Published online Dec 26, 2024. doi: 10.4330/wjc.v16.i12.689
Revised: October 4, 2024
Accepted: November 1, 2024
Published online: December 26, 2024
Processing time: 174 Days and 5.4 Hours
The maintenance of intracellular and extracellular adenosine triphosphate (ATP) levels plays a pivotal role in cardiac function. In recent years, burgeoning at
Core Tip: Understanding the mechanisms behind adenosine triphosphate (ATP)-induced cell death (AICD) is crucial for addressing various cardiovascular diseases. AICD, triggered by elevated extracellular ATP levels, differs from other forms of cell death and has emerged as a significant contributor to conditions such as myocardial ischemia-reperfusion injury, sepsis-induced cardiomyopathy, and diabetic cardiomyopathy. This review explores the physiological roles of ATP in the cardiovascular system and delves into the molecular and metabolic mechanisms underlying AICD. Identifying therapeutic targets to mitigate AICD holds promise for treating cardiovascular diseases, although challenges remain. This review provides valuable insights and strategies for preventing and managing cardiovascular disorders.
- Citation: Wang W, Wang XM, Zhang HL, Zhao R, Wang Y, Zhang HL, Song ZJ. Molecular and metabolic landscape of adenosine triphosphate-induced cell death in cardiovascular disease. World J Cardiol 2024; 16(12): 689-706
- URL: https://www.wjgnet.com/1949-8462/full/v16/i12/689.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i12.689
Over the past decade, the Committee on Cell Death Nomenclature has diligently crafted a comprehensive delineation of cell demise, integrating various perspectives encompassing morphology, biochemistry, and functionality[1]. Studies have made novel insights into the mechanisms governing diverse cell death mechanisms. Research has elucidated the intricate interplay of apoptosis, necrotic apoptosis, pyroptosis, and apoptosis in the etiology of cardiovascular disorders[2]. Adenosine triphosphate (ATP) serves as a multifaceted signaling molecule within cells, as it assumes a pivotal role in cellular energy metabolism. There has been a recent surge in investigations delving into ATP-induced cell death (AICD). AICD represents a distinct mode of cellular demise elicited by heightened extracellular ATP (eATP) levels, distinguishing it from conventional forms of cell death like apoptosis and necrosis. Nonetheless, the specific methods and modalities behind AICD continue to be unresolved [3].
Intracellular ATP typically maintains a delicate equilibrium, serving as a pivotal currency for energy transfer, signaling cascades, and cellular metabolism. Both external stimuli or internal insults can perturb this balance, leading to disruptions in intracellular ATP homeostasis, eATP release, and ultimately cellular demise[2]. Concurrently, AICD causes the release of inflammatory mediators, inducing local or systemic inflammatory cascades and causing metabolic dysregulation. Among the myriad metabolic alterations observed in cardiovascular diseases, lipid metabolism disorders prominently stand out [4]. Additionally, lipid metabolism contributes to the deposition of heat-sensitive proteins during disease onset, underscoring the intricate interplay between lipid metabolism and thermal protein deposition. Never
During AICD, alterations in phospholipid distribution across the cell membrane are observed alongside disruptions in ATP homeostasis, culminating in membrane destabilization and rupture. This phenomenon is intricately linked to cellular damage and inflammation in cardiovascular pathologies[7]. Investigations have elucidated the mechanism underlying ATP-mediated T cell demise through P2X7 receptor (P2X7R) activation[8]. Consequently, P2X7R expression emerges as a pivotal determinant of AICD, not only offering insights into the immunomodulatory mechanisms underlying cardiovascular diseases but also presenting novel avenues for therapeutic intervention[9,10].
To date, substantial evidence underscores the intricate association between AICD and cardiovascular disease pathogenesis, implicating inflammatory responses, cellular damage, and immune dysregulation as pivotal mediators. In this comprehensive review, we elucidate the intricate regulation of ATP homeostasis and delineate the underlying mechanisms of lipid metabolism. Moreover, we delve into the progression of AICD in cardiovascular pathologies and explore its potential implications in the context of arrhythmias.
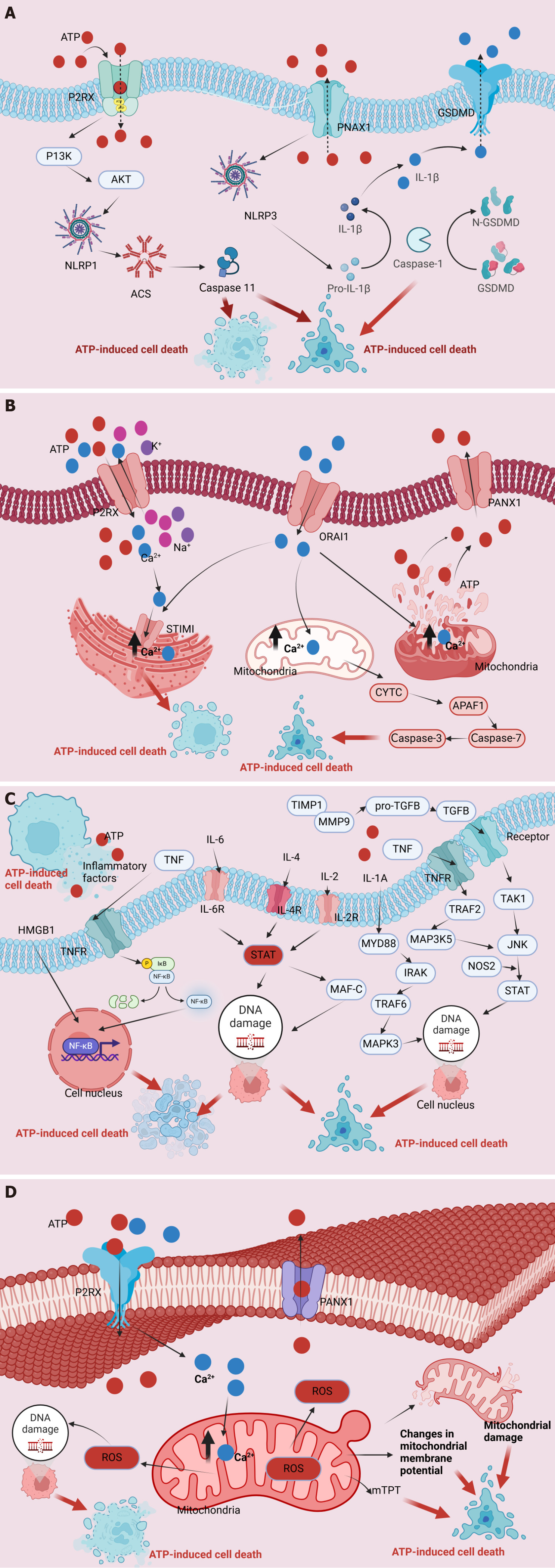
As a multifaceted signaling molecule, ATP orchestrates pivotal biological activities within cellular microenvironments, including metabolic processes, signal transduction cascades, and energy transfer. The intricate balance of intracellular ATP levels, termed ATP homeostasis, is meticulously maintained through the interplay of synthesis and utilization processes within cells. However, this equilibrium can be disrupted following internal injuries or external stimuli, leading to elevated extracellular ATP (eATP) levels and subsequent intracellular ATP release[11]. This perturbation results in AICD, mediated by several well-elucidated mechanisms and regulatory pathways. ATP primarily engages with extracellular P2R proteins, particularly the P2X7R family, triggering a cascade of events that includes the activation of associated receptors such as NOD-like receptor family pyrin domain-containing protein 1 (NLRP1) and NLRP3. This activation cascade meticulously coordinates apoptotic signals, encompassing caspases-1, -3, and -11, while also implicating necrotic effectors, such as gasdermin E and gasdermin D, ultimately leading to cellular demise[2]. Secondarily, ATP’s interaction with ion channels on the cell membrane modulates ion balance, notably through P2X7R activation-induced opening of ion channels, leading to intracellular calcium ion (Ca2+) accumulation within various cellular compartments, including the Golgi apparatus and mitochondria. This aberrant Ca2+ influx induces nuclear DNA damage, precipitating cellular demise. Additionally, ATP triggers mitochondrial dysfunction, evident in the loss of mitochondrial membrane potential, disruption of the mitochondrial respiratory chain, production of reactive oxygen species (ROS), and the disruption of mitochondrial membrane permeability. These aberrations culminate in cellular demise. Moreover, ATP induces immune-inflammatory responses and cell death pathways, leading to the release of inflammatory mediators such as interleukin (IL)-1β, IL-18, tumor necrosis factor (TNF)-α, IL-2, IL-4, IL-6, IL-10, C-C chemokine ligand 5, and CXC motif chemokine ligand 2, ultimately driving cell death (Figure 1)[12].
Upon external stimuli or internal injury, elevated eATP levels and intracellular ATP release induces cell demise. ATP induces cellular demise by inducing mitochondrial membrane potential loss through membrane K+/Na+ imbalance, mitochondrial respiratory chain disruption, ROS production, and linear membrane permeability alterations. As a result, it coordinates immune-inflammatory reactions, initiates cell death pathways and AICD, leading to the secretion of inflammatory mediators including IL-1β, IL-18, TNF-α, IL-2, IL-4, IL-6, IL-10, C-C chemokine ligand 5, and CXC motif chemokine ligand 2, ultimately resulting in cellular demise[2]. Additionally, P2 receptor-mediated ATP exerts an anti-apoptotic effect, involving pathways such as phosphoinositide 3-kinase, extracellular signal regulated kinase 1 and 2, mitoKATP, and nitric oxide synthase pathway[13]. Moreover, the production of ROS and oxidative stress serve as central mechanisms responsible for cellular damage and dysfunction. Sirtuin 6 (SIRT6), a member of the sirtuin family of NAD+-dependent class III deacetylases, holds a pivotal role in resisting oxidative stress. SIRT6 upregulates AMP/ATP levels and activates the adenosine 5’-monophosphate-activated protein kinase (AMPK)-forkhead box O3α (FoxO3α) axis, triggering the expression of downstream antioxidant genes, such as manganese superoxide dismutase and catalase. This process alleviates intracellular oxidative stress and confers protection against ischemic heart injury[14]. Furthermore, myocardial ischemia-reperfusion injury (IRI) involves multiple mechanisms, including ROS production, changes in cellular osmotic pressure, and inflammatory reactions. Calcium overload, oxygen level fluctuations, and mitochondrial ROS are major contributors to the irreversible opening of the mitochondrial permeability transition pore (mPTP). These processes are intricately associated with NLRP3 inflammasome activation, governing the maturation and secretion of IL-1β and IL-18[15]. Consequently, upregulation of the caspase-1 pathway and IL-18 release further exacerbates cell death. Moreover, endothelial dysfunction occurs regardless of myocardial IRI presence, resulting from oxygen level fluctuations, reduced nitric oxide production, and excessive ROS generation. This ultimately leads to the expression of adhesion molecules and leukocyte infiltration. The central role of the NLRP3 inflammasome in modulating coronary blood flow alterations via endothelial dysfunction underscores its significance in ischemic heart disease pathology[16].
Additionally, ATP interacts with peripheral purine type 2 receptors, specifically P2X7R, while simultaneously activating associated receptors, such as NLRP1 and NLRP3. This activation triggers apoptotic signals involving caspase-1, caspase-3, and caspase-11, and involves necrotic proteins like gasdermin E and gasdermin D[2]. Caspase-1 has emerged as a molecular target with the potential to impede cardiovascular disease progression, notably heart failure (HF), owing to its pivotal role in fostering inflammation and cardiomyocyte loss. Studies suggest that left ventricular assist device implantation modulates caspase-1 expression levels, thus altering inflammatory and apoptotic aspects of the heart. Inflammation appears pivotal in modulating caspase-1 signaling and its downstream effects, including apoptosis. However, caspase-1 deficiency exacerbates myocardial hypertrophy in renal ischemia-reperfusion mouse models[17,18]. Additionally, inflammation assumes a crucial role in HF onset, progression, and prognosis. The NLRP3 inflammatory complex serves as a pivotal hub in chronic inflammatory responses, fostering the generation of pro-inflammatory cytokines IL-1β and IL-18, thereby exacerbating inflammation. Thus, inhibition of downstream factors of the NLRP3 inflammatory complex and its signaling pathway holds promise as a novel intervention strategy for HF treatment[19].
However, pharmacological inhibition of eATP or genetic ablation of P2X7Rs disrupts the function of the myocardial NLRP3 inflammatory complex during stress overload, highlighting the pivotal role of the ATP/P2X7 axis in cardiac inflammation and hypertrophy. eATP induces hypertrophic alterations in cardiomyocytes via an NLRP3- and IL-1β-dependent mechanism. Research on the sympathetic nervous system indicates that sympathetic efferent nerves are the main source of eATP. The depletion of ATP released by sympathetic efferent nerves and the elimination of cardiac afferent nerves or lipophilic β receptors lead to reduced cardiac eATP levels, subsequently inhibiting the activation of the NLRP3 inflammatory complex, IL-1β production, and adaptive myocardial hypertrophy in response to pressure overload[20].
Moreover, the chloride/bicarbonate ion exchangers AE1, AE2, and AE3 are integral membrane proteins involved in pH regulation across vertebrate tissues, modulated by neurohormonal regulation. Co-expression of AE1 and AE3 in cardiomyocytes facilitates purine agonist ATP-induced cation exchange. ATP stimulates the phosphorylation of tyrosine residues on AE1, leading to the activation of Fyn tyrosine kinase and the binding of Fyn and FAK to AE1. Inhibiting Src-family kinases in vivo using compounds like genistein, herbimycin A, or ST638 effectively blocks ATP-triggered AE1 activation. Microinjection of anti-C-terminal Src kinase 1 antibodies or recombinant C-terminal Src kinase, which inhibits Src-family kinase activation, significantly reduces ATP-induced AE1 activation. Moreover, microinjection of anti-FAK antibodies and expression of Phe397 FAK dominant negative mutants in cardiomyocytes impede purine-induced AE1 activation. As a result, tyrosine kinases have emerged as crucial regulators in the acute modulation of intracellular pH and cellular function, particularly in the excitation-contraction coupling of cardiomyocytes[21]. Mild mitochondrial uncoupling in cardiomyocytes triggered by uncoupling agents prompts signal transducer and activator of transcription 3 (STAT3) activation and ATP upregulation. However, excessive mitochondrial uncoupling results in STAT3 inhibition, ATP depletion, and subsequent cellular damage. The development of mitochondrial uncoupling agents with a precisely calibrated dose window that induces mild uncoupling represents a promising approach for enhancing cardiac protection[22].
The human heart relies on a diverse range of energy substrates to maintain its normal contractile function. Under physiological conditions, glucose and long-chain fatty acids (FAs) serve as the primary substrates involved in cardiometabolic processes. However, during stress, there is a shift in substrate preference towards glucose or FAs, which has been implicated in heart disease[23,24]. Research indicates that the pannexin-1 channel is responsible for releasing ATP, subsequently activating fibroblasts within the heart[25]. When cardiac fibroblasts are exposed to ATP or its non-hydrolyzed analog benzoyl ATP, they undergo apoptosis. Similarly, TNF-α, a cytokine linked to the advancement of chronic HF, exacerbates cell death. Similar effects were observed in a murine cardiac muscle cell line, where TNF-α counteracted the decrease in P2X(6) mRNA expression typically seen with prolonged exposure to agonists. This indicates that TNF-α disrupts a protective mechanism intended to prevent calcium overload and eventual calcium-dependent cell death by inhibiting ATP-induced P2X6 desensitization[26]. Moreover, stromal interaction molecule 1, a well-known calcium detector within the endoplasmic reticulum calcium reservoir, is increasingly acknowledged as a crucial factor in regulating cardiac hypertrophy and diabetic cardiomyopathy[27,28]. Consequently, a range of proteins involved in regulating cellular ATP homeostasis play crucial roles in AICD[16-20,23-26,29-58] (Table 1).
| Gene | Function | Role in AICD | Effects of genetic deletion or overexpression | Ref. |
| P2RX7 | Inflammation and immune regulation, neurotransmission, apoptosis and autophagy | Activates inflammatory mediators and increases calcium ions | Its activation is closely related to the development of cardiac diseases such as cardiomyopathy, myocardial infarction and myocarditis | [29] |
| CASP3 | Execution stage of apoptosis | CASP3 cleavage by CASP1/4/5/11 forms pores, releasing proinflammatory cytokines | Caspase contributes to the progressive decline in systolic function observed in heart failure by facilitating the degradation of myofibrillar protein. Therefore, the selective inhibition of CASP3’s proteolytic function may offer a promising strategy for mitigating or reversing the effects of heart failure | [30] |
| PANX1 | Widely involved in ATP and ion permeability, can effectively reduce CCI induced mechanical pain and thermal hyperalgesia | P2X7 activation opens PANX1 channels, releasing ATP and triggering cell death pathways | PANX1 channels release ATP, which then activates fibroblasts in the heart and promotes the development of cardiac fibrosis after myocardial infarction. PANX1 deficiency results in atrioventricular block, delayed ventricular depolarization, significantly prolonged QT interval and rate-corrected QT interval, and an increased incidence of atrial fibrillation following intraatrial burst stimulation | [25,31] |
| NLRP3 | It plays an important role in inflammation and immune responses and can sense various stimuli inside and outside the cell | Upon activation by stimulatory signals, NLRP3 forms an inflammasome, triggering CASP1 activation. This in turn leads to the release of cytokines and apoptosis | Involved in the process of ischemia-reperfusion injury and endothelial dysfunction, affecting the changes of coronary blood flow; participate in chronic inflammatory response and myocardial hypertrophy, accelerate the production of pro-inflammatory cytokines, leading to the occurrence and development of heart failure | [16,19,20] |
| CASP1 | Membrane hyperpolarization; mitochondrial depolarization and positive regulation of IL-1α production | CASP1 triggers the processing of cytokines, pyrosis, and inflammation, thereby orchestrating the inflammatory response | Involved in inflammation and loss of heart muscle cells. LVAD implantation may alter the inflammatory and apoptotic characteristics of the heart by regulating CASP1 expression levels. CASP1 deficiency resulted in more obvious myocardial hypertrophy in renal ischemia-reperfusion mice | [17,18] |
| P2RY1 | Activates downstream signals | P2RY1 has the capacity to elevate calcium ion levels within the Golgi apparatus | P2RY1 gene is associated with the development of heart disease and the response to anticoagulant therapy. Meanwhile, the polymorphism of P2RY1 gene is associated with the onset age of myocardial infarction, which may have a protective effect or influence the progression of myocardial infarction | [32] |
| P2RY11 | Immune regulation, neurotransmission, insulin secretion | It plays a role in immune inflammatory mechanisms | The P2RY11 gene is implicated in the regulation and repair of inflammatory processes in the heart. Enhanced expression of this gene may facilitate myocardial fibrosis and play a crucial role in the restoration of cardiac function following acute myocardial infarction | [20] |
| ORAI1 | Calcium ion coupling is involved in the activation and proliferation of immune cells | Increased intracellular calcium ions | The ORAI1 gene plays an important role in the heart, especially in cardiac diseases such as cardiac hypertrophy and heart failure, and is involved in regulating the flow of calcium ions in cardiomyocytes, affecting the systolic and diastolic functions of the heart | [33] |
| STIM1 | Calcium ion sensor. It is involved in immune cell activation, muscle contraction and cell cycle regulation | STIM1 responds to ATP-induced calcium influx by activating ORAI1, thereby contributing to cell death | STIM1 plays a pivotal role in regulating SOCE and Ca2+ storage replenishment, crucial for heart development and growth. Additionally, the STIM1 gene modulates energy substrate preferences in the heart, with implications for metabolic disorders like cardiac hypertrophy and diabetic cardiomyopathy. Elucidating its molecular mechanisms could lead to the discovery of novel therapeutic targets for the prevention and treatment of cardiac metabolic diseases | [23,24] |
| CASP8 | Modulating apoptosis | CASP8 causes apoptosis | It is involved in apoptosis and cytokine processing and is crucial for heart development and hematopoietic function. Lack of CASP8 leads to defects in heart muscle development and a decrease in hematopoietic progenitor cells | [34] |
| CASP9 | Modulating apoptosis (programmed cell death) | CASP9 causes apoptosis | The CASP9 gene is involved in mitochondria-mediated apoptosis in the heart. As an inhibitor of CASP9, HAX-1 protein protects cardiomyocytes from apoptosis and maintains cardiac function | [35] |
| CASP7 | The executive stage of catalytic apoptosis | CASP7 causes apoptosis | Inhibition of CASP7 can reduce myocardial infarction size and apoptosis, providing a new strategy for the treatment of myocardial ischemia | [36] |
| P2RX3 | Involved in the conduction of sensory neurons and the perception of pain | NA | It is involved in pain signal transduction caused by myocardial ischemia and is a potential therapeutic target | [37,38] |
| NLRP1 | Regulates inflammation and immune response | Upon activation, NLRP1 triggers CASP1 activation, leading to the induction of pyroptosis and the release of IL-1β and IL-18 | NLRP1 gene is closely related to cardiovascular diseases. The NLRP1 inflammatory complex expressed by NLRP1 gene is involved in the pathogenesis of cardiovascular diseases and may be a potential therapeutic target | [39] |
| P2RX4 | Involved in cellular signaling | P2RX4 promotes AICD (pyroptosis) through the activation of the NLRP3 inflammasome, resulting in the production of IL-1β and IL-18 | The P2RX4 gene in the heart may influence blood pressure and kidney function by regulating vascular tension | [40] |
| P2RX5 | Involved in neurotransmission and pain regulation | NA | P2RX5 gene may be related to varicose veins and synaptic vesicles in the heart, and it is involved in cardiac development and functional regulation | [41] |
| SAPK | Involved in cellular stress response and inflammation regulation | ATP triggers cell death through SAPK pathways, modulating apoptosis, necrosis, and stress signaling mechanisms | It plays a role in regulating cardiomyocyte hypertrophy and apoptosis. MiR-350 induces cardiomyocyte hypertrophy by inhibiting the SAPK pathway, suggesting that the SAPK gene is a key regulator of pathologic heart hypertrophy and apoptosis | [42] |
| p38 MAPK | It is involved in cell signaling, cell stress response, inflammation regulation, apoptosis and other biological processes | ATP stimulates p38MAPK, ultimately leading to cell death via apoptosis and necrosis | It is involved in the regulation of cardiomyocyte proliferation, apoptosis and hypertrophy. Involved in the regulation of stress response and cardiomyocyte differentiation, its balance in terms of protective and deleterious effects affects cardiac function | [43] |
| ASK1 | It regulates biological processes such as cell survival and death, inflammatory response, cell stress response, and oxidative stress | Elevated levels of ATP trigger cellular stress, activating ASK1 and subsequent downstream pathways, ultimately leading to cell death | ASK1 activation can lead to hypertrophy, fibrosis and dysfunction of the heart | [44] |
| NOX2 | It plays a crucial role in the generation of reactive oxygen species within cells, thereby regulating physiological processes including cell signaling, immune response, and oxidative stress | ATP stimulates NOX2 activation, leading to ROS production, which induces oxidative stress and potentially triggers cell death | Increased NOX2 activity may lead to diaphragmatic dysfunction, which can trigger symptoms of heart failure | [45] |
| Bax | It is involved in regulating biological processes such as cell development, immune response and tumor suppression | Elevated levels of ATP trigger Bax activation, resulting in mitochondrial dysfunction and apoptotic cell death | It is involved in the process of myocardial apoptosis induced by ischemia | [46] |
| MLC | It plays a pivotal role in regulating muscle contraction and movement, thereby influencing biological processes including cell morphology and motility | Depletion of ATP impairs muscle contraction by compromising myosin function and cellular viability | Reduced MLC expression is associated with the pathogenesis of heart failure | [47] |
| ROCK I | It orchestrates biological processes encompassing cell morphology, polarity, and contraction, integral to functions like cell migration, muscle contractility, and cytoskeletal remodeling | ATP stimulates P2X7Rs, triggering apoptosis through the Rho/ROCK pathway, potentially involving ROCK I | It plays a vital role in signal transduction and regulation within cardiomyocytes; involvement in the regulation of Cav 3.2 channels and stabilization of HIF-1α may increase the risk of arrhythmia during ischemia | [48,49] |
| ERK1/2 | It is involved in the regulation of biological processes such as cell growth, proliferation, differentiation and cell survival, and affects cell signaling and cell fate determination | ERK1/2 promotes cell survival and opposes apoptosis, yet sustained activation can ultimately trigger cell death. By activating the ERK1/2 pathway, it plays a pivotal role in determining cell fate | Signaling pathways involved in adaptive or adaptive remodeling; involved in cardiomyocyte hypertrophy and survival | [50,51] |
| P2X6 | It is involved in the regulation of biological processes such as cell signaling, apoptosis and inflammatory response, and may play a role in neurotransmitter release and pain transmission | Activation may elevate calcium levels, potentially initiating cell death mechanisms | P2X6 gene is up-regulated in chronic heart failure, and its activation may be involved in the pathological process of chronic heart failure | [26] |
| CYTC | The electron transport molecules in the mitochondrial respiratory chain are involved in cellular respiration and energy production, as well as regulating the process of apoptosis | During cellular stress, the release of cytochrome c from mitochondria initiates the apoptotic process | Phosphorylation at Thr50 increases with aging, which can inhibit cardiomyocyte apoptosis, especially apoptosis caused by hypoxia/reoxidation, and protect cardiac function | [52] |
| TNF-α | It plays a crucial role in regulating biological processes encompassing inflammation, immune response, and apoptosis, thereby exerting significant influence on inflammatory conditions, immune disorders, and tumor progression | ATP triggers cell death by activating TNF-α and initiating apoptosis or necroptosis pathways. In response to ATP, immune cells produce TNF-α, thereby amplifying the cellular response | The TNF-α gene plays a key role in heart failure, promoting inflammation and cell damage. Increased expression of TNF-α in failing hearts correlates with disease severity and is a potential therapeutic target | [53] |
| P2RY5 | It is involved in cell signaling, skin development, pigmentation and other biological processes, which may be related to hair follicle development and skin pigment distribution regulation | NA | In the heart, it may be associated with inflammation and Crohn’s disease activity index, and its expression level may be associated with cardiac dysfunction | [54] |
| P2RY14 | It plays a pivotal role in regulating biological processes including immune and inflammatory responses, potentially contributing to the activation of immune cells and the release of inflammatory mediators | NA | P2RY14 gene may be involved in the inflammatory process of ischemic acute kidney injury in the heart, and its expression changes are related to the development of AKI after cardiac surgery, which may be a potential therapeutic target for preventing and alleviating ischemic AKI | [55] |
| P2RY13 | It regulates cellular immune response, participates in the regulation of inflammatory response and immune cell activation, and plays a significant role in immune regulation and inflammatory processes | P2Y13 may play a significant role in ADP receptors, primarily implicated in maintaining ATP homeostasis | Variations in the P2RY13 gene are associated with cardiovascular risk markers that may affect heart health | [56] |
| P2RY12 | It plays a crucial role in platelet aggregation, thrombosis, and hemostasis, thereby contributing significantly to blood coagulation and vascular repair processes | P2Y12 may play a role in ADP receptors, mainly involved in ATP homeostasis | The receptor encoded by the P2RY12 gene regulates platelet aggregation in the heart, preventing clots from forming. The use of P2Y12 inhibitors protects the heart and reduces the risk of myocardial infarction and reperfusion injury | [57] |
| P2RY6 | It is integral to cell signaling and inflammation regulation, potentially contributing to the activation of immune cells and the secretion of inflammatory mediators | P2Y6 may play a role in calcium signaling processes | In hypertrophic cardiomyopathy, P2RY6 gene-associated lncRNAs exhibit significant upregulation and may regulate cardiac growth, serving as potential biomarkers and therapeutic targets for hypertrophic cardiomyopathy | [58] |
The coordinated activation of various gene networks involving energy usage, mitochondrial ATP synthesis, heart muscle contraction, and ion movement is essential for preserving normal heart function. Transcriptional regulators, such as estrogen-related receptors (ERRs), play pivotal roles in coordinating these gene networks, regulating cellular metabolism, and contraction mechanisms. ERRs, particularly ERRα and ERRγ, have emerged as critical regulators of cardiac function, as their deficiency leads to cardiac dysfunction, especially under increased workload conditions. Intriguingly, in diabetic cardiomyopathy, metabolic inflexibility is linked to increased mitochondrial FA oxidation and ERRγ expression, hinting at a possible role of persistent ERRγ expression in cardiogenic outcomes[27]. Furthermore, studies have revealed the regulatory role of pannexin-1 half-channel activity by eATP-sensitive P2X7Rs. Nonetheless, the precise mechanisms governing how eATP-sensitive P2X7Rs regulate the opening and closing of Px1 half-channels remain largely elusive. Evidence suggests that under pathological conditions like ischemia, P2X7R activation leads to the opening of Px1 half-channels, resulting in the influx of large amounts of extracellular Ca2+ and the subsequent release of intracellular ATP, ultimately culminating in cell death[28]. Furthermore, the seamless provision of energy is paramount for maintaining the normal contractile and relaxation functions of the heart. Therefore, metabolic disorders and impaired mitochondrial bioenergy, leading to disruptions in ATP production, are implicated in various heart diseases[59].
IRI represents a prevalent and life-threatening clinical complication affecting various organs, including the heart, liver, kidneys, and brain[60]. Myocardial IRI is characterized by multifaceted mechanisms, including the generation of ROS, alterations in cellular osmotic balance, and inflammatory responses. Excessive calcium, variations in oxygen levels, and the generation of mitochondrial ROS collectively leads to the permanent opening of the mPTP, resulting in harmful effects. ROS generation and subsequent oxidative stress are key mechanisms responsible for cellular damage and dysfunction during cardiac IRI. These processes are intricately connected to NLRP3 inflammasome activation, which facilitates cell demise by enhancing the caspase-1 pathway and IL-18 secretion[15].
NLRP3 belongs to the nucleotide-binding domain (NOD)-like receptor family and is expressed by various immune and non-immune cells. When activated, NLRP3, together with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase-1, come together to create the NLRP3 inflammasome complex. This assembly regulates inflammation by cleaving pro-inflammatory cytokines IL-1β and IL-18, promoting pyroptotic cell death[61]. Significantly, targeting the NLRP3 inflammasome holds promise as a therapeutic strategy for ischemic stroke, with MCC950 demonstrating potential clinical efficacy[62]. Moreover, in hypertensive target organ damage, various triggers such as oxidative stress and inflammation activate the NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines that worsen tissue damage and dysfunction[63].
Research using heterozygous SIRT6 knockout [SIRT6 (+/-)] mice and cardiomyocyte models in vitro has elucidated SIRT6’s role in modulating oxidative stress and myocardial damage during IRI. Partial loss of SIRT6 exacerbates myocardial damage, ventricular remodeling, and oxidative stress. In mice subjected to myocardial I/R, restoring SIRT6 expression via direct cardiomyocyte injection of adenovirus vectors to reexpress it rescues the adverse effects of SIRT6 knockout on ischemic hearts. Partial SIRT6 deletion hinders myocardial function recovery after I/R. Importantly, SIRT6 increases AMP/ATP levels, activates the AMPK-FoxO3α axis, and boosts the expression of downstream antioxidant genes, such as manganese superoxide dismutase and catalase. This sequence mitigates intracellular oxidative stress, leading to the protective effect against ischemic heart damage. Thus, SIRT6 activation of FoxO3α in an AMP/ATP-driven, AMPK-dependent manner enhances antioxidant defense mechanisms and suppresses oxidative stress, thereby shielding the heart from IRI[14].
Furthermore, investigations have demonstrated that the reversal of calcium ion entry into cardiac cells can lead to a decrease in mechanical function, disruption of cell ultrastructure, depletion of ATP levels, increase in intracellular calcium ions, and initiation of cell apoptosis. Intracellular calcium overload influences various pathways involved in the apoptotic cascade. Exposure of the heart to a brief period without calcium followed by reintroduction of calcium results in significant structural and functional changes in the myocardium, a phenomenon commonly known as the “calcium paradox”. The heart experiencing the calcium paradox serves as an exemplary model for understanding the mechanisms of cellular injury caused by intracellular calcium overload at the cardiomyocyte level after reoxygenation following hypoxia or ischemia. A study aimed to determine whether cardiomyocytes undergo apoptosis after 5 minutes of calcium depletion followed by 30 minutes of calcium restoration. It is important to note that cardiomyocytes subjected to 30 minutes of ischemia followed by 60 minutes of reperfusion have exhibited apoptotic cell death[64].
Diabetes is a common comorbidity in cardiovascular disease, heightening the heart’s susceptibility to IRI. As a result, individuals with diabetes often have a worse prognosis following acute myocardial infarction compared to those without diabetes. Importantly, diabetes exacerbates myocardial IRI by activating the NADPH oxidase pathway in an AMPK-dependent manner, ultimately resulting in different types of programmed cell death[65,66]. Additionally, diabetic cardiomyopathy, a condition marked by heart muscle dysfunction regardless of coronary artery disease and hypertension, is worsened by diabetes. Mitochondrial dysfunction emerges as a key feature of diabetic cardiomyopathy, with mitochondria exerting varied effects on cardiomyocyte function, including oxidative stress, metabolic shifts, intracellular signaling, and cell death. Normally, damaged mitochondria undergo mitophagy, a process that breaks down dysfunctional mitochondria for lysosomal degradation. However, impaired mitophagy leads to the buildup of dysfunctional mitochondria, resulting in cardiomyocyte death[60,67].
Type 2 diabetes mellitus (T2DM) is a rapidly spreading condition, with cardiovascular issues being the leading cause of death among diabetic patients. Prolonged high blood sugar levels impair vascular function by affecting the function of vascular smooth muscle cells (VSMCs) and intracellular calcium dynamics. To investigate intracellular calcium signaling in VSMCs from Zucker diabetic obese rats, Fura-2/AM calcium imaging was performed. The findings revealed that T2DM reduces calcium release from the sarcoplasmic reticulum while increasing the activity of store-operated channels. Additionally, key calcium export mechanisms (SERCA, PMCA, and NCX) show heightened activity during the initial stages of ATP-induced calcium transients. However, during later stages, calcium entry increases alongside a decrease in NCX, SERCA, and PMCA activity, resulting in a shortened decay time of ATP-induced calcium transients during the early phase and an increased amplitude during the subsequent plateau. Elevated cytoplasmic calcium levels in VSMCs may contribute to vascular dysfunction associated with T2DM[68].
Sepsis stands as a prominent global cause of mortality and morbidity. Autophagy is a cellular process that facilitates the degradation and recycling of damaged organelles and proteins, and it is posited to confer a protective effect against sepsis-induced myocardial dysfunction (SIMD). Experimental models of septicemia were established in male Sprague-Dawley rats via cecal ligation and puncture. Assessment of cardiac damage involved examining serum markers, echocardiographic parameters, histological analysis with hematoxylin and eosin staining, evaluating cardiac mitochondrial health using transmission electron microscopy, measuring ATP and mitochondrial DNA levels, and quantifying cardiac oxidative stress using REDOX markers in cardiac tissue samples. To assess gene and protein expression levels, real-time polymerase chain reaction and western blotting techniques were utilized. Chromatin co-immunoprecipitation and quantitative real-time polymerase chain reaction were utilized to analyze the binding of histone deacetylase (HDAC) to the phosphatase and tensin homolog (PTEN) promoter and the histone acetylation level of the PTEN promoter.
The results revealed that valproic acid (VPA) alleviated mitochondrial impairment, oxidative stress, and inflammation in septic rats, thereby reducing SIMD by enhancing myocardial autophagy levels. This effect was mediated by VPA-induced autophagy, which downregulated PTEN expression through HDAC1 and HDAC3 in septic rat myocardial tissue. Furthermore, VPA promoted myocardial autophagy by upregulating PTEN expression and inhibiting the protein kinase B/mammalian target of rapamycin pathway, thereby ameliorating SIMD[69]. Moreover, research has highlighted the protective effects of irisin against both acute and chronic myocardial injury. Treatment with irisin mitigated cardiomyocyte death and myocardial dysfunction induced by lipopolysaccharide (LPS). Mechanistically, LPS exposure induced mitochondrial oxidative damage, resulting in ATP depletion in cardiomyocytes and activating apoptosis through caspase. Conversely, irisin preserved mitochondrial function by inhibiting LPS-induced mitochondrial fission mediated by dynamin-related protein 1. Notably, irisin restored the c-Jun N-terminal kinase-large tumor suppressor kinase 2 signaling pathway associated with dynamin-related protein 1-mediated mitochondrial fission activation induced by LPS, suggesting its potential as a promising therapeutic approach for SIMD[70]. Furthermore, exogenous carbon monoxide can regulate mitochondrial energy metabolism by influencing the expression of peroxisome proliferator-activated receptor-gamma coactivator 1-alpha, nuclear respiratory factor 1, and mitochondrial transcription factor A. As a result, it improved cardiac function in sepsis[71].
M-iPSC-CMs were obtained from a patient harboring a mitochondrial 16S rRNA gene (MT-RNR2). Hypertrophic cardiomyopathy (HCM) represents a condition characterized by cardiac hypertrophy, diastolic dysfunction, and sudden cardiac death, particularly prevalent among young individuals. The involvement of mitochondrial DNA mutations in HCM pathogenesis has been recognized. Induced pluripotent stem cell-derived cardiomyocytes have diminished mitochondrial protein levels, thereby resulting in mitochondrial dysfunction and ultrastructural aberrations. Simultaneously, the mutation resulted in a decrease in the ATP/ADP ratio and mitochondrial membrane potential, ultimately leading to an increased intracellular Ca2+ concentration, a characteristic feature of various HCM-specific electrophysiological abnormalities[72]. Furthermore, phosphorus-31 magnetic resonance spectroscopy studies conducted in rats revealed a significant impairment in cardiac energy metabolism, characterized by a reduced phosphocreatine to ATP ratio (-31%, P < 0.05)[73]. The MYBPC3 gene, which encodes myocardial myosin-binding protein C, stands as the predominant genetic factor underlying HCM. Remarkably, myocardial fibrosis (MF) emerged as a pivotal player in HCM development. Nevertheless, the precise mechanism by which mutant MYBPC3 contributes to MF remains unclear. A model featuring R495Q mutant pigs was established using cytosine base editing technology, leading to early onset MF shortly after birth. Intriguingly, the “heart-specific” MYBPC3 gene was transcribed and expressed at the protein level not only in cardiac fibroblasts across different species but also in NIH3T3 fibroblasts. CRISPR-mediated ablation of Mybpc3 in NIH3T3 fibroblasts triggered nuclear factor κB signaling pathway activation, resulting in enhanced expression of transforming growth factor-beta 1 and other proinflammatory genes. Increased levels of transforming growth factor-beta 1 led to the upregulation of hypoxia-inducible factor-1 alpha and its downstream glycolytic targets, such as GLUT1, PFK, and LDHA. This resulted in enhanced aerobic glycolysis and elevated ATP production rates, accelerating cardiac fibroblast activation and ultimately contributing to HCM progression[74].
Approximately one-third of individuals afflicted with mitochondrial disease experience some manifestation of cardiomyopathy, often characterized by symptoms such as HF and arrhythmias. The primary source of ATP production occurs via oxidative phosphorylation of FAs and carbohydrates within the mitochondrial respiratory chain[75]. Mitochondria serve as the principal ATP suppliers, crucial for fulfilling the heart muscle’s energy requirements to sustain continuous electrical activity and contractile function. Emerging evidence suggests that mitochondrial dysfunction can deleteriously affect cardiac electrical function by reducing ATP synthesis and triggering excessive ROS production. This disrupts intracellular ion homeostasis and membrane excitability, ultimately increasing the risk of arrhythmias[76]. Furthermore, ventricular fibrillation is closely associated with myocardial ischemia. Sudden cardiac death can be the initial clinical presentation of myocardial ischemia or infarction in approximately 20%-25% of patients. Fatal arrhythmias often result from a complex sequence of pathophysiological abnormalities, arising from intricate interactions among coronary vascular events, myocardial injury, changes in autonomic tone, metabolic conditions, and cardiac ion status. The timing of ischemic onset also plays a crucial role, with a substantial surge in ventricular arrhythmias typically observed within the first few minutes, persisting for about 30 minutes[77].
In large animal hearts, regional ischemia generally induces two distinct stages of ventricular arrhythmia. The first stage (1A), occurring around 5 to 7 minutes after perfusion cessation, is characterized by membrane depolarization, slight acidification in intracellular and extracellular spaces, and minor disturbances in membrane potential. The subsequent stage of ventricular arrhythmia (1B) emerges between 20 and 30 minutes post-perfusion cessation, during which ischemia-induced changes in K+ and pH stabilize. The onset of arrhythmia in this stage is presumed to be associated with electrolytic coupling between cells, evident from the rapid rise in tissue impedance preceding arrhythmia. Research has demonstrated that interventions like ischemic preconditioning can attenuate the effects of subsequent ischemia by postponing the emergence of electrolytic coupling between cells, thereby delaying the occurrence of ischemia-induced arrhythmias[78]. Additionally, acute ischemia triggers the opening of K(ATP) channels, inducing cardiomyocyte acidosis and hypoxia, resulting in significant repolarization dispersion across the boundary region. Concurrently, abnormalities in intracellular Ca2+ handling manifest within the initial minutes of acute myocardial ischemia, potentially serving as a significant contributor to arrhythmogenesis in individuals with coronary artery disease[77].
Due to its crucial role in heart disease pathogenesis, AICD holds significant promise as a therapeutic target in the cardiovascular field. Here, we present an overview of diverse small molecules that impede AICD pathways and discuss their potential applications across various heart disease models[30,36,63,79-109] (Table 2). Persistent low-level inflammation is a fundamental factor in various diseases, particularly cardiovascular conditions. While efforts to address inflammation in cardiovascular disease are still in their early stages, they are an area of significant interest. P2X7R, an ATP-activated ion channel, stands out as a promising target for the development of new drugs, primarily involved in regulating inflammatory responses and cell death mechanisms[110]. Due to its pivotal function in inflammation and immune responses, P2X7R stands out as a promising target for treating inflammatory conditions. Research has shown that Rhein hinders ATP/BZATP-triggered calcium increase, pore formation, ROS production, reduced phagocytosis, IL-1β release, and cell death by blocking P2X7Rs in rat peritoneal macrophages[111]. Stimulation of P2X7 and the resulting increase in IL-1β and IL-18 levels are linked to the development of several cardiovascular conditions, such as high blood pressure, artery hardening, tissue damage from restricted blood flow followed by restoration, and heart weakening. However, medications that block P2X7 have shown effectiveness in lowering blood pressure in individuals with hypertension and slowing down artery hardening in experimental animals. Trials in clinical settings have revealed that drugs inhibiting IL-1β and IL-18 can notably lower the likelihood of major negative heart events, including heart attacks and HF[79]. Additionally, P2X7 stands out among P2X receptors because it can operate as both a typical receptor activated by a molecule and a channel that allows substances to pass through, causing cell death when exposed to ATP for extended periods[112]. Furthermore, mild disruption of mitochondrial coupling provides protective effects against various diseases. However, identifying mild disruption induced by chemical agents remains uncertain. Research has shown that typical chemical agents such as FCCP, niacinamide, and BAM15 induce two-phase changes in STAT3 activity in heart muscle cells - boosting STAT3 at low concentrations while suppressing it at high concentrations, albeit with different ranges of doses. Low doses of these agents activate STAT3 by slightly increasing mitochondrial ROS production and subsequently activating JAK/STAT3 in heart muscle cells. Conversely, high doses of these agents lead to STAT3 suppression, reduced ATP production, and heart muscle cell death. Excessive disruption triggers STAT3 inhibition through excessive mitochondrial ROS production and reduced AMPK activation induced by ATP. Low doses of mitochondrial uncoupling agents alleviate doxorubicin-induced STAT3 inhibition and heart muscle cell death, with STAT3 activation playing a crucial role in the cardiac protective effects of these agents. Mild disruption of mitochondrial coupling in heart muscle cells by these agents is characterized by STAT3 activation and increased ATP levels. Conversely, excessive disruption leads to STAT3 inhibition, decreased ATP levels, and cellular damage. Developing mitochondrial uncoupling agents with an optimal dose range to induce mild disruption represents a promising approach for protecting the heart[22].
| Drug | Mechanism | Targets | Ref. |
| P2X7 antagonist | Inhibit P2RX7 function | High blood pressure; atherosclerosis | [79] |
| IL-1β and IL-18 inhibitors | Inhibit the release of IL-1β and IL-18 | Myocardial infarction and heart failure | [79] |
| Caspase-3 inhibitors | Inhibit the proteolysis of caspase-3 | Reduces or reverses heart failure | [30] |
| S-propranolol | Decreased caspase-3 activity | I/R injury | [80] |
| Spirolactone | Inhibits alpha-adrenergic vasoconstriction in the arteries | Drug-resistant hypertension | [81] |
| Prosulfanilone and carbenolone | Blocking thrombin-induced calcein outflow and reducing Ca2+ inflow, ATP release, platelet aggregation, and thrombosis at the in vitro arterial shear rate | Arterial thrombus | [82] |
| Curcumin, resveratrol, notoginseng lactone and allicin | Inhibition of NLRP3 inflammasome | Hypertension TOD | [63] |
| Pubescenoside A active compound | It inhibited NLRP3 inflammatory activation and induced Nrf2 signaling pathway | I/R injury | [83] |
| Resveratrol (PIC) | TG storage and caspase 1 activity were inhibited | Atherosclerosis | |
| MRS-2179 | Inhibit platelet aggregation | Thrombotic syndrome | [84,85] |
| MRS2500 | Inhibit P2RY1 | Thrombus | [86] |
| NF157 | Inhibit inositol phosphate accumulation | I/R injury | [87] |
| SKF96365 | The entry of orai1 Ca2+ was inhibited | Atherosclerosis | [88] |
| ML9 | Inhibition of STIM1 | Hypertrophy and Ca2+ overload due to I/R; cardiomyocyte death | [89] |
| TDCPP | Decreased STIM1 expression of and increased GSK3β phosphorylation | I/R injury | [90] |
| MMPSI | Selective inhibition of caspase 3/7 | Myocardial ischemic injury | [36] |
| Acetyl-tyr-val-ala-asp chloromethyl ketone | They blocked caspase activation | Myocardial injury induced by ischemia and reperfusion; myocardial infarction | [91] |
| Hypericin | Up-regulation of autophagy after myocardial infarction | Myocardial infarction | [92] |
| MRS-2339 | Activated the heart P2X receptor | Heart failure | [93] |
| Propofol | Induced autophagy | I/R injury | [94] |
| Carvedilol | Novel vasodilator beta-adrenergic receptor antagonist and potent antioxidant | Myocardial I/R induced apoptosis | [95] |
| Midazolam | Inhibit p38 MAPK | Myocardial I/R injury | [96] |
| Ulinastatin | Inhibit inflammation, oxidative stress and apoptosis | Chronic heart failure | [97] |
| Kaempferol | Inhibition of ASK1 | Cardiac hypertrophy | [98] |
| KN-93 | Inhibition of NOX2 | Cardiac remodeling and heart failure | [99] |
| Acacetin | Inhibit oxidative stress, inflammation and apoptosis | Diabetic cardiomyopathy | [100] |
| CETP inhibitor | Elevated phosphorylation levels of vascular myosin light chain and myosin phosphatase target subunit 1, a protein that promotes contractility, along with enhanced reactive ROS production | Hypertension | [101] |
| Fasudil | ROCKI inhibition | Coronary vasospasm, angina pectoris, hypertension, heart failure | [102,103] |
| Isosteviol (STV) | ERK1/2 is selectively activated in cells exposed to stress | Myocardial ischemia-reperfusion | [103] |
| Adriamycin (DOX) | Induced oxidative stress | Heart failure | [105] |
| Plasminogen activator inhibitor 1 | Release the pro-inflammatory cytokine TNF-α | Thromboembolism complication | [106] |
| Rosuvastatin | MG53 up-regulation was induced | Myocarditis | [107] |
| Na+/H+ exchanger 1 | Catalyze increased intracellular Na uptake | Hypertrophy of heart; heart failure | [108] |
| Prasugrel | Inhibit P2RY12 | ST-segment elevation myocardial infarction following primary percutaneous coronary intervention | [109] |
Studies indicate that simultaneous exposure to LPS and ATP leads to pronounced ASC speck formation, caspase-1 activation, cell death, and ROS production. Inhibiting the ATP-gated purinergic receptor P2X7 significantly reduces caspase-1 activation, while sodium vanadate effectively induces caspase-1 activation. Moreover, adjunctive therapy with ethanol reverses caspase-1 activation, ASC speck formation, and ROS production triggered by LPS and ATP. In HepG2 cells, both LPS and ATP signaling are required for ASC speck formation and caspase-1 induction. Additionally, P2X7 may play a critical role in inflammasome activation, and ethanol’s acute effects on the inflammasome may involve reduced ROS production, thereby enhancing tyrosine phosphatase activity[113].
Moreover, another investigation demonstrated that CORM-3 effectively impedes NLRP3 inflammasome activation by obstructing the interaction between NLRP3 and the adaptor protein ASC, thereby alleviating myocardial dysfunction in septic mice[15]. Moreover, when J774 cells are stimulated with LPS and ATP, they display characteristics akin to pyroptosis, including increased expression of IL-1β mRNA and protein, activation of caspase-1, assembly of the inflammasome, and cell death. Cathelicidin LL-37 (LL-37) effectively inhibits LPS/ATP-induced IL-1β expression, caspase-1 activation, inflammasome assembly, and cell death. Notably, LL-37 disrupts the binding of LPS to target cells and reduces ATP-induced/P2X7-mediated caspase-1 activation. These findings suggest that LL-37 can counteract LPS activity and suppress P2X7 response to ATP, thereby mitigating LPS/ATP-induced pyroptosis. Hence, leveraging LL-37’s dual actions on LPS binding and P2X7 activation may present novel strategies for managing sepsis[114].
P2X7R assumes a pivotal function in diverse pathological states linked to tissue damage and inflammation, rendering human P2X7R an appealing therapeutic target. Through evaluation of human P2X7R-mediated Ca2+ responses, three compounds (C23, C40, and C60) were identified from a pool of 73 top-ranked compounds. These compounds underwent additional characterization utilizing Ca2+ imaging, patch clamp current recording, YO-PRO-1 uptake, and propyl iodide cell death assay. The findings revealed that all three compounds effectively inhibited BZATP-induced Ca2+ response and demonstrated potent protective effects against AICD[115]. Moreover, the anti-inflammatory effects of P2X7R antagonists stem from their ability to inhibit P2X7R-mediated secretion of pro-inflammatory cytokines from activated macrophages. P2X7R antagonists reliably hinder ATP-triggered casein release, a phenomenon not observed in P2X7R(-/-) mouse macrophages and unrelated to cellular apoptosis. Nevertheless, our findings indicate that P2X7R activation may independently contribute to tissue injury by facilitating protease release, distinct from its pro-inflammatory actions mediated by IL-1 cytokines[116]. Furthermore, recent studies have shown that exposure of HeLa cells to interferon-gamma leads to increased expression of P2X7 mRNA and full-length protein, altering ATP-dependent calcium flux and rendering the cells susceptible to ATP-induced apoptosis. Importantly, P2X7 antagonists hold promise in attenuating this apoptotic reaction[117].
In summary, AICD plays a prominent role in the pathogenesis of cardiovascular disease, contributing to tissue damage, inflammation, and adverse remodeling. Understanding the molecular and metabolic landscape of AICD provides valuable insights into disease mechanisms and identifies potential therapeutic targets. Future research efforts should focus on addressing the limitations, advancing our understanding of these pathways, and developing targeted interventions to improve clinical outcomes in cardiovascular patients. Continued exploration of small molecules, biologics, and gene-based therapies targeting AICD pathways may lead to the development of innovative treatments for cardiovascular diseases. Conducting well-designed clinical trials to evaluate the efficacy and safety of novel therapeutic interventions targeting AICD is essential for translating preclinical findings into clinical practice.
| 1. | Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, García-Sáez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jäättelä M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, López-Otín C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Muñoz-Pinedo C, Nagata S, Nuñez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3672] [Cited by in RCA: 4906] [Article Influence: 613.3] [Reference Citation Analysis (0)] |
| 2. | Wang W, Zhang H, Sandai D, Zhao R, Bai J, Wang Y, Wang Y, Zhang Z, Zhang HL, Song ZJ. ATP-induced cell death: a novel hypothesis for osteoporosis. Front Cell Dev Biol. 2023;11:1324213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Zhang HL, Sandai D, Zhang ZW, Song ZJ, Babu D, Tabana Y, Dahham SS, Adam Ahmed Adam M, Wang Y, Wang W, Zhang HL, Zhao R, Barakat K, Harun MSR, Shapudin SNM, Lok B. Adenosine triphosphate induced cell death: Mechanisms and implications in cancer biology and therapy. World J Clin Oncol. 2023;14:549-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 4. | Qiu Y, Shi YN, Zhu N, Zhang S, Zhang CJ, Gu J, He P, Dai AG, Qin L. A Lipid Perspective on Regulated Pyroptosis. Int J Biol Sci. 2023;19:2333-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 5. | Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, Huang J, Wang F, Zhou F, Zhang L. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19:971-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 499] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 6. | He X, Fan X, Bai B, Lu N, Zhang S, Zhang L. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol Res. 2021;165:105447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Ryoden Y, Segawa K, Nagata S. Requirement of Xk and Vps13a for the P2X7-mediated phospholipid scrambling and cell lysis in mouse T cells. Proc Natl Acad Sci U S A. 2022;119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 9. | Ryoden Y, Nagata S. The XK plasma membrane scramblase and the VPS13A cytosolic lipid transporter for ATP-induced cell death. Bioessays. 2022;44:e2200106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Winzer R, Serracant-Prat A, Brock VJ, Pinto-Espinoza C, Rissiek B, Amadi M, Eich N, Rissiek A, Schneider E, Magnus T, Guse AH, Diercks BP, Koch-Nolte F, Tolosa E. P2X7 is expressed on human innate-like T lymphocytes and mediates susceptibility to ATP-induced cell death. Eur J Immunol. 2022;52:1805-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 11. | Hirata Y, Nada Y, Yamada Y, Toyama T, Fukunaga K, Hwang GW, Noguchi T, Matsuzawa A. Elaidic Acid Potentiates Extracellular ATP-Induced Apoptosis via the P2X(7)-ROS-ASK1-p38 Axis in Microglial Cell Lines. Biol Pharm Bull. 2020;43:1562-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Zhang HL, Doblin S, Zhang ZW, Song ZJ, Dinesh B, Tabana Y, Saad DS, Adam Ahmed Adam M, Wang Y, Wang W, Zhang HL, Wu S, Zhao R, Khaled B. Elucidating the molecular basis of ATP-induced cell death in breast cancer: Construction of a robust prognostic model. World J Clin Oncol. 2024;15:208-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Feliu C, Peyret H, Poitevin G, Cazaubon Y, Oszust F, Nguyen P, Millart H, Djerada Z. Complementary Role of P2 and Adenosine Receptors in ATP Induced-Anti-Apoptotic Effects Against Hypoxic Injury of HUVECs. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Wang XX, Wang XL, Tong MM, Gan L, Chen H, Wu SS, Chen JX, Li RL, Wu Y, Zhang HY, Zhu Y, Li YX, He JH, Wang M, Jiang W. SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms. Basic Res Cardiol. 2016;111:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | Zhang W, Tao A, Lan T, Cepinskas G, Kao R, Martin CM, Rui T. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol. 2017;112:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Pagliaro P, Penna C. Inhibitors of NLRP3 Inflammasome in Ischemic Heart Disease: Focus on Functional and Redox Aspects. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 17. | Prescimone T, D'Amico A, Caselli C, Cabiati M, Viglione F, Caruso R, Verde A, Del Ry S, Trivella MG, Giannessi D. Caspase-1 transcripts in failing human heart after mechanical unloading. Cardiovasc Pathol. 2015;24:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Trentin-Sonoda M, Fratoni FM, da Cruz Junho CV, Silva WC, Panico K, Carneiro-Ramos MS. Caspase-1 as Molecular Key in Cardiac Remodeling during Cardiorenal Syndrome Type 3 in the Murine Model. Curr Mol Med. 2019;20:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Higashikuni Y, Liu W, Numata G, Tanaka K, Fukuda D, Tanaka Y, Hirata Y, Imamura T, Takimoto E, Komuro I, Sata M. NLRP3 Inflammasome Activation Through Heart-Brain Interaction Initiates Cardiac Inflammation and Hypertrophy During Pressure Overload. Circulation. 2023;147:338-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 20. | Miquelestorena-Standley E, da Silva AVV, Monnier M, Chadet S, Piollet M, Héraud A, Lemoine R, Bochaton T, Derumeaux G, Roger S, Ivanes F, Angoulvant D. Human peripheral blood mononuclear cells display a temporal evolving inflammatory profile after myocardial infarction and modify myocardial fibroblasts phenotype. Sci Rep. 2023;13:16745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Pucéat M, Roche S, Vassort G. Src family tyrosine kinase regulates intracellular pH in cardiomyocytes. J Cell Biol. 1998;141:1637-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Gao JL, Zhao J, Zhu HB, Peng X, Zhu JX, Ma MH, Fu Y, Hu N, Tai Y, Xuan XC, Dong DL. Characterizations of mitochondrial uncoupling induced by chemical mitochondrial uncouplers in cardiomyocytes. Free Radic Biol Med. 2018;124:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Rosenberg P, Katz D, Bryson V. SOCE and STIM1 signaling in the heart: Timing and location matter. Cell Calcium. 2019;77:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Liu P, Yang Z, Wang Y, Sun A. Role of STIM1 in the Regulation of Cardiac Energy Substrate Preference. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303:H1208-H1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Banfi C, Ferrario S, De Vincenti O, Ceruti S, Fumagalli M, Mazzola A, D' Ambrosi N, Volontè C, Fratto P, Vitali E, Burnstock G, Beltrami E, Parolari A, Polvani G, Biglioli P, Tremoli E, Abbracchio MP. P2 receptors in human heart: upregulation of P2X6 in patients undergoing heart transplantation, interaction with TNFalpha and potential role in myocardial cell death. J Mol Cell Cardiol. 2005;39:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Lasheras J, Pardo R, Velilla M, Poncelas M, Salvatella N, Simó R, Ruiz-Meana M, Zamora M, Villena JA. Cardiac-Specific Overexpression of ERRγ in Mice Induces Severe Heart Dysfunction and Early Lethality. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Iwabuchi S, Kawahara K. Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem Int. 2011;58:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Bin Dayel AF, Alonazi AS, Alshammari TK, Alrasheed NM. P2X7 purinergic receptor: A potential target in heart diseases (Review). Mol Med Rep. 2023;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Yang B, Ye D, Wang Y. Caspase-3 as a therapeutic target for heart failure. Expert Opin Ther Targets. 2013;17:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Petric S, Klein S, Dannenberg L, Lahres T, Clasen L, Schmidt KG, Ding Z, Donner BC. Pannexin-1 Deficient Mice Have an Increased Susceptibility for Atrial Fibrillation and Show a QT-Prolongation Phenotype. Cell Physiol Biochem. 2016;38:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Ignatovica V, Latkovskis G, Peculis R, Megnis K, Schioth HB, Vaivade I, Fridmanis D, Pirags V, Erglis A, Klovins J. Single nucleotide polymorphisms of the purinergic 1 receptor are not associated with myocardial infarction in a Latvian population. Mol Biol Rep. 2012;39:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Luo R, Gomez AM, Benitah JP, Sabourin J. Targeting Orai1-Mediated Store-Operated Ca(2+) Entry in Heart Failure. Front Cell Dev Biol. 2020;8:586109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Kruidering M, Evan GI. Caspase-8 in apoptosis: the beginning of "the end"? IUBMB Life. 2000;50:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Han Y, Chen YS, Liu Z, Bodyak N, Rigor D, Bisping E, Pu WT, Kang PM. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res. 2006;99:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Chapman JG, Magee WP, Stukenbrok HA, Beckius GE, Milici AJ, Tracey WR. A novel nonpeptidic caspase-3/7 inhibitor, (S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin reduces myocardial ischemic injury. Eur J Pharmacol. 2002;456:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Zhang C, Li G, Liang S, Xu C, Zhu G, Wang Y, Zhang A, Wan F. Myocardial ischemic nociceptive signaling mediated by P2X3 receptor in rat stellate ganglion neurons. Brain Res Bull. 2008;75:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Pijacka W, Moraes DJ, Ratcliffe LE, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JF. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med. 2016;22:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 39. | Liao Y, Liu K, Zhu L. Emerging Roles of Inflammasomes in Cardiovascular Diseases. Front Immunol. 2022;13:834289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Menzies RI, Unwin RJ, Dash RK, Beard DA, Cowley AW Jr, Carlson BE, Mullins JJ, Bailey MA. Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Front Physiol. 2013;4:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Hansen MA, Bennett MR, Barden JA. Distribution of purinergic P2X receptors in the rat heart. J Auton Nerv Syst. 1999;78:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Ge Y, Pan S, Guan D, Yin H, Fan Y, Liu J, Zhang S, Zhang H, Feng L, Wang Y, Xu R, Yin JQ. MicroRNA-350 induces pathological heart hypertrophy by repressing both p38 and JNK pathways. Biochim Biophys Acta. 2013;1832:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Romero-Becerra R, Santamans AM, Folgueira C, Sabio G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 44. | Jiang L, Ren L, Guo X, Zhao J, Zhang H, Chen S, Le S, Liu H, Ye P, Chen M, Xia J. Dual-specificity Phosphatase 9 protects against Cardiac Hypertrophy by targeting ASK1. Int J Biol Sci. 2021;17:2193-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Kumar RA, Hahn D, Kelley RC, Muscato DR, Shamoun A, Curbelo-Bermudez N, Butler WG, Yegorova S, Ryan TE, Ferreira LF. Skeletal muscle Nox4 knockout prevents and Nox2 knockout blunts loss of maximal diaphragm force in mice with heart failure with reduced ejection fraction. Free Radic Biol Med. 2023;194:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Borutaite V. AMPK, MAPK and Bax in the heart: some questions answered. Biochem J. 2008;412:e15-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Ogut O, Brozovich FV. The potential role of MLC phosphatase and MAPK signalling in the pathogenesis of vascular dysfunction in heart failure. J Cell Mol Med. 2008;12:2158-2164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 659] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 49. | González-Rodríguez P, Falcón D, Castro MJ, Ureña J, López-Barneo J, Castellano A. Hypoxic induction of T-type Ca(2+) channels in rat cardiac myocytes: role of HIF-1α and RhoA/ROCK signalling. J Physiol. 2015;593:4729-4745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Tarone G, Sbroggiò M, Brancaccio M. Key role of ERK1/2 molecular scaffolds in heart pathology. Cell Mol Life Sci. 2013;70:4047-4054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Sbroggiò M, Bertero A, Velasco S, Fusella F, De Blasio E, Bahou WF, Silengo L, Turco E, Brancaccio M, Tarone G. ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1. J Cell Sci. 2011;124:3515-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Li F, Sun H, Lin X, Li Q, Zhao D, Cheng Z, Liu J, Fan Q. Increased cytochrome C threonine 50 phosphorylation in aging heart as a novel defensive signaling against hypoxia/reoxygenation induced apoptosis. Aging (Albany NY). 2022;14:5699-5709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 53. | Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 54. | Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis. 2009;15:971-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Battistone MA, Mendelsohn AC, Spallanzani RG, Allegretti AS, Liberman RN, Sesma J, Kalim S, Wall SM, Bonventre JV, Lazarowski ER, Brown D, Breton S. Proinflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice. J Clin Invest. 2020;130:3734-3749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 56. | Verdier C, Ruidavets JB, Genoux A, Combes G, Bongard V, Taraszkiewicz D, Galinier M, Elbaz M, Ferrières J, Martinez LO, Perret B. Common p2y(13) polymorphisms are associated with plasma inhibitory factor 1 and lipoprotein(a) concentrations, heart rate and body fat mass: The GENES study. Arch Cardiovasc Dis. 2019;112:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Cohen MV, Downey JM. What Are Optimal P2Y12 Inhibitor and Schedule of Administration in Patients With Acute Coronary Syndrome? J Cardiovasc Pharmacol Ther. 2020;25:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Guo Q, Wang J, Sun R, Gu W, He Z, Chen Q, Liu W, Chen Y, Wang J, Zhang Y. Identification of circulating hub long noncoding RNAs associated with hypertrophic cardiomyopathy using weighted correlation network analysis. Mol Med Rep. 2020;22:4637-4644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Ramachandra CJA, Chua J, Cong S, Kp MMJ, Shim W, Wu JC, Hausenloy DJ. Human-induced pluripotent stem cells for modelling metabolic perturbations and impaired bioenergetics underlying cardiomyopathies. Cardiovasc Res. 2021;117:694-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20:7-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 738] [Article Influence: 246.0] [Reference Citation Analysis (0)] |
| 61. | Shen HH, Yang YX, Meng X, Luo XY, Li XM, Shuai ZW, Ye DQ, Pan HF. NLRP3: A promising therapeutic target for autoimmune diseases. Autoimmun Rev. 2018;17:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 62. | Wang H, Zhong D, Chen H, Jin J, Liu Q, Li G. NLRP3 inflammasome activates interleukin-23/interleukin-17 axis during ischaemia-reperfusion injury in cerebral ischaemia in mice. Life Sci. 2019;227:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Liao X, Han Y, Shen C, Liu J, Wang Y. Targeting the NLRP3 inflammasome for the treatment of hypertensive target organ damage: Role of natural products and formulations. Phytother Res. 2023;37:5622-5638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 64. | Xu YJ, Saini HK, Zhang M, Elimban V, Dhalla NS. MAPK activation and apoptotic alterations in hearts subjected to calcium paradox are attenuated by taurine. Cardiovasc Res. 2006;72:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Lejay A, Fang F, John R, Van JA, Barr M, Thaveau F, Chakfe N, Geny B, Scholey JW. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol. 2016;91:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 66. | Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang Z, Yao W. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J Cell Mol Med. 2020;24:6670-6679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 67. | Chang X, Li Y, Cai C, Wu F, He J, Zhang Y, Zhong J, Tan Y, Liu R, Zhu H, Zhou H. Mitochondrial quality control mechanisms as molecular targets in diabetic heart. Metabolism. 2022;137:155313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 68. | Moreno-Salgado A, Coyotl-Santiago N, Moreno-Vazquez R, Lopez-Teyssier M, Garcia-Carrasco M, Moccia F, Berra-Romani R. Alterations of the Ca(2+) clearing mechanisms by type 2 diabetes in aortic smooth muscle cells of Zucker diabetic fatty rat. Front Physiol. 2023;14:1200115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 69. | Shi X, Liu Y, Zhang D, Xiao D. Valproic acid attenuates sepsis-induced myocardial dysfunction in rats by accelerating autophagy through the PTEN/AKT/mTOR pathway. Life Sci. 2019;232:116613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 70. | Tan Y, Ouyang H, Xiao X, Zhong J, Dong M. Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperones. 2019;24:595-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 71. | Wang X, Qin W, Qiu X, Cao J, Liu D, Sun B. A novel role of exogenous carbon monoxide on protecting cardiac function and improving survival against sepsis via mitochondrial energetic metabolism pathway. Int J Biol Sci. 2014;10:777-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Li S, Pan H, Tan C, Sun Y, Song Y, Zhang X, Yang W, Wang X, Li D, Dai Y, Ma Q, Xu C, Zhu X, Kang L, Fu Y, Xu X, Shu J, Zhou N, Han F, Qin D, Huang W, Liu Z, Yan Q. Mitochondrial Dysfunctions Contribute to Hypertrophic Cardiomyopathy in Patient iPSC-Derived Cardiomyocytes with MT-RNR2 Mutation. Stem Cell Reports. 2018;10:808-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Luedde M, Flögel U, Knorr M, Grundt C, Hippe HJ, Brors B, Frank D, Haselmann U, Antony C, Voelkers M, Schrader J, Most P, Lemmer B, Katus HA, Frey N. Decreased contractility due to energy deprivation in a transgenic rat model of hypertrophic cardiomyopathy. J Mol Med (Berl). 2009;87:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Zou X, Ouyang H, Lin F, Zhang H, Yang Y, Pang D, Han R, Tang X. MYBPC3 deficiency in cardiac fibroblasts drives their activation and contributes to fibrosis. Cell Death Dis. 2022;13:948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Pavez-Giani MG, Cyganek L. Recent Advances in Modeling Mitochondrial Cardiomyopathy Using Human Induced Pluripotent Stem Cells. Front Cell Dev Biol. 2021;9:800529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Yang KC, Bonini MG, Dudley SC Jr. Mitochondria and arrhythmias. Free Radic Biol Med. 2014;71:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 77. | Luqman N, Sung RJ, Wang CL, Kuo CT. Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007;119:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Cascio WE, Yang H, Muller-Borer BJ, Johnson TA. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. J Electrocardiol. 2005;38:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Shokoples BG, Paradis P, Schiffrin EL. P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2021;41:186-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 80. | Jia X, Zhang L, Mao X. S-propranolol protected H9C2 cells from ischemia/reperfusion-induced apoptosis via downregultion of RACK1 Gene. Int J Clin Exp Pathol. 2015;8:10335-10344. [PubMed] |
| 81. | Good ME, Chiu YH, Poon IKH, Medina CB, Butcher JT, Mendu SK, DeLalio LJ, Lohman AW, Leitinger N, Barrett E, Lorenz UM, Desai BN, Jaffe IZ, Bayliss DA, Isakson BE, Ravichandran KS. Pannexin 1 Channels as an Unexpected New Target of the Anti-Hypertensive Drug Spironolactone. Circ Res. 2018;122:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 82. | Taylor KA, Wright JR, Vial C, Evans RJ, Mahaut-Smith MP. Amplification of human platelet activation by surface pannexin-1 channels. J Thromb Haemost. 2014;12:987-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Cheng Y, Cheng L, Gao X, Chen S, Wu P, Wang C, Liu Z. Covalent modification of Keap1 at Cys77 and Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3 inflammasome activation in myocardial ischemia-reperfusion injury. Theranostics. 2021;11:861-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 84. | Baurand A, Gachet C. The P2Y(1) receptor as a target for new antithrombotic drugs: a review of the P2Y(1) antagonist MRS-2179. Cardiovasc Drug Rev. 2003;21:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Lenain N, Freund M, Léon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1:1144-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Djerada Z, Peyret H, Dukic S, Millart H. Extracellular NAADP affords cardioprotection against ischemia and reperfusion injury and involves the P2Y11-like receptor. Biochem Biophys Res Commun. 2013;434:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Liang SJ, Zeng DY, Mai XY, Shang JY, Wu QQ, Yuan JN, Yu BX, Zhou P, Zhang FR, Liu YY, Lv XF, Liu J, Ou JS, Qian JS, Zhou JG. Inhibition of Orai1 Store-Operated Calcium Channel Prevents Foam Cell Formation and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:618-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Shaikh S, Troncoso R, Mondaca-Ruff D, Parra V, Garcia L, Chiong M, Lavandero S. The STIM1 inhibitor ML9 disrupts basal autophagy in cardiomyocytes by decreasing lysosome content. Toxicol In Vitro. 2018;48:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | He X, Li S, Fang X, Liao Y. TDCPP protects cardiomyocytes from hypoxia-reoxygenation injury induced apoptosis through mitigating calcium overload and promotion GSK-3β phosphorylation. Regul Toxicol Pharmacol. 2018;92:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Holly TA, Drincic A, Byun Y, Nakamura S, Harris K, Klocke FJ, Cryns VL. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 92. | Yang Y, Li J, Rao T, Fang Z, Zhang J. The role and mechanism of hyperoside against myocardial infarction in mice by regulating autophagy via NLRP1 inflammation pathway. J Ethnopharmacol. 2021;276:114187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Shen JB, Cronin C, Sonin D, Joshi BV, Gongora Nieto M, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy: implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1077-H1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Li H, Zhang X, Tan J, Sun L, Xu LH, Jiang YG, Lou JS, Shi XY, Mi WD. Propofol postconditioning protects H9c2 cells from hypoxia/reoxygenation injury by inducing autophagy via the SAPK/JNK pathway. Mol Med Rep. 2018;17:4573-4580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, Louden C, Gu JL, Kumar S, Poste G, Ruffolo RR Jr, Feuerstein GZ. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res. 1998;82:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Zhou W, Cai D. Midazolam suppresses ischemia/reperfusion-induced cardiomyocyte apoptosis by inhibiting the JNK/p38 MAPK signaling pathway. Can J Physiol Pharmacol. 2022;100:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Li L, Hao J, Jiang X, Li P, Sen H. Cardioprotective effects of ulinastatin against isoproterenol-induced chronic heart failure through the PI3KAkt, p38 MAPK and NF-κB pathways. Mol Med Rep. 2018;17:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Feng H, Cao J, Zhang G, Wang Y. Kaempferol Attenuates Cardiac Hypertrophy via Regulation of ASK1/MAPK Signaling Pathway and Oxidative Stress. Planta Med. 2017;83:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 99. | Ni Y, Deng J, Bai H, Liu C, Liu X, Wang X. CaMKII inhibitor KN-93 impaired angiogenesis and aggravated cardiac remodelling and heart failure via inhibiting NOX2/mtROS/p-VEGFR2 and STAT3 pathways. J Cell Mol Med. 2022;26:312-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Song F, Mao YJ, Hu Y, Zhao SS, Wang R, Wu WY, Li GR, Wang Y, Li G. Acacetin attenuates diabetes-induced cardiomyopathy by inhibiting oxidative stress and energy metabolism via PPAR-α/AMPK pathway. Eur J Pharmacol. 2022;922:174916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Rios FJ, Lopes RA, Neves KB, Camargo LL, Montezano AC, Touyz RM. Off-Target Vascular Effects of Cholesteryl Ester Transfer Protein Inhibitors Involve Redox-Sensitive and Signal Transducer and Activator of Transcription 3-Dependent Pathways. J Pharmacol Exp Ther. 2016;357:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Shimokawa H, Satoh K. 2015 ATVB Plenary Lecture: translational research on rho-kinase in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2015;35:1756-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7:657-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 104. | Abdul KSM, Faiz N, Jovanović A, Tan W. Isosteviol Protects H9c2 Cells Against Hypoxia-reoxygenation by Activating ERK1/2. Cardiovasc Hematol Disord Drug Targets. 2021;21:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 105. | Yuan Y, Fan S, Shu L, Huang W, Xie L, Bi C, Yu H, Wang Y, Li Y. Exploration the Mechanism of Doxorubicin-Induced Heart Failure in Rats by Integration of Proteomics and Metabolomics Data. Front Pharmacol. 2020;11:600561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Morrow GB, Whyte CS, Mutch NJ. A Serpin With a Finger in Many PAIs: PAI-1's Central Function in Thromboinflammation and Cardiovascular Disease. Front Cardiovasc Med. 2021;8:653655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 107. | Zhuang J, Cheng G, Huang J, Guo H, Lai Y, Wang J, Shan Z, Zheng S. Rosuvastatin exerts cardioprotective effect in lipopolysaccharide-mediated injury of cardiomyocytes in an MG53-dependent manner. BMC Cardiovasc Disord. 2022;22:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 108. | Xia H, Zahra A, Jia M, Wang Q, Wang Y, Campbell SL, Wu J. Na(+)/H(+) Exchanger 1, a Potential Therapeutic Drug Target for Cardiac Hypertrophy and Heart Failure. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 109. | Rafique AM, Nayyar P, Wang TY, Mehran R, Baber U, Berger PB, Tobis J, Currier J, Dave RH, Henry TD. Optimal P2Y12 Inhibitor in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Network Meta-Analysis. JACC Cardiovasc Interv. 2016;9:1036-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 110. | Pislyagin E, Kozlovskiy S, Menchinskaya E, Chingizova E, Likhatskaya G, Gorpenchenko T, Sabutski Y, Polonik S, Aminin D. Synthetic 1,4-Naphthoquinones inhibit P2X7 receptors in murine neuroblastoma cells. Bioorg Med Chem. 2021;31:115975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Hu F, Xing F, Zhu G, Xu G, Li C, Qu J, Lee I, Pan L. Rhein antagonizes P2X7 receptor in rat peritoneal macrophages. Sci Rep. 2015;5:14012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Innocenti B, Pfeiffer S, Zrenner E, Kohler K, Guenther E. ATP-induced non-neuronal cell permeabilization in the rat inner retina. J Neurosci. 2004;24:8577-8583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Hörauf JA, Kany S, Janicova A, Xu B, Vrdoljak T, Sturm R, Dunay IR, Martin L, Relja B. Short Exposure to Ethanol Diminishes Caspase-1 and ASC Activation in Human HepG2 Cells In Vitro. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Hu Z, Murakami T, Suzuki K, Tamura H, Kuwahara-Arai K, Iba T, Nagaoka I. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism. PLoS One. 2014;9:e85765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 115. | Caseley EA, Muench SP, Fishwick CW, Jiang LH. Structure-based identification and characterisation of structurally novel human P2X7 receptor antagonists. Biochem Pharmacol. 2016;116:130-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185:2611-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 117. | Welter-Stahl L, da Silva CM, Schachter J, Persechini PM, Souza HS, Ojcius DM, Coutinho-Silva R. Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNgamma in human epithelial cells. Biochim Biophys Acta. 2009;1788:1176-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/