recorded, with a minimum score of 5 considered acceptable (Supplementary Table 1).
All statistical analyses were conducted with Open Meta-Analyst software. For each study, pooled effect sizes (hazard ratio or odds ratio) with 95% confidence intervals were determined. The Cochran Q and I2 statistics were used to estimate study heterogeneity. Considering substantial heterogeneity (I2 > 50%), random-effects models (DerSimonian-Laird technique) were used. The leave-one-out strategy was used for sensitivity analysis, and funnel plot asymmetry was used for publishing bias analysis by visual inspection. Subgroup analysis was also performed based on sample size, study types, and mean age of the included patient population. The statistical significance level was set at P ≤ 0.05.
Thirteen studies were included in our study[2,4-15], encompassing a total of 3628 patients (Figure 1). Table 1 presents baseline characteristics, including study design, year the study was conducted, mean age of the population included in the study, diagnostic modality used to diagnose AD and its subtype, and clinical outcomes.
| Ref. | Year | Country | Study design | Mean age/median age | Male | Total AD cases | Type of AD | Total cases with mortality in AD | Diagnostic technique |
| Feng et al[15] | 2022 | China | Prospective cohort | 51.86 ± 10.76 | 396 (87.26) | 470 | Type A | 151 | CT angiography |
| Wang et al[10] | 2022 | China | Retrospective cohort | 54 | 121 (75.6) | 160 | Type A | 36 | Aorta angiography with multidetector CT |
| Zhang et al[9] | 2021 | China | Retrospective cohort | 52.76 ± 11.73 | 172 (76.8) | 224 | Type A | 33 | CTA, and color doppler echocardiography |
| Keskin et al[13] | 2021 | Turkey | Retrospective cross-sectional | 61 ± 12 | 99 (65.6) | 151 | Type A | 35 | Contrast-enhanced CTA or MRA |
| Liu et al[5] | 2021 | China | Retrospective cohort | 52 | 326 (89.8) | 363 | Type B | 26 | Multidetector contrast-enhanced CT |
| Xie et al[4] | 2021 | China | Retrospective cohort | Survived: 50.67 ± 11.49, died: 52.47 ± 12.52 | 279 | 345 | Type A and Type B | 75 | CT/MRI |
| Zhang et al[11] | 2020 | China | Retrospective cohort | 50 ± 12 | 149 (80) | 186 | Type A | 40 | CT |
| Yang et al[7] | 2020 | China | Retrospective cohort | Training set: 50.10 ± 11.58, validation set: 51.55 ± 10.62 | 536 | 703 | Type A | 235 | CTA or MRA |
| Guo et al[14] | 2019 | China | Prospective cohort | Survived: 52.0 ± 13.0, died: 52.1 ± 10.2 | 73 | 109 | Type A and type B | 31 | Contrast-enhanced CT |
| Itagaki et al[2] | 2018 | Japan | Retrospective cohort | 64.5 | 143 (54.58) | 262 | Type A | 23 | Contrast-enhanced CT |
| Li et al[8] | 2017 | China | Retrospective | 51.1 ± 13.1 | 262 (79.6) | 329 | Type A | 66 | CTA |
| Huang et al[6] | 2015 | China | Prospective cohort | 48.5 ± 11.5 | 161 (75.9) | 212 | Type A | 27 | Multidetector CT |
| Wen et al[12] | 2013 | China | Prospective cohort | Survived: 48.9 ± 7.6, died: 48.6 ± 7.6 | 96 | 114 | Type A and Type B | 31 | Chest radiography, transthoracic or transesophageal echocardiography and contrast-enhanced CT |
We discovered that the pooled prevalence of AD-related in-hospital mortality (ADIM) was 20% (95%CI: 15%-25%). In the analyzed data, higher DD values were associated with a higher risk of ADIM compared to lower DD values. This finding was supported by the statistical significance of both the unadjusted effect size (effect size: 1.94, 95%CI: 1.34-2.8, P < 0.01) and the adjusted effect size (effect size: 1.12, 95%CI: 1.05-1.19, P < 0.01) (Figure 2). However, the included studies displayed high heterogeneity and publication bias on visual inspection by funnel plot asymmetry (Supplementary Figure 1).
Subgroup analyses were conducted to delve deeper into the relationship between DD values and ADIM (Supple
AD is associated with a remarkably high mortality rate (27%)[1], yet it frequently is underdiagnosed. The findings of this systematic review and meta-analysis add to our understanding of the predictive usefulness of DD levels in AD. In-hospital mortality was found to be more likely in AD patients with elevated DD, and similar findings were observed across subgroups, i.e., studies with larger sample numbers, prospective studies, and studies with mean ages under 60. DD, a degradation product of cross-linked fibrin, when elevated, indicates the activation of coagulation and fibrinolytic systems. Damage to the aortic wall in AD triggers the coagulation cascade and subsequent fibrinolysis, which raises DD levels[16]. The results of this meta-analysis lend support to the notion that higher DD levels may indicate a more severe AD pathology or more extensive dissection, thus increasing the likelihood of adverse outcomes, particularly in-hospital mortality.
Our study revealed that younger patients (< 60 years old) had higher odds of ADIM than elderly patients (> 60 years old). Younger patients may have fewer comorbidities and better overall health, thereby implying a direct association of DD levels’ impact on mortality risk. DD levels increase with age, possibly due to a higher prevalence of comorbidities, and this might reduce the clinical significance of DD assay in the elderly[17]. Consequently, a higher cut-off may be more appropriate in older patients predicting mortality in AD[18]. The landscape of risk factors for AD varies between young and elderly. Younger AD patients may be at risk of worse outcomes due to the underpinning effect of connective tissue problems or hereditary factors that could cause more severe AD.
The meta-analysis showed that studies with larger sample sizes were associated with an increased risk of ADIM with elevated DD. Larger sample sizes have greater statistical power and accuracy, thereby reinforcing the predictive value of DD. As a potential key biomarker, DD can be employed in conjunction with existing recognized risk variables to enhance risk prediction in AD patients[19]. Our meta-analysis supports the idea of including DD in risk prediction models for outcomes of AD. This could assist clinicians in identifying individuals who might benefit from more aggressive management strategies, including interventions or intensive monitoring, as well as those at higher risk of unfavorable outcomes, necessitating optimal resource utilization in healthcare settings.
Comorbidities, DD levels, and AD-related outcomes can all interact in a complex, multivariate manner. The precise mechanisms and interactions between all of these factors are not fully understood and may vary depending on patient characteristics, disease severity, and other clinical factors. Cardiovascular comorbidities like hypertension, diabetes, and coronary artery disease aid in the onset and progression of AD by inducing structural changes in the aorta and promoting inflammation. Elevated DD levels may indicate the extent of aortic damage and thrombus development in AD. However, the specificity of elevated DD levels diminishes in conditions such as pregnancy, cancer, recent surgery, or trauma[20]. Cardiovascular comorbidities and higher-than-normal DD levels have both been linked to a higher risk of mortality, surgical complications, longer hospital admissions, and worse long-term survival in AD patients. The complicated and multifaceted mechanisms underlying the association between these factors and mortality in AD necessitate careful management and monitoring of DD levels and cardiovascular comorbidities to achieve optimal short and long-term outcomes.
DD testing is common in many clinical settings and is a popular biomarker for evaluating inflammation, coagulation, and fibrinolysis. DD is more affordable, readily available, and easier to assess than other biomarkers examined in the context of AD prognosis, such as troponins, brain natriuretic peptide, and C-reactive protein. Our study suggests that DD could be an effective biomarker for predicting in-hospital mortality in patients with AD, making it a crucial tool in clinical practice with significant implications on risk assessment, clinical judgment, and cost-effectiveness.
Our study presents several limitations that should be taken into consideration when interpreting the findings. The inclusion of observational studies introduces the potential for selection bias, measurement bias, and confounding in the individual studies incorporated in this meta-analysis. Furthermore, the quality of the included studies could vary, which might have an impact on the robustness and generalizability of the results. Moderate to high heterogeneity among the included studies, as indicated by I2 statistics, raises concerns about the consistency of findings. The pooled estimates may be impacted as a result of variations in study design, patient demographics, and methodology. Although heterogeneity was taken into account using random-effects models, subgroup analysis, and sensitivity analysis, caution ought to be used when interpreting the pooled results. A notable limitation is the lack of data on the etiology of AD. While this meta-analysis demonstrates an association between DD and in-hospital mortality in AD, it does not establish causation or elucidate underlying mechanisms. Future research is essential to elucidate the role of DD in AD prognosis and validate the findings of this meta-analysis, including prospective cohort studies and mechanistic investigations. Additionally, our meta-analysis focused on short-term outcomes, specifically in-hospital mortality. Long-term mortality and other important outcomes such as morbidity, quality of life, and healthcare resource utilization were not explored. Further investigation is needed to understand the relationship between DD and other clinically significant outcomes in AD.
According to this systematic review and meta-analysis, elevated DD levels are linked to a higher risk of in-hospital mortality in patients with AD. DD may be a useful prognostic biomarker for AD patients, and its incorporation into risk prediction models could enhance their accuracy and predictive capability. Despite some limitations, this review underscores the potential of DD as an advanced and cost-effective biomarker for evaluating in-hospital mortality in AD patients. However, further prospective validation studies are needed to establish the clinical utility of DD in risk stratification and management of AD patients. Subsequent investigations could explore the synergistic effects of DD with other biomarkers to improve risk prediction models and investigate the therapeutic implications of DD in AD management. Overall, these findings contribute to our understanding of AD prognosis and offer guidance for current and future clinical practice and research in this field.
| 1. | Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, Harris KM, Hutchinson S, O'Gara P, Suzuki T, Nienaber CA, Eagle KA; IRAD Investigators. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation. 2018;137:1846-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 948] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 2. | Itagaki R, Kimura N, Mieno M, Hori D, Itoh S, Akiyoshi K, Yuri K, Tanno K, Kawahito K, Yamaguchi A. Characteristics and Treatment Outcomes of Acute Type A Aortic Dissection With Elevated D-Dimer Concentration. J Am Heart Assoc. 2018;7:e009144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Gorla R, Erbel R, Kahlert P, Tsagakis K, Jakob H, Mahabadi AA, Schlosser T, Eggebrecht H, Bossone E, Jánosi RA. Diagnostic role and prognostic implications of D-dimer in different classes of acute aortic syndromes. Eur Heart J Acute Cardiovasc Care. 2017;6:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Xie N, Zhang W, Li H, Zhou J, Yang X, Zou L, Wan Z. Admission Values of Plasma Biomarkers Predict the Short-Term Outcomes in Acute Aortic Dissection. Heart Surg Forum. 2021;24:E048-E054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Liu J, Liu W, Ma W, Chen L, Liang H, Fan R, Zeng H, Geng Q, Yang F, Luo J. Prognostic dynamic nomogram integrated with metabolic acidosis for in-hospital mortality and organ malperfusion in acute type B aortic dissection patients undergoing thoracic endovascular aortic repair. BMC Cardiovasc Disord. 2021;21:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Huang B, Yang Y, Lu H, Zhao Z, Zhang S, Hui R, Fan X. Impact of d-Dimer Levels on Admission on Inhospital and Long-Term Outcome in Patients With Type A Acute Aortic Dissection. Am J Cardiol. 2015;115:1595-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Yang G, Zhou Y, He H, Pan X, Li X, Chai X. A nomogram for predicting in-hospital mortality in acute type A aortic dissection patients. J Thorac Dis. 2020;12:264-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Li ZD, Liu Y, Zhu J, Wang J, Lu FL, Han L, Xu ZY. Risk factors of pre-operational aortic rupture in acute and subacute Stanford type A aortic dissection patients. J Thorac Dis. 2017;9:4979-4987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Chen T, Chen Q, Min H, Nan J, Guo Z. Development and evaluation of an early death risk prediction model after acute type A aortic dissection. Ann Transl Med. 2021;9:1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Wang M, Luo L, Xia X, Jiang J, Zhang L, Ge G, Dong N. A simple model predicting in-hospital death in patients with type A acute aortic dissection. Perfusion. 2022;37:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Xu X, Lu Y, Guo L, Ma L. Preoperative uric acid predicts in-hospital death in patients with acute type a aortic dissection. J Cardiothorac Surg. 2020;15:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Wen D, Du X, Dong JZ, Zhou XL, Ma CS. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart. 2013;99:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Keskin HA, Kurtul A, Esenboğa K, Çiçek MC, Katırcıoğlu SF. Prognostic nutritional index predicts in-hospital mortality in patients with acute Stanford type A aortic dissection. Perfusion. 2021;36:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Guo T, Zhou X, Zhu A, Peng W, Zhong Y, Chai X. The Role of Serum Tenascin-C in Predicting In-Hospital Death in Acute Aortic Dissection. Int Heart J. 2019;60:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Feng W, Wang Q, Li C, Wu J, Kuang J, Yang J, Fan R. Significant Prediction of In-hospital Major Adverse Events by D-Dimer Level in Patients With Acute Type A Aortic Dissection. Front Cardiovasc Med. 2022;9:821928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Xu Y, Liang S, Liang Z, Huang C, Luo Y, Liang G, Wang W. Admission D-dimer to lymphocyte counts ratio as a novel biomarker for predicting the in-hospital mortality in patients with acute aortic dissection. BMC Cardiovasc Disord. 2023;23:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Linkins LA, Takach Lapner S. Review of D-dimer testing: Good, Bad, and Ugly. Int J Lab Hematol. 2017;39 Suppl 1:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, Rutschmann OT, Sanchez O, Jaffrelot M, Trinh-Duc A, Le Gall C, Moustafa F, Principe A, Van Houten AA, Ten Wolde M, Douma RA, Hazelaar G, Erkens PM, Van Kralingen KW, Grootenboers MJ, Durian MF, Cheung YW, Meyer G, Bounameaux H, Huisman MV, Kamphuisen PW, Le Gal G. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 626] [Article Influence: 52.2] [Reference Citation Analysis (1)] |
| 19. | Tian L, Fan X, Zhu J, Liang Y, Li J, Yang Y. Plasma D-dimer and in-hospital mortality in patients with Stanford type A acute aortic dissection. Blood Coagul Fibrinolysis. 2014;25:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Weitz JI, Fredenburgh JC, Eikelboom JW. A Test in Context: D-Dimer. J Am Coll Cardiol. 2017;70:2411-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 389] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/