Published online Aug 26, 2021. doi: 10.4330/wjc.v13.i8.361
Peer-review started: March 21, 2021
First decision: May 5, 2021
Revised: May 26, 2021
Accepted: July 16, 2021
Article in press: July 16, 2021
Published online: August 26, 2021
Processing time: 154 Days and 22.5 Hours
Coexistent coronary artery disease is commonly seen in patients undergoing transcatheter aortic valve implantation (TAVI). Previous studies showed that pre-TAVI coronary revascularisation was not associated with improved outcomes, challenging the clinical value of routine coronary angiogram (CA).
To assess whether a selective approach to perform pre-TAVI CA is safe and feasible.
This was a retrospective non-randomised single-centre analysis of consecutive patients undergoing TAVI. A selective approach for performing CA tailored to patient clinical need was developed. Clinical outcomes were compared based on whether patients underwent CA. The primary endpoint was a composite of all-cause mortality, myocardial infraction, repeat CA, and re-admission with heart failure.
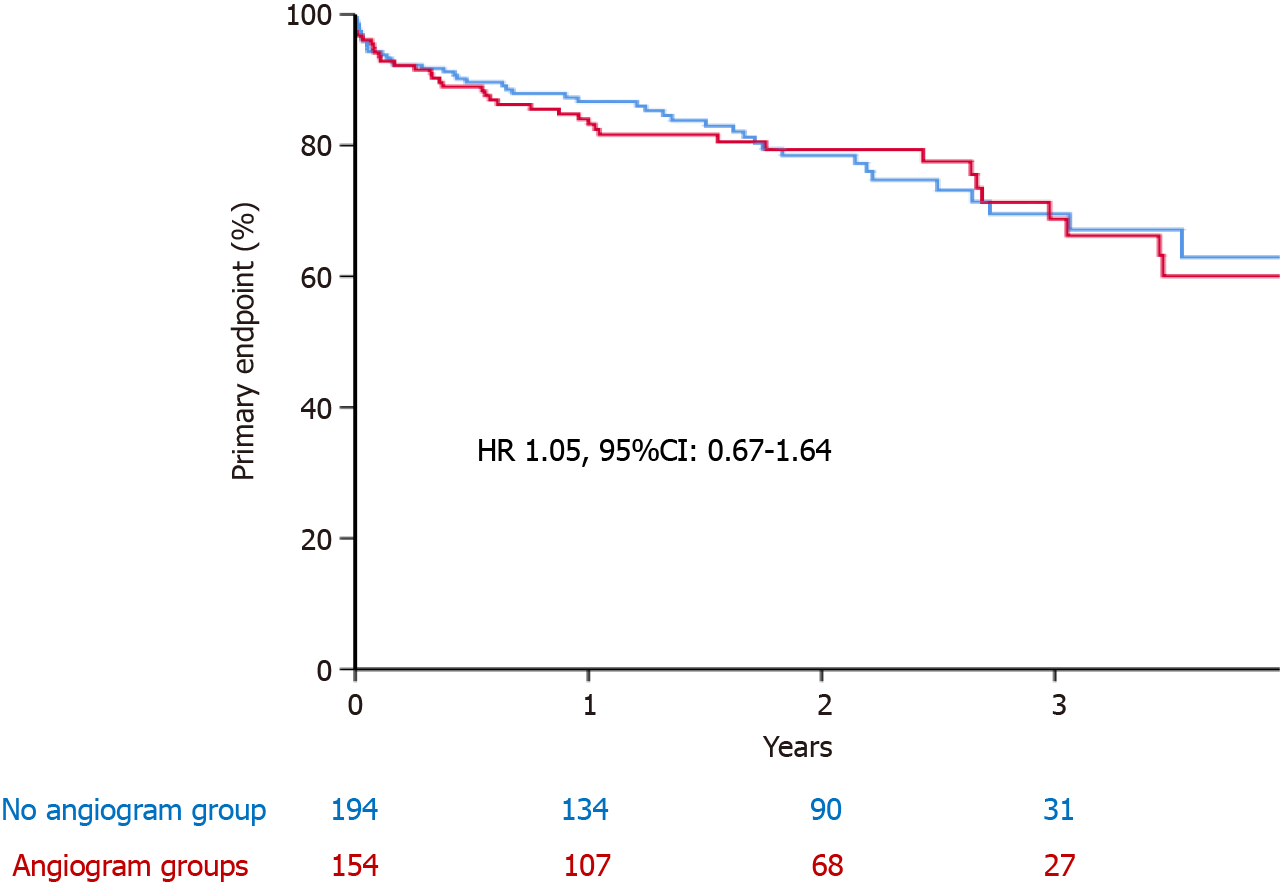
Of 348 patients (average age 81 ± 7 and 57% male) were included with a median follow up of 19 (9-31) mo. One hundred and fifty-four (44%) patients, underwent CA before TAVI procedure. Patients who underwent CA were more likely to have previous myocardial infarction (MI) and previous percutaneous revascularisation. The primary endpoint was comparable between the two group (22.6% vs 22.2%; hazard ratio 1.05, 95%CI: 0.67-1.64, P = 0.82). Patients who had CA were less likely to be readmitted with heart failure (P = 0.022), but more likely to have repeat CA
Selective CA is a feasible and safe approach. The clinical value of routine CA should be challenged in future randomised trials
Core Tip: Previous studies showed that pre-transcatheter aortic valve implantation coronary revascularisation was not associated with improved outcomes, challenging the clinical value of routine coronary angiogram (CA). A selective approach for performing CA tailored to patient clinical need was developed. In 348 patients, the primary endpoint of all-cause mortality, myocardial infraction, repeat CA, and re-admission with heart failure was comparable between patients who underwent CA vs no CA (22.6% vs 22.2%; hazard ratio 1.05, 95%CI: 0.67-1.64, P = 0.82). Patients who had CA were less likely to be readmitted with heart failure (P = 0.022), but more likely to have repeat CA (P = 0.002) and myocardial infarction (P = 0.007). The clinical value of routine CA should be challenged in future randomised trials.
- Citation: Beska B, Manoharan D, Mohammed A, Das R, Edwards R, Zaman A, Alkhalil M. Role of coronary angiogram before transcatheter aortic valve implantation. World J Cardiol 2021; 13(8): 361-371
- URL: https://www.wjgnet.com/1949-8462/full/v13/i8/361.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i8.361
Aortic stenosis is the most common valve disease requiring intervention in Europe and North America and is largely a disease of older adults[1]. Transcatheter aortic valve implantation (TAVI) is now a standard treatment option for management of severe aortic stenosis[1,2]. Given the ageing population and increasing prevalence of disease, the volume of patients requiring valve intervention is likely to increase. Moreover, a paradigm shift towards the use of TAVI in lower-risk patients is becoming more evident[3,4].
Coexistent coronary artery disease (CAD) is commonly seen in those with severe aortic stenosis, with shared traditional cardiovascular risk factors[5,6]. Whether re
The lack of data showing consistent benefits in pre-TAVI revascularisation cha
This was a retrospective observational analysis of consecutive patients undergoing TAVI at the Freeman Hospital in Newcastle-upon-Tyne over 4 years. Patients with inaccessible electronic follow-up were excluded. The TAVI programme is a regional service and ascertaining clinical follow up was an essential criterion to be included in this study.
The TAVI procedure and valve choice was left to the operators’ discretion. Clinical, echocardiographic and procedural characteristics were prospectively entered into a dedicated TAVR database which was retrospectively interrogated. All patients had an echocardiogram and computed tomography (CT) prior to TAVI procedure.
A selective approach to perform CA was developed during the study period. This was tailored to patient’s clinical status and invasive angiogram was performed if: (1) Patient reported typical exertional chest pain which was relieved at rest and was suggestive of angina; (2) Impaired left ventricle systolic function (ejection fraction ≤ 50%), particularly if there were regional wall motion abnormalities; or (3) Extensive calcifications (> 70% of lumen diameter stenosis) involving the proximal segments of left or right coronary arteries detected on CT as part of TAVI work up.
Coronary revascularisation was recommended prior to TAVI, if patients had angio
The primary endpoint was a combination of all-cause mortality, myocardial infarction (MI), repeat CA, and re-admission to hospital with heart failure. Secondary endpoints included the individual or combination of two components of the primary endpoint. Procedural MI was excluded, and only spontaneous MI was included. Repeat CA after TAVI was indicated in the presence of symptoms, signs of ischaemia, or elevated cardiac biomarkers. Flow limiting lesion was defined as degree of stenosis ≥ 70% on epicardial coronary artery of ≥ 2.5 mm in size.
Mortality data were provided by the Office of National Statistics. Other clinical endpoints were retrieved using dedicated electronic databases for clinical follow up. MI events were cross-checked by evaluating troponin measurements and referral letter for invasive CA. Heart failure readmissions were similarly assessed using reported chest X-ray.
Data were assessed for normality of distribution using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± SD or as median accompanied by inter
Of 480 patients undergoing TAVI, 348 (73%) patients with accessible electronic records were included in this analysis. Patients were followed for a median of 19 (9-31) mo.
The average age was 81 ± 7 and 57% of patients were male. Two-thirds of patients were markedly symptomatic with NYHA class III/IV. 295 (85%) patients received balloon expanding valve and 34 (10%) had self-expanding valves. Less than half of the cohort, 154 (44%) patients, underwent coronary angiography before TAVI procedure. Almost three-quarters of patients had their TAVI procedure in an elective setting. There were no differences between patients who did or did not undergo CA in terms of age, gender, cardiovascular risk, body mass index, kidney function, or other vascular disease. Baseline clinical characteristics stratified according to CA pre-TAVI are presented in Table 1. Patients who underwent CA were more likely to have previous MI (14% vs 8%, P = 0.07), and previous percutaneous coronary intervention (21% vs 10%, P = 0.007), but less likely to have previous coronary artery bypass graft (7% vs 17%, P = 0.03). There were no reported complications with any of the cases that underwent coronary angiography.
| Whole group (n = 348) | No coronary angiogram group | Coronary angiogram group | P value | |
| Age (mean ± SD) | 81 ± 7 | 82 ± 7 | 81 ± 7 | NS |
| Male gender, n (%) | 197 (57) | 109 (56) | 88 (57) | NS |
| Obesity, n (%) | 83 (34) | 43 (31) | 40 (37) | NS |
| Hypertension, n (%) | 136 (55) | 79 (57) | 57 (53) | NS |
| NYHA III/IV, n (%) | 232 (67) | 127 (66) | 105 (68) | NS |
| Diabetes, n (%) | 68 (20) | 39 (20) | 29 (19) | NS |
| Smoking history, n (%) | 166 (48) | 94 (49) | 72 (47) | NS |
| Previous MI, n (%) | 38 (11) | 16 (8) | 22 (14) | NS |
| Previous PCI, n (%) | 52 (15) | 20 (10) | 32 (21) | < 0.01 |
| Previous CABG, n (%) | 43 (12) | 33 (17) | 10 (7) | < 0.01 |
| COPD, n (%) | 58 (17) | 34 (18) | 24 (16) | NS |
| CVA/TIA, n (%) | 29 (8) | 14 (7) | 15 (10) | NS |
| AF, n (%) | 83 (24) | 47 (24) | 36 (23) | NS |
| PVD, n (%) | 55 (16) | 24 (12) | 31 (20) | < 0.05 |
| Creatinine (mean ± SD) | 112 ± 97 | 114 ± 117 | 110 ± 65 | NS |
| Elective admission, n (%) | 271 (78) | 149 (77) | 122 (79) | NS |
Echocardiographic and procedural characteristics are presented in Table 2. There were no differences between the two groups in gradients, valve area, or left ventricle function. Transfemoral approach was performed in the majority of cases (89%). Patients who underwent CA were more likely to have undergone an alternative access TAVI i.e., via subclavian, trans axillary, apical, or direct aortic. Similarly, general anaesthesia was more frequently used in CA vs no CA groups (12% vs 2%, P < 0.001)
| Whole group | No coronary angiogram group | Coronary angiogram group | P value | |
| Peak gradient (mean ± SD) | 74 ± 23 | 73 ± 22 | 74 ± 24 | NS |
| Mean gradient (mean ± SD) | 43 ±15 | 44 ±15 | 43 ±15 | NS |
| Valve area (mean ± SD) | 0.70 ± 0.20 | 0.71 ± 0.18 | 0.69 ± 0.22 | NS |
| Preserved LV function, n (%) | 274 (79) | 158 (81) | 116 (75) | NS |
| Moderate MR, n (%) | 35 (10) | 20 (10) | 15 (10) | NS |
| PA pressure (mean ± SD) | 39 ± 20 | 40 ± 15 | 39 ± 25 | NS |
| Indication for AR or mixed AoV disease, n (%) | 19 (6) | 9 (5) | 10 (7) | NS |
| Bioprosthetic valve, n (%) | 17 (5) | 14 (7) | 3 (2) | < 0.05 |
| GA, n (%) | 23 (7) | 4 (2) | 19 (12) | < 0.01 |
| BAV, n (%) | 34 (10) | 15 (8) | 19 (12) | NS |
| Transfemoral approach, n (%) | 311 (89) | 183 (94) | 128 (83) | < 0.01 |
| Balloon-expanding, n (%) | 295 (85) | 161 (83) | 134 (87) | NS |
| Valve size (> 23 mm), n (%) | 222 (64) | 125 (64) | 97 (63) | NS |
The primary endpoint of all-cause mortality, MI, repeat CA, and re-admission to hospital with heart failure was comparable between the two groups [22.6% vs 22.2%; hazard ratio (HR) 1.05, 95%CI: 0.67-1.64, P = 0.82] (Figure 1 and Table 3). All-cause mortality was comparable between the two groups (19% vs 17%; HR 1.18, 95%CI: 0.71-1.95, P = 0.52). Patients who had CA were less likely to be readmitted with heart failure (1.3% vs 6.2%, P = 0.022) but more likely to have repeat CA (5.8% vs 0.5%, P = 0.003). Likewise, patients in the CA group had higher rate of subsequent MI (3.9% vs 1.0%, P = 0.07), although this did not reach statistical significance.
| Whole group | No coronary angiogram group | Coronary angiogram group | P value | |
| Primary endpoint, n (%) | 78 (22.4) | 44 (22.6) | 33 (22.2) | NS |
| Death, n (%) | 63 (18.1) | 37 (19.0) | 26 (17.0) | NS |
| Myocardial infarction, n (%) | 8 (2.3) | 2 (1.0) | 6 (3.9) | NS |
| Readmission with heart failure, n (%) | 14 (4) | 12 (6.2) | 2 (1.3) | < 0.05 |
| Coronary angiogram post valve intervention, n (%) | 10 (2.9) | 1 (0.5) | 9 (5.8) | < 0.01 |
| Death & myocardial infarction, n (%) | 69 (19.8) | 38 (19.5) | 31 (20.3) | NS |
| Death & readmission with heart failure, n (%) | 70 (20.1) | 43 (22.1) | 27 (17.6) | NS |
| Myocardial infarction & readmission with heart failure, n (%) | 21 (6) | 13 (6.7) | 8 (5.2) | NS |
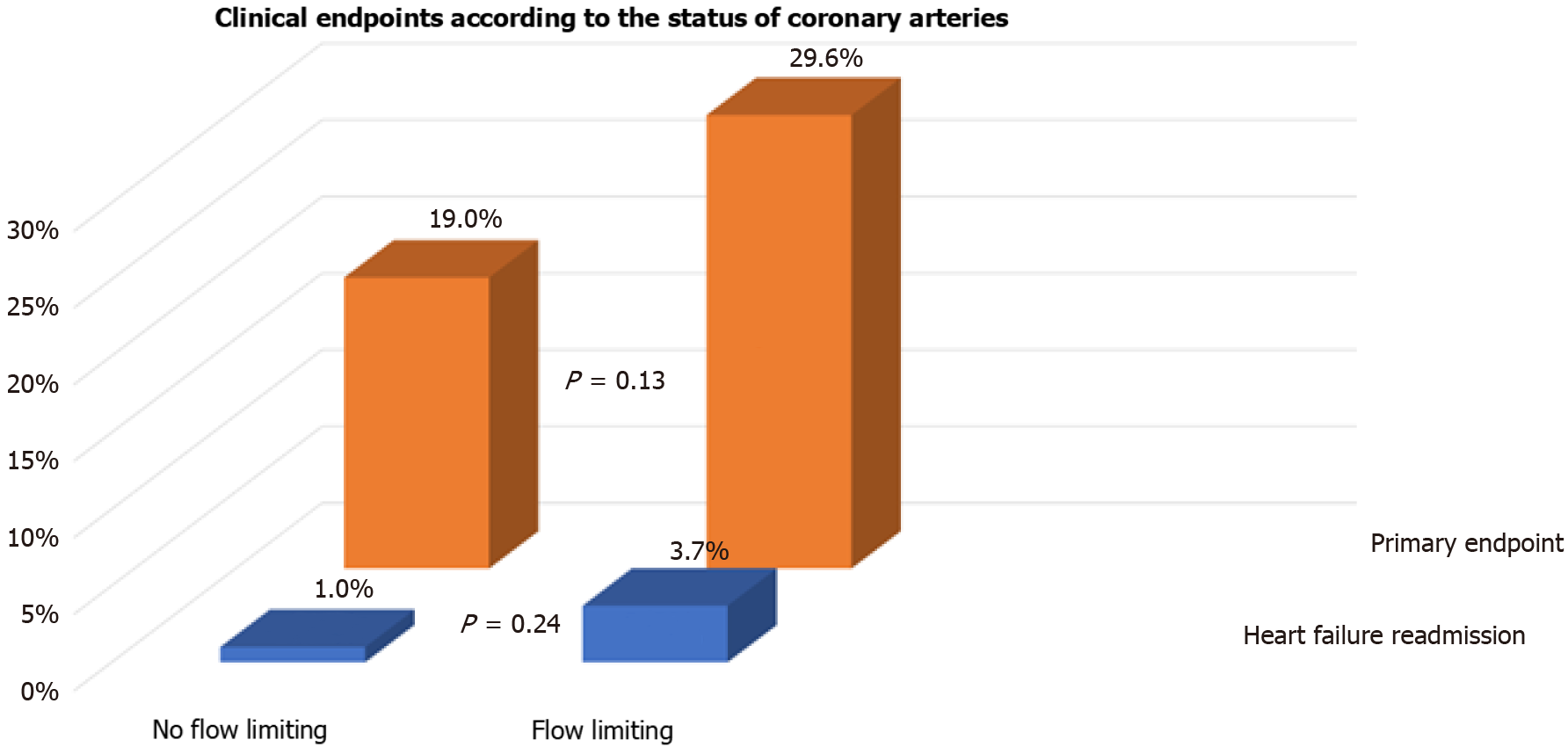
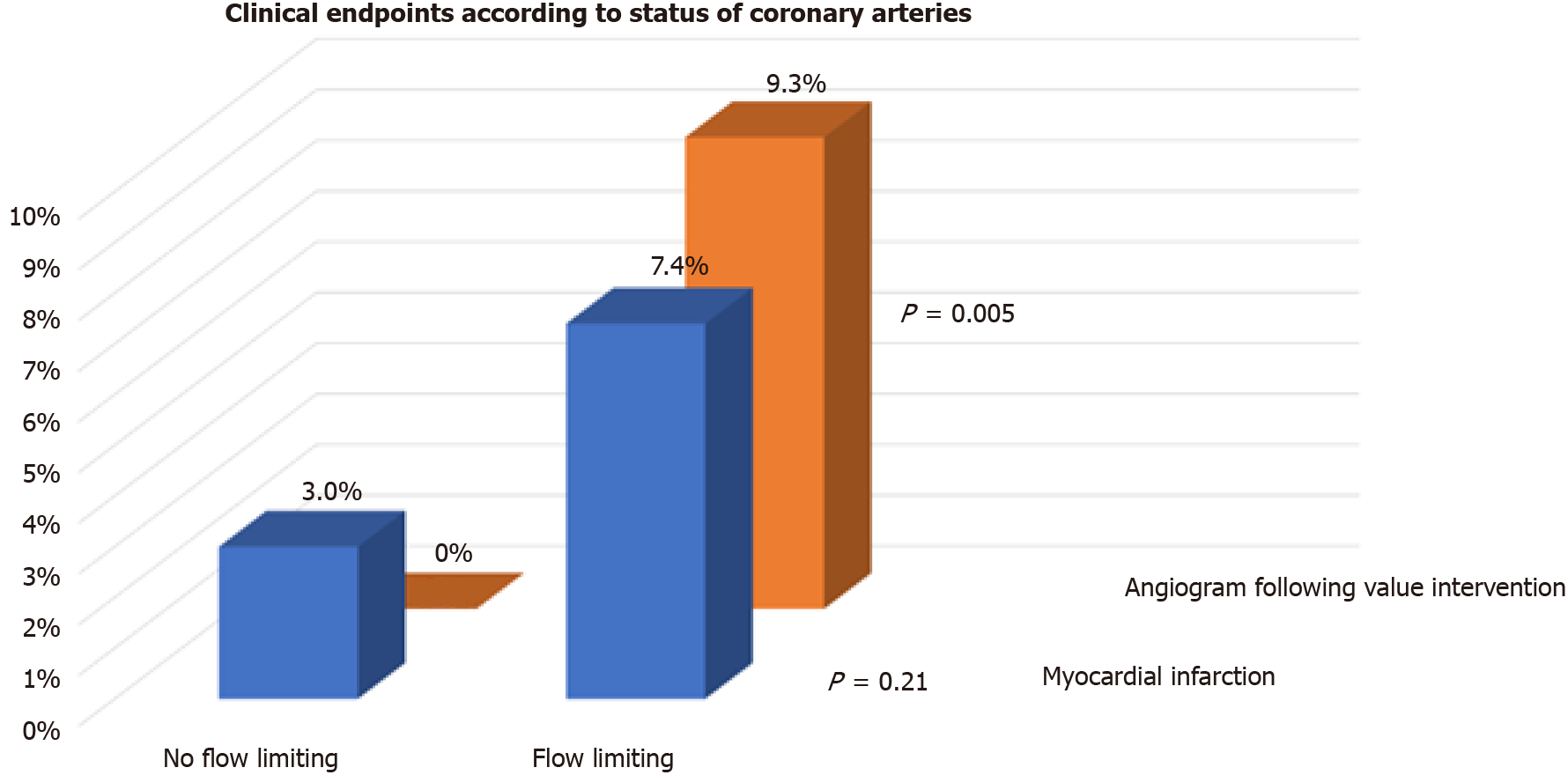
We also assessed whether flow limiting lesions on CA before TAVI were associated with events post TAVI. One-third (54/154) of patients had flow limiting lesions on CA before TAVI. There were no differences in the incidence of the primary endpoint (29.6% vs 19.0%, P = 0.13), readmission with heart failure (3.7% vs 1.0%, P = 0.24), or spontaneous MI (7.4% vs 3.0%, P = 0.21) between patients with flow limiting vs those with no flow limiting lesions on CA, respectively (Figures 2 and 3). Repeat CA following TAVI was statistically more frequent in those with flow limiting lesions (9.3% vs 0%, P = 0.005) (Figure 3). Two patients had percutaneous coronary inter
The main finding of this study was that our devised approach of selective CA tailored to patient clinical characteristics was safe and feasible over a relatively short follow up. Second, a composite of all-cause mortality, MI, repeat CA, and re-admission to hospital with heart failure was comparable irrespective of CA before TAVI procedure and importantly, there were no differences based on the obstructive nature of coronary lesions on CA before TAVI. Third, patients with flow limiting lesions pre-TAVI were more likely to have repeat CA following TAVI.
There is a strong association between CAD and aortic stenosis. In almost 16000 patients undergoing TAVI in the German Aortic Valve Registry, 50% of patients were reported to have concomitant coronary artery disease[13]. The management of CAD in this setting is controversial with previous studies highlighting the prognostic role of CAD in patients undergoing TAVI[14]. Moreover, observational data suggested a potential caveat when coronary arteries were not revascularised by demonstrating an association between rapid pacing and adverse outcomes in TAVI patients[15,16]. Coronary revascularisation was proposed to mitigate ventricular stunning and reduce prolong hypotension during rapid pacing, although this needs to be confirmed in randomised trials[17].
Nonetheless, percutaneous coronary intervention was not consistently associated with a reduction in adverse events following TAVI[10,18]. Recently, the percutaneous coronary intervention prior to TAVI (ACTIVATION) trial showed no difference in one-year survival or readmission to hospital according to whether coronary revascularisation was performed before TAVI[19,20]. This was the first randomised trial to test this hypothesis and challenges the current recommendation of performing routine coronary revascularisation for proximal CAD before TAVI[1,2]. Moreover, Snow et al[21] showed that the management of aortic stenosis and concomitant CAD can be effectively managed by TAVI alone[21]. Importantly, our data are consistent with the results of these studies, and questions the need for CA, and revascularisation, before TAVI.
The current study suggests that the criteria for selective CA before TAVI is a safe and feasible. Patients with history of exertional chest pain suggestive of angina, left ventricle dysfunction, or extensive calcification on CT were deemed suitable for CA before TAVI. Up to 40% of patients with aortic stenosis may report angina symptoms and coronary revascularisation is indicated to relieve symptoms and improve quality of life. The presence of left ventricular dysfunction maybe related to CAD and sug
Routine CA subjects patients to additional invasive procedure with associated risks, albeit small[23]. Contrast-induced nephropathy is a recognised risk with CA and is increased in the elderly and in patients with renal dysfunction[24,25]. These features are commonly observed in patients undergoing TAVI, and may exacerbate the renal risk associated following TAVI procedure itself[26]. Moreover, routine CA would add further delays to patients who are waiting for definitive valve intervention. There was an increase in mortality risk of almost 4% for each month delay while waiting for TAVI procedure[27].
Access to coronary arteries following TAVI has been reported to be challenging[28-30]. This was highlighted with self-expanding compared to balloon-expanding valves[31]. Therefore, it may be argued that upfront revascularisation of coronary lesions pre-TAVI would overcome the need to access the coronary arteries following TAVI. Nonetheless, these studies were of small sample size with success rate in engaging coronaries ranging from 50% to 100%[32]. Better understanding of the relationship between the bioprosthetic valve, particularly leaflets commissures, and coronary ostia would allow selective CA following TAVI[33].
Coronary events are relatively uncommon following TAVI. In our series, MI was reported in 2.3% of cases which was comparable to other contemporary studies[34]. Importantly, subsequent CAs did not always demonstrate culprit coronary lesions that required revascularisation. This should not be surprising since other mechanisms of MI were proposed such as coronary embolism secondary to subclinical leaflet thrombosis, late migration of the bioprosthetic valve, impaired coronary flow dy
Our study has several limitations. This was a retrospective single centre study associated with the inherent limitations of the design of the study. We had to exclude almost 25% of patients who underwent TAVI in our centre as their post procedural follow up could not be ascertained. Clinical endpoints were not adjudicated and were defined according to hospital discharge letters. This bias may have been mitigated by including hard events such as mortality and heart failure admissions.
Selective CA is a feasible and safe approach. It is not associated with high adverse outcomes. The role of CA before TAVI is increasingly undermined by the low ischaemic event rate. Additionally, the disconnect between CAD and subsequent cardiac events question the pre-emptive approach of coronary revascularisation in TAVI. Large randomised trials are required to test this hypothesis in the future.
Routine coronary revascularisation pre-transcatheter aortic valve implantation (TAVI) was not associated with improved outcomes, yet, coronary angiogram (CA) is still performed as part of TAVI work up.
The lack of data showing consistent benefits in pre-TAVI revascularisation challenges the need for routine invasive coronary angiogram before TAVI procedure.
To assess whether a selective approach to perform pre-TAVI CA is safe and feasible.
Retrospective analysis of consecutive patients undergoing TAVI who underwent CA vs those who did not was performed. Decision to undergo CA pre-TAVI was tailored to patients clinical characteristics.
The primary endpoint was a composite of all-cause mortality, myocardial infraction, repeat CA, and re-admission with heart failure was comparable between the two groups.
Selective CA is a feasible and safe approach. The clinical value of routine CA should be challenged in future randomised trials.
Future randomised clinical trials are required to test whether selective CA is safe.
| 1. | Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4598] [Cited by in RCA: 4470] [Article Influence: 496.7] [Reference Citation Analysis (0)] |
| 2. | Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease : A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Nucl Cardiol. 2017;24:1759-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR; PARTNER 3 Investigators. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3668] [Cited by in RCA: 3719] [Article Influence: 531.3] [Reference Citation Analysis (0)] |
| 4. | Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ; Evolut Low Risk Trial Investigators. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2802] [Cited by in RCA: 2831] [Article Influence: 404.4] [Reference Citation Analysis (0)] |
| 5. | Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1397] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 7. | Beach JM, Mihaljevic T, Svensson LG, Rajeswaran J, Marwick T, Griffin B, Johnston DR, Sabik JF 3rd, Blackstone EH. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2013;61:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Lund O, Nielsen TT, Pilegaard HK, Magnussen K, Knudsen MA. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 1990;100:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Mullany CJ, Elveback LR, Frye RL, Pluth JR, Edwards WD, Orszulak TA, Nassef LA Jr, Riner RE, Danielson GK. Coronary artery disease and its management: influence on survival in patients undergoing aortic valve replacement. J Am Coll Cardiol. 1987;10:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Lateef N, Khan MS, Deo SV, Yamani N, Riaz H, Virk HUH, Khan SU, Hedrick DP, Kanaan A, Reed GW, Krishnaswamy A, Puri R, Kapadia SR, Kalra A. Meta-Analysis Comparing Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation With Versus Without Percutaneous Coronary Intervention. Am J Cardiol. 2019;124:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Kotronias RA, Kwok CS, George S, Capodanno D, Ludman PF, Townend JN, Doshi SN, Khogali SS, Généreux P, Herrmann HC, Mamas MA, Bagur R. Transcatheter Aortic Valve Implantation With or Without Percutaneous Coronary Artery Revascularization Strategy: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Abdel-Wahab M, Mostafa AE, Geist V, Stöcker B, Gordian K, Merten C, Richardt D, Toelg R, Richardt G. Comparison of outcomes in patients having isolated transcatheter aortic valve implantation versus combined with preprocedural percutaneous coronary intervention. Am J Cardiol. 2012;109:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Walther T, Hamm CW, Schuler G, Berkowitsch A, Kötting J, Mangner N, Mudra H, Beckmann A, Cremer J, Welz A, Lange R, Kuck KH, Mohr FW, Möllmann H; GARY Executive Board. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol. 2015;65:2173-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 14. | Sankaramangalam K, Banerjee K, Kandregula K, Mohananey D, Parashar A, Jones BM, Jobanputra Y, Mick S, Krishnaswamy A, Svensson LG, Kapadia SR. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Fefer P, Bogdan A, Grossman Y, Berkovitch A, Brodov Y, Kuperstein R, Segev A, Guetta V, Barbash IM. Impact of Rapid Ventricular Pacing on Outcome After Transcatheter Aortic Valve Replacement. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Axell RG, White PA, Giblett JP, Williams L, Rana BS, Klein A, O'Sullivan M, Davies WR, Densem CG, Hoole SP. Rapid Pacing-Induced Right Ventricular Dysfunction Is Evident After Balloon-Expandable Transfemoral Aortic Valve Replacement. J Am Coll Cardiol. 2017;69:903-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Axell RG, Giblett JP, White PA, Klein A, Hampton-Til J, O'Sullivan M, Braganza D, Davies WR, West NEJ, Densem CG, Hoole SP. Stunning and Right Ventricular Dysfunction Is Induced by Coronary Balloon Occlusion and Rapid Pacing in Humans: Insights From Right Ventricular Conductance Catheter Studies. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Khawaja MZ, Asrress KN, Haran H, Arri S, Nadra I, Bolter K, Wilson K, Clack L, Hancock J, Young CP, Bapat V, Thomas M, Redwood S. The effect of coronary artery disease defined by quantitative coronary angiography and SYNTAX score upon outcome after transcatheter aortic valve implantation (TAVI) using the Edwards bioprosthesis. EuroIntervention. 2015;11:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Khawaja MZ, Wang D, Pocock S, Redwood SR, Thomas MR. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: study protocol for a randomized controlled trial. Trials. 2014;15:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Leclercq F, Robert P, Labour J, Lattuca B, Akodad M, Macia JC, Gervasoni R, Roubille F, Gandet T, Schmutz L, Nogue E, Nagot N, Albat B, Cayla G. Prior balloon valvuloplasty versus DIRECT transcatheter Aortic Valve Implantation (DIRECTAVI): study protocol for a randomized controlled trial. Trials. 2017;18:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Snow TM, Ludman P, Banya W, DeBelder M, MacCarthy PM, Davies SW, Di Mario C, Moat NE. Management of concomitant coronary artery disease in patients undergoing transcatheter aortic valve implantation: the United Kingdom TAVI Registry. Int J Cardiol. 2015;199:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Michail M, Ihdayhid AR, Comella A, Thakur U, Cameron JD, McCormick LM, Gooley RP, Nicholls SJ, Mathur A, Hughes AD, Ko BS, Brown AJ. Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in Patients With Severe Aortic Stenosis: The CAST-FFR Study. Circ Cardiovasc Interv. 2021;14:e009586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Al-Hijji MA, Lennon RJ, Gulati R, El Sabbagh A, Park JY, Crusan D, Kanwar A, Behfar A, Lerman A, Holmes DR, Bell M, Singh M. Safety and Risk of Major Complications With Diagnostic Cardiac Catheterization. Circ Cardiovasc Interv. 2019;12:e007791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. 2016;102:638-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 25. | Solomon RJ, Natarajan MK, Doucet S, Sharma SK, Staniloae CS, Katholi RE, Gelormini JL, Labinaz M, Moreyra AE; Investigators of the CARE Study. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189-3196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 231] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Rahman MS, Sharma R, Brecker SJD. Transcatheter aortic valve implantation in patients with pre-existing chronic kidney disease. Int J Cardiol Heart Vasc. 2015;8:9-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Malaisrie SC, McDonald E, Kruse J, Li Z, McGee EC Jr, Abicht TO, Russell H, McCarthy PM, Andrei AC. Mortality while waiting for aortic valve replacement. Ann Thorac Surg. 2014;98:1564-1570; discussion 1570-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Boukantar M, Gallet R, Mouillet G, Belarbi A, Rubimbura V, Ternacle J, Dubois-Rande JL, Teiger E. Coronary Procedures After TAVI With the Self-Expanding Aortic Bioprosthesis Medtronic CoreValve™, Not an Easy Matter. J Interv Cardiol. 2017;30:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Vilalta V, Asmarats L, Ferreira-Neto AN, Maes F, de Freitas Campos Guimarães L, Couture T, Paradis JM, Mohammadi S, Dumont E, Kalavrouziotis D, Delarochellière R, Rodés-Cabau J. Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2018;11:2523-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Faroux L, Munoz-Garcia E, Serra V, Alperi A, Nombela-Franco L, Fischer Q, Veiga G, Donaint P, Asmarats L, Vilalta V, Chamandi C, Regueiro A, Gutiérrez E, Munoz-Garcia A, Garcia Del Blanco B, Bach-Oller M, Moris C, Armijo G, Urena M, Fradejas-Sastre V, Metz D, Castillo P, Fernandez-Nofrerias E, Sabaté M, Tamargo M, Del Val D, Couture T, Rodes-Cabau J. Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2020;13:e008620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Blumenstein J, Kim WK, Liebetrau C, Gaede L, Kempfert J, Walther T, Hamm C, Möllmann H. Challenges of coronary angiography and intervention in patients previously treated by TAVI. Clin Res Cardiol. 2015;104:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Faroux L, Guimaraes L, Wintzer-Wehekind J, Junquera L, Ferreira-Neto AN, Del Val D, Muntané-Carol G, Mohammadi S, Paradis JM, Rodés-Cabau J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:362-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 33. | Yudi MB, Sharma SK, Tang GHL, Kini A. Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018;71:1360-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 34. | Alkhalil M, Brennan P, McQuillan C, Jeganathan R, Manoharan G, Owens CG, Spence MS. Flow, Reflected by Stroke Volume Index, Is a Risk Marker in High-Gradient Aortic Stenosis Patients Undergoing Transcatheter Aortic Valve Replacement. Can J Cardiol. 2020;36:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Latsios G S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY