Published online Sep 26, 2020. doi: 10.4330/wjc.v12.i9.450
Peer-review started: June 28, 2020
First decision: June 15, 2020
Revised: June 28, 2020
Accepted: September 15, 2020
Article in press: September 15, 2020
Published online: September 26, 2020
Processing time: 110 Days and 21.9 Hours
Patients with vasospastic angina (VSA) sometimes experience prolonged chest symptoms. The clinical characteristics of these patients have not been clarified.
To investigate the clinical characteristics of prolonged VSA patients.
This study included 167 patients with VSA diagnosed by spasm provocation tests (SPTs) using acetylcholine, which recorded the frequencies of positive reactions to a low dose of acetylcholine (L-ACh), total occlusion due to spasm (TOC), focal spasm, and the unavoidable use of nitroglycerin (unavoidable-NTG) during SPTs. The patients underwent a medical interview that investigated the maximum duration and frequency of chest symptoms as well as the frequencies of variant angina and other serious symptoms. The patients were divided into two groups based on the maximal duration: The short-duration group (< 15 min; n = 114) and the long-duration group (≥ 15 min; n = 53). They were also divided into two groups based on the frequency of chest symptoms: The low-frequency group (< 4/mo; n = 88) and the high-frequency group (≥ 4/mo; n = 79).
The long-duration group showed higher frequencies of other serious symptoms (P < 0.001) and variant angina (P < 0.05) as well as higher frequencies of spasm induction by L-ACh (P < 0.05), TOC (P < 0.05), focal spasm (P < 0.01), and unavoidable-NTG (P < 0.01) than the short-duration group. These parameters did not differ significantly between the low-frequency and high-frequency groups.
These findings suggest that patients with VSA who experience prolonged chest symptoms may have more severe characteristics of VSA.
Core Tip: We have sometimes experienced patients with vasospastic angina (VSA) who had prolonged chest symptoms (≥ 15 min). We showed that such VSA patients had higher frequencies of other serious symptoms and variant angina as well as higher frequencies of spasm induction by a low dose of acetylcholine, total occlusion due to spasm, focal spasm, and unavoidable use of nitroglycerin in the spasm provocation test (SPT). On the other hand, the frequency of chest symptoms did not influence these findings in the SPT. Prolonged chest symptoms may be related to more severe characteristics of VSA.
- Citation: Teragawa H, Oshita C, Orita Y. Clinical significance of prolonged chest pain in vasospastic angina. World J Cardiol 2020; 12(9): 450-459
- URL: https://www.wjgnet.com/1949-8462/full/v12/i9/450.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i9.450
Some patients with vasospastic angina (VSA) experience prolonged chest symptoms[1]. The guidelines for VSA[2] note that chest pain in patients with VSA usually occurs at rest, with pain persisting for several minutes up to approximately 15 min. The guidelines also noted that chest pain due to coronary spasms often persisted longer than that during exercise and that these attacks are sometimes accompanied by cold sweats and disturbances of consciousness. However, the clinical characteristics of patients with VSA who experience these prolonged chest symptoms have yet to be clarified. We investigated the clinical characteristics of such patients.
This observational, retrospective study included patients with VSA diagnosed by spasm provocation tests (SPTs) who attended our institution from 2011 to 2015 (n = 251). We applied the following exclusion criteria: Patients without chest symptoms, such as those with only syncope (n = 8) or heart failure (n = 15), and those with an unclear duration of chest symptoms (n = 38). During the study period, spasm provocation at our institution was performed first in the right coronary artery (RCA). We therefore also excluded patients for whom spasm provocation could not be performed in the RCA because of its small size or the inability to place a catheter into the ostium of the RCA (n = 23). Finally, 167 patients were enrolled in the present study. The protocol of the study was approved by the ethics committee of our institution. Written informed consent was obtained from all of the patients.
The patients and their families underwent detailed medical interviews that established the maximum duration of chest symptoms and the frequency of chest symptoms per month. The maximum duration of chest symptoms was determined as follows: It was 5 min when the patients and their families answered “several minutes”, and it was 20 min when they answered “from 10 to 20 min”. In addition, the interviews recorded whether the patients experienced cold sweats or syncope[3,4], which were considered to be symptoms accompanying VSA. The patients were divided into two sets of two groups according to the maximum duration and frequency of their chest symptoms. The long-duration and short-duration groups comprised patients with maximum durations of symptoms of ≥ 15 min and < 15 min, respectively. The cut-off value of 15 min was in accordance with the guidelines for VSA[2]. The median frequency of symptoms was four times per month. The high-frequency and low-frequency groups were comprised of patients whose frequencies of symptoms were > 4 times/month and ≤ 4 times/month, respectively.
An SPT was performed using the methods described previously[5]. In brief, after the initial coronary angiogram (CAG), 20 and 50 μg doses of acetylcholine (ACh) were injected into the RCA. When coronary spasm was not induced by 50 μg of ACh, a maximum dose of 80 μg of ACh was infused into the RCA. CAG was obtained just after coronary spasms were induced or the maximum ACh infusion was finished. If a coronary spasm was induced but improved spontaneously, an SPT of the left coronary artery (LCA) was then performed without an intracoronary injection of nitroglycerin (NTG) into the RCA. In such cases, once the SPT for the LCA was finished, CAG was repeated following an NTG injection into the RCA. If the coronary spasm provoked by ACh infusion into the RCA was prolonged or severe enough to induce hemodynamic instability, an intracoronary injection of 0.3 mg of NTG was applied to relieve the spasms.
An SPT of the LCA was then performed. The SPT of the LCA was performed by infusing 50 and 100 μg doses of ACh into the LCA using a similar method. If coronary spasm was not induced by 100 μg of ACh, a maximum of 200 μg of ACh was infused into the LCA. CAG was performed just after a coronary spasm was provoked or the maximum ACh infusion was finished. An intracoronary injection of 0.3 mg of NTG was administered, followed by the final CAG for the LCA.
We adopted the use of an autoinjector as described previously[5]. When unstable hemodynamics continued, small doses of intracoronary or intravenous catecholamines were infused. In this study, low doses of ACh (L-ACh) were considered to be 20 μg for the RCA and 50 μg for the LCA. The diameters of the coronary artery were measured as described previously[5]. Lesions with > 20% stenosis were defined as atherosclerotic lesions. Because an association between myocardial bridges and VSA has been reported[6,7], we assessed whether a myocardial bridge, which was defined as the systolic narrowing of the coronary artery diameter by > 20% compared to that in diastole, was present.
We defined variant angina (VA) as angina with a recorded spontaneous ST elevation on ECG. VSA was defined as ≥ 90% narrowing of the coronary arteries on angiograms during the provocation accompanied by the presence of usual chest pain and/or the presence of ST-segment deviation on ECG[2]. A focal spasm was defined as a transient vessel narrowing of > 90% localized to the major coronary arteries. A diffuse spasm was defined as a 90% diffuse vasoconstriction observed in ≥ 2 adjacent coronary segments of the coronary arteries[8]. Multivessel spasms were defined as coronary spasms that occurred in ≥ 2 major coronary arteries. For multivessel spasms, we could not assess when the subsequent SPT was negative after an unavoidable use of NTG. For the present study, data for each patient were collected based on the frequency of the following test and the other events: Spasm provocation induced by L-ACh, total occlusion of the coronary artery due to spasm (TOC), unavoidable administration of NTG into the RCA, severe complications accompanied by prolonged unstable hemodynamics requiring intravenous catecholamines, ventricular fibrillation, and pulseless ventricular tachycardia. In addition, the use of coronary vasodilators was assessed when the patient attended the hospital before admission for CAG.
The patient was asked about his or her current smoking status, and any family history of coronary artery disease (FH-CAD) was recorded. Hypertension, dyslipidemia, diabetes mellitus and chronic kidney disease were defined based on the standard definitions described in previous papers[5]. A patient was defined as an alcohol drinker if he or she consumed alcohol one or more times a week.
The left ventricular ejection fraction was measured using cardiac ultrasonography. In the majority of studied patients (n = 158), flow-mediated dilation (FMD) as an endothelium-dependent function and NTG-induced dilation (NID) as an endothelium-independent function were measured as previously described[9].
After discharge, the patients were followed up at our institution as far as possible, and all studied patients visited for at least one follow-up. One hundred twenty-two patients have been followed through the final check-up in 2019. Eight patients died during the follow-up period, and the remaining 37 patients were followed through 2018.
Among the 122 patients with a recent follow-up (73%), the follow-up examinations included information about the patients’ medications from their medication notebooks. We assessed the number of coronary vasodilators used and the number of angina attacks (per month) experienced in the previous 3 mo. Cardiac events related to VSA were recorded for each patient, including readmission for angina or other cardiovascular diseases and death from cardiac and noncardiac causes. The major adverse cardiac event (MACE) was defined as death from cardiac causes or readmission for cardiovascular causes.
Data are presented as the mean ± SD or medians with interquartile ranges for nonnormally distributed data and noncontinuous variables, respectively. Baseline characteristics of the groups were compared using Student’s unpaired t-tests, Wilcoxon signed-rank tests, or χ2 analysis, as appropriate. Survival was analyzed by the Kaplan-Meier survival curve method with log-rank tests.
The statistical analyses were performed using JMP Ver. 14 (SAS Institute Inc., United States). A P value < 0.05 was considered statistically significant.
There were 114 patients in the short-duration group, 53 patients in the long-duration group, 88 patients in the low-frequency group, and 79 patients in the high-frequency group (Table 1). There were no significant differences in patient characteristics between the short-duration and long-duration groups. The only significant difference between the low-frequency and high-frequency groups was the presence of an FH-CAD, which was higher in the high-frequency group than in the low-frequency group. There was no significant relationship between the maximum duration and the frequency of chest symptoms. There were no significant differences between the groups regarding FMD and NID.
| Short-duration | Long-duration | P value | Low-frequency | High-frequency | P value | |
| No. | 114 | 53 | 88 | 79 | ||
| Age (yr) | 67 ± 10 | 67 ± 11 | 0.8401 | 67 ± 11 | 68 ± 10 | 0.6669 |
| Male/female | 58/56 | 28/25 | 0.8142 | 40/48 | 46/33 | 0.0991 |
| BMI (kg/m2) | 24.3 ± 4.1 | 24.2 ± 3.6 | 0.7171 | 24.2 ± 3.8 | 24.5 ± 4.1 | 0.5697 |
| Coronary risk factors (%) | ||||||
| Current smoker | 24 (21) | 9 (17) | 0.5385 | 17 (19) | 16 (20) | 0.8796 |
| Hypertension | 81 (71) | 38 (72) | 0.9316 | 64 (73) | 55 (70) | 0.6578 |
| Dyslipidemia | 76 (67) | 29 (58) | 0.1368 | 56 (64) | 49 (62) | 0.8297 |
| Diabetes mellitus | 31 (27) | 9 (17) | 0.1501 | 19 (19) | 23 (29) | 0.1386 |
| Alcohol consumption (%) | 40 (35) | 23 (43) | 0.3025 | 34 (39) | 29 (37) | 0.7995 |
| Family history of CAD (%) | 26 (23) | 10 (19) | 0.5645 | 13 (15) | 23 (29) | 0.0244 |
| CKD (%) | 34 (30) | 16 (30) | 0.9619 | 21 (24) | 29 (37) | 0.0704 |
| Taking statins (%) | 50 (44) | 20 (38) | 0.4554 | 34 (39) | 36 (46) | 0.3646 |
| LVEF on UCG (%) | 66 ± 10 | 67 ± 9 | 0.5752 | 66 ± 9 | 67 ± 10 | 0.7165 |
| FMD (%, n) | 3.9 ± 3.1 (107) | 3.4 ± 3.2 (51) | 0.3200 | 3.9 ± 2.8 (82) | 3.5 ± 3.5 (76) | 0.4872 |
| NID (%, n) | 15.2 ± 7.0 (107) | 14.5 ± 6.4 (51) | 0.5795 | 14.8 ± 6.5 (82) | 15.1 ± 7.1 (76) | 0.8295 |
The frequencies of chest symptoms at rest and during exercise did not differ among the four groups (Table 2). The median values of the maximum duration of chest symptoms were 30 min for the long-duration group and 6 min for the short-duration group. The median frequency of chest symptoms did not differ between these two groups (both 4 times/mo). The median frequency of chest symptoms was 12 times/mo for the high-frequency group and 2 times/mo for the low-frequency group. The median values of the maximum duration of chest symptoms did not differ between these two groups (both 10 min). The frequencies of VA and other serious symptoms, including cold sweats and syncope, were higher in the long-duration group than in the short-duration group (P = 0.0369 and P < 0.0001, respectively). The frequency of VA and other serious symptoms did not differ between the low- and high-frequency groups. The number of patients taking vasodilators before admission was similar across the four groups.
| Short-duration | Long-duration | P value | Low-frequency | High-frequency | P value | |
| Chest symptoms | ||||||
| Only at rest (%) | 88 (77) | 48 (91) | 0.0814 | 75 (85) | 61 (77) | 0.2360 |
| At rest and exercise (%) | 12 (11) | 1 (2) | 4 (5) | 9 (11) | ||
| Only during exercise (%) | 14 (12) | 4 (8) | 9 (14) | 9 (11) | ||
| Maximum duration (min) | 6 (5, 10) | 30 (20, 60) | < 0.0001 | 10 (6, 20) | 10 (5, 10) | 0.3633 |
| Frequency (/mo) | 4 (2, 12) | 4 (2, 10) | 0.1833 | 2 (1, 4) | 12 (8, 20) | < 0.0001 |
| Frequency of VA (%) | 0 (0) | 2 (4) | 0.0369 | 2 (3) | 0 (0) | 0.1776 |
| Other serious symptoms (%) | 3 (3) | 13 (25) | < 0.0001 | 12 (14) | 4 (5) | 0.0602 |
| No. taking vasodilators before admission | 0.5 (0, 1) | 0 (0, 1) | 0.1786 | 0 (0, 1) | 0 (0, 1) | 0.4895 |
Table 3 summarizes the angiographic and SPT findings. The frequencies of atherosclerotic changes and the presence of a myocardial bridge did not differ significantly among the groups. There were no significant differences in the angiographic and SPT-related parameters between the low- and high-frequency groups. However, spasm induction by L-ACh, TOC, focal spasm, and the unavoidable use of NTG were significantly higher in the long-duration group than in the short-duration group (P = 0.0444, P = 0.0113, P = 0.0006, and P = 0.0062, respectively). The frequency of multivessel spasms did not differ significantly between these two groups. Severe complications were experienced by 12 patients (7%), including ventricular fibrillation in one patient and unstable hemodynamics in eleven. The frequency of severe complications did not differ between the long- and short-duration groups.
| Short-duration | Long-duration | P value | Low-frequency | High-frequency | P value | |
| CAG | ||||||
| Atherosclerotic change (%) | 73 (64) | 34 (64) | 0.9884 | 53 (60) | 54 (68) | 0.7747 |
| Myocardial bridge (%) | 11 (11) | 5 (9) | 0.9646 | 9 (10) | 7 (9) | 0.7645 |
| SPT | ||||||
| Low dose of ACh (%) | 26 (23) | 20 (38) | 0.0444 | 26 (30) | 20 (25) | 0.5414 |
| Total occlusion (%) | 7 (6) | 10 (19) | 0.0113 | 6 (7) | 11 (14) | 0.1295 |
| Multivessel spasm (%) | 63 (62) | 30 (70) | 0.3189 | 50 (62) | 48 (68) | 0.4500 |
| (No.) | (n = 102) | (n = 50) | (n = 81) | (n = 71) | ||
| Focal/diffuse spasm | 12/102 | 17/36 | 0.0006 | 15/73 | 14/65 | 0.9083 |
| RCA spasm (%) | 78 (68) | 39 (74) | 0.4977 | 61 (69) | 56 (71) | 0.8252 |
| Unavoidable use of NTG (%) | 25 (32) | 23 (59) | 0.0062 | 20 (34) | 28 (49) | 0.096 |
| LAD spasm (%) | 96 (94) | 49 (98) | 0.2833 | 75 (93) | 70 (99) | 0.0783 |
| (No.) | (n = 102) | (n = 50) | (n = 81) | (n = 71) | ||
| Severe complications (%) | 7 (6) | 5 (9) | 0.4430 | 5 (6) | 7 (9) | 0.4211 |
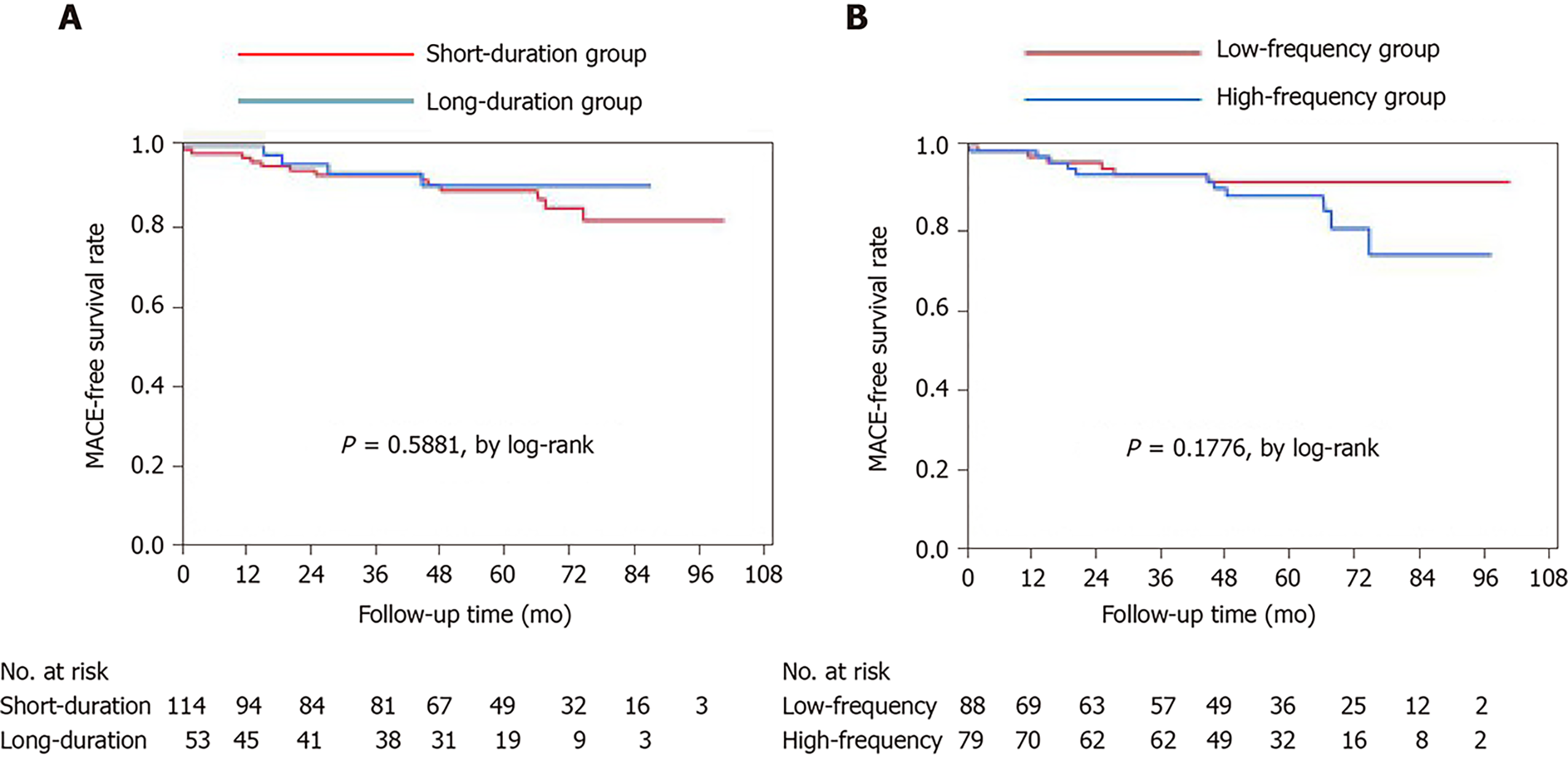
The numbers of patients taking vasodilators at discharge were similar in the four groups (Table 4). The median period of follow-up was 58 mo. Of the 167 patients, 122 (73%) were followed up at our institution through 2019, with no differences in the numbers and periods of follow-up among the groups. The median numbers of angina attacks and numbers of patients taking vasodilators were not significantly different among the groups. None of the patients experienced cardiac death during follow-up. The frequencies of noncardiac death and cardiac events requiring readmission for angina and heart failure or valvular heart disease did not differ among the four groups. Among all studied patients, the Kaplan-Meier survival curves showed no significant differences in the incidence of MACE among the groups (Figure 1).
| Short-duration | Long-duration | P value | Low-frequency | High-frequency | P value | |
| No. taking vasodilators at discharge | 1 (1, 1) | 1 (1, 1) | 0.9199 | 1 (1, 1) | 1 (1, 1) | 0.7862 |
| Median period of follow-up (mo) | 58 (36, 75) | 56 (34, 67) | 0.3505 | 54 (20, 75) | 59 (41, 71) | 0.4678 |
| Follow-up | ||||||
| No. of recent follow-ups (a, %) | 82 (71.9) | 40 (75.5) | 0.7547 | 60 (68.2) | 62 (78.5) | 0.2434 |
| No. of deaths during follow-up (b, %) | 5 (4.4) | 3 (5.6) | 4 (4.5) | 4 (5.0) | ||
| No. of follow-ups before 2018 (c, %) | 27 (23.7) | 10 (18.9) | 24 (27.3) | 13 (16.5) | ||
| No. of anginal attacks (/mo) in patients (a) | 0 (0, 1) | 0 (0, 1) | 0.4574 | 0 (0, 1) | 0 (0, 2.5) | 0.1305 |
| No. taking vasodilators among patients (a) | ||||||
| No. taking vasodilators among patients (a) at follow-up | 1 (1, 2) | 1 (1, 2) | 0.2131 | 1 (1, 2) | 1 (1, 2) | 0.7408 |
| Prognosis among patients (a) and (b) | ||||||
| Cardiac death (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Noncardiac death (%) | 5 (6) | 3 (7) | 0.7837 | 4 (6) | 4 (6) | 0.9642 |
| Cardiac events (%) | 13 (15) | 4 (9) | 0.3695 | 6 (9) | 11 (17) | 0.2176 |
This study investigated the clinical characteristics of patients with VSA who experienced prolonged chest symptoms lasting ≥ 15 min, including their symptoms, SPT-related parameters, and prognosis, and compared these with those of patients whose chest symptoms lasted < 15 min. The results showed that the VSA patients who experienced longer-duration chest symptoms had more serious symptoms and that they were more likely to have VA. In the SPT, these patients were more likely to experience spasms induced by L-ACh, TOC, focal spasms, and the unavoidable use of NTG. Thus, the maximum duration of episodes of chest symptoms may provide important information regarding higher VSA activity.
We performed a similar comparison in the same patient group between those who experienced chest symptoms fewer than four times per month (the median frequency of symptoms) and those who experienced symptoms more frequently. This did not show any significant differences. In addition, the maximum duration and frequency of chest symptoms had no influence on prognosis or the chest symptoms reported during follow-up.
There has been little investigation into the relationship between the maximum duration of chest symptoms and VSA activity. Myocardial ischemia related to the organic stenosis of a coronary artery is generally induced by an increase in oxygen demand relative to the amount of oxygen that can be supplied[10]. This mismatch in oxygen demand and supply can be caused by increases in blood pressure and heart rate due to exercise, anger, and low temperatures.
When a patient with organic coronary stenosis experiences chest symptoms, these can usually be controlled by reducing the factor causing the increase in oxygen demand. Thus, chest symptoms in patients with organic coronary stenosis are typically relieved within 15 min. Regarding VSA, exercise[11], smoking[12], hyperventilation[13], and alcohol consumption[14] are recognized as specific inducers of VSA. However, for most VSA patients, the onset of a coronary spasm is not triggered by a specific factor. Thus, chest symptoms in VSA patients may persist longer. In this study, we looked for factors that may contribute to longer chest symptoms, but we were unable to identify any.
Peripheral endothelial dysfunction has been shown to be associated with coronary endothelial dysfunction[15]. We therefore investigated peripheral endothelial function in most of the patients in this study, but the results suggested that this did not account for the longer-duration chest symptoms. However, there was a significant finding that a family history of coronary artery disease was more common among the VSA patients who experienced more frequent chest symptoms. We cannot account for this finding, and further investigation is needed.
It has been reported that cold sweating is a serious symptom in VSA[3,4], and it is widely recognized that VA is a significantly higher activity of VSA[16,17]. The presence of atherosclerotic changes[16], spasm induction by L-ACh[18], TOC[18], focal spasms[8,19] and multivessel spasm[16] in the SPT have been reported as factors associated with higher VSA activity or a poor prognosis. In the present study, the patients who experienced longer-duration chest symptoms had higher frequencies of spasms induced by L-ACh, TOC, and focal spasm. However, the frequencies of atherosclerotic changes and multivessel spasm did not differ from those of the patients who did not experience long-duration symptoms. In addition, the frequency of the unavoidable use of NTG was higher in the VSA patients who experienced longer-duration chest symptoms, although spasm provocation in the RCA was similar between the two groups.
Given the higher frequencies of these serious symptoms and SPT findings, the presence of longer-duration chest symptoms in patients with VSA is undoubtedly an important indicator suggestive of higher VSA activity. Conversely, classifying the patients according to the frequency of their chest symptoms showed no association with higher VSA activity. This may have been because the classification according to the median value of the frequency of chest symptoms may have been insufficient or because the frequency of chest symptoms in patients with VSA may not be a good indicator of VSA activity.
In this study, neither the maximum duration nor the frequency of chest symptoms showed associations with the numbers of patients using vasodilators, frequency of chest symptoms, or cardiac events such as readmission for cardiovascular disease or cardiac death. The low follow-up rate of the patients in this study and our aggressive medication approach may have contributed to these results. These results may also have been affected by the types of vasodilators used, the timing and frequency of their use, and whether they were brand-new or generic types of vasodilator[18,20]. However, even when these factors are taken into consideration, the severity and/or degree of chest symptoms before SPT may not reflect the long-term prognosis.
The present study has some clinical implications. Longer-duration chest symptoms are undoubtedly a clinically important sign for detecting patients with higher VSA activity. The patients with longer-duration chest symptoms exhibited high frequencies during the SPTs of several findings suggestive of a higher VSA activity; thus, provocation in these patients should be performed carefully, starting with a very low dose of ACh. At our institution, based on the duration of chest symptoms and/or other serious symptoms, we start the SPTs with a dose of ACh of 10 µg for the RCA and of 20 µg for the LCA.
The present study had several limitations. First, the SPTs started with the RCA. However, the guidelines for VSA[2] have recommended starting the SPT with the LCA. Thus, some of the results of the present study may not be generalizable to all patients with VSA.
Second, the definitions of duration and frequency of chest symptoms adopted in the present study are not universally accepted or consistently applied. The cutoff duration of 15 min used in this study was based on the VSA guidelines[2]. Conversely, the cutoff value used for the frequency of chest symptoms was simply the median value of four per month. In addition, the durations and frequencies of the symptoms were determined by questionnaires and thus may not be accurate. In addition, silent myocardial ischemia due to coronary spasm has been reported[11]; therefore, the symptom-dependent assessment of the degree of VSA activity may not be completely accurate.
Third, the unavoidable use of NTG was not determined by any objective parameters but by the judgment of the CAG operator. Finally, the rate of follow-up was not high, at 73%, and the results of the follow-up should be assessed in light of this low follow-up rate.
In conclusion, approximately 30% of the patients with VSA experienced chest symptoms that persisted longer than 15 min. These patients exhibited higher VSA activity. When taking the medical histories of patients with VSA, the cardiologist should record not only the frequency of chest symptoms but also their maximum duration.
Patients with vasospastic angina (VSA) sometimes experience prolonged chest symptoms compared with patients with atherosclerotic coronary sclerosis.
The clinical characteristics of VSA patients who have prolonged chest symptoms have not been clarified.
The objective of the present study was to clarify the clinical characteristics, including the results of the spasm provocation test (SPT) and prognosis, of VSA patients with prolonged chest symptoms.
This study included 167 patients with VSA diagnosed by SPT using acetylcholine and recorded the frequencies of positive reactions to a low dose of acetylcholine (L-ACh), total occlusion due to spasm (TOC), focal spasm, and the unavoidable use of nitroglycerin (unavoidable-NTG) during the SPT. The patients underwent a medical interview that investigated the maximum duration and frequency of chest symptoms as well as the frequencies of variant angina and other serious symptoms. The patients were divided into two groups based on the maximal duration: The short-duration group (< 15 min; n = 114) and the long-duration group (≥ 15 min; n = 53). They were also divided into two groups based on the frequency of chest symptoms: The low-frequency group (< 4/month; n = 88) and the high-frequency group (≥ 4/month; n = 79). Furthermore, prognosis including major cardiovascular events was investigated in the studied patients.
The long-duration group showed higher frequencies of other serious symptoms (P < 0.001) and variant angina (P < 0.05) as well as higher frequencies of spasm induction by L-ACh (P < 0.05), TOC (P < 0.05), focal spasm (P < 0.01), and unavoidable-NTG (P < 0.01) than the short-duration group. These parameters did not differ significantly between the low-frequency and high-frequency groups. On the other hand, neither the duration nor frequency of chest symptoms influenced the prognosis in the studied patients.
These findings suggest that patients with VSA who experience prolonged chest symptoms may have more severe characteristics of VSA. Cardiologists should keep this in mind and be more careful in performing the SPT in such patients.
We would like to thank Ms. Akemi Seno for her secretarial help.
| 1. | Teragawa H, Oshita C, Ueda T. The Significance of Recognizing Myocardial Bridge in the Coronary Spasm Diagnosis in Myocardial Infarction with Nonobstructive Coronary Arteries. Intern Med. 2020;59:89-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 3. | Nishiyama C, Iwami T, Kawamura T, Kitamura T, Tanigawa K, Sakai T, Hayashida S, Nishiuchi T, Hayashi Y, Hiraide A. Prodromal symptoms of out-of-hospital cardiac arrests: a report from a large-scale population-based cohort study. Resuscitation. 2013;84:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Sueda S, Kohno H. Impact of pharmacological spasm provocation test in patients with a history of syncope. Heart Vessels. 2018;33:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Teragawa H, Oshita C, Ueda T. History of gastroesophageal reflux disease in patients with suspected coronary artery disease. Heart Vessels. 2019;34:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Teragawa H, Oshita C, Ueda T. The Myocardial Bridge: Potential Influences on the Coronary Artery Vasculature. Clin Med Insights Cardiol. 2019;13:1179546819846493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Teragawa H, Fukuda Y, Matsuda K, Hirao H, Higashi Y, Yamagata T, Oshima T, Matsuura H, Chayama K. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol. 2003;26:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, Ohba K, Tsujita K, Kojima S, Tayama S, Hokimoto S, Matsui K, Sugiyama S, Yamabe H, Ogawa H. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013;2:e000227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Teragawa H, Kato M, Kurokawa J, Yamagata T, Matsuura H, Chayama K. Usefulness of flow-mediated dilation of the brachial artery and/or the intima-media thickness of the carotid artery in predicting coronary narrowing in patients suspected of having coronary artery disease. Am J Cardiol. 2001;88:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Sandoval Y, Jaffe AS. Type 2 Myocardial Infarction: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:1846-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 11. | Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 167] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Sugiishi M, Takatsu F. Cigarette smoking is a major risk factor for coronary spasm. Circulation. 1993;87:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Nakao K, Ohgushi M, Yoshimura M, Morooka K, Okumura K, Ogawa H, Kugiyama K, Oike Y, Fujimoto K, Yasue H. Hyperventilation as a specific test for diagnosis of coronary artery spasm. Am J Cardiol. 1997;80:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Mizuno Y, Harada E, Morita S, Kinoshita K, Hayashida M, Shono M, Morikawa Y, Murohara T, Nakayama M, Yoshimura M, Yasue H. East asian variant of aldehyde dehydrogenase 2 is associated with coronary spastic angina: possible roles of reactive aldehydes and implications of alcohol flushing syndrome. Circulation. 2015;131:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol. 2005;28:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm Association. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62:1144-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Ahn JM, Lee KH, Yoo SY, Cho YR, Suh J, Shin ES, Lee JH, Shin DI, Kim SH, Baek SH, Seung KB, Nam CW, Jin ES, Lee SW, Oh JH, Jang JH, Park HW, Yoon NS, Cho JG, Lee CH, Park DW, Kang SJ, Lee SW, Kim J, Kim YH, Nam KB, Lee CW, Choi KJ, Song JK, Kim YH, Park SW, Park SJ. Prognosis of Variant Angina Manifesting as Aborted Sudden Cardiac Death. J Am Coll Cardiol. 2016;68:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Teragawa H, Oshita C, Ueda T. Coronary spasm: It's common, but it's still unsolved. World J Cardiol. 2018;10:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Kim DW, Her SH, Ahn Y, Shin DI, Han SH, Kim DS, Choi DJ, Kwon HM, Gwon HC, Jo SH, Rha SW, Baek SH. Clinical outcome according to spasm type of single coronary artery provoked by intracoronary ergonovine tests in patients without significant organic stenosis. Int J Cardiol. 2018;252:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Goto-Semba R, Fujii Y, Ueda T, Oshita C, Teragawa H. Increased frequency of angina attacks caused by switching a brand-name vasodilator to a generic vasodilator in patients with vasospastic angina: Two case reports. World J Cardiol. 2018;10:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sueda S S-Editor: Gong ZM L-Editor: A P-Editor: Li JH