Published online Sep 26, 2020. doi: 10.4330/wjc.v12.i9.437
Peer-review started: February 28, 2020
First decision: May 28, 2020
Revised: June 11, 2020
Accepted: August 24, 2020
Article in press: August 24, 2020
Published online: September 26, 2020
Processing time: 205 Days and 20.2 Hours
Repolarization heterogeneity (RH) is an intrinsic property of ventricular myocardium and the reason for T-wave formation on electrocardiogram (ECG). Exceeding the physiologically based RH level is associated with appearance of life-threatening ventricular arrhythmias and sudden cardiac death. In this regard, an accurate and comprehensive evaluation of the degree of RH parameters is of importance for assessment of heart state and arrhythmic risk. This review is devoted to comprehensive consideration of RH phenomena in terms of electrophysiological processes underlying RH, cardiac electric field formation during ventricular repolarization, as well as clinical significance of RH and its reflection on ECG parameters. The formation of transmural, apicobasal, left-to-right and anterior-posterior gradients of action potential durations and end of repolarization times resulting from the heterogenous distribution of repolarizing ion currents and action potential morphology throughout the heart ventricles, and the different sensitivity of myocardial cells in different ventricular regions to the action of pharmacological agents, temperature, frequency of stimulation, etc., are being discussed. The review is focused on the fact that RH has different aspects – temporal and spatial, global and local; ECG reflection of various RH aspects and their clinical significance are being discussed. Strategies for comprehensive assessment of ventricular RH using different ECG indices reflecting various RH aspects are presented.
Core Tip: A comprehensive assessment of ventricular repolarization process is an important part of electrocardiogram (ECG) diagnostics. First of all, the increased repolarization heterogeneity is associated with arrhythmogenesis. Besides, repolarization disturbances reflect the degree of electric remodeling of myocardium related to heart failure degree and mortality. We herein discuss the electrophysiological basis for repolarization heterogeneity and the factors that modulate it. We demonstrate that repolarization heterogeneity has various aspects – temporal and spatial, global and local, and there is a need in different ECG-indices to evaluate all the aspects.
- Citation: Arteyeva NV. Dispersion of ventricular repolarization: Temporal and spatial. World J Cardiol 2020; 12(9): 437-449
- URL: https://www.wjgnet.com/1949-8462/full/v12/i9/437.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i9.437
Repolarization process is cardinally different from depolarization. During depolarization, the elementary (cellular) electric field generators responsible for QRS complex formation are concentrated in narrow (approximately 0.8-1 mm) regions of space that separates the excited myocardium (cells with peak action potential) from unexcited one (cells with resting potential)[1]. In contrast, during repolarization the elementary electric generators are dispersed in almost the entire volume of the ventricles, with small gradients in membrane potential between the neighbouring cells. All ventricular cells, the repolarization of which is not yet completed, contribute to cardiac electric field generation.
T-wave is a result of repolarization heterogeneity (RH) – non-simultaneous end-of-repolarization in different ventricular layers and regions. This heterogeneity arises from: (1) Different activation times; and (2) Different action potential duration (APD) of ventricular cells, due to the heterogeneous distribution of repolarizing currents[2]. The global RH in the heart ventricles is defined by the areas of the earliest and the latest repolarization – the difference in end-of-repolarization times in these areas and in their location (temporal and spatial heterogeneity, correspondingly).
In normal heart, physiological heterogeneities in structure, electrical and mechanical activity are crucial for normal, efficient excitation and pumping[3]. Due to multiple reasons (impaired function of outward K+ currents in cardiac myocytes, which may be caused by genetic defects or result from various acquired pathophysiological conditions, including electrical remodelling in cardiac disease, ion channel modulation by clinically used pharmacological agents, and systemic electrolyte disorders seen in heart failure, such as hypokalaemia), the level of RH could increase[4].
Exceeding the physiologically reasonable level of RH could lead to the development of life-threatening ventricular arrhythmias[4-6]. In this regard, an accurate and comprehensive evaluation of RH on the basis of electrocardiogram (ECG) is of importance. This review focuses on various aspects of RH (temporal and spatial, global and local) – their electophysiological basis, ECG reflection and clinical significance.
The reason for different action potential morphology and different sensitivity of myocardial cells to the action of pharmacological agents, temperature, frequency of stimulation, etc. is the heterogenous distribution of repolarizing ion currents throughout the heart ventricles. There are differences in repolarizing currents across ventricular walls[7,8], between the left and the right ventricles, between the apex and the base of the ventricles, and between anterior and posterior ventricular surface[9,10].
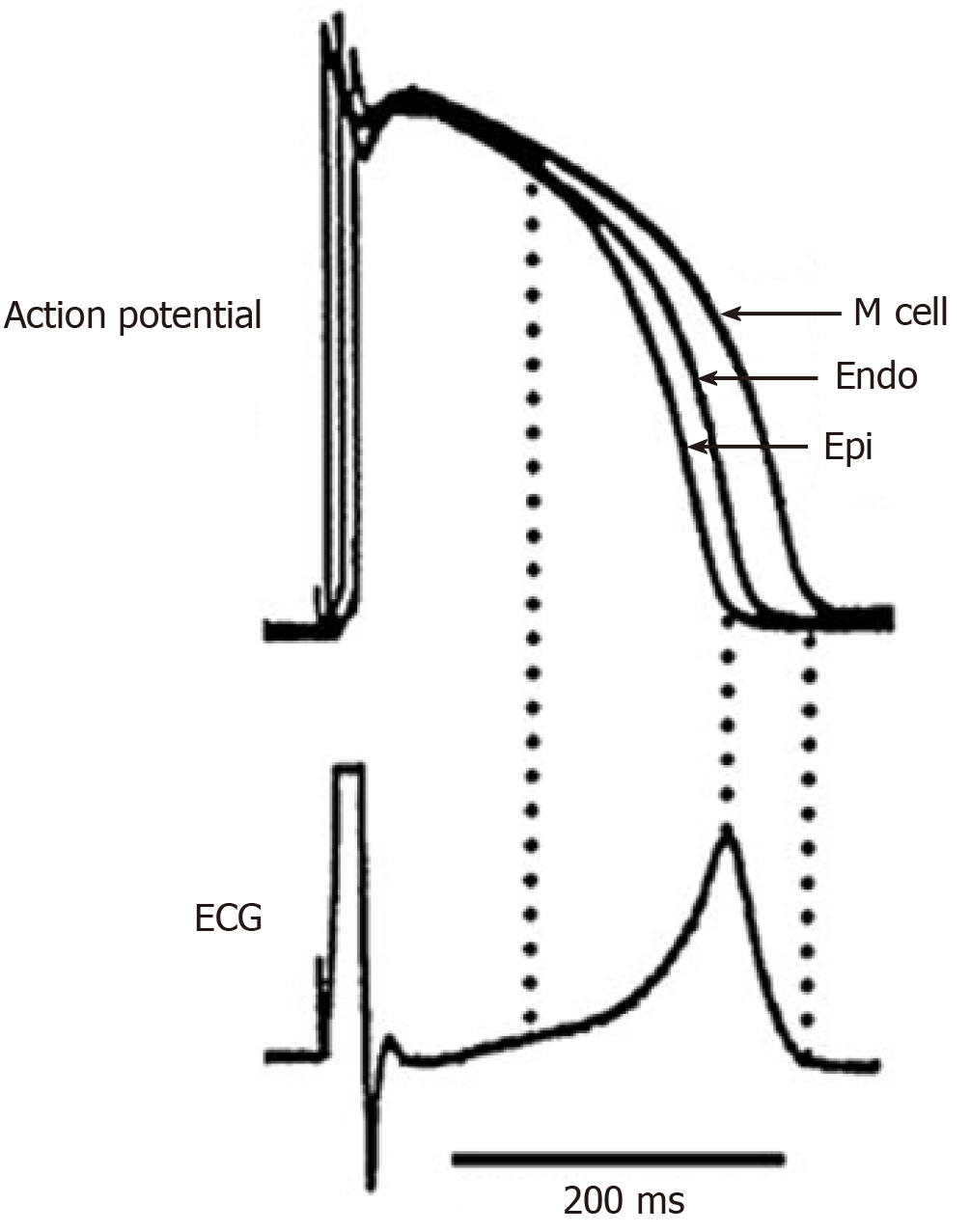
In transmural plane, in vitro studies revealed three types of cells: Epicardial (with the shortest APD), endocardial and M-cells with the longest APD, belonging to the deep layers of the myocardium (Figure 1)[7,8]. In interventricular septum, M-cells were less pronounced than in the free walls of the ventricles[11]. In epicardial and M-cells, the morphology of phase 1 is characterized by a prominent transient outward current (Ito)-mediated notch responsible for the ‘spike and dome’ morphology[8]. M cells are distinguished from the other cell types in that they display a smaller slowly activating delayed rectifier current (IKs), but a larger late sodium current (late INa) and sodium-calcium exchange current (INa-Ca). These ionic distinctions underlie the longer APD and steeper APD-rate relationship of the M-cells, which is more pronounced in the presence of antiarrhythmic agents with class III actions[8].
In vivo experiments did not confirm the existence of M-cells and a substantial transmural APD gradient[12-14]. This fact can be explained, firstly, by electrotonic interaction in myocardium in vivo, which partially eliminates intrinsic differences in the electrophysiological properties of the cells across ventricular wall[15,16]. Secondly, M-cells can be functionally detected at a low frequency of stimulation, while at physiological frequencies, transmural electrophysiological differences between the cells are significantly reduced[17,18]. It should also be noted that APD recorded in vivo is always significantly less than those recorded in vitro[14].
At the same time, in vivo as well as in vitro studies confirm the existence of apicobasal, anteroposterior and left-to-right differences in repolarizing ion currents[12,19-22]. Apico-basal differences were found in the expression of those channel proteins which are involved in mediation of the transient outward K(+) current and the slow delayed rectifier K(+) current: Expression of Kv1.4, KChIP2, KvLQT1 and MinK was significantly higher in apical than in basal myocardium in both canine and human hearts[19]. Prominent differences in the magnitude of the I(to) 1-mediated action potential notch were found in cells isolated from the right and the left canine ventricular epicardium; the influence of this current, although small, is more important in the left ventricle[20-22].
Transmural APD gradient is mostly pronounced in isolated myocardial cells and wedge preparations extracted from different ventricular regions – left ventricle[23-25], right ventricle[26], interventricular septum[27,28]; it is resulted from APD differences between epi- and M-cells (in vitro), and between epi- and endo cells (in vivo). The magnitude of transmural APD gradient recorded in vitro reached 100 ms and more[24], and it depended on the wall thickness (the largest transmural APD gradient was recorded in the interventricular septum, the smallest one - in the right ventricle[28,29]) and location (transmural APD gradient was different at the apex and at the base of the ventricles[23]).
The transmural APD gradient is even attributed a key role in T-wave formation and it is assumed as “the symbol of repolarization dispersion"[30,31]. Although, this is true only for a ventricular wedge preparation (Figure 1), but in the whole heart dispersion of repolarization (DOR) and T-wave are resulted from several gradients[32-34]. In vivo experiments did not reveal a substantial transmural APD gradient in the heart ventricles[12-14].
Apicobasal gradient was detected in almost all animal and humans studies. However, its direction was found to be different in various species and sometimes controversial. APD recorded at the apex were longer than those recorded at the base of the ventricles in human[35-37], rabbit[38,39], dog[12,13], and pig[40]. In other studies, the apical APD were shorter than the basal ones in rabbit[41], pig[42], guinea pig[43], rats[44,45], and chicken[46]. The controversial direction of apicobasal APD gradient in the same species can be explained by the high sensitivity of repolarization to temperature conditions, which could vary in different studies. In some cases, apicobasal gradient was dominating and responsible for cardiac electric field formation[47,48].
Along with the transmural and apicobasal gradients, the left-to-right gradient was revealed in human and several animal species. APD in the right ventricle were longer than in the left ventricle in human[49], rabbit[50], pig[40] and guinea pig[51]. The opposite interventricular gradient was recorded in dog[52,53] and rat[44,45].
Activation sequence affects RH in two ways. First, it contributes to repolarization sequence, because end of repolarization time of myocardial cell is a sum of activation time and APD, and repolarization gradients are combinations of activation and APD gradients. Second, activation sequence can directly effect on APD magnitude, especially at heart stimulation. APD were longer in the center of stimulation, and decreased towards the periphery[57]. The transfer of stimulus from endo- to epicardium prolonged epicardial APD and shortened endocardial APD, and, correspondingly, changed the transmural repolarization gradient[16,58,59]. The reversed activation sequence mostly affected APD of M cells[58]. Thus, earlier activation was associated with longer APD. Nevertheless, the relationship between early activation and longer APD is ambiguous: In rabbit hearts, repolarization sequence in general corresponded to those of depolarization, i.e., the shorter APD were associated with the earlier activation times[60].
Repolarization gradients in the heart ventricles responsible for T-wave genesis are formed as a result of superimposed gradients of activation times and APD. Nevertheless, the magnitudes of APD gradients usually exceed the magnitudes of activation gradients, therefore APD gradients determine the sequence of repolarization to a greater extent, and changes in repolarization occur almost always because of APD changes.
The analysis of contribution of different parts of the canine heart ventricles to dispersion in repolarization times showed that transmural gradient contributed only 13% to the total DOR, while apicobasal, interventricular, and anterior-posterior gradients contributed the remaining 87%[54]. Simulation studies support that transmural, apicobasal, interventricular and anteroposterior repolarization gradients are all essential to T-wave genesis[32-34].
Repolarization is rather sensitive than depolarization to the changes in external and internal conditions such as fluctuations in temperature, concentration of various ions, heart rate, electrical remodeling associated with various pathologies. Inhomogeneous changes in action potentials’ morphology modify and amplify the temporal and/or spatial heterogeneity of repolarization. Exceeding the physiologically based level of RH can lead to the development of life-threatening ventricular arrhythmias[5,6]. In this regard, the analysis of both temporal and spatial RH parameters is of importance.
In experimental diabetes mellitus, there were substantial changes in spatial but not in temporal repolarization gradients. In mice, there were increased apicobasal and left-to-right gradients[61]; in rabbit, apicobasal gradient was decreased but a large anteroposteral gradient arised[62-64].
At electrical heart stimulation, the location of stimulus effected on APD and, correspondingly, on repolarization gradients: APD were longer in the center of stimulation, and decreased towards the periphery[57,59,65].
In Tako-Tsubo cardiomyopathy, the ischemic-like Wellens’ ECG pattern coincides and quantitatively correlates with apicobasal gradient of myocardial edema as evidenced by using cardiovascular magnetic resonance imaging[66]; dynamic negative T-waves and QTc prolongation are likely to reflect the edema-induced transient inhomogeneity and an increased RH between apical and basal left ventricular regions. An increase in apicobasal repolarization gradient on endo- and epicardium was also found in patients with cardiomyopathy and ventricular arrhythmia vulnerability[67]. In Brugada syndrome, APD shortening in the right ventricle strengthens the left-to-right repolarization gradient and spatial RH[68].
In hypertrophic cardiomyopathy, ECG analysis allowed to reveal the mechanism of cardiomyopathy: Ionic remodelling and action potential prolongation in hypertrophied apical and septal areas (T-wave inversion with normal QRS complex), or abnormal Purkinje-myocardial coupling causing abnormal QRS morphology in leads V4-V6[69].
In hypothermia, which is used for protection of myocardium from hypoxic injury, APD of all myocardial cells, including conducting system and pacemakers, prolong nonuniformly as a result of an increase in repolarizing currents[70,71]; the nonuniform APD prolongation leads to the increase in both temporal and spatial RH[5,72,73]. Epicardial APD prolong to the larger extent than endocardial ones, resulting in the inversion of transmural repolarization gradient at hypothermia[30]. Apicobasal, left-to-right and anteroposteral repolarization gradients were inversed at hypothermia, too[73]. Earlier, T-wave inversion at hypothermia was associated with the inversion of transmural[30] or apicobasal[73] repolarization gradients. The recent in silico studies demonstrated that transmural repolarization gradient do not play a crucial role in the cardiac electric field inversion under hypothermia, and the inversion of epicardial repolarization gradients (apicobasal, anterior-posterior and interventricular) causes T-wave inversion regardless of transmural gradient direction[74].
In hypoxia/ischemia, APD shortening is associated with electrolyte imbalance in conditions of oxygen supply termination/limitation[75], and increase in extracellular potassium concentration[76]. Hyperkalemia leads to sodium channels’ inactivation and slower conduction velocity[77], as well as to shorter repolarization, since it enhances potassium currents[77,78]. In addition, APD shortening at hypoxia may be associated with the release of catecholamines, which enhance the calcium-dependent chlorine current ICl (Ca) and activate the cAMP-dependent chlorine current ICl (cAMP)[79]. In vitro studies showed that subepicardial layers were more sensible to ischemia than subebdocardial ones[80,81], although in vivo there were no transmural differences in response to ischemia[82]. At ischemia, a significant increase in left-to-right repolarization gradient was observed[83]. In general, ischemia enhanced both temporal and spatial RH[84].
The quantitative temporal measure of RH is DOR – the time difference between the earliest and the latest end of repolarization in the heart ventricles. A number of experimental studies demonstrated that an increased DOR promotes arrhythmogenic substrate formation[4-6]. Table 1 summarizing ECG-indices with their ability to evaluate the degree and the nature of ventricular RH and the degree of arrhythmic risk.
| Repolarization heterogeneity aspect | T-wave index | Cut-off values (arrhythmogenesis) |
| Maximal end of repolarization/maximal APD values | QT | 450 ms (males), 460 ms (females)[119] |
| Maximal end of repolarization/maximal APD values in conditions of QRS widening | JTend | |
| The proportion between the minimal and the maximal APD | Tpeak-Tend/QT | 0.22[122]; 0.23[123]; 0.31[114] |
| T-wave symmetry | ≤ 1.7[98] | |
| The global repolarization dispersion | Tpeak-Tend | ≥ 103.3 ± 17.4 ms[86,87]; ≥ 142 ms[114] |
| T-wave amplitude and T-wave area, calculated on the basis of T-vector or Root Mean Square ECG | ||
| Ventricular gradient, or QRS-T integral | ||
| Local differences in repolarization dispersion | Tpeak-Tend dispersion | 35 ms[113]; 42 ms[114] |
| Lead-to-lead differences in Tpeak and Tend instants between adjacent leads | ||
| Local differences in the end of repolarization times | QT dispersion | 39 ms[114]; 93 ms[124] |
| Early repolarization (plateau phase) | JTpeak | |
| The difference in the spatial sequence of depolarization and repolarization | Spatial QRS-T angle | 135°[89,94-99] |
| The general direction of repolarization sequence | T-vector projection onto the heart ventricles | |
| T-loop complexity | ||
| The relative magnitudes of apicobasal, anterior-posterior and left-to-right repolarization gradients | Ratio between X, Y, Z components of T-vector | |
| The location or the areas of the shortest and the longest APD | T-vector projection onto the heart ventricles | |
| Electrical instability of ventricular myocardium at cellular level | Macrovolt and microvolt T-wave alternans | |
| Beat-to-beat T-vector variability |
The most “traditional”, but perhaps the least accurate index of DOR is QT interval dispersion. Because of the low reproducibility of clinical data, almost two decades ago it was concluded that QT dispersion gives a poor assessment of DOR[85,86]. From theoretical viewpoint, QT dispersion reflects local differences in the latest (T-wave end), but not the earliest repolarization; thus, it reflects DOR only partially.
The more accurate index of DOR is Tpeak-Tend interval - a useful arrhythmic risk stratification tool in a wide variety of pathologies[87-89]. It was proven both experimentally and in silico that Tpeak-Tend directly reflects DOR magnitude[56,90-92]. Although, a serious problem in using Tpeak-Tend for diagnostics is the discrepancy between the cut-off values resulting from different T-end determining method (baseline or tangent) as well as different number of ECG leads involved in calculations. In some studies, Tpeak-Tend was not a predictor of arrhythmia[93,94]; however, this does not decease its clinical significance, but suggests that mechanisms of triggering arrhythmias are not necessarily associated with increased DOR, and the search for new arrhythmogenic indices should be continued. The alternative relative assessments of DOR magnitude are T-wave amplitude, width, area and symmetry[95-99] (Table 1).
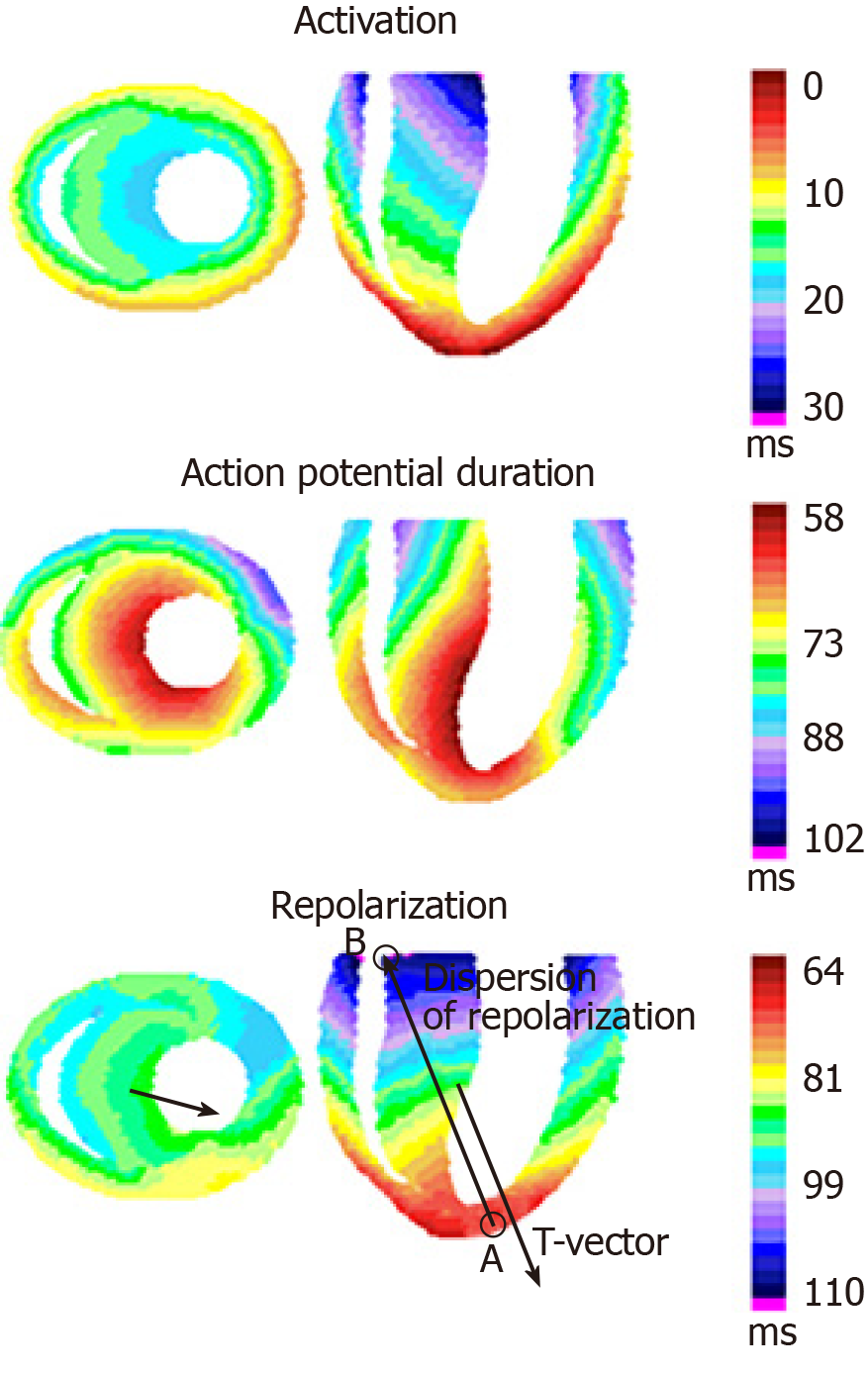
Traditionally, the term DOR is associated with temporal RH. However, since the regions of early and late repolarization differ both in time and location, DOR is a vectorial parameter, directed from point A (the region of the earliest end of repolarization) to point B (the region of the latest end of repolarization) (Figure 2). The spatial characteristic of RH is T-vector of vectorcardiogram – a three-dimensional total electric vector of ventricular repolarization, which can be calculated on the basis of standard ECG set[100].
T-vector amplitude is not directly equal to DOR: The first is calculated in mV, and the second in ms. However, from physical viewpoint, T-vector amplitude must be proportional to DOR magnitude, and the relationship between T-vector components (Tx, Ty and Tz) must reflect the proportion between ventricular repolarization gradients in corresponding directions.
T-vector direction reflects the general sequence of repolarization, but in the opposite way: T-vector is oriented from the regions of late repolarization towards the regions of early repolarization (Figure 2). Substantial changes in T-vector direction, even if DOR magnitude is within normal range (e.g., experimental Diabetes Mellitus[61-64]), indicate a large-scale electrical heart remodeling.
T-vector provides important information in addition to “scalar” DOR value[101]: The amplitudes of cardiac potentials’ peaks and the time of their occurrence on ECG depend on lead location, while vectorcardiogram provides objective, “weighted” values; Ventricular gradient (three-dimensional QRS-T integral) reflects the distribution of the action potentials’ morphology in the heart ventricles[102]; ST-vector reflects the presence and peculiarities of ischemia; A distorted, twisted T-loop (the trajectory of T-vector projections on anatomical planes during ventricular repolarization) indicates pathological repolarization, while normal T-loop has a correct smoothed shape[103-105].
Besides T-vector direction itself, the angle between T-vector and QRS-vector (QRS-T angle) is highly informative regarding spatial RH[106,107]. In healthy people, repolarization is practically opposite to depolarization, and QRS-T angle is relatively small (≤ 105°)[101,108]. An increased QRS-T angle (≥ 135°) indicates the changes in repolarization sequence, and, correspondingly, the changes in repolarization gradients resulted from electrophysiological disturbances in ventricular myocardium – the altered distribution if ion channels and action potentials’ durations[105,109]. An increased QRS-T angle was shown to be the most reliable predictor of the risk of life-threatening arrhythmias and death from heart disease compared with other ECG parameters[105,109-111].
DOR magnitude along with T-vector reflects the total (global) temporal and spatial repolarization pattern in the heart ventricles, but do not reflect the local electrophysiological heterogeneities. At the same time, increase in local RH may be more relevant for arrhythmia development than increase in global DOR: The regions with the greatest local repolarization time differences often serve as sources for ectopic beats and Torsade de pointes[111-113].
The same condition (e.g., myocardial ischemia) can lead to the increase in both local and global DOR, and in such a case the global and local repolarization changes are hardly distinguishable, and specific novel markers for local DOR magnitude are need. Dispersion of Tpeak-Tend interval (the difference between the earliest Tpeak and the latest Tend among 12 standard leads) was proposed as a possible specific marker for the local DOR[114,115]. Besides, mathematical simulations showed that local increase in DOR can be expressed in increased lead-to‑lead differences in Tpeak and Tend instants between adjacent anatomically ordered standard leads [aVL, I, aVR(-), II, aVF, III, and V1-V6], even if global DOR, Tpeak-Tend interval and Tpeak-Tend dispersion are within a normal range[116].
In some cases, indices characterizing duration and morphology of action potentials (QT, JTpeak and JTend intervals)[117-120], as well as electrical instability of ventricular myocardium at cellular level (macrovolt and microvolt T-wave alternans, beat-to-beat T-vector variability)[121,122] may be of clinical importance (Table 1).
Both temporal (the time difference between the earliest and the latest end of repolarization in the whole ventricles, and the local differences in end of repolarization times) and spatial (the general direction of ventricular repolarization sequence and the relative magnitudes of repolarization gradients) heterogeneity of ventricular repolarization are of clinical importance. The complex use of different ECG indices (Tpeak-Tend interval and its dispersion, T-vector and T-loop parameters, QRS-T angle, etc.) provides information about temporal and spatial, global and local characteristics of ventricular repolarization for better heart state assessment.
| 1. | Vander Ark CR, Reynolds EW. An experimental study of propagated electrical activity in the canine heart. Circ Res. 1970;26:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 744] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 3. | Dressler FF, Brado J, Odening KE. Electromechanical heterogeneity in the heart: A key to long QT syndrome? Herzschrittmacherther Elektrophysiol. 2018;29:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Osadchii OE. Role of abnormal repolarization in the mechanism of cardiac arrhythmia. Acta Physiol (Oxf). 2017;220 Suppl 712:1-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Han J, Moe GK. Nonuniform Recovery of Excitability in Ventricular Muscle. Circ Res. 1964;14:44-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 653] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Nanke T, Nakazawa K, Arai M, Ryuu S, Osada K, Sakurai T, Miyake F. Clinical significance of the dispersion of the activation--recovery interval and recovery time as markers for ventricular fibrillation susceptibility in patients with Brugada syndrome. Circ J. 2002;66:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Brahmajothi MV, Morales MJ, Reimer KA, Strauss HC. Regional localization of ERG, the channel protein responsible for the rapid component of the delayed rectifier, K+ current in the ferret heart. Circ Res. 1997;81:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Strom M, Wan X, Poelzing S, Ficker E, Rosenbaum DS. Gap junction heterogeneity as mechanism for electrophysiologically distinct properties across the ventricular wall. Am J Physiol Heart Circ Physiol. 2010;298:H787-H794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Morita ST, Zipes DP, Morita H, Wu J. Analysis of action potentials in the canine ventricular septum: no phenotypic expression of M cells. Cardiovasc Res. 2007;74:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Bauer A, Becker R, Karle C, Schreiner KD, Senges JC, Voss F, Kraft P, Kuebler W, Schoels W. Effects of the I(Kr)-blocking agent dofetilide and of the I(Ks)-blocking agent chromanol 293b on regional disparity of left ventricular repolarization in the intact canine heart. J Cardiovasc Pharmacol. 2002;39:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JM, Plotnikov AN, Shlapakova IN, Danilo P, Tijssen JG, Rosen MR. Repolarization gradients in the canine left ventricle before and after induction of short-term cardiac memory. Circulation. 2005;112:1711-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Voss F, Opthof T, Marker J, Bauer A, Katus HA, Becker R. There is no transmural heterogeneity in an index of action potential duration in the canine left ventricle. Heart Rhythm. 2009;6:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Joyner RW. Modulation of repolarization by electrotonic interactions. Jpn Heart J. 1986;27 Suppl 1:167-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Myles RC, Bernus O, Burton FL, Cobbe SM, Smith GL. Effect of activation sequence on transmural patterns of repolarization and action potential duration in rabbit ventricular myocardium. Am J Physiol Heart Circ Physiol. 2010;299:H1812-H1822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 294] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res. 1993;72:671-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 266] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Szentadrassy N, Banyasz T, Biro T, Szabo G, Toth BI, Magyar J, Lazar J, Varro A, Kovacs L, Nanasi PP. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res. 2005;65:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Di Diego JM, Sun ZQ, Antzelevitch C. I(to) and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548-H561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Medei E, Marocolo M, Rodrigues Dde C, Arantes PC, Takiya CM, Silva J, Rondinelli E, Goldenberg RC, de Carvalho AC, Nascimento JH. Chronic treatment with anabolic steroids induces ventricular repolarization disturbances: cellular, ionic and molecular mechanism. J Mol Cell Cardiol. 2010;49:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Martin CA, Siedlecka U, Kemmerich K, Lawrence J, Cartledge J, Guzadhur L, Brice N, Grace AA, Schwiening C, Terracciano CM, Huang CL. Reduced Na(+) and higher K(+) channel expression and function contribute to right ventricular origin of arrhythmias in Scn5a+/- mice. Open Biol. 2012;2:120072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Sekiya S, Ichikawa S, Tsutsumi T, Harumi K. Nonuniform action potential durations at different sites in canine left ventricle. Jpn Heart J. 1983;24:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res. 2010;106:981-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 25. | Di Diego JM, Sicouri S, Myles RC, Burton FL, Smith GL, Antzelevitch C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J Mol Cell Cardiol. 2013;54:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Li GR, Feng J, Yue L, Carrier M. Transmural heterogeneity of action potentials and Ito1 in myocytes isolated from the human right ventricle. Am J Physiol. 1998;275:H369-H377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Ramakers C, Stengl M, Spätjens RL, Moorman AF, Vos MA. Molecular and electrical characterization of the canine cardiac ventricular septum. J Mol Cell Cardiol. 2005;38:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Sicouri S, Glass A, Ferreiro M, Antzelevitch C. Transseptal dispersion of repolarization and its role in the development of Torsade de Pointes arrhythmias. J Cardiovasc Electrophysiol. 2010;21:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Wan X, Bryant SM, Hart G. A topographical study of mechanical and electrical properties of single myocytes isolated from normal guinea-pig ventricular muscle. J Anat. 2003;202:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Higuchi T, Nakaya Y. T wave polarity related to the repolarization process of epicardial and endocardial ventricular surfaces. Am Heart J. 1984;108:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Antzelevitch C. Transmural dispersion of repolarization and the T wave. Cardiovasc Res. 2001;50:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Okada J, Washio T, Maehara A, Momomura S, Sugiura S, Hisada T. Transmural and apicobasal gradients in repolarization contribute to T-wave genesis in human surface ECG. Am J Physiol Heart Circ Physiol. 2011;301:H200-H208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Arteyeva NV, Azarov JE, Vityazev VA, Shmakov DN. Action potential duration gradients in the heart ventricles and the cardiac electric field during ventricular repolarization (a model study). J Electrocardiol. 2015;48:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Zheng Y, Wei D, Zhu X, Chen W, Fukuda K, Shimokawa H. Transmural, interventricular, apicobasal and anteroposterior action potential duration gradients are all essential to the genesis of the concordant and realistic T wave: A whole-heart model study. J Electrocardiol. 2016;49:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Franz MR, Bargheer K, Rafflenbeul W, Haverich A, Lichtlen PR. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 1987;75:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 213] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Cowan JC, Hilton CJ, Griffiths CJ, Tansuphaswadikul S, Bourke JP, Murray A, Campbell RW. Sequence of epicardial repolarisation and configuration of the T wave. Br Heart J. 1988;60:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Meijborg VM, Conrath CE, Opthof T, Belterman CN, de Bakker JM, Coronel R. Electrocardiographic T wave and its relation with ventricular repolarization along major anatomical axes. Circ Arrhythm Electrophysiol. 2014;7:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama I. Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilideclass III agents. Cardiovasc Res. 1999;43:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Dressler FF, Bodi I, Menza M, Moss R, Bugger H, Bode C, Behrends JC, Seemann G, Odening KE. Interregional electro-mechanical heterogeneity in the rabbit myocardium. Prog Biophys Mol Biol. 2017;130:344-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Newton JC, Johnson PL, Justice RK, Smith WM, Ideker RE. Estimated global epicardial distribution of activation rate and conduction block during porcine ventricular fibrillation. J Cardiovasc Electrophysiol. 2002;13:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Azarov JE, Shmakov DN, Vityazev VA, Roshchevskaya IM, Roshchevsky MP. Activation and repolarization patterns in the ventricular epicardium under sinus rhythm in frog and rabbit hearts. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Kongstad O, Xia Y, Liu Y, Liang Y, Olsson B, Yuan S. Ventricular repolarization sequences on the epicardium and endocardium. Monophasic action potential mapping in healthy pigs. J Electrocardiol. 2012;45:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Kanai A, Salama G. Optical mapping reveals that repolarization spreads anisotropically and is guided by fiber orientation in guinea pig hearts. Circ Res. 1995;77:784-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Watanabe T, Delbridge LM, Bustamante JO, McDonald TF. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983;52:280-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Donohoe P, Hendry BM, Walgama OV, Bertaso F, Hopster DJ, Shattock MJ, James AF. An altered repolarizing potassium current in rat cardiac myocytes after subtotal nephrectomy. J Am Soc Nephrol. 2000;11:1589-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Kharin SN. Depolarisation and repolarisation sequences of ventricular epicardium in chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Integr Physiol. 2004;137:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Noble D, Cohen I. The interpretation of the T wave of the electrocardiogram. Cardiovasc Res. 1978;12:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Kongstad O, Xia Y, Liang Y, Hertervig E, Ljungström E, Olsson B, Yuan S. Epicardial and endocardial dispersion of ventricular repolarization. A study of monophasic action potential mapping in healthy pigs. Scand Cardiovasc J. 2005;39:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Chen PS, Moser KM, Dembitsky WP, Auger WR, Daily PO, Calisi CM, Jamieson SW, Feld GK. Epicardial activation and repolarization patterns in patients with right ventricular hypertrophy. Circulation. 1991;83:104-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Nishimura M, Watanabe Y, Toda H. The genesis of bifid T waves: experimental demonstration in isolated perfused rabbit hearts. Int J Cardiol. 1984;6:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Poelzing S, Veeraraghavan R. Heterogeneous ventricular chamber response to hypokalemia and inward rectifier potassium channel blockade underlies bifurcated T wave in guinea pig. Am J Physiol Heart Circ Physiol. 2007;292:H3043-H3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Coronel R, Opthof T, Plotnikov AN, Wilms-Schopman FJ, Shlapakova IN, Danilo P, Sosunov EA, Anyukhovsky EP, Janse MJ, Rosen MR. Long-term cardiac memory in canine heart is associated with the evolution of a transmural repolarization gradient. Cardiovasc Res. 2007;74:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Opthof T, Janse MJ, Meijborg VM, Cinca J, Rosen MR, Coronel R. Dispersion in ventricular repolarization in the human, canine and porcine heart. Prog Biophys Mol Biol. 2016;120:222-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?: Repolarization Gradients in the Intact Heart. Circ Arrhythm Electrophysiol. 2009;2:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Janse MJ, Coronel R, Opthof T, Sosunov EA, Anyukhovsky EP, Rosen MR. Repolarization gradients in the intact heart: transmural or apico-basal? Prog Biophys Mol Biol. 2012;109:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Arteyeva NV, Goshka SL, Sedova KA, Bernikova OG, Azarov JE. What does the T(peak)-T(end) interval reflect? An experimental and model study. J Electrocardiol. 2013;46:296.e1-296.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Osaka T, Kodama I, Tsuboi N, Toyama J, Yamada K. Effects of activation sequence and anisotropic cellular geometry on the repolarization phase of action potential of dog ventricular muscles. Circulation. 1987;76:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Fish JM, Di Diego JM, Nesterenko V, Antzelevitch C. Epicardial activation of left ventricular wall prolongs QT interval and transmural dispersion of repolarization: implications for biventricular pacing. Circulation. 2004;109:2136-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Srinivasan NT, Orini M, Simon RB, Providência R, Khan FZ, Segal OR, Babu GG, Bradley R, Rowland E, Ahsan S, Chow AW, Lowe MD, Taggart P, Lambiase PD. Ventricular stimulus site influences dynamic dispersion of repolarization in the intact human heart. Am J Physiol Heart Circ Physiol. 2016;311:H545-H554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Vaykshnorayte MA, Ovechkin AO, Azarov JE. The effect of diabetes mellitus on the ventricular epicardial activation and repolarization in mice. Physiol Res. 2012;61:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Sedova KA, Vaykshnorayte MA, Ovechkin AO, Kneppo P, Bernikova OG, Vityazev VA, Azarov JE. Ventricular electrical heterogeneity in experimental diabetes mellitus: effect of myocardial ischemia. Physiol Res. 2016;65:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Ovechkin AO, Vaykshnorayte MA, Sedova K, Shumikhin KV, Arteyeva NV, Azarov JE. Functional role of myocardial electrical remodeling in diabetic rabbits. Can J Physiol Pharmacol. 2015;93:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Sedova KA, Azarov JE, Arteyeva NV, Ovechkin AO, Vaykshnorayte MA, Vityazev VA, Bernikova OG, Shmakov DN, Kneppo P. Mechanism of electrocardiographic T-wave flattening in diabetes mellitus: experimental and simulation study. Physiol Res. 2017;66:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Tsvetkova AS, Kibler NA, Nuzhny VP, Shmakov DN, Azarov JE. Acute effects of pacing site on repolarization and haemodynamics of the canine ventricles. Europace. 2011;13:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Perazzolo Marra M, Zorzi A, Corbetti F, De Lazzari M, Migliore F, Tona F, Tarantini G, Iliceto S, Corrado D. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens' ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm. 2013;10:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 66. | Chauhan VS, Downar E, Nanthakumar K, Parker JD, Ross HJ, Chan W, Picton P. Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy: a human in vivo study. Am J Physiol Heart Circ Physiol. 2006;290:H79-H86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Antzelevitch C. Drug-induced spatial dispersion of repolarization. Cardiol J. 2008;15:100-121. [PubMed] |
| 68. | Lyon A, Bueno-Orovio A, Zacur E, Ariga R, Grau V, Neubauer S, Watkins H, Rodriguez B, Mincholé A. Electrocardiogram phenotypes in hypertrophic cardiomyopathy caused by distinct mechanisms: apico-basal repolarization gradients vs. Purkinje-myocardial coupling abnormalities. Europace. 2018;20:iii102-iii112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Sperelakis N, Lehmkuhl D. Effects of temperature and metabolic poisons on membrane potentials of cultured heart cells. Am J Physiol. 1967;213:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Kiyosue T, Arita M, Muramatsu H, Spindler AJ, Noble D. Ionic mechanisms of action potential prolongation at low temperature in guinea-pig ventricular myocytes. J Physiol. 1993;468:85-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 71. | Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 525] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 72. | Azarov JE, Shmakov DN, Vityazev VA, Roshchevskaya IM, Arteyeva NV, Kharin SN, Roshchevsky MP. Ventricular repolarization pattern under heart cooling in the rabbit. Acta Physiol (Oxf). 2008;193:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Arteyeva NV, Azarov JE. The Role of Transmural Repolarization Gradient in the Inversion of Cardiac Electric Field: Model Study of ECG in Hypothermia. Ann Noninvasive Electrocardiol. 2017;22:e12360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 472] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 75. | Hill JL, Gettes LS. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980;61:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 284] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res. 1997;35:256-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 215] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Yan GX, Yamada KA, Kléber AG, McHowat J, Corr PB. Dissociation between cellular K+ loss, reduction in repolarization time, and tissue ATP levels during myocardial hypoxia and ischemia. Circ Res. 1993;72:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Ruiz Petrich E, Schanne OF, Ponce Zumino A. Electrophysiological responses to ischemia and reperfusion. EXS. 1996;76:115-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Kimura S, Bassett AL, Kohya T, Kozlovskis PL, Myerburg RJ. Simultaneous recording of action potentials from endocardium and epicardium during ischemia in the isolated cat ventricle: relation of temporal electrophysiologic heterogeneities to arrhythmias. Circulation. 1986;74:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Lukas A, Antzelevitch C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current. Circulation. 1993;88:2903-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 172] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, Pugsley W, Kallis P. Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res. 2001;50:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Pandit SV, Kaur K, Zlochiver S, Noujaim SF, Furspan P, Mironov S, Shibayama J, Anumonwo J, Jalife J. Left-to-right ventricular differences in I(KATP) underlie epicardial repolarization gradient during global ischemia. Heart Rhythm. 2011;8:1732-1739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Salama G, Kanai AJ, Huang D, Efimov IR, Girouard SD, Rosenbaum DS. Hypoxia and hypothermia enhance spatial heterogeneities of repolarization in guinea pig hearts: analysis of spatial autocorrelation of optically recorded action potential durations. J Cardiovasc Electrophysiol. 1998;9:164-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36:1749-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 414] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 85. | Rautaharju PM, Mason JW, Akiyama T. New age- and sex-specific criteria for QT prolongation based on rate correction formulas that minimize bias at the upper normal limits. Int J Cardiol. 2014;174:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 86. | Antzelevitch C, Di Diego JM, Argenziano M. Tpeak-Tend as a predictor of ventricular arrhythmogenesis. Int J Cardiol. 2017;249:75-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Tse G, Yan BP. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace. 2017;19:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 88. | Coronel R. Complexity and the interpretation of results. Heart Rhythm. 2009;6:528-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P, Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 90. | Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol. 2008;41:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 91. | Xia Y, Liang Y, Kongstad O, Holm M, Olsson B, Yuan S. Tpeak-Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J Interv Card Electrophysiol. 2005;14:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Porthan K, Viitasalo M, Toivonen L, Havulinna AS, Jula A, Tikkanen JT, Väänänen H, Nieminen MS, Huikuri HV, Newton-Cheh C, Salomaa V, Oikarinen L. Predictive value of electrocardiographic T-wave morphology parameters and T-wave peak to T-wave end interval for sudden cardiac death in the general population. Circ Arrhythm Electrophysiol. 2013;6:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 93. | Smetana P, Schmidt A, Zabel M, Hnatkova K, Franz M, Huber K, Malik M. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol. 2011;44:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 94. | Arteyeva NV, Azarov JE. Effect of action potential duration on Tpeak-Tend interval, T-wave area and T-wave amplitude as indices of dispersion of repolarization: Theoretical and simulation study in the rabbit heart. J Electrocardiol. 2017;50:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Mainardi L, Sassi R. Some theoretical results on the observability of repolarization heterogeneity on surface ECG. J Electrocardiol. 2013;46:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 96. | Végh EM, Engels EB, van Deursen CJ, Merkely B, Vernooy K, Singh JP, Prinzen FW. T-wave area as biomarker of clinical response to cardiac resynchronization therapy. Europace. 2016;18:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Fuller MS, Sándor G, Punske B, Taccardi B, MacLeod RS, Ershler PR, Green LS, Lux RL. Estimates of repolarization dispersion from electrocardiographic measurements. Circulation. 2000;102:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Sasaki A, Takimiya A, Arai T, Song Y, Nakajima S, Muto K, Ibukiyama C. Abnormalities of T waves in effort angina pectoris patients at rest evaluated by spatial velocity electrocardiogram. Jpn Heart J. 1996;37:879-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 99. | Kors JA. Lead transformations and the dipole approximation: Practical applications. J Electrocardiol. 2015;48:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 100. | Man S, Maan AC, Schalij MJ, Swenne CA. Vectorcardiographic diagnostic & prognostic information derived from the 12-lead electrocardiogram: Historical review and clinical perspective. J Electrocardiol. 2015;48:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Draisma HH, Schalij MJ, van der Wall EE, Swenne CA. Elucidation of the spatial ventricular gradient and its link with dispersion of repolarization. Heart Rhythm. 2006;3:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Hartl DL. Genetic dissection of segregation distortion II. Mechanism of suppression of distortion by certain inversions. Genetics. 1975;539-547. [PubMed] |
| 103. | De Ambroggi L, Aimè E, Ceriotti C, Rovida M, Negroni S. Mapping of ventricular repolarization potentials in patients with arrhythmogenic right ventricular dysplasia: principal component analysis of the ST-T waves. Circulation. 1997;96:4314-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Zabel M, Malik M. Practical use of T wave morphology assessment. Card Electrophysiol Rev. 2002;6:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Dilaveris P, Gialafos E, Pantazis A, Synetos A, Triposkiadis F, Gialafos J. The spatial QRS-T angle as a marker of ventricular repolarisation in hypertension. J Hum Hypertens. 2001;15:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Voulgari C, Pagoni S, Tesfaye S, Tentolouris N. The spatial QRS-T angle: implications in clinical practice. Curr Cardiol Rev. 2013;9:197-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Scherptong RW, Henkens IR, Man SC, Le Cessie S, Vliegen HW, Draisma HH, Maan AC, Schalij MJ, Swenne CA. Normal limits of the spatial QRS-T angle and ventricular gradient in 12-lead electrocardiograms of young adults: dependence on sex and heart rate. J Electrocardiol. 2008;41:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Kors JA, Kardys I, van der Meer IM, van Herpen G, Hofman A, van der Kuip DA, Witteman JC. Spatial QRS-T angle as a risk indicator of cardiac death in an elderly population. J Electrocardiol. 2003;36 Suppl:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women's Health Initiative. Circulation. 2006;113:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 110. | Dunnink A, Stams TRG, Bossu A, Meijborg VMF, Beekman JDM, Wijers SC, De Bakker JMT, Vos MA. Torsade de pointes arrhythmias arise at the site of maximal heterogeneity of repolarization in the chronic complete atrioventricular block dog. Europace. 2017;19:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Yoon N, Patocskai B, Antzelevitch C. Epicardial Substrate as a Target for Radiofrequency Ablation in an Experimental Model of Early Repolarization Syndrome. Circ Arrhythm Electrophysiol. 2018;11:e006511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 112. | Verrier RL, Huikuri H. Tracking interlead heterogeneity of R- and T-wave morphology to disclose latent risk for sudden cardiac death. Heart Rhythm. 2017;14:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, Quiñones Pérez MA, Fayad Rodríguez Y. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 382] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 114. | Xianpei W, Sha W, Chuanyu G, Juanjuan Y, Chong C, Yongen S, Yu F, Zhenhao L. Tpeak-Tend dispersion as a predictor for malignant arrhythmia events in patients with vasospastic angina. Int J Cardiol. 2017;249:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Arteyeva NV, Azarov JE. ECG markers of local but not global increase in dispersion of ventricular repolarization (simulation study). J Electrocardiol. 2020;60:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Zareba W, McNitt S, Polonsky S, Couderc JP. JT interval: What does this interval mean? J Electrocardiol. 2017;50:748-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Zulqarnain MA, Qureshi WT, O'Neal WT, Shah AJ, Soliman EZ. Risk of Mortality Associated With QT and JT Intervals at Different Levels of QRS Duration (from the Third National Health and Nutrition Examination Survey). Am J Cardiol. 2015;116:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | Mazzanti A, Maragna R, Vacanti G, Monteforte N, Bloise R, Marino M, Braghieri L, Gambelli P, Memmi M, Pagan E, Morini M, Malovini A, Ortiz M, Sacilotto L, Bellazzi R, Monserrat L, Napolitano C, Bagnardi V, Priori SG. Interplay Between Genetic Substrate, QTc Duration, and Arrhythmia Risk in Patients With Long QT Syndrome. J Am Coll Cardiol. 2018;71:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 119. | Postema PG, Wilde AA. The measurement of the QT interval. Curr Cardiol Rev. 2014;10:287-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 120. | Aro AL, Kenttä TV, Huikuri HV. Microvolt T-wave Alternans: Where Are We Now? Arrhythm Electrophysiol Rev. 2016;5:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Klingenheben T, Hohnloser SH. Clinical value of T-wave alternans assessment. Card Electrophysiol Rev. 2002;6:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Sucu M, Ucaman B, Ozer O, Altas Y, Polat E. Novel Ventricular Repolarization Indices in Patients with Coronary Slow Flow. J Atr Fibrillation. 2016;9:1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 123. | Zhao Z, Yuan Z, Ji Y, Wu Y, Qi Y. Left ventricular hypertrophy amplifies the QT, and Tp-e intervals and the Tp-e/ QT ratio of left chest ECG. J Biomed Res. 2010;24:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Magrì D, Santolamazza C, Limite L, Mastromarino V, Casenghi M, Orlando P, Pagannone E, Musumeci MB, Maruotti A, Ricotta A, Oliviero G, Piccirillo G, Volpe M, Autore C. QT spatial dispersion and sudden cardiac death in hypertrophic cardiomyopathy: Time for reappraisal. J Cardiol. 2017;70:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iacoviello M S-Editor: Yan JP L-Editor: A P-Editor: Li JH