Published online May 26, 2024. doi: 10.4330/wjc.v16.i5.231
Revised: January 29, 2024
Accepted: April 9, 2024
Published online: May 26, 2024
Processing time: 186 Days and 18.6 Hours
The use of anticoagulation therapy could prove to be controversial when trying to balance ischemic stroke and intracranial bleeding risks in patients with concurrent cerebral amyloid angiopathy (CAA) and atrial fibrillation (AF). In fact, CAA is an age-related cerebral vasculopathy that predisposes patients to intracerebral hemorrhage. Nevertheless, many AF patients require oral systemic dose-adjusted warfarin, direct oral anticoagulants (such as factor Xa inhibitors) or direct thrombin inhibitors to control often associated with cardioembolic stroke risk. The prevalence of both CAA and AF is expected to rise, due to the aging of the po
Core Tip: The use of anticoagulation therapy could prove to be controversial when trying to balance ischemic stroke and intracranial bleeding risks in patients with concurrent cerebral amyloid angiopathy (CAA) and atrial fibrillation (AF). This review aims to provide an overview of the management of patients with concomitant AF and CAA and proposes the implementation of a risk-based decision-making algorithm.
- Citation: Fusco L, Palamà Z, Scarà A, Borrelli A, Robles AG, De Masi De Luca G, Romano S, Sciarra L. Management of cerebral amyloid angiopathy and atrial fibrillation: We are still far from precision medicine. World J Cardiol 2024; 16(5): 231-239
- URL: https://www.wjgnet.com/1949-8462/full/v16/i5/231.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i5.231
In the last two decades, the management of thromboembolic risk in atrial fibrillation (AF) has dramatically improved, with a continuous reduction of thromboembolic events. Almost all aspects of AF management are based on clear evidence, covering treatment of underlying cardiovascular conditions, rate control or rhythm control, and anticoagulation. However, some clinical settings are yet to be covered by evidence. In particular, the management of patients with both high bleeding and stroke risks could present strong uncertainties in clinical practice. This is the case in patients with concomitant cerebral amyloid angiopathy (CAA) and AF that pose challenges for the risk-benefit balance of anticoagulation therapy. AF, not considered directly life-threatening, is associated with increased cardiovascular diseases (such as heart failure, and pacemaker implantation), ischemic risk (such as stroke) and an overall increased risk of death. Ischemic risk is often reduced by anticoagulants. However, patients with CAA have a high risk of intracerebral hemorrhage (ICH), worsened by anticoagulation therapy[1-3]. This clinical dilemma is increasingly becoming a common scenario, due to the aging of the population. CAA and AF are frequent diseases in older age groups. Both these diseases have seen massive rises in incidence over recent years; with a prevalence of around 5% for moderate to severe CAA in cognitively normal elderly and around 20% for AF in subjects aged > 80 years. In fact, myocardial fibrosis and atrial remodeling play an important role as substrates for AF in the aging heart as well as CAA results from the cerebrovascular deposition of β-amyloid protein in the aging brain. Although CAA could be undetected, based on these epidemiological data, in the elderly population a large cohort has concurrent AF and CAA. In the absence of any specific recommendations regarding anticoagulation management in patients with CAA and AF, all specialists should balance the risk-benefit of each patient’s treatment on an individual basis. Many clinical guidelines recommend using validated scores for bleeding and stroke risk. HAS-BLED (hypertension; abnormal renal/liver function; stroke; bleeding history or predisposition; labile international normalized ratio; elderly, age ≥ 65 years; and drugs/alcohol concomitantly) is the common score to predict the risk of major bleeding secondary to anticoagulation, whereas the CHA2DS2-VASc score is used to assess the 1-year risk of stroke in patients with AF and to determine whether anticoagulation therapy is indicated[4,5]. However, these current risk scores might be of limited value for a specific subset of populations, such as patients with concurrent AF and CAA. Better bleeding risk assessment is essential in each older subject. We believe that the increasing impact of CAA in clinical practice and its well-known association with ICH cannot be ignored while establishing whether to start or continue anticoagulant therapies. A search of the literature was carried out to create this review, which aims to provide a clinically oriented update on anticoagulation management for patients with concomitant CAA and AF, focusing on the importance of further factors involved in the risk profile. Specifically, we propose the implementation of a risk-based decision-making algorithm in order to provide a more accurate precision-medicine approach.
CAA is a cerebrovascular disorder characterized by the presence of amyloid β-peptide (Aβ) deposits within media and adventitia of small to medium-sized arteries in the cortex and leptomeninges[6,7]. This is a chronic degenerative process that causes changes in blood vessels: loss of smooth muscle cells, fibrinoid necrosis and simultaneous accumulation of Aβ40 species, an eosinophilic hyaline material. That deposition in blood vessel walls can lead to lobar ICH and to smaller areas of bleeding, including cerebral microbleeds (CMBs) and cortical superficial siderosis (cSS). In addition to ICH, CAA can also cause ischemic events such as microinfarcts. Other important clinical manifestations of CAA may include cognitive impairments, transient neurological symptoms, and inflammatory leukoencephalopathy[1,2]. This clinical and pathological heterogeneity could indicate related but distinct phenotypes of CAA, reflected in different clinical syndromes[2-6]. The most common localization is the occipital cortex followed by the frontal, temporal, and parietal cortices[8,9]. Some studies suggest that parieto-occipital regions are related to the most severe CAA[10].
Although the conclusive diagnosis of CAA requires histopathological confirmation, this disease is associated with characteristic magnetic resonance imaging (MRI) biomarkers, including cSS, CMBs, centrum semiovale perivascular spaces, and white matter hyperintensity[2-9]. At present the possible-probable diagnosis is based on a set of pathological parameters, clinical aspects and MRI findings that are termed the Boston criteria 2.0[10,11]. This latest version of diagnostic criteria updates the previous Boston criteria and Modified Boston Criteria (proposed in 1995 and 2010, respectively)[11]. These new criteria take into account hemorrhagic and nonhemorrhagic imaging findings in patients aged ≥ 50 years with spontaneous ICH; transient focal neurological episodes; cognitive impairment or dementia without the presence of deeper hemorrhagic or other causes of hemorrhagic lesions. Probable CAA requires the presence of at least two strictly lobar hemorrhagic lesions (ICH, cortical CMBs, and cSS or convexity subarachnoid hemorrhage), or at least the presence of a strictly lobar hemorrhagic lesion plus one white matter features[10,11].
Given that the patients with CAA are at notably higher risk of ICH[2], improving accuracy for the diagnosis of CAA is crucial to assess individual bleeding risk and determining the risk-benefit balance for the anticoagulation therapy[12-14]. CAA is one of the most common causes of spontaneous ICH and accounts for 15%-20% of lobar ICHs that are preferentially localized in cortical and subcortical regions[2,15-18]. CAA is also an increasingly recognized cause of recurrent ICH with an annual risk of 9%-10%[18,19]. Within 5 years after the first ICH, 25% of patients with CAA have a new episode.
Some genetic and imaging data have recently shown to be strong predictors of future ICH in patients with CAA and they can help to better stratify the individual bleeding risk such as the presence of cSS, CMBs and apolipoprotein E (ApoE) allele status[12]. cSS represents the hemosiderin deposition in the subarachnoid space that results from extravasation of blood from leptomeningeal vessels. It has been shown to be the strongest predictor of future ICH[12-20] and could represent an independent risk factor for the development of dementia post-ICH[12]. Another important finding for ICH risk stratification is the number and the presence of CMBs. Recent literature has confirmed the association between the presence and number of CMBs and increased incidence of ICH[12-21]. Genetic factors play an important role in the pathophysiology of CAA, such as ApoE genotype; ApoE polymorphisms are associated with an increased risk of lobar ICH[12]. In those carrying ApoE4/E4 and ApoE2/E4, it appears that the E4 allele enhances amyloid deposition in blood vessels in a dose-dependent fashion and the E2 allele is related to the severity of vascular pathology, promoting vessel rupture[12-22].
AF is one of the most common sustained cardiac arrhythmias, and is associated with increased thromboembolic risk[23,24], and, compared to sinus rhythm, it has higher mortality[24]. The rate of stroke (without anticoagulation therapy) can amount to 20% a year, based on the patient’s comorbidities[25]. In a large observational cohort, the risk of ischemic stroke in non-anticoagulated subjects with AF ranged from 0.2% to 14.4% yearly. The mortality rates post-stroke ranged from 15% during the first month to 50% during the following 5 years, and the recurrence rate of stroke was high over that period[26]. Antithrombotic prophylaxis with vitamin K antagonists (VKAs) and direct-acting oral anticoagulants (DOACs) is the cornerstone approach to controlling this ischemic risk[5], and it has demonstrated strong protection against first-ever stroke and recurrent events[26-28]. Before the advent of DOACs, warfarin, a VKA, showed effectiveness and safety with a reduction of overall stroke risk by 64%[29,30]. However, there are several practical limitations to using VKAs. These include residual ischemic risk, its narrow therapeutic window, difficulties in achieving it, and monitoring anticoagulation serum levels[26,31,32]. International guidelines have recently prioritized the use of DOACs over VKA for the aforementioned reasons[33].
These new agents do not require frequent therapeutic monitoring due to a predictable pharmacokinetic profile, rapid onset of action, and fewer drug and food interactions[34-35]. These novel drugs have been compared to warfarin in randomized trials and have demonstrated equivalent or improved efficacy in reducing cardioembolic stroke risk in patients with AF, with a lower incidence of ICH[36-39].
At present, several factors can predict stroke in AF: clinical, electrical, genetic and biological markers[4,40]. However, in the current guidelines, the risk stratification model recommended for stroke prediction in patients with AF is the CHA2DS2-VASc score which is based on clinical data[5]. This represents an updated score that has been validated and suggested in the most recent guidelines. Patients with a CHA2DS2-VASc score of ≥ 2 have a stroke risk of around 2.5% annually and should use DOACs or VKAs to reduce this risk. Lower scores are considered to represent a low risk of stroke, with an event rate of around 1% annually. This tool, which helps clinicians to quickly estimate risk relying on only a short set of clinical features (congestive heart failure; hypertension; age ≥ 75 years; diabetes; stroke; vascular disease; age 65-74 years; and sex category), could be insufficient and inaccurate in complex patients that present with other ischemic risk factors[41-43].
The most difficult dilemmas occur when patients with AF and concomitant CAA have an indication for antithrombotic therapy. This is because evaluating the interaction of the many factors involved may prove challenging. Unfortunately, this scenario is becoming progressively common in clinical practice. The prevalence of CAA and AF is strongly age related[1-3,44,45] and increasing rapidly due to improved diagnostic procedures and aging of the global population[2]. The prevalence of moderate-to-severe CAA based on pathology and imaging was around 5% in cognitively normal elderly and up to 50% in patients with lobar ICH[1]. The prevalence of AF is around 1% for individuals younger than 60 years, around 12% for those aged 75-84 years, and up to 20% for people aged > 85 years[2,3,46,47]. The main issue is that in some cases antithrombotic strategies could theoretically do more harm than good.
Even in subjects with low baseline ICH risk, anticoagulation therapy is significantly associated with a higher risk of ICH and ICH-related mortality. Specifically, warfarin increases the risk of ICH by five times compared to placebo, while DOACs showed a 2-3-fold increase in ICH risk[4,47-49]. In a recent systematic review and meta-analysis, all DOACs showed a lower risk of ICH than VKA compared with VKAs, dabigatran reduced the risk of ICH by 60%, apixaban by 57%, edoxaban by 56% and rivaroxaban by 41%[50]. Anticoagulation-associated ICH has a worse outcome than non-OAC ICH, with 50% mortality[47,48]. As discussed above, some providers recommend using CHA2DS2-VASC for ischemic risk score as well as different bleeding scores to determine the risk of major bleeding events secondary to anticoagulation[5,47,51]. The most commonly used is the HAS-BLED score. This bleeding risk score has shown to have moderate predictive abilities and to perform better than other scores[51-53]. However, this approach may be insufficient to estimate the risk of ICH in CAA, as some markers of ICH risk factors such as cSS or lobar CMBs are not systematically included[47-54].
Possible strategies for bleeding risk reduction: management of comorbidities and additional therapies: The correct management of patients with concomitant AF and CAA, besides anticoagulation therapy, includes controlling cardiovascular risk factors (hypertension and statin use) and considering alternatives to anticoagulation [i.e., left atrial appendage closure (LAAC)].
Hypertension is a well-known risk factor for both ischemic and bleeding events and its rigorous control can reduce rates of ICH in primary and secondary prevention[55]. It is plausible that hypertension could play an important role in altering the forces upon the vessel wall, leading to the rupture of the vessel and changing perivascular clearance[48]. A subanalysis of a progress trial showed a risk reduction of 77% in patients with a probable diagnosis of CAA with intensive treatment of hypertension[56], confirming the results from other studies that show a net benefit in patients with CAA to keep blood pressure (BP) < 120/80 mmHg[57]. A single-center observational cohort study also demonstrated an association between BP and the risk of recurrent ICH. The association between elevated BP and ICH recurrence appeared to become stronger with the worsening severity of hypertension[58]. Visit-to-visit BP variable parameters have been shown to be related to the evolution of CMBs and white matter lesions, which could explain the association between high BP and ICH risk[59]. These data strongly suggest the importance of BP control in patients with CAA[58]. Future research could help to individualize the BP-lowering strategy in patients with CAA.
Strong scientific evidence shows the established benefits of statins on cardiac and cerebrovascular diseases, for both primary and secondary prevention[59-63]. However, some studies have suggested a relationship between the use of statins and an increased risk of cerebral hemorrhage. A prospective, randomized, placebo-controlled trial of stroke prevention by aggressive reduction in cholesterol levels demonstrated a beneficial effect of statins on the risk of recurrent stroke, along with an increase in the incidence of ICH in the statin arm compared to the placebo arm[60]. A retrospective review in patients with spontaneous ICH demonstrated an association between statin treatment and an increased number of microbleeds[64]. A recent analysis that used a decision analytic model calculated that statin therapy is predicted to raise the annual probability of lobar ICH recurrence from 14% to 22%[65]. In contrast, many studies have demonstrated a positive effect of statin therapy on ICH risk[66]. A recent nationwide observational study showed that statins were associated with a lower risk of ICH compared with non-statin therapy[67]. Another nationwide follow-up cohort study observed that patients with ICH after therapy with hydrophilic statins had a significantly lower risk of recurrent ICH compared to subjects using lipophilic statins[68]. Currently, in patients with CAA and clear indication for statin therapy according to guidelines, there is no evidence to avoid statin treatment[12].
Most of the thromboembolism in AF that causes ischemic stroke originates from the LAA[69,70]. Closure of the LAA represents a safe and effective strategy for stroke prevention, targeting patients with high bleeding risk[12], and a few clinical data support some devices for percutaneous LAAC. A recent observational cohort study demonstrated that, in subjects with CAA, LAAC was a safe and valid approach to stroke prevention, even without long-term anticoagulant therapy[71]. Nonsurgical LAA closure with a Watchman device has been shown not to be inferior to anticoagulation therapy for ischemic stroke prevention, with an 85% reduction in hemorrhagic stroke[71]. The net benefit of this strategy can be assessed in large prospective studies but at present there is no conclusive evidence.
Discussion and implementation of risk stratification: Aging of the global population and higher survival rates have led to a substantial increase in the prevalence of cerebrovascular diseases associated with chronic cardiac conditions. As a result, clinical practitioners have to deal with increasingly complex decision-making when administering therapy to patients with both high bleeding and ischemic risks. In addition, current guidelines do not set out any clear-cut recommendations as to how to balance the risk-benefit of antithrombotics in a specific subgroup of patients. The suggested risk scores and decisional algorithm do not consider some neurological conditions that can increase hemorrhagic risk. The risk of OAC-ICH in patients with CAA can be calculated based on several clinical and MRI findings. In addition, recent evidence has demonstrated that an integrated care approach in patients with a chronic complex conditions such as AF is associated with an overall benefit in terms of cardiovascular outcomes[72-75]. For those reasons, the international guidelines recommend a holistic approach to managing the AF population. This includes multidisciplinary team care, the use of technology, and comprehensive management of patients’ conditions (e.g., comorbidity and cardiovascular risk factors)[76,77]. This approach called the AF better care pathway, has three main components: A covers anticoagulation therapy to avoid stroke; B means better symptom management; and C includes optimization of cardiovascular and other comorbidity risks. The first component encompasses the optimal management of anticoagulation which means maintaining a stable time in the therapeutic range (higher than 65%-70%) for warfarin treatment and appropriate dosage for DOAC treatment. Symptoms management is included in the second component. The last component covers control of cardiometabolic and lifestyle risk factors[78]. Many studies have shown that there is a serious suboptimal prescription of DOACs, especially in elderly patients. The frequent underuse of oral anticoagulants in this high-risk population could be due to many reasons, including worries about cerebral bleeding[79-81]. Older patients, often affected by multiple comorbidities, could complicate the therapeutic decision-making process in optimizing the risks and benefits of OAC[82]. Deeper knowledge about the bleeding risk of each subject can help clinicians better assess the risk/benefit ratio for anticoagulation treatment.
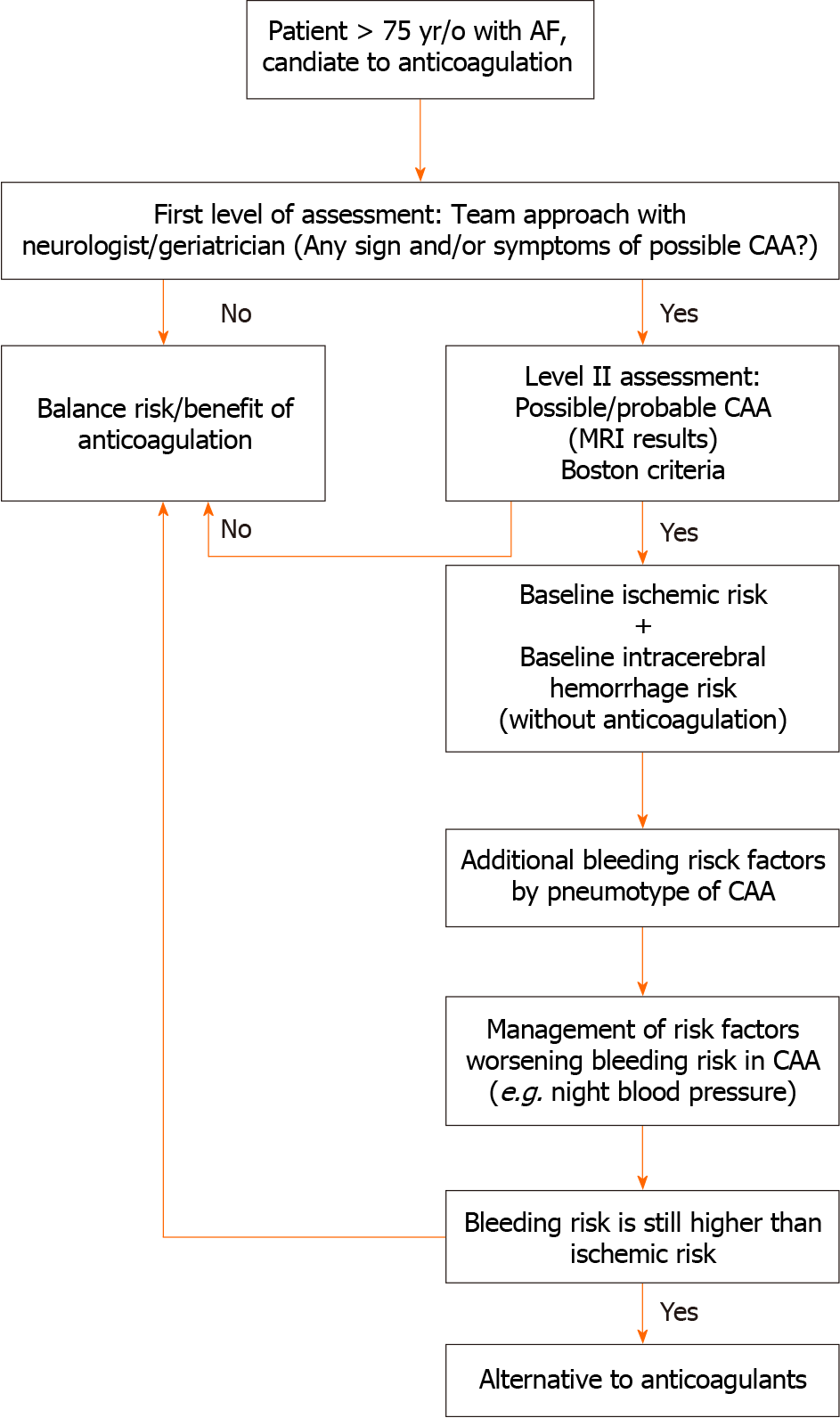
The expansion of an integrated chronic care program could lead to a change in the risk/benefit balance of anticoagulation therapy by encompassing a more accurate evaluation of neurological conditions that can worsen the bleeding risk. We think that CAA may be one of the additional bleeding factors that ought to be suspected in everyday clinical evaluation in elderly people who are candidates for anticoagulation therapy. To go further in this direction, we emphasize the need for an integrated approach with neurological and geriatric assessment, by systematically integrating the probable presence of CAA in elderly candidates for anticoagulation therapy. Randomized trials are urgently needed to evaluate the exact weight of emergent ICH risk factors, implement risk scores, and identify viable ways of decreasing this risk. For the latter, the following ought to be assessed systematically in patients aged > 75 years (Figure 1): (1) Baseline risk of ICH without anticoagulation; (2) additional ICH risk (by phenotype) in patients with possible/probable CAA; (3) ischemic stroke risk without anticoagulation; (4) management of BP; and (5) evaluation of alternatives to anticoagulation discussion with patients.
Evidence increasingly points to difficulties/limitations in stroke and bleeding risk assessments in AF and CAA. Current guidelines and risk scores do not consider all of the factors involved. Precise identification of these risk factors and their magnitude of risk will promote patient-tailored management for ischemic stroke prevention in the setting of AF. This review aims to reduce the gap between guidelines and precision medicine, helping clinicians in the management of anticoagulation solutions.
| 1. | Battistin U, AlQassim N, Hallak Y, Mohammed M, Hasan A, Oluwole OJ. Cerebral Amyloid Angiopathy and Atrial Fibrillation: An up to Date Case Report. Neurohospitalist. 2022;12:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Cannistraro RJ, Meschia JF. The Clinical Dilemma of Anticoagulation Use in Patients with Cerebral Amyloid Angiopathy and Atrial Fibrillation. Curr Cardiol Rep. 2018;20:106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Kelly J. New horizons: managing antithrombotic dilemmas in patients with cerebral amyloid angiopathy. Age Ageing. 2021;50:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Ding WY, Harrison S, Gupta D, Lip GYH, Lane DA. Stroke and Bleeding Risk Assessments in Patients With Atrial Fibrillation: Concepts and Controversies. Front Med (Lausanne). 2020;7:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6942] [Article Influence: 1388.4] [Reference Citation Analysis (1)] |
| 6. | Kuhn J, Sharman T. Cerebral Amyloid Angiopathy. 2023 Jun 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 7. | Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 8. | Magaki S, Tang Z, Tung S, Williams CK, Lo D, Yong WH, Khanlou N, Vinters HV. The effects of cerebral amyloid angiopathy on integrity of the blood-brain barrier. Neurobiol Aging. 2018;70:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140:1829-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 10. | Charidimou A, Boulouis G, Frosch MP, Baron JC, Pasi M, Albucher JF, Banerjee G, Barbato C, Bonneville F, Brandner S, Calviere L, Caparros F, Casolla B, Cordonnier C, Delisle MB, Deramecourt V, Dichgans M, Gokcal E, Herms J, Hernandez-Guillamon M, Jäger HR, Jaunmuktane Z, Linn J, Martinez-Ramirez S, Martínez-Sáez E, Mawrin C, Montaner J, Moulin S, Olivot JM, Piazza F, Puy L, Raposo N, Rodrigues MA, Roeber S, Romero JR, Samarasekera N, Schneider JA, Schreiber S, Schreiber F, Schwall C, Smith C, Szalardy L, Varlet P, Viguier A, Wardlaw JM, Warren A, Wollenweber FA, Zedde M, van Buchem MA, Gurol ME, Viswanathan A, Al-Shahi Salman R, Smith EE, Werring DJ, Greenberg SM. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022;21:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 452] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 11. | Du Y, Zhang W, Locatelli M, Simister RJ, Jäger HR, Werring DJ. The Boston criteria version 2.0 increase the proportion of lobar intracerebral haemorrhage classified as probable cerebral amyloid angiopathy. J Neurol. 2023;270:3243-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Kozberg MG, Perosa V, Gurol ME, van Veluw SJ. A practical approach to the management of cerebral amyloid angiopathy. Int J Stroke. 2021;16:356-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | An SJ, Kim TJ, Yoon BW. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J Stroke. 2017;19:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 667] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 14. | Weber SA, Patel RK, Lutsep HL. Cerebral amyloid angiopathy: diagnosis and potential therapies. Expert Rev Neurother. 2018;18:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Mehndiratta P, Manjila S, Ostergard T, Eisele S, Cohen ML, Sila C, Selman WR. Cerebral amyloid angiopathy-associated intracerebral hemorrhage: pathology and management. Neurosurg Focus. 2012;32:E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | van Etten ES, Gurol ME, van der Grond J, Haan J, Viswanathan A, Schwab KM, Ayres AM, Algra A, Rosand J, van Buchem MA, Terwindt GM, Greenberg SM, Wermer MJ. Recurrent hemorrhage risk and mortality in hereditary and sporadic cerebral amyloid angiopathy. Neurology. 2016;87:1482-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2482] [Article Influence: 155.1] [Reference Citation Analysis (0)] |
| 18. | Pinho J, Almeida FC, Araújo JM, Machado Á, Costa AS, Silva F, Francisco A, Quintas-Neves M, Ferreira C, Soares-Fernandes JP, Oliveira TG. Sex-Specific Patterns of Cerebral Atrophy and Enlarged Perivascular Spaces in Patients with Cerebral Amyloid Angiopathy and Dementia. AJNR Am J Neuroradiol. 2023;44:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Garg A, Ortega-Gutierrez S, Farooqui M, Nagaraja N. Recurrent intracerebral hemorrhage in patients with cerebral amyloid angiopathy: a propensity-matched case-control study. J Neurol. 2022;269:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Charidimou A, Boulouis G, Greenberg SM, Viswanathan A. Cortical superficial siderosis and bleeding risk in cerebral amyloid angiopathy: A meta-analysis. Neurology. 2019;93:e2192-e2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Greenberg SM. Cerebral microbleeds and prediction of intracranial haemorrhage. Lancet Neurol. 2021;20:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Marini S, Crawford K, Morotti A, Lee MJ, Pezzini A, Moomaw CJ, Flaherty ML, Montaner J, Roquer J, Jimenez-Conde J, Giralt-Steinhauer E, Elosua R, Cuadrado-Godia E, Soriano-Tarraga C, Slowik A, Jagiella JM, Pera J, Urbanik A, Pichler A, Hansen BM, McCauley JL, Tirschwell DL, Selim M, Brown DL, Silliman SL, Worrall BB, Meschia JF, Kidwell CS, Testai FD, Kittner SJ, Schmidt H, Enzinger C, Deary IJ, Rannikmae K, Samarasekera N, Al-Shahi Salman R, Sudlow CL, Klijn CJM, van Nieuwenhuizen KM, Fernandez-Cadenas I, Delgado P, Norrving B, Lindgren A, Goldstein JN, Viswanathan A, Greenberg SM, Falcone GJ, Biffi A, Langefeld CD, Woo D, Rosand J, Anderson CD; International Stroke Genetics Consortium. Association of Apolipoprotein E With Intracerebral Hemorrhage Risk by Race/Ethnicity: A Meta-analysis. JAMA Neurol. 2019;76:480-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Ruigómez A, Johansson S, Wallander MA, Edvardsson N, García Rodríguez LA. Risk of cardiovascular and cerebrovascular events after atrial fibrillation diagnosis. Int J Cardiol. 2009;136:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Lilli A, Di Cori A, Zacà V. Thromboembolic risk and effect of oral anticoagulation according to atrial fibrillation patterns: A systematic review and meta-analysis. Clin Cardiol. 2017;40:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Furie KL, Goldstein LB, Albers GW, Khatri P, Neyens R, Turakhia MP, Turan TN, Wood KA; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Peripheral Vascular Disease. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:3442-3453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Wu J, Zhang Y, Liao X, Lei Y. Anticoagulation Therapy for Non-valvular Atrial Fibrillation: A Mini-Review. Front Med (Lausanne). 2020;7:350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016;388:806-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 28. | Wu J, Alsaeed ES, Barrett J, Hall M, Cowan C, Gale CP. Prescription of oral anticoagulants and antiplatelets for stroke prophylaxis in atrial fibrillation: nationwide time series ecological analysis. Europace. 2020;22:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3181] [Cited by in RCA: 3800] [Article Influence: 316.7] [Reference Citation Analysis (1)] |
| 30. | Ababneh M, Nasser SA, Rababa'h A, Ababneh F. Warfarin adherence and anticoagulation control in atrial fibrillation patients: a systematic review. Eur Rev Med Pharmacol Sci. 2021;25:7926-7933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Hawkins D. Limitations of traditional anticoagulants. Pharmacotherapy. 2004;24:62S-65S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol. 2018;3:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 2025] [Article Influence: 289.3] [Reference Citation Analysis (0)] |
| 34. | Oliphant CS, Jacobs A, Kabra R, Das P. Novel oral anticoagulants for the prevention and treatment of thromboembolism. Future Cardiol. 2013;9:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Gunawardena T. Direct oral anticoagulants: A review for the non-specialist. Hematol Rep. 2021;13:9239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7917] [Cited by in RCA: 8205] [Article Influence: 482.6] [Reference Citation Analysis (0)] |
| 37. | Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4144] [Cited by in RCA: 3924] [Article Influence: 301.8] [Reference Citation Analysis (1)] |
| 38. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6075] [Cited by in RCA: 6701] [Article Influence: 446.7] [Reference Citation Analysis (9)] |
| 39. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 7064] [Article Influence: 470.9] [Reference Citation Analysis (2)] |
| 40. | Jagadish PS, Kabra R. Stroke Risk in Atrial Fibrillation: Beyond the CHA(2)DS(2)-VASc Score. Curr Cardiol Rep. 2019;21:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Jia X, Levine GN, Birnbaum Y. The CHA(2)DS(2)-VASc score: Not as simple as it seems. Int J Cardiol. 2018;257:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Dudziñska-Szczerba K, Kułakowski P, Michałowska I, Baran J. Association Between Left Atrial Appendage Morphology and Function and the Risk of Ischaemic Stroke in Patients with Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2022;11:e09. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 43. | Bianconi E, Del Freo G, Salvadori E, Barbato C, Formelli B, Pescini F, Pracucci G, Sarti C, Cesari F, Chiti S, Diciotti S, Gori AM, Marzi C, Fainardi E, Giusti B, Marcucci R, Bertaccini B, Poggesi A. Can CHA(2)DS(2)-VASc and HAS-BLED Foresee the Presence of Cerebral Microbleeds, Lacunar and Non-Lacunar Infarcts in Elderly Patients With Atrial Fibrillation? Data From Strat-AF Study. Front Neurol. 2022;13:883786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, Reynolds K, Go AS. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 45. | Li T, Huang Y, Cai W, Chen X, Men X, Lu T, Wu A, Lu Z. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. 2020;11:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 46. | Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 542] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 47. | Wagner G, Smeikal M, Gisinger C, Moertl D, Nopp S, Gartlehner G, Pabinger I, Ohrenberger G, Ay C. Epidemiology, risk profile, management, and outcome in geriatric patients with atrial fibrillation in two long-term care hospitals. Sci Rep. 2022;12:18725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 48. | Gurol ME. Nonpharmacological Management of Atrial Fibrillation in Patients at High Intracranial Hemorrhage Risk. Stroke. 2018;49:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Ma T, Liu C, Jiang T, Qin H, Wu R, Zhou P. Comparative risk for intracranial hemorrhage related to new oral anticoagulants: A network meta-analysis. Medicine (Baltimore). 2021;100:e24522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Wu T, Lv C, Wu L, Chen W, Lv M, Jiang S, Zhang J. Risk of intracranial hemorrhage with direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Neurol. 2022;269:664-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Zhu W, He W, Guo L, Wang X, Hong K. The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin Cardiol. 2015;38:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 52. | Gao X, Cai X, Yang Y, Zhou Y, Zhu W. Diagnostic Accuracy of the HAS-BLED Bleeding Score in VKA- or DOAC-Treated Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8:757087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 53. | Caldeira D, Costa J, Fernandes RM, Pinto FJ, Ferreira JJ. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2014;40:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Ward R, Ponamgi S, DeSimone CV, English S, Hodge DO, Slusser JP, Graff-Radford J, Rabinstein AA, Asirvatham SJ, Holmes D Jr. Utility of HAS-BLED and CHA(2)DS(2)-VASc Scores Among Patients With Atrial Fibrillation and Imaging Evidence of Cerebral Amyloid Angiopathy. Mayo Clin Proc. 2020;95:2090-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Block F, Dafotakis M. Cerebral Amyloid Angiopathy in Stroke Medicine. Dtsch Arztebl Int. 2017;114:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Arima H, Tzourio C, Anderson C, Woodward M, Bousser MG, MacMahon S, Neal B, Chalmers J; PROGRESS Collaborative Group. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke. 2010;41:394-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 57. | Li W, Jin C, Vaidya A, Wu Y, Rexrode K, Zheng X, Gurol ME, Ma C, Wu S, Gao X. Blood Pressure Trajectories and the Risk of Intracerebral Hemorrhage and Cerebral Infarction: A Prospective Study. Hypertension. 2017;70:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 58. | Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, Rosand J. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. JAMA. 2015;314:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 59. | Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z, Wu J, Zeng J, Yao C, Huang Y; CASISP Study Group. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 1932] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 61. | Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 729] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 62. | Karam JG, Loney-Hutchinson L, McFarlane SI; Stroke Prevetion by Aggressive Reduction in Cholestrol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack: The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. J Cardiometab Syndr. 2008;3:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 63. | Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1187] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 64. | Haussen DC, Henninger N, Kumar S, Selim M. Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage. Stroke. 2012;43:2677-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Westover MB, Bianchi MT, Eckman MH, Greenberg SM. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Rudolph DA, Hald SM, García Rodríguez LA, Möller S, Hallas J, Goldstein LB, Gaist D. Association of Long-term Statin Use With the Risk of Intracerebral Hemorrhage: A Danish Nationwide Case-Control Study. Neurology. 2022;99:e711-e719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Åsberg S, Eriksson M. Statin therapy and the risk of intracerebral haemorrhage: a nationwide observational study. Int J Stroke. 2015;10 Suppl A100:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Tai SY, Lin FC, Lee CY, Chang CJ, Wu MT, Chien CY. Statin use after intracerebral hemorrhage: a 10-year nationwide cohort study. Brain Behav. 2016;6:e00487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Ibebuogu UN, Schafer JH, Schwade MJ, Waller JL, Sharma GK, Robinson VJB. Useful indices of thrombogenesis in the exclusion of intra-cardiac thrombus. Echocardiography. 2020;37:86-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Zhan Y, Joza J, Al Rawahi M, Barbosa RS, Samuel M, Bernier M, Huynh T, Thanassoulis G, Essebag V. Assessment and Management of the Left Atrial Appendage Thrombus in Patients With Nonvalvular Atrial Fibrillation. Can J Cardiol. 2018;34:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, Horton RP, Buchbinder M, Neuzil P, Gordon NT, Holmes DR Jr; PREVAIL and PROTECT AF Investigators. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017;70:2964-2975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 787] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 72. | Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P, Hendriks JML. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart. 2017;103:1947-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 73. | Hendriks JML, Tieleman RG, Vrijhoef HJM, Wijtvliet P, Gallagher C, Prins MH, Sanders P, Crijns HJGM. Integrated specialized atrial fibrillation clinics reduce all-cause mortality: post hoc analysis of a randomized clinical trial. Europace. 2019;21:1785-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet. 2017;390:1873-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 75. | Hendriks JM, Gallagher C, Middeldorp ME, Lau DH, Sanders P. Risk factor management and atrial fibrillation. Europace. 2021;23:ii52-ii60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Kozieł M, Yang PS, Guo Y, Lip GYH, Proietti M. Adherence to the 'Atrial Fibrillation Better Care' Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes-A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb Haemost. 2022;122:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 77. | Sepehri Shamloo A, Dagres N, Hindricks G. 2020 ESC guidelines on atrial fibrillation: Summary of the most relevant recommendations and innovations. Herz. 2021;46:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 500] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 79. | Kozieł M, Simovic S, Pavlovic N, Kocijancic A, Paparisto V, Music L, Trendafilova E, Dan AR, Kusljugic Z, Dan GA, Lip GYH, Potpara TS. Adherence to the ABC (Atrial fibrillation Better Care) pathway in the Balkan region: the BALKAN-AF survey. Pol Arch Intern Med. 2020;130:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Franchi C, Antoniazzi S, Proietti M, Nobili A, Mannucci PM; SIM-AF Collaborators. Appropriateness of oral anticoagulant therapy prescription and its associated factors in hospitalized older people with atrial fibrillation. Br J Clin Pharmacol. 2018;84:2010-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Ding M, Fratiglioni L, Johnell K, Fastbom J, Ljungdahl M, Qiu C. Atrial fibrillation and use of antithrombotic medications in older people: A population-based study. Int J Cardiol. 2017;249:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Ko D, Lin KJ, Bessette LG, Lee SB, Walkey AJ, Cheng S, Kim E, Glynn RJ, Kim DH. Trends in Use of Oral Anticoagulants in Older Adults With Newly Diagnosed Atrial Fibrillation, 2010-2020. JAMA Netw Open. 2022;5:e2242964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Sawadogo W, United States S-Editor: Liu H L-Editor: Kerr C P-Editor: Yuan YY