Published online Feb 26, 2024. doi: 10.4330/wjc.v16.i2.80
Peer-review started: October 10, 2023
First decision: December 18, 2023
Revised: January 2, 2024
Accepted: February 2, 2024
Article in press: February 2, 2024
Published online: February 26, 2024
Processing time: 133 Days and 9.7 Hours
Acute myocardial infarction (AMI) is a severe cardiovascular disease caused by the blockage of coronary arteries that leads to ischemic necrosis of the myocar
To construct a nomogram model for forecasting pre-hospital delay (PHD) likelihood in patients with AMI and to assess the precision of the nomogram model in predicting PHD risk.
A retrospective cohort design was employed to investigate predictive factors for PHD in patients with AMI diagnosed between January 2022 and September 2022. The study included 252 patients, with 180 randomly assigned to the development group and the remaining 72 to the validation group in a 7:3 ratio. Independent risk factors influencing PHD were identified in the development group, leading to the establishment of a nomogram model for predicting PHD in patients with AMI. The model's predictive performance was evaluated using the receiver operating characteristic curve in both the development and validation groups.
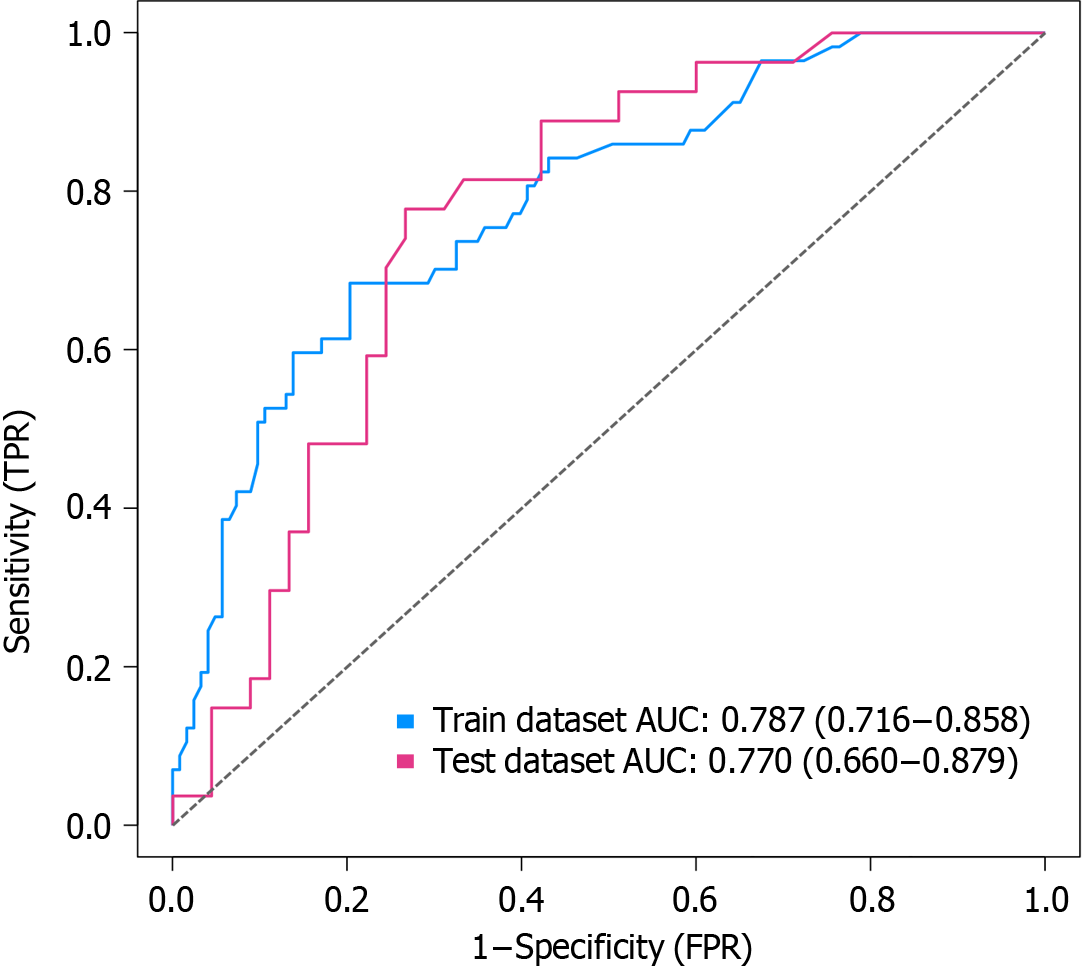
Independent risk factors for PHD in patients with AMI included living alone, hyperlipidemia, age, diabetes mellitus, and digestive system diseases (P < 0.05). A nomogram model incorporating these five predictors accurately predicted PHD occurrence. The receiver operating characteristic curve analysis indicated area under the receiver operating characteristic curve values of 0.787 (95% confidence interval: 0.716–0.858) and 0.770 (95% confidence interval: 0.660-0.879) in the development and validation groups, respectively, demonstrating the model's good discriminatory ability. The Hosmer–Lemeshow goodness-of-fit test revealed no statistically significant disparity between the anticipated and observed incidence of PHD in both development and validation cohorts (P > 0.05), indicating satisfactory model calibration.
The nomogram model, developed with independent risk factors, accurately forecasts PHD likelihood in AMI individuals, enabling efficient identification of PHD risk in these patients.
Core Tip: The study developed a nomogram model to predict pre-hospital delay (PHD) in acute myocardial infarction (AMI) patients. Independent risk factors for PHD were identified, and a nomogram was constructed using these predictors. The model showed good discriminatory ability and satisfactory calibration. This nomogram can effectively identify PHD risk in AMI patients.
- Citation: Cao JY, Zhang LX, Zhou XJ. Development and validation of a nomogram model for predicting the risk of pre-hospital delay in patients with acute myocardial infarction. World J Cardiol 2024; 16(2): 80-91
- URL: https://www.wjgnet.com/1949-8462/full/v16/i2/80.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i2.80
Acute myocardial infarction (AMI), a cardiovascular condition often stemming from coronary artery disease, arises when the abrupt blockage of the coronary artery disrupts blood circulation, leading to localized myocardial ischemic necrosis. Existing research underscores the critical impact of timely medical contact, revealing that every hour of delay increases the risk of death in patients with AMI by approximately 10%[1]. Swift restoration of blood flow in the infarct-related artery is pivotal for effective AMI treatment, with the efficacy of infarct-related artery recanalization treatment being significantly time-dependent. The shorter the duration between AMI onset and treatment, the more favorable the therapeutic outcomes[2].
The temporal aspect of AMI treatment encompasses pre-hospital delay time (PDT) and in-hospital delay time[3,4]. While measures such as optimizing hospital emergency procedures and establishing green channels have successfully reduced in-hospital delay time[5], PDT remains a challenge due to various influencing factors. Creating a universally applicable treatment protocol to minimize PDT proves challenging, given the diverse circumstances. Treating all patients with pre-hospital AMI with efficient measures poses a logistical challenge, potentially straining first aid resources and impacting the treatment of other emergency cases.
Therefore, the crux of shortening PDT lies in the identification of high-risk patients with AMI and the swift implementation of emergency measures[6]. This study aims to delve into the determinants of pre-hospital delay (PHD) in patients with AMI, with a specific focus on constructing a robust risk prediction model. The ultimate objective was to gain insights and leverage them for devising targeted and efficient strategies to mitigate PDT in patients with AMI, ensuring prompt and effective care.
This retrospective cohort study spanned from January 2022 to September 2022 and focused on patients with AMI admitted to a tertiary hospital in Anhui Province, China. The inclusion criteria mandated that patients were transported by ambulance and diagnosed with AMI based on clinical symptoms, electrocardiogram, and myocardial enzyme dynamic observation. The exclusion criteria comprised patients with missing data or mental disorders. The study received approval from the Institutional Review Board (IRB) of the research institution (ID: 2023-RE-124), and due to its retrospective nature, informed consent requirements were exempted by the IRB.
Data encompassed diverse factors such as age, sex, coronary artery lesions, Killip classification, family monthly income, body mass index, stent count, comorbidities (hypertension, cerebral infarction, hyperlipidemia, diabetes, renal insufficiency, digestive system diseases), Timi classification, medical expenses payment method, history of AMI, onset time (daytime: 8:00–17:00, nighttime: 17:01–07:59), living arrangements, work status, heart failure, education level, marital status, alcohol consumption, smoking, atrial fibrillation—totaling 26 indicators. Predefined as the interval from myocardial infarction symptom onset to seeking medical care, PDT was set at 6 h, a threshold for thrombolytic therapy[7]. Thus, patients were categorized into two groups: PDT ≤ 6 h (non-PHD) and PDT > 6 h (PHD).
Data analysis utilized Epidata 3.1 and SPSS 23.0. Quantitative variables with normal distribution were presented as mean ± standard deviation; non-normally distributed variables were denoted as M(P25, P75), signifying the median value alongside the 25th and 75th percentiles. Counting data were expressed as case numbers and percentages. Independent risk factors influencing PHD were identified through univariate and multivariate logistic regression analysis. The nomogram model was constructed using R software (version 4.0.1), and its predictive efficacy was assessed using the receiver operating characteristic (ROC) curve. The degree of calibration of the nomogram model was evaluated through calibration curve and Hosmer–Lemeshow goodness-of-fit tests. Clinical applicability was assessed through decision curve analysis (DCA). P < 0.05 was considered statistically significant.
Out of the total 252 patients with AMI, 84 (33.33%) experienced PHD. The study population was randomly divided into a development group (n = 180) and a validation group (n = 72) in a 7:3 ratio. Upon comparing demographic and clinical characteristics between the two groups, no statistically significant disparities were observed (P > 0.05), between both groups, indicating homogeneity (Table 1).
| Variable | Category | Total | Development group | Validation group | P value |
| n | 252 | 180 | 72 | ||
| Sex (%) | Male | 197 (78.2) | 142 (78.9) | 55 (76.4) | 0.664 |
| Female | 55 (21.8) | 38 (21.1) | 17 (23.6) | ||
| Age, mean (SD) | 61.09 (14.55) | 60.94 (14.82) | 61.46 (13.94) | 0.798 | |
| Killip grade (%) | 1 | 195 (77.4) | 141 (78.3) | 54 (75.0) | 0.914 |
| 2 | 42 (16.7) | 29 (16.1) | 13 (18.1) | ||
| 3 | 10 (4.0) | 7 (3.9) | 3 (4.2) | ||
| 4 | 5 (2.0) | 3 (1.7) | 2 (2.8) | ||
| Monthly household income (%) | < 3000 yuan | 125 (49.6) | 82 (45.6) | 43 (59.7) | 0.125 |
| 3000–5000 yuan | 85 (33.7) | 66 (36.7) | 19 (26.4) | ||
| > 5000 yuan | 42 (16.7) | 32 (17.8) | 10 (13.9) | ||
| Number of stents placed, mean (SD) | 1.47 (0.91) | 1.40 (0.89) | 1.64 (0.94) | 0.059 | |
| Number of coronary artery lesions, mean (SD) | 1.89 (0.79) | 1.83 (0.79) | 2.03 (0.79) | 0.078 | |
| Combined with hypertension (%) | No | 114 (45.2) | 88 (48.9) | 26 (36.1) | 0.066 |
| Yes | 138 (54.8) | 92 (51.1) | 46 (63.9) | ||
| Combined with cerebral infarction (%) | No | 227 (90.1) | 166 (92.2) | 61 (84.7) | 0.072 |
| Yes | 25 (9.9) | 14 (7.8) | 11 (15.3) | ||
| Combined with hyperlipidemia (%) | No | 214 (84.9) | 156 (86.7) | 58 (80.6) | 0.221 |
| Yes | 38 (15.1) | 24 (13.3) | 14 (19.4) | ||
| Diabetes mellitus (%) | No | 164 (65.1) | 121 (67.2) | 43 (59.7) | 0.259 |
| Yes | 88 (34.9) | 59 (32.8) | 29 (40.3) | ||
| Combined with renal insufficiency (%) | No | 227 (90.1) | 162 (90.0) | 65 (90.3) | 0.947 |
| Yes | 25 (9.9) | 18 (10.0) | 7 (9.7) | ||
| Combined digestive system diseases (%) | No | 217 (86.1) | 155 (86.1) | 62 (86.1) | 1 |
| Yes | 35 (13.9) | 25 (13.9) | 10 (13.9) | ||
| Timi classification (%) | 0 | 136 (54.0) | 100 (55.6) | 36 (50.0) | 0.491 |
| 1 | 15 (6.0) | 11 (6.1) | 4 (5.6) | ||
| 2 | 27 (10.7) | 21 (11.7) | 6 (8.3) | ||
| 3 | 74 (29.4) | 48 (26.7) | 26 (36.1) | ||
| Payment method (%) | Self-paid | 17 (6.7) | 11 (6.1) | 6 (8.3) | 0.525 |
| Medical insurance reimbursement | 235 (93.3) | 169 (93.9) | 66 (91.7) | ||
| History of acute myocardial infarction (%) | No | 242 (96.0) | 175 (97.2) | 67 (93.1) | 0.126 |
| Yes | 10 (4.0) | 5 (2.8) | 5 (6.9) | ||
| Time of onset (%) | Daytime | 110 (43.7) | 73 (40.6) | 37 (51.4) | 0.117 |
| Nighttime | 142 (56.3) | 107 (59.4) | 35 (48.6) | ||
| Living alone (%) | No | 227 (90.1) | 162 (90.0) | 65 (90.3) | 0.947 |
| Yes | 25 (9.9) | 18 (10.0) | 7 (9.7) | ||
| Type of residence (%) | Rural | 94 (37.3) | 67 (37.2) | 27 (37.5) | 0.967 |
| Town | 158 (62.7) | 113 (62.8) | 45 (62.5) | ||
| Regular jobs (%) | No | 170 (67.5) | 120 (66.7) | 50 (69.4) | 0.671 |
| Yes | 82 (32.5) | 60 (33.3) | 22 (30.6) | ||
| History of heart failure (%) | No | 227 (90.1) | 165 (91.7) | 62 (86.1) | 0.183 |
| Yes | 25 (9.9) | 15 ( 8.3) | 10 (13.9) | ||
| Education level (%) | Junior high school and below | 153 (60.7) | 108 (60.0) | 45 (62.5) | 0.321 |
| Senior high school | 45 (17.9) | 36 (20.0) | 9 (12.5) | ||
| University and above | 54 (21.4) | 36 (20.0) | 18 (25.0) | ||
| Marriage (%) | Married | 228 (90.5) | 165 (91.7) | 63 (87.5) | 0.114 |
| Divorce or widow | 20 (7.9) | 11 ( 6.1) | 9 (12.5) | ||
| Unmarried | 4 (1.6) | 4 (2.2) | 0 (0.0) | ||
| Drinking (%) | No | 177 (70.2) | 125 (69.4) | 52 (72.2) | 0.663 |
| Yes | 75 (29.8) | 55 (30.6) | 20 (27.8) | ||
| Smoking (%) | No | 133 (52.8) | 93 (51.7) | 40 (55.6) | 0.576 |
| Yes | 119 (47.2) | 87 (48.3) | 32 (44.4) | ||
| Body mass index, mean (SD) | 24.46 (3.35) | 24.54 (3.37) | 24.25 (3.33) | 0.526 | |
| Combined with atrial fibrillation (%) | No | 236 (93.7) | 169 (93.9) | 67 (93.1) | 0.806 |
| Yes | 16 (6.3) | 11 (6.1) | 5 (6.9) |
In the development group, univariate and multivariate Logistic regression methods were employed to identify risk factors associated with post-hospital discharge (PHD) in patients with AMI. Univariate analysis revealed significant associations (P < 0.05) with PHD for variables like living alone, nighttime onset, combined hypertension and hyperlipidemia, urban residence, age, combined diabetes, smoking, combined digestive system diseases, and female sex. Multivariate analysis identified living alone, hyperlipidemia, advancing age, diabetes, and digestive system diseases as independent risk factors for PHD in patients with AMI (P < 0.05), as detailed in Table 2.
| Variables | Univariate logistic regression | Multivariate logistic regression | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| BMI | 0.956 (0.867, 1.050) | 0.35 | ||
| Killip Grade 1 | Reference | |||
| Killip Grade 2 | 1.061 (0.429, 2.464) | 0.894 | ||
| Killip Grade 3 | 3.143 (0.666, 16.536) | 0.145 | ||
| Killip Grade 4 | 4.714 (0.440, 103.073) | 0.211 | ||
| Timi Grade 0 | Reference | |||
| Timi Grade 1 | 1.855 (0.501, 6.615) | 0.337 | ||
| Timi Grade 2 | 0.890 (0.294, 2.420) | 0.826 | ||
| Timi Grade 3 | 1.012 (0.474, 2.109) | 0.975 | ||
| Living alone | 3.059 (1.138, 8.476) | 0.027 | 4.654 (1.386, 16.957) | 0.015 |
| Daytime onset | Reference | |||
| Nighttime onset | 2.831 (1.434, 5.853) | 0.004 | 2.200 (0.968, 5.188) | 0.064 |
| Combined with atrial fibrillation | 1.875 (0.520, 6.497) | 0.317 | ||
| Combined with hypertension | 2.862 (1.493, 5.649) | 0.002 | 1.990 (0.906, 4.473) | 0.089 |
| Combined with hyperlipidemia | 2.467 (1.024, 5.957) | 0.042 | 3.151 (1.095, 9.373) | 0.035 |
| Have a regular job | 0.791 (0.395, 1.541) | 0.497 | ||
| Number of diseased coronary artery | 1.109 (0.743, 1.656) | 0.61 | ||
| Marital status: married | Reference | |||
| Marriage: Divorced or widowed | 0.792 (0.168, 2.864) | 0.739 | ||
| Marriage: Unmarried | 0.704 (0.034, 5.648) | 0.764 | ||
| Prior history of AMI | 3.361 (0.542, 26.068) | 0.191 | ||
| Family monthly income < 3000 yuan | Reference | |||
| The monthly income of the family = 3000–5000 yuan | 0.922 (0.449, 1.872) | 0.822 | ||
| Family monthly income > 5000 yuan | 1.560 (0.660, 3.636) | 0.304 | ||
| Type of residence: town | 0.387 (0.201, 0.737) | 0.004 | 0.576 (0.258, 1.281) | 0.175 |
| Complicated with cerebral infarction | 1.691 (0.533, 5.112) | 0.353 | ||
| Age | 1.043 (1.020, 1.069) | < 0.001 | 1.034 (1.004, 1.065) | 0.027 |
| Complicated with renal insufficiency | 1.845 (0.668, 4.959) | 0.225 | ||
| Complicated with diabetes | 3.211 (1.662, 6.278) | 0.001 | 3.208 (1.466, 7.228) | 0.004 |
| Education level is junior high school or below | Reference | |||
| High school education | 1.669 (0.766, 3.610) | 0.193 | ||
| Education level is university or above | 0.417 (0.146, 1.035) | 0.076 | ||
| Smoking | 0.404 (0.207, 0.771) | 0.007 | 0.682 (0.279, 1.652) | 0.395 |
| Complicated with digestive system diseases | 2.733 (1.154, 6.533) | 0.022 | 3.937 (1.433, 11.236) | 0.009 |
| Complicated with heart failure | 1.490 (0.478, 4.355) | 0.471 | ||
| Female sex | 2.737 (1.309, 5.753) | 0.007 | 1.419 (0.531, 3.776) | 0.482 |
| Drinking | 0.841 (0.413, 1.659) | 0.622 | ||
| Payment method: medical insurance reimbursement | 1.252 (0.347, 5.886) | 0.747 | ||
| Number of stents placed | 1.300 (0.916, 1.856) | 0.142 | ||
Construction of the nomogram prediction model for PHD risk in the development group involved utilizing the "rms" package in R software. Five predictors were identified through multivariate Logistic regression analysis, as depicted in Figure 1. The nomogram developed in this study is a valuable tool for assessing the risk of PHD in patients with AMI. The interpretation of the nomogram involves a systematic process. Each indicator in the nomogram corresponds to a specific vertical line that ascends from the respective score on the horizontal axis labeled "Points." Along each indicator's line, a specific score is assigned based on the patient's characteristics. Four specific scores from different indicators are added together to calculate the total score.
The total score is located on the horizontal axis labeled "Total Points." A vertical line is drawn downward from the total score to intersect with the axis labeled "Risk of Pre-hospital Delay." The value corresponding to the intersection point indicates the estimated risk of pre-hospital delay for the patient. As an example, consider a 65-year-old patient with the following scores: age (65 years): 60 points, hyperlipidemia: 27.5 points, digestive system diseases: 35 points, living with family (not living alone): 0 points. The total score for this patient would be 122.5 points. Locating 122.5 points on the "Total Points" axis and drawing a line downward intersects with the "Risk of Pre-hospital Delay" axis. The corresponding value on the "Risk of Pre-hospital Delay" axis (e.g., 0.67 points) indicates the estimated risk of pre-hospital delay for this patient.
To evaluate the discrimination performance, the nomogram model's area under the ROC curve was calculated. In the development group, the area under the ROC curve was 0.787 (95% confidence interval: 0.716–0.858), and in the validation group, it was 0.770 (95% confidence interval: 0.660–0.879). These results signify favorable discrimination, as illustrated in Figure 2.
The calibration curve and the Hosmer–Lemeshow goodness of fit test were used to assess the calibration degree of the nomogram model. The results from the Hosmer–Lemeshow goodness of fit test indicated no statistically significant deviation between the predicted probability of PHD from the nomogram model and the actual occurrence in both the development and validation groups (P > 0.05). This implies that the nomogram model exhibits favorable calibration, as depicted in Figure 3.
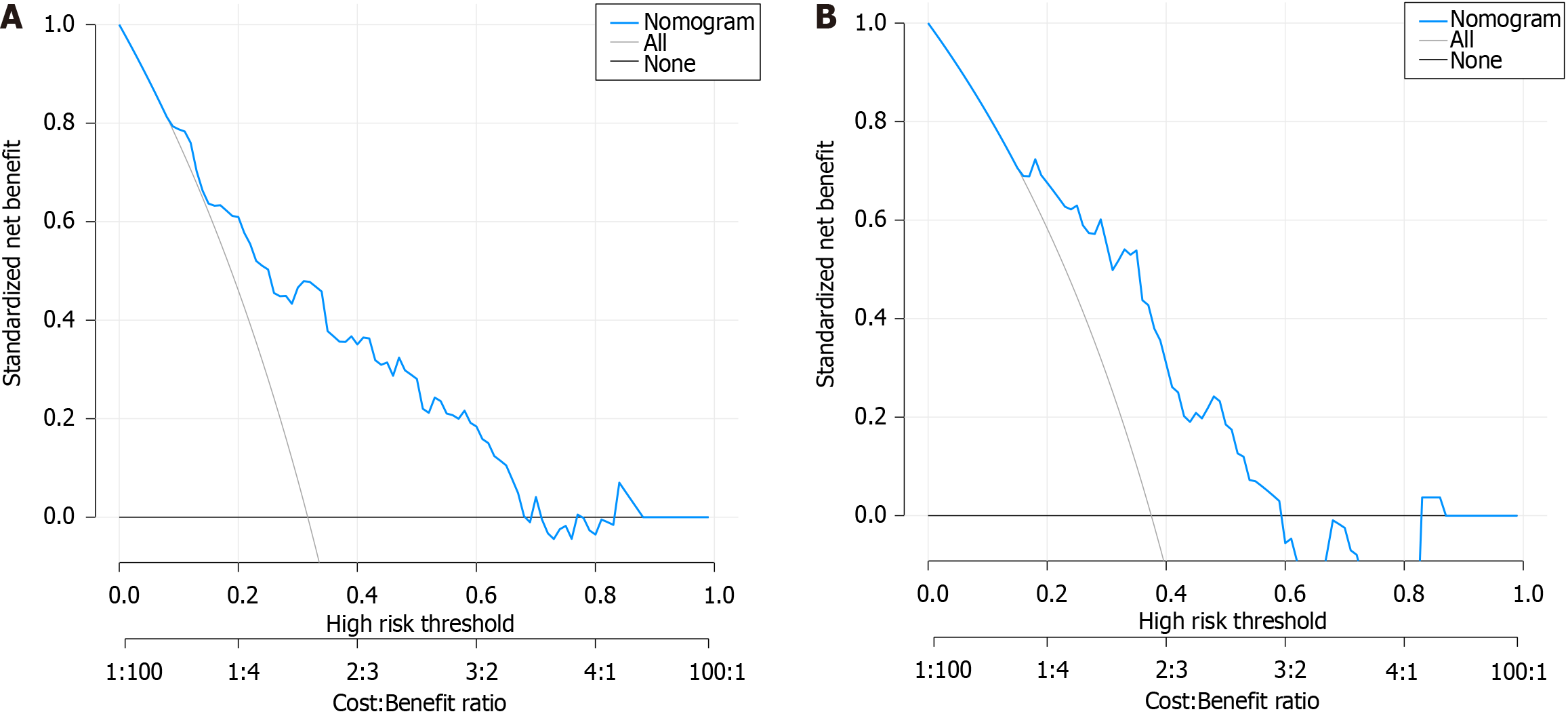
The clinical applicability of the nomogram model was assessed using the DCA curve. The DCA curve analysis indicated that the nomogram model achieved the highest clinical net rate when the threshold probability of PHD in the development group ranged from 0.09 to 0.68 and that in the validation group ranged from 0.16 to 0.59. This performance surpassed both "full intervention" and "no intervention" schemes, affirming the favorable clinical applicability of the nomogram model, as shown in Figure 4.
AMI represents a common emergency with high morbidity and mortality, particularly affecting a younger demographic, posing a substantial threat to patients' lives[8]. Studies have underscored the significance of recanalization in restoring cardiovascular supply, enhancing cardiac oxygen delivery, and improving patient prognosis[9]. However, the success of reperfusion therapy is contingent upon its timeliness; the earlier the patient reaches the hospital, the better the treatment outcomes[10]. Despite the recognized importance of prompt intervention, delays in treatment time, including PHD and in-hospital delay, contribute to a significant proportion of cases where myocardial infarction patients fail to receive timely treatment[11]. Currently, 30%-40% of patients with AMI experience PHD, presenting a challenge due to numerous interference factors that impede the development of a unified and efficient treatment process[12,13]. To address this challenge and reduce the time interval from symptom onset to percutaneous coronary intervention, it is imperative to identify individuals at high risk before discharge and administer specific interventions to enhance the efficacy of treatment for myocardial infarction. In the present study, potential risk factors influencing the occurrence of post-hospital discharge were identified through univariate analysis and subsequently adjusted using multivariate logistic regression analysis. Consequently, five distinct risk factors were identified as independent predictors of PHD in patients with AMI: advanced age, living alone, comorbidity with digestive system diseases, comorbidity with hyperlipidemia, and comorbidity with diabetes.
This study has provided evidence supporting the role of age as an independent determinant of PHD in patients with AMI, aligning with findings reported by Ouellet et al[14]. Atypical symptoms in the elderly were identified as a significant cause of PHD[15]. Older patients, often burdened with comorbidities such as hypertension, diabetes, and cerebrovascular disease, may present with atypical symptoms and symptoms of pre-infarction angina pectoris, contri
The challenges posed by China's burgeoning aging population, marked by a rising number of elderly individuals residing alone and empty nesters, necessitate innovative approaches to address healthcare concerns, particularly in cases of acute and severe illnesses[17,18]. For patients living alone, the potential delay in medical treatment due to the inability to take necessary self-help measures, especially in cases affecting their activities, poses a serious risk, with potential life-threatening consequences. In this context, the study highlights the potential utility of remote monitoring systems, such as smart watches and bracelets, to control the health of elderly individuals living alone in real-time. This technological intervention holds promise in effectively reducing the risk of PHD in patients living alone, thereby enhancing timely medical intervention and improving outcomes.
The study also brings attention to the significant impact of co-occurring digestive system diseases as an autonomous risk factor for PHD in patients with AMI. Diseases like chronic gastritis and gastrointestinal ulcers are identified for their potential to cause pain and mask symptoms of myocardial infarction[19], leading to missed treatment opportunities. To address this, the study advocates for tailored AMI health education for patients with digestive system diseases, particularly ulcer diseases. Concrete measures proposed include the preparation of a basic knowledge manual about heart disease, emphasizing causes, symptoms, and preventive measures. Additionally, the involvement of medical staff for in-home health talks, encouraging participation in mutual support groups, and imparting knowledge on developing good daily habits are suggested strategies to empower the elderly living alone with digestive system diseases to better understand and cope with heart disease.
Furthermore, the study substantiates a positive association between hyperlipidemia and the susceptibility to PHD among patients with AMI. This finding aligns with a cross-sectional investigation on PHD in patients with AMI in Saudi Arabia, as reported by Alahmadi et al[20]. Hyperlipidemia, a common cause of AMI, is elucidated as a contributor to atherosclerosis, leading to the formation of lipid plaques on arterial walls. The instability of these plaques can result in rupture and thrombus formation, culminating in the occurrence of AMI. The study underscores the multifaceted impact of hyperlipidemia on vascular function, inducing endothelial cell injury and inflammatory reactions, ultimately affecting the occurrence and prognosis of AMI and increasing the risk of PHD. Previous studies[21-23] have highlighted prolonged PDT in patients with AMI with diabetes, aligning with the current study's findings indicating that diabetes can increase the risk of PHD in patients with AMI. The study by Ängerud et al[24] emphasized a significantly longer median PDT in diabetic patients compared to non-diabetic patients (75 min), suggesting the need for targeted strategies to address the unique challenges faced by this subgroup. Additionally, the study in China proposed that the atypical presentation of chest pain symptoms in diabetic patients, with a higher proportion of NSTEMI patients, contributes to the PHD risk in this population[25].
The cumulative evidence from this study highlights the necessity for comprehensive strategies addressing the diverse risk factors influencing PHD in patients with AMI, including technological interventions, tailored education programs, and targeted management approaches for specific comorbidities. The integration of clinical prediction models into medical practice, particularly nomograms, has become pivotal in risk and benefit assessments, providing a more objective and precise means of information acquisition[26,27]. Within cardiovascular research, nomograms have proven effective in predicting various outcomes, ranging from in-hospital mortality to the risk of specific complications post-diagnosis[28-31]. Despite these advancements, there exists a notable gap in literature pertaining to nomogram studies specifically examining the risk of post-hospital discharge in patients with AMI. Addressing this void, our study utilized univariate and multivariate Logistic regression to identify independent risk factors for PHD in patients with AMI, culminating in the development of a nomogram model tailored to predict this risk. Notably, our findings underscore the nomogram model's favorable calibration, discrimination, and clinical applicability, as evidenced by the Hosmer-Lemeshow goodness of fit test, ROC curve analysis, and DCA curve.
The nomogram model developed in this study fills a crucial gap in predicting the risk of PHD in patients with AMI, providing a valuable tool for rapid risk assessment and more targeted treatment strategies. By identifying high-risk patients, the nomogram facilitates swift and effective intervention, ultimately enhancing patient prognosis.
However, it is imperative to acknowledge certain limitations inherent in this study. The retrospective nature of the research introduces potential issues such as incomplete data, low data quality, and case selection bias, which may impact result accuracy. Additionally, the study's reliance on data from a single medical institution raises concerns about its representativeness for broader populations. The limited sample size and consideration of only a subset of predictors further necessitate caution in generalizing the results. Future endeavors should prioritize large-scale, multi-center, and multi-regional studies to enhance result representativeness and generalizability.
In conclusion, this study, despite its limitations, successfully identified five independent risk factors associated with PHD in patients with AMI. The subsequent construction of a nomogram model exhibited robust predictive value, offering valuable insights for pre-hospital treatment strategies in patients with AMI and mitigating the risk of PHD. The results emphasize the significance of incorporating nomograms into clinical practice for enhanced risk assessment and tailored interventions in the context of AMI.
Acute myocardial infarction (AMI), a lethal heart condition, results from coronary artery blockages that cause myocardial ischemia and necrosis. Treatment delays heighten death risks, making prompt medical response critical. This study focuses on reducing pre-hospital delays by identifying high-risk AMI patients, developing a risk prediction model, and implementing tailored strategies for timely care.
The timely management of AMI is crucial for improving patient outcomes, yet pre-hospital delay time (PDT) poses a significant challenge, leading to increased morbidity and mortality rates. This research is motivated by the need to understand the determinants of PDT in AMI patients and develop a robust risk prediction model. By identifying high-risk individuals and implementing targeted strategies to reduce PDT, this study aims to enhance the delivery of prompt and effective care. Its significance lies in addressing a critical knowledge gap in cardiovascular medicine and offering practical solutions to optimize AMI treatment outcomes for future research in this field.
The main objective is to investigate determinants of pre-hospital delay (PHD) in AMI patients and construct a risk prediction model. Realizing these objectives has significant implications for future research in this field, allowing refinement of models, development of evidence-based guidelines, and optimization of AMI treatment strategies for improved patient outcomes.
This retrospective cohort study investigated determinants of PHD in AMI patients and developed a risk prediction model. Data on 26 indicators were collected from AMI patients admitted to a tertiary hospital in Anhui Province, China. Statistical analysis involved logistic regression, nomogram modeling, receiver operating characteristic curve analysis, calibration tests, and decision curve analysis. The study contributes to advancing AMI management research.
This study identified risk factors for post-hospital discharge in acute myocardial infarction patients. Living alone, hyperlipidemia, age, diabetes, and digestive system diseases were significant predictors. A nomogram model accurately predicted the risk of post-hospital discharge. This model can help healthcare professionals identify high-risk patients and provide targeted interventions, but further validation is needed in larger populations.
This study concludes that the newly developed nomogram model, incorporating independent risk factors, accurately predicts the likelihood of post-hospital discharge in acute myocardial infarction patients. This model offers a valuable tool for efficiently identifying individuals at risk of post-hospital discharge, providing potential benefits for targeted interventions and improved patient outcomes in clinical practice.
Future research should address limitations of the retrospective design, limited sample size, and subset of predictors. Large-scale, multi-center studies with comprehensive data are needed to enhance generalizability. Exploring additional risk factors and refining predictive models can improve accuracy for forecasting post-hospital discharge outcomes in acute myocardial infarction patients, benefiting clinical decision-making.
| 1. | Beaudoin FL, Straube S, Lopez J, Mello MJ, Baird J. Prescription opioid misuse among ED patients discharged with opioids. Am J Emerg Med. 2014;32:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Xu WH, Liu XM. Influencing factors and coping strategies of prehospital delay in patients with acute myocardial infarction. Cardio-cerebrovascular Disease Prevention and Treatment. 2022;22:79-83. [DOI] [Full Text] |
| 3. | Du LF, Lian HW, Ma QB. Distribution of delay time of emergency percutaneous coronary intervention in the patients with ST-segment elevation myocardial infarction. Chinese J Emerg Med. 2016;36:146-149. [DOI] [Full Text] |
| 4. | Fu R, Song CX, Dou KF, Yang JG, Xu HY, Gao XJ, Liu QQ, Xu H, Yang YJ. Differences in symptoms and pre-hospital delay among acute myocardial infarction patients according to ST-segment elevation on electrocardiogram: an analysis of China Acute Myocardial Infarction (CAMI) registry. Chin Med J (Engl). 2019;132:519-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Imamura T. Optimal risk stratification and therapeutic strategy for acute myocardial infarction. Clin Cardiol. 2021;44:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Gebhard CE, Gebhard C, Maafi F, Bertrand MJ, Stähli BE, Maredziak M, Bengs S, Haider A, Zhang ZW, Smith DC, Ly HQ. Impact of summer season on pre-hospital time delays in women and men undergoing primary percutaneous coronary intervention. Sci Total Environ. 2019;656:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Fang XY, Albarqouni L, von Eisenhart Rothe AF, Hoschar S, Ronel J, Ladwig KH. Is denial a maladaptive coping mechanism which prolongs pre-hospital delay in patients with ST-segment elevation myocardial infarction? J Psychosom Res. 2016;91:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Wang HJ, Dou KF. Consensus of China experts on the evaluation index system of medical quality in adults with acute ST-segment elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:849-856. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Chen GY, Li KL, Tan JM. Clinical study of recombinant human pro-urokinase for injection in the treatment of acute ST-segment elevation myocardial infarction. Medical Innovation of China. 2017;14:130-132. [DOI] [Full Text] |
| 10. | Tarantini G, Van de Werf F, Bilato C, Gersh B. Primary percutaneous coronary intervention for acute myocardial infarction: Is it worth the wait? The risk-time relationship and the need to quantify the impact of delay. Am Heart J. 2011;161:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Tang YF, Wang XH, Zhang P, Cao X, Yang HH. Risk factors and assessment tools for delay in seeking medical care in patients with acute coronary syndrome. PLA Journal of Nursing. 2015;32:25-28. [DOI] [Full Text] |
| 12. | Guo JJ, Qin LJ, Zang ST, Ren Y. Research progress on influencing factors and intervention measures of prehospital delay in patients with acute myocardial infarction. Chinese J Emerg Med. 2020;29:1498-1502. [DOI] [Full Text] |
| 13. | Xia F, Ding N, Miao ZL, Yang D, Han Y. Effect of prehospital delay on cardiac function in patients with acute anterior myocardial infarction undergoing emergency percutaneous coronary intervention. The Journal of Practical Medicine. 2021;37:1824-1826. [DOI] [Full Text] |
| 14. | Ouellet GM, Geda M, Murphy TE, Tsang S, Tinetti ME, Chaudhry SI. Prehospital Delay in Older Adults with Acute Myocardial Infarction: The ComprehenSIVe Evaluation of Risk Factors in Older Patients with Acute Myocardial Infarction Study. J Am Geriatr Soc. 2017;65:2391-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Lee SH, Kim HK, Jeong MH, Lee JM, Gwon HC, Chae SC, Seong IW, Park JS, Chae JK, Hur SH, Cha KS, Kim HS, Seung KB, Rha SW, Ahn TH, Kim CJ, Hwang JY, Choi DJ, Yoon J, Joo SJ, Hwang KK, Kim DI, Oh SK; KAMIR Investigators. Pre-hospital delay and emergency medical services in acute myocardial infarction. Korean J Intern Med. 2020;35:119-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Liu DX, Gao P, Duan YW, Wang YQ, Yin NN, Han YP. Analysis of influencing factors of delay in hospitalization in patients with acute ST segment elevation myocardial infarction. Hebei Pharmaceutical. 2021;43:264-266. [DOI] [Full Text] |
| 17. | Xiang YB, Yin LX, Zuo ML, Shuai P, Zou JL. Effects of living alone and co-living on cardiovascular disease indexes in 829 elderly people aged 60 years and over in Sichuan Province. Occupational Health and Injury. 2019;34:70-74. |
| 18. | Wu HX, Ye Q. Causative analysis and nursing strategy of pre-hospital delayed treatment in elderly patients with acute myocardial infarction. General Nursing. 2017;15:2113-2115. [DOI] [Full Text] |
| 19. | Yan WJ. Misdiagnosis of acute myocardial infarction with atypical initial symptoms. Clinical Misdiagnosis & Mistherapy. 2017;30:28-32. [DOI] [Full Text] |
| 20. | Alahmadi AF, ALSaedi MF, Alahmadi AE, Alharbi MG, Alharbi IH, Radman Al-Dubai SA. Pre-hospital delay among patients with acute myocardial infarction in Saudi Arabia. A cross-sectional study. Saudi Med J. 2020;41:819-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Wah W, Pek PP, Ho AF, Fook-Chong S, Zheng H, Loy EY, Chua TS, Koh TH, Chow KY, Earnest A, Pang J, Ong ME. Symptom-to-door delay among patients with ST-segment elevation myocardial infarction in Singapore. Emerg Med Australas. 2017;29:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Peng YG, Feng JJ, Guo LF, Li N, Liu WH, Li GJ, Hao G, Zu XL. Factors associated with prehospital delay in patients with ST-segment elevation acute myocardial infarction in China. Am J Emerg Med. 2014;32:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Li PW, Yu DS. Predictors of pre-hospital delay in Hong Kong Chinese patients with acute myocardial infarction. Eur J Cardiovasc Nurs. 2018;17:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ängerud KH, Thylén I, Sederholm Lawesson S, Eliasson M, Näslund U, Brulin C; SymTime Study Group. Symptoms and delay times during myocardial infarction in 694 patients with and without diabetes; an explorative cross-sectional study. BMC Cardiovasc Disord. 2016;16:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Fu R, Li SD, Song CX, Yang JA, Xu HY, Gao XJ, Xu Y, Zeng JP, Li JN, Dou KF, Yang YJ. Clinical significance of diabetes on symptom and patient delay among patients with acute myocardial infarction-an analysis from China Acute Myocardial Infarction (CAMI) registry. J Geriatr Cardiol. 2019;16:395-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Kurman RJ, Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 215] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Pan X, Yang W, Chen Y, Tong L, Li C, Li H. Nomogram for predicting the overall survival of patients with inflammatory breast cancer: A SEER-based study. Breast. 2019;47:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Jia J, Tao SM. Risk prediction model of in-hospital mortality in critically ill patients with acute ST-segment elevation myocardial infarction based on plasma osmolality. Journal of Kunming Medical University. 2022;43:58-65. [DOI] [Full Text] |
| 29. | Zhou CL, Jin XY, Zhao J, Ning HG, Zhang XM, Ren C, Liu LM, Zhou LH, Liu LN. Study on nomogram prediction model of heart failure after PCI in patients with acute myocardial infarction. Journal of Cardiovascular Rehabilitation Medicine. 2022;31:586-590. [DOI] [Full Text] |
| 30. | Wei RJ. Construction of risk prediction model for malignant ventricular rhythm in early acute myocardial infarction. Guilin Medical College. 2022:12-15. |
| 31. | Zhang BB, He T, Wu N, Ren YQ, Zhang JY, Jiang WJ, Li BG. Risk factors of new onset atrial fibrillation in patients with acute myocardial infarction and construction of nomogram model for risk prediction. Journal of Practical Cardio-cerebral-pulmonary Vascular Diseases. 2021;29:14-18. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bloomfield D, United States S-Editor: Liu JH L-Editor: A P-Editor: Zhao S