Published online Jan 27, 2026. doi: 10.4240/wjgs.v18.i1.115285
Revised: October 29, 2025
Accepted: December 4, 2025
Published online: January 27, 2026
Processing time: 100 Days and 16.2 Hours
Staging laparoscopy (SL) is a valuable tool for detecting occult peritoneal or hepatic metastases in patients with gastroesophageal junction (GEJ) cancer, es
To evaluate the DP of SL in identifying abdominal metastatic disease in patients with clinically resectable GEJ tumors.
Systematic review and meta-analysis in accordance with PRISMA 2020 guidelines. A comprehensive PubMed search was performed up to March 29, 2024, using Medical Subject Headings terms related to GEJ, laparoscopy, and cancer staging. Inclusion criteria: Studies assessing SL in patients with GEJ tumors. Primary outcome was the rate of upstaging to stage IV due to peritoneal or hepatic metastases. Secondary outcomes included details on techniques, patient characteristics, and procedural factors. Risk of bias was evaluated using Risk of Bias in Non-randomised Studies of Interventions and certainty of evidence with Grading of Recommendations, Assessment, Development and Evaluation.
Eighteen studies involving 1591 patients were included. SL upstaged 22% of patients (95% confidence interval: 17-27) to stage IV due to occult metastatic disease. The pooled positivity rates were positive peritoneal malignancy (17.5%), peritoneal carcinomatosis (13%), malignant peritoneal cytology (9%), and hepatic metastases (9.2%). SL avoided unnecessary surgery in 19.8% of cases. Subgroup analysis revealed consistent performance in Siewert II tumors (DP = 13%, I2 = 0), while in Siewert I tumors it was more heterogeneous (DP = 18%, I2 = 93.7%). Only five studies reported complications, mostly minor, with no procedure-related mortality. No comorbidities, carcinomatosis scoring, conversion to open surgery, complications of follow-up, readmissions, postoperative length of stay, or delay in initiating neoadjuvant therapy were recorded.
SL improves staging accuracy in GEJ cancers, especially Siewert II. Despite heterogeneity and limited data stratification, SL may guide therapeutic decisions and help avoid unnecessary or futile surgeries.
Core Tip: Staging laparoscopy (SL) upstaged nearly one in five patients with clinically resectable gastroesophageal junction cancer by revealing occult peritoneal or hepatic metastases. This finding underlines the value of SL in refining staging and guiding treatment decisions. Diagnostic performance was especially high in Siewert II tumors, without any significant heterogeneity. However, key procedural and patient-related data were not appropriately reported across studies. These results support the routine use of SL in staging gastroesophageal junction tumors and highlight the urgent need for standardized reporting to improve risk stratification and clinical outcomes in this challenging and heterogeneous cancer subgroup.
- Citation: de la Plaza Llamas R, Ribera Díaz D, Betancor Díaz P, Díaz Candelas DA, Latorre-Fragua RA, Gorini L, Arellano González R, Gemio del Rey IA. Staging laparoscopy in esophagogastric junction cancer: Systematic review and meta-analysis. World J Gastrointest Surg 2026; 18(1): 115285
- URL: https://www.wjgnet.com/1948-9366/full/v18/i1/115285.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v18.i1.115285
Esophagogastric junction (GEJ) tumors have traditionally been defined as those tumors that originate within 5 cm proximal or distal to the gastric cardia. They are subdivided into three types according to the Siewert classification: Siewert 1, originating in the distal esophagus, usually in an area of intestinal metaplasia (Barrett’s esophagus); Siewert 2, arising from the gastric cardia, and originating in the gastric epithelium or intestinal metaplasia; and Siewert 3, arising from gastric tissue distal to the cardia[1]. Thus, Siewert 1 are located between 1 cm and 5 cm above the cardia, Siewert 2 is between 1 cm above and 2 cm below the cardia, and Siewert 3 between 2 cm and 5 cm below the cardia.
Although the Siewert system continues to be used to classify the different areas of the GEJ, the 8th edition of the American Joint Committee on Cancer (AJCC)-tumor-node-metastasis (TNM) classifies GEJ tumors as those with their epicenter between 2 cm proximal and 2 cm distal to the cardia. The definition includes some Siewert 1 tumors and all Siewert 2 tumors, but excludes Siewert 3 tumors. A tumor with a more proximal epicenter is defined as an esophageal tumor (including some Siewert 1 tumors), and a more distal tumor is defined as a gastric tumor (including all Siewert 3 tumors). This prognostic classification suggests that, theoretically, the approach to Siewert 1 and 2 might be the same as for esophageal cancers, with the possibility of subtotal esophagectomy, and that in Siewert 3 tumors a gastrectomy can be considered, extended to the distal esophagus if necessary[2].
Staging laparoscopy (SL) plays a fundamental role in the treatment of cancers of the gastrointestinal and gynecological tracts. By means of a relatively non-invasive intervention, it allows the detection of positive peritoneal malignancy (PPM), an umbrella term that includes positive peritoneal carcinomatosis or macroscopic carcinomatosis (PPC), ascites with positive peritoneal cytology (PPCyt), and positive peritoneal wash cytology (PPWCyt). SL can also detect hepatic metastatic disease (HMD) not identified by previous diagnostic tests. All these SL findings are considered distant metastases and upstage the tumor to stage IV in the AJCC-TNM classification (8th edition)[3]. They also change the indication for surgery; unnecessary laparotomies and resections are avoided, and patients are assigned to palliative treatment with chemotherapy and/or radiotherapy.
The technical aspects of SL have changed since its inception, and even today there is no consensus on the best approach. Currently, in most cases, the procedure consists primarily of two techniques: Macroscopic assessment of metastases with biopsies, and peritoneal lavage with saline solution to confirm cytology. If present, ascitic fluid can be aspirated. Although the areas for macroscopic inspection and saline instillation are not standardized, procedures have emerged in recent years that seek greater diagnostic sensitivity. One of the approaches that has shown the best results is the four-step procedure proposed in 2019 by Liu et al[4].
The European Society for Medical Oncology guidelines recommend performing SL in all patients with stage IB-III gastric cancers considered potentially resectable, including Siewert III, in view of its reported sensitivity of 84.6% and specificity of 100%. These rates of detection are higher than those obtained with other diagnostic techniques such as computed tomography (CT), endoscopic ultrasound, or positron emission tomography-CT scan[5]. According to these same guidelines, in Siewert I and Siewert II, SL should be performed in locally advanced cases (T3-T4) with involvement of the cardia. Approximately 15% are positive for peritoneal metastases[6].
The practical guidelines of the National Comprehensive Cancer Network recommend performance of SL in gastric cancers with T3 and/or N+ involvement (positive lymph nodes) and also in cases of T1B or higher if surgery or preoperative chemotherapy is considered[7].
Various indices can be used to assess the outcomes of carcinomatosis in SL. One of the most useful is the Peritoneal Carcinomatosis Index, which has prognostic and therapeutic value.
The primary objective of this study was to determine the usefulness of SL in GEJ tumors for identifying peritoneal and/or hepatic cancer in patients with clinically resectable disease. The secondary objectives were to record the characteristics of the studies included, country, time period, preoperative tumor staging, indication for intervention, patient characteristics, description of the technique, postoperative assessment, and follow-up.
The research question was developed in accordance with the PICO format: In patients with clinically resectable adenocarcinoma of the GEJ (P), does SL (I), compared to conventional imaging techniques (C), improve the detection of metastatic disease and enable oncological upstaging (O)?
This study was carried out in accordance with the PRISMA 2020 guidelines[8] and its explanatory guide[9] and complied with the various items included in the checklist. The statistical review of the study was performed by a biomedical statistician.
The search strategy included the following terms: An unlimited search was conducted in PubMed, updated on March 29, 2024. The following Medical Subject Headings terms were used: Esophagogastric junction; laparoscopy and tumor neoplasm staging, with the addition of the corresponding entry terms.
[(Esophagogastric Junction) OR (Gastroesophageal Junction) OR (Cancer of Esophagus) OR (Cancer of the Esophagus) OR (Esophageal Cancer) OR (Esophagus Cancer) OR (Esophagus Neoplasm) OR (Neoplasms, Esophageal) OR (Siewert)] AND [(Laparoscopy) OR (Laparoscopic Assisted Surgery) OR (Laparoscopic Surgery) OR (Laparoscopic Surgical Procedure) OR (Laparoscopic Surgical Procedures) OR (Peritoneoscopy) OR (Procedure, Laparoscopic Surgical) OR (Procedures, Laparoscopic Surgical) OR (Surgery, Laparoscopic) OR (Surgical Procedure, Laparoscopic) OR (Surgical Procedures, Laparoscopic)] AND [(Neoplasm Staging) OR (Cancer Staging) OR (Staging, Neoplasm) OR (TNM Classification) OR (TNM Staging) OR (TNM Staging System) OR (Tumor Staging) OR (“Staging laparoscopy”)].
The inclusion criterion was the description of patients with GEJ tumors who underwent SL at diagnosis. Exclusion criteria included failure to specify the SL results for the subgroup of patients with GEJ tumors, systematic reviews or meta-analyses, and isolated clinical cases.
The article selection process was conducted by two researchers and was divided into three parts: (1) Selection of articles based on their titles; (2) Appraisal of abstracts; and (3) Appraisal of full article. In the event of a discrepancy, a third researcher resolved the issue.
For the data collection process, the presence or absence of the study variables was recorded in an Excel spreadsheet. The data to be studied were previously defined, and the spreadsheet was created before the text reading began. Texts in languages other than English were translated using the program UPDF v1.7.
The following variables in the selected articles were analyzed to investigate the primary objective: Diagnostic performance (DP) of SL, defined as the number of patients upstaged to stage IV due to the presence of PPC, PPCyt, and PPWCyt; and HMD, to assess disease dissemination. To evaluate the secondary objectives of the study, the variables shown in Table 1 were analyzed. To determine which data from each study were eligible, the articles were read in full. Variables were recorded as specified or unspecified in each study (Table 2).
| Characteristics | Classification |
| Study characteristics | Year |
| Country | |
| Study design | |
| Study period | |
| Patient characteristics | Number of patients |
| Sex | |
| Mean age | |
| Reported comorbidities and scoring systems (e.g., ASA, Charlson Comorbidity Index, Clinical Frailty Scale, POSSUM, etc.) | |
| Siewert classification type | |
| Indication for staging laparoscopy (T, N) | |
| Technical description | Number of surgical ports used |
| Camera angle employed | |
| Pneumoperitoneum pressure | |
| Quantification of carcinomatosis, if present | |
| Abdominal regions inspected | |
| Sites of peritoneal lavage sampling | |
| Timing of lavage | |
| Lavage volume instilled and aspirated | |
| Duration of the procedure | |
| Use of intraoperative ultrasound | |
| Conversion to laparotomy | |
| Postoperative assessment | Presence of complications |
| Sources used to evaluate complications | |
| Use of complication classification/scoring systems | |
| Follow-up duration | |
| Postoperative hospital stay | |
| Readmission | |
| Interval between SL and initiation of neoadjuvant therapy or definitive surgery | |
| Characteristics | Specified | Not specified | ||
| n | Population | n | Population | |
| Comorbidities | 0 | 0 | 18 | 1591 |
| Indications for laparoscopy (based on T and N) | 1 | 41 | 17 | 1550 |
| TNM version used | 5 | 540 | 13 | 1051 |
| Siewert type considered for inclusion | 7 | 668 | 11 | 923 |
| Method of tumor localization for Siewert classification | 11 | 911 | 7 | 680 |
| Patient position during procedure | 2 | 84 | 16 | 1507 |
| Type of access to peritoneal cavity | 3 | 234 | 15 | 1357 |
| Number of surgical ports | 14 | 1258 | 4 | 333 |
| Type of camera | 4 | 347 | 14 | 1244 |
| Pneumoperitoneum pressure | 1 | 43 | 17 | 1548 |
| Substance used for peritoneal lavage | 8 | 766 | 4 | 418 |
| Volume of lavage fluid | 8 | 766 | 4 | 418 |
| Timing of peritoneal lavage during surgery | 4 | 237 | 8 | 947 |
| Use of intraoperative ultrasound | 7 | 399 | 11 | 1192 |
| Carcinomatosis measured with scores or indices | 0 | 0 | 18 | 1591 |
| Study design | 14 | 1385 | 4 | 206 |
| Study period specified (months) | 15 | 1255 | 3 | 336 |
| Regions inspected | 9 | 567 | 9 | 1024 |
| Regions sampled for lavage | 2 | 117 | 10 | 1067 |
| Volume of aspirated lavage | 1 | 81 | 11 | 1103 |
| Surgical time | 4 | 309 | 14 | 1282 |
| Positive macroscopic carcinomatosis | 15 | 1453 | 3 | 138 |
| Positive hepatic metastases | 6 | 434 | 12 | 1157 |
| Positive ascites | 1 | 43 | 12 | 1184 |
| Cytology performed on lavage | 9 | 826 | 3 | 358 |
| Conversion to open surgery | 0 | 0 | 18 | 1591 |
| Data recording methodology | 8 | 1052 | 10 | 539 |
| Evaluation of complications | 5 | 381 | 13 | 1210 |
| Use of complication scoring system | 0 | 0 | 18 | 1591 |
| Postoperative length of stay | 0 | 0 | 18 | 1591 |
| Performed as outpatient surgery | 0 | 0 | 18 | 1591 |
| Follow-up duration | 0 | 0 | 18 | 1591 |
| Readmissions | 0 | 0 | 18 | 1591 |
| Days from surgery to oncological treatment | 0 | 0 | 18 | 1591 |
In the event that the SL results were not fully specified, the number of patients who tested positive for the intervention was indicated, but the question of whether the assessment was made macroscopically, in ascitic fluid, or in HMD was not recorded. Patients appeared as unspecified in these subgroups, but they were included in the DP assessment.
Patients who tested positive for both PPC and PPCyt were classified as positive for PPC. This was only necessary in one of the studies. In cases where it was not specified which patients overlapped, all were counted in the respective groups (PPC, PPCyt, PPWCyt, and HMD), and the number of patients in whom the therapeutic approach was modified was recorded under the heading of those no longer defined as resectable after SL. This means that the sum of the different positive sections in the SL may be greater than the final number of patients whose staging was modified. However, this was only necessary in one of the studies.
The overall certainty of the evidence for the main outcomes was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. Five domains were considered (risk of bias, inconsistency, indirectness, imprecision, and publication bias), and the certainty of evidence was classified as high, moderate, low, or very low[10].
The main results are shown in Table 3, which indicates the data not specified in each study. Other tables show the number of studies that included/did not include information for specific variables.
| Ref. | No. of patients | Study type | Country | Inspection areas | Peritoneal lavage areas | Positive in macroscopic carcinomatosis | Positive in liver metastases | Positive in ascites cytology | Positive in peritoneal lavage | Positive peritoneal malignancy | Patients who became unresectable |
| Halle-Smith et al[13], 2024 | 396 | R | United Kingdom | NS | NS | 29 | NS | NS | 29 | 58 | 58 |
| Mitchell et al[14], 2023 | 79 | R | United States | NS | NS | NS | NS | NS | NS | NS | 12 |
| Strandby et al[15], 2020 | 81 | P | Denmark | NS | 1, 2 | 6 | NS | NS | 4 | 10 | 10 |
| Mirza et al[16], 2016 | 212 | R | United Kingdom | 2, 3, 4, 9, 10 | NS | 27 | NS | NS | NS | 27 | 27 |
| Strandby et al[17], 2016 | 171 | R | Denmark | NS | NP | 9 | 4 | NP | NP | 9 | 13 |
| Simon et al[18], 2016 | 41 | R | France | 1, 2, 3, 4, 11, 12, 13 | NS | NS | NS | NS | NS | NS | 5 |
| Bhatti et al[19], 2014 | 60 | R | Pakistan | NS | NS | 5 | NS | NS | 12 | 17 | 17 |
| Munasinghe et al[20], 2013 | 36 | R | United Kingdom | 4, 5, 8, 11, 12, 13, 14 | 1, 2, 3 | 2 | NS | NS | 3 | 5 | 5 |
| Nath et al[21], 2008 | 48 | P | United Kingdom | NS | NS | 3 | NS | NS | 4 | 7 | 7 |
| Sarela et al[22], 2006 | 105 | NS | United States | NS | NS | 40 | NS | NS | NS | 40 | 40 |
| Clements et al[23], 2004 | 45 | NS | United Kingdom | NS | NP | 4 | NS | NP | NP | 4 | 4 |
| Menon and Dehn[24], 2003 | 43 | P | United Kingdom | 4, 8, 9, 15 | NP | 6 | 5 | 1 | NP | 7 | 12 |
| Nieveen van Dikjum et al[25], 1999 | 36 | NS | Netherlands | 4, 13, 14 | NS | 11 | NS | NS | NS | 11 | 11 |
| Romijn et al[26], 1998 | 20 | NS | Netherlands | 4, 5, 9, 16, 17 | NP | 4 | 2 | NP | NP | 4 | 6 |
| Stein et al[27], 1997 | 72 | P | Germany | 4, 7, 8, 13, 17, 18 | NS | 8 | 13 | NS | 12 | 20 | 33 |
| O´brien et al[28], 1995 | 39 | P | Ireland | NS | NP | 8 | 1 | NP | NP | 8 | 8 |
| Dagnini et al[29], 1986 | 89 | R | Italy | 4, 5, 6, 16, 19 | NP | 27 | 15 | NP | NP | 27 | 42 |
| Bemelman et al[30], 1995 | 18 | P | Netherlands | 4, 8, 17 | NS | NS | NS | NS | 0 | NS | 5 |
The risk of bias in the studies included was formally assessed with the Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I) tool, since all were observational (prospective or retrospective). Domains evaluated included selection of participants, classification of interventions, missing data, measurement of outcomes, and selective reporting[11].
In addition to ROBINS-I and GRADE, the methodological quality of DP was assessed using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool[12]. However, its application to our dataset has limitations, since many of the studies were observational and were not designed as classic studies of diagnostic accuracy. This article was not prospectively registered.
Regarding the statistical analysis, the results were presented in tables when looking for associations between certain variables, and in the form of an effect diagram or forest plot for the results of the meta-analysis of the number of patients who tested positive for different outcomes (PPC, PPCyt, PPWCyt, HMD, and DP).
For the analysis of relationships, the χ2 test was used to relate two qualitative variables, the nonparametric Mann-Whitney U test to compare a numerical variable between two groups, and the nonparametric Spearman’s Rho correlation coefficient between two numerical variables.
A random-effects meta-analysis was used, as all meta-analyses showed heterogeneity between studies. This heterogeneity was measured using the I2 coefficient. A sensitivity analysis was performed to identify factors associated with this heterogeneity, using the Siewert coefficient as a possible cause. Subgroups were therefore created based on this factor and on the two main variables (DP and PPC).
Statistical analyses were performed using STATA/SE v16.0, and P < 0.05 were considered statistically significant. Variables were described using the frequency distribution for qualitative variables and the mean, standard deviation, minimum and maximum values, median, and interquartile range for quantitative variables. For the meta-analysis, the percentage of PPM, PPC, HMD, and DP were used as effect measures. Regarding the certainty or confidence in the body of evidence used, in all the articles selected the results and methodologies were clearly described and allowed for replication. Only articles with sufficient information were considered.
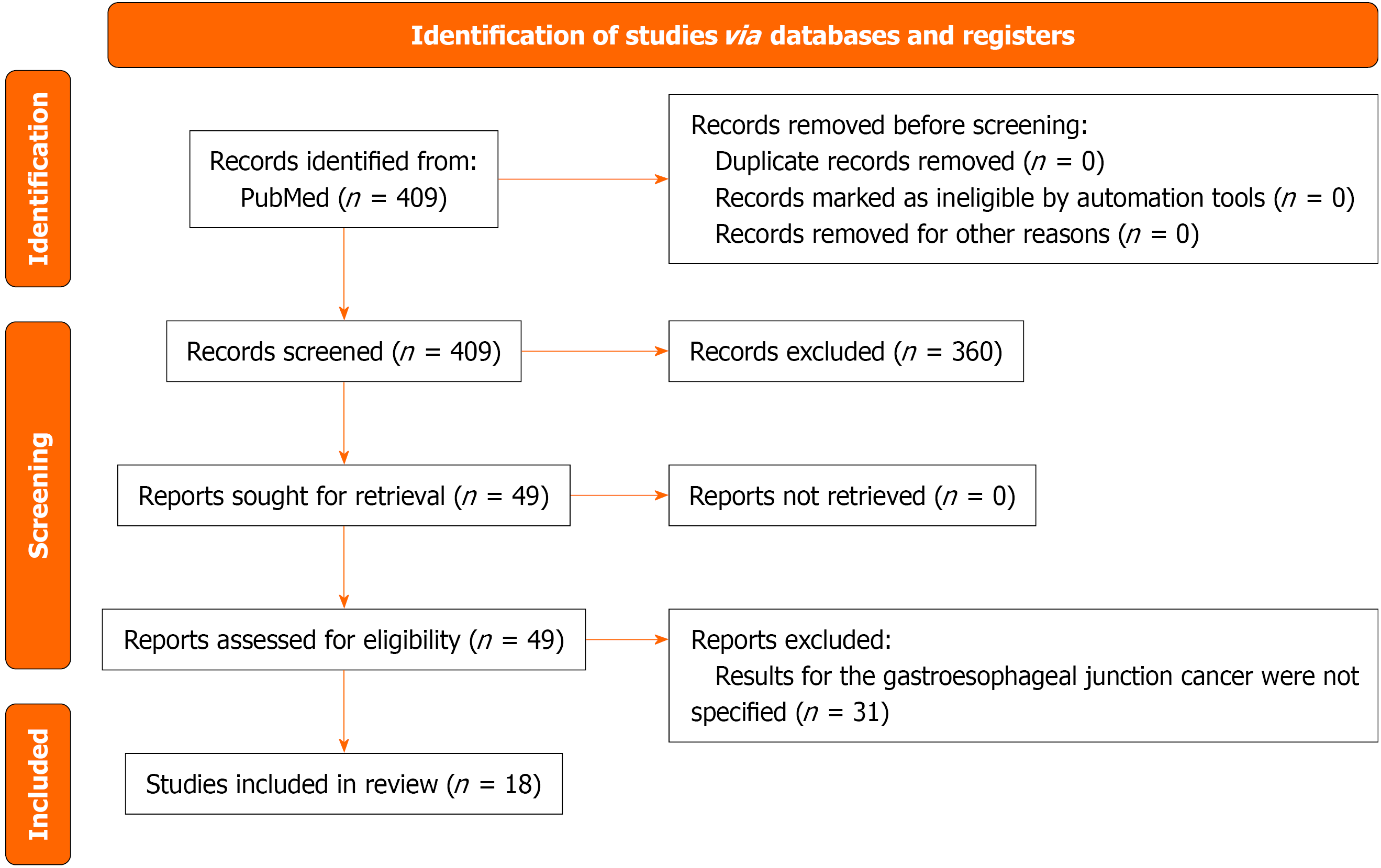
The PRISMA 2020 flow diagram is shown in Figure 1. In all, 18 studies including 1591 patients were analyzed. SL upstaged 22% of patients to stage IV [95% confidence interval (CI): 17-27].
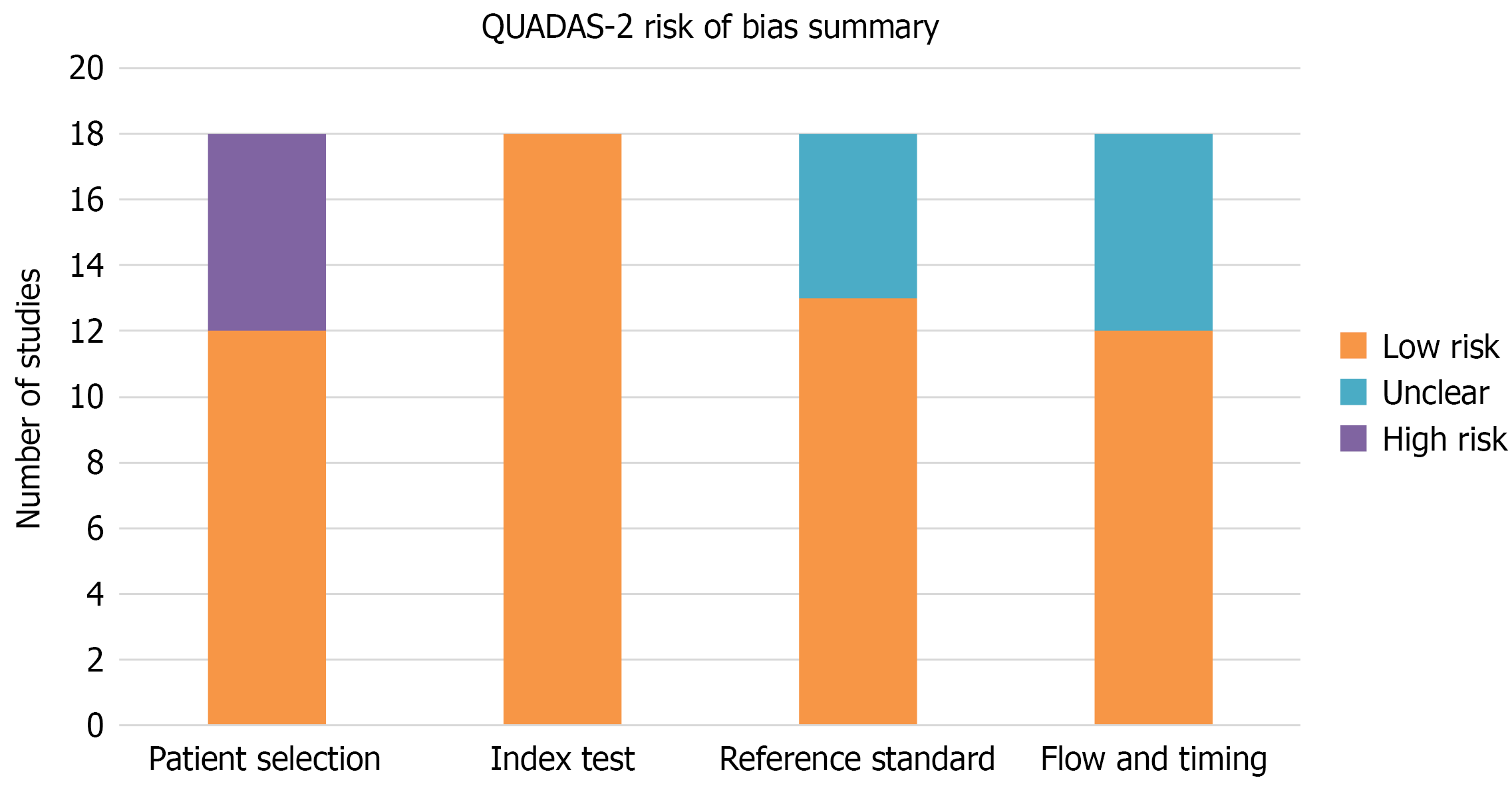
Risk of bias assessment: Of the 18 studies, 10 (56%) were judged to present a low risk of bias, seven (39%) moderate risk, and one (5%) a high risk according to ROBINS-I. The main concerns were related to the retrospective design and incomplete reporting of variables (Table 4).
| Ref. | Design | Participant selection | Intervention classification | Missing data | Outcome measurement | Selective reporting | Overall risk |
| Mitchell et al[14], 2023 | Retrospective | Moderate | Moderate | Moderate | Low | Moderate | Moderate |
| Halle-Smith et al[13], 2024 | Prospective | Low | Low | Low | Low | Low | Low |
| Strandby et al[15], 2020 | Prospective | Low | Low | Low | Low | Low | Low |
| Strandby et al[17], 2016 | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Mirza et al[16], 2016 | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Simon et al[18], 2016 | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Bhatti et al[19], 2014 | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Munasinghe et al[20], 2013 | Prospective | Low | Low | Low | Low | Low | Low |
| Nath et al[21], 2008 | Prospective | Low | Low | Low | Low | Low | Low |
| Sarela 2006[22] | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Clements et al[23], 2004 | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Menon and Dehn[24], 2003 | Prospective | Low | Low | Low | Low | Low | Low |
| Nieveen van Dikjum et al[25], 1999 | Prospective | Low | Low | Low | Low | Low | Low |
| Romijn et al[26], 1998 | Prospective | Low | Low | Low | Low | Low | Low |
| Stein et al[27], 1997 | Prospective | Low | Low | Moderate | Low | Low | Low |
| O´brien et al[28], 1995 | Prospective | Low | Moderate | Moderate | Low | Low | Low |
| Bemelman et al[30], 1995 | Prospective | Low | Low | Low | Low | Low | Low |
| Dagnini et al[29], 1986 | Retrospective | High | High | High | Moderate | High | High |
QUADAS-2 analysis showed a low risk of bias for the SL index test in all studies. The main concerns arose in patient selection (six studies being recorded as high risk) and flow/timing (unclear also in six studies). Reference standards were frequently unclear due to the lack of explicit reporting (Table 5). A global QUADAS-2 traffic-light plot is provided (Figure 2).
| Ref. | Patient selection | Index test (laparoscopy) | Reference standard | Flow and timing |
| Mitchell et al[14], 2023 | Low | Low | Low | Low |
| Halle-Smith et al[13], 2024 | Low | Low | Low | Low |
| Strandby et al[15], 2020 | Low | Low | Low | Low |
| Strandby et al[17], 2016 | High | Low | Unclear | Unclear |
| Mirza et al[16], 2016 | High | Low | Unclear | Unclear |
| Simon et al[18], 2016 | Low | Low | Low | Low |
| Bhatti et al[19], 2014 | High | Low | Low | Unclear |
| Munasinghe et al[20], 2013 | Low | Low | Low | Low |
| Nath et al[21], 2008 | Low | Low | Unclear | Unclear |
| Sarela et al[22], 2006 | High | Low | Unclear | Unclear |
| Clements et al[23], 2004 | High | Low | Unclear | Unclear |
| Menon and Dehn[24], 2003 | Low | Low | Low | Low |
| Nieveen van Dikjum et al[25], 1999 | Low | Low | Low | Low |
| Romijn et al[26], 1998 | Low | Low | Low | Low |
| Stein et al[27], 1997 | Low | Low | Low | Low |
| O´brien et al[28], 1995 | Low | Low | Low | Low |
| Bemelman et al[30], 1995 | Low | Low | Low | Low |
| Dagnini et al[29], 1986 | High | Low | Unclear | Unclear |
The characteristics and most relevant results from the studies included are shown in Table 3[13-30].
A total of 1591 patients underwent SL. Of these, 315 (19.8%) were no longer considered resectable after SL, avoiding further surgical interventions. Regarding positive results for PPC, PPCyt, PPWCyt, and HMD, only a few studies provided separate data (Table 6). These studies reported malignancy, as follows: PPM in 17.5%, PPC in 13%, PPWCyt in 9%, and HMD in 9.2% (Table 6).
| Description | Number of studies | Patients (n) | Positive | Negative | ||
| n | % | n | % | |||
| Total patients with staging laparoscopy identifying disseminated disease | 18 | 1591 | 315 | 19.8 | 1276 | 80.2 |
| Studies specifying positive peritoneal metastases | 15 | 1453 | 254 | 17.5 | 1199 | 82.5 |
| Studies specifying positive peritoneal carcinomatosis | 15 | 1453 | 189 | 13 | 1264 | 87 |
| Studies specifying positive peritoneal washing cytology | 7 | 711 | 64 | 9 | 647 | 91 |
| Studies specifying hepatic metastases detected | 6 | 434 | 40 | 9.2 | 394 | 90.8 |
Only nine articles reported the areas inspected in SL[16,18,20,24-27,29,30]. The most frequently explored was the liver, present in all nine articles, followed by the hepatoduodenal ligament, in four articles[18,20,25,27]. In general, the areas inspected by the studies varied widely (Table 3).
The data that the selected articles specified for the variables studied in this meta-analysis appear in Table 2. The variables that were not specified in any of the articles were: Comorbidities, carcinomatosis measured with scores or indices, conversion to open surgery, use of a complication scoring system, postoperative length of stay, performance as outpatient surgery, follow-up duration and days from surgery to oncological treatment (Table 2).
Most of the articles did not mention subgroups by age or sex, and few specified whether cancers were gastric, esophageal, or GEJ. In the four articles that did define these data, 156 patients were male and 51 females, with mean ages of 55 years and 66 years respectively.
Regarding the methodology applied for data collection in different studies, only eight of the articles specified the source of the data: Six obtained data from databases, and two from retrospective reviews of medical records. As regards the Siewert classification, only seven articles specified the Siewert type they took as the basis for defining a GEJ tumor. None of the articles considered Siewert 3 as GEJ, but many of them included Siewert 3 in the group of gastric tumors.
Focusing now on specific aspects of the procedure, eight articles reported the performance of peritoneal lavage and described some of its characteristics. In all cases, physiological saline solution was used as the lavage agent, the most common volume being 500 mL and the range from 150 mL to 1000 mL. Surgical time was specified in four of the articles, with mean times of 32 minutes, 32 minutes, 34 minutes, and 60 minutes.
Seven studies referred to intraoperative ultrasound: Four performed it, and three did not. Only five studies mentioned complications post-intervention. Two reported no associated morbidity or mortality, one no major complication, and the other two reported a complication rate of 4% (without specifying their nature) and a surgical site infection rate of 3.5%. None mentioned any specific scales for assessing complications.
In the analysis of the relationship between the main outcomes (DP, PPM, PPC, HMD) and the main variables under study (Siewert type, number of areas examined, peritoneal lavage, lavage volume, ultrasound), none of the results obtained were statistically significant, mainly due to the small number of articles that mentioned most of the variables used (Tables 7, 8, 9, 10, and 11).
| Variable | Siewert 1 | Siewert 2 | P value | ||||||||||||||
| n | mean | SD | Min | Max | p50 | p25 | p75 | n | mean | SD | Min | Max | p50 | p25 | p75 | ||
| Macroscopic carcinomatosis | 3 | 14.67 | 10.69 | 8 | 27 | 9 | 8 | 27 | 2 | 3.5 | 0.71 | 3 | 4 | 3.5 | 3 | 4 | 0.200 |
| Hepatic metastases | 2 | 8.5 | 6.36 | 4 | 13 | 8.5 | 4 | 13 | 0 | - | - | - | - | - | - | - | - |
| Peritoneal malignancy | 3 | 12.67 | 12.9 | 2 | 27 | 9 | 2 | 27 | 2 | 5.5 | 2.12 | 4 | 7 | 5.5 | 4 | 7 | 0.800 |
| Staging changes | 4 | 19.5 | 12.79 | 5 | 33 | 20 | 9 | 30 | 3 | 7.67 | 4.04 | 4 | 12 | 7 | 4 | 12 | 0.228 |
| Variable | Number of inspected areas | |
| Correlation coefficient | P value | |
| Staging changes | 0.316 | 0.684 |
| Peritoneal malignancy | -0.632 | 0.135 |
| Macroscopic carcinomatosis | 0.316 | 0.684 |
| Hepatic metastases | 0.316 | 0.684 |
| Peritoneal lavage performed | n | mean | SD | Min | Max | p50 | p25 | p75 | P value |
| Not performed | 6 | 9.83 | 8.66 | 4 | 27 | 7.5 | 4 | 9 | 0.999 |
| Performed or not specified | 9 | 14.67 | 18.24 | 1 | 58 | 7 | 4 | 17 |
| Variable | Lavage volume | |
| Correlation coefficient | P value | |
| Peritoneal malignancy | 0.559 | 0.248 |
| Staging changes | 0.067 | 0.886 |
| Ultrasound performed | n | Mean | SD | Min | Max | p50 | p25 | p75 | P value |
| Yes | 3 | 5.67 | 4.73 | 2 | 11 | 4 | 2 | 11 | 0.700 |
| No | 3 | 8 | 1 | 7 | 9 | 8 | 7 | 9 |
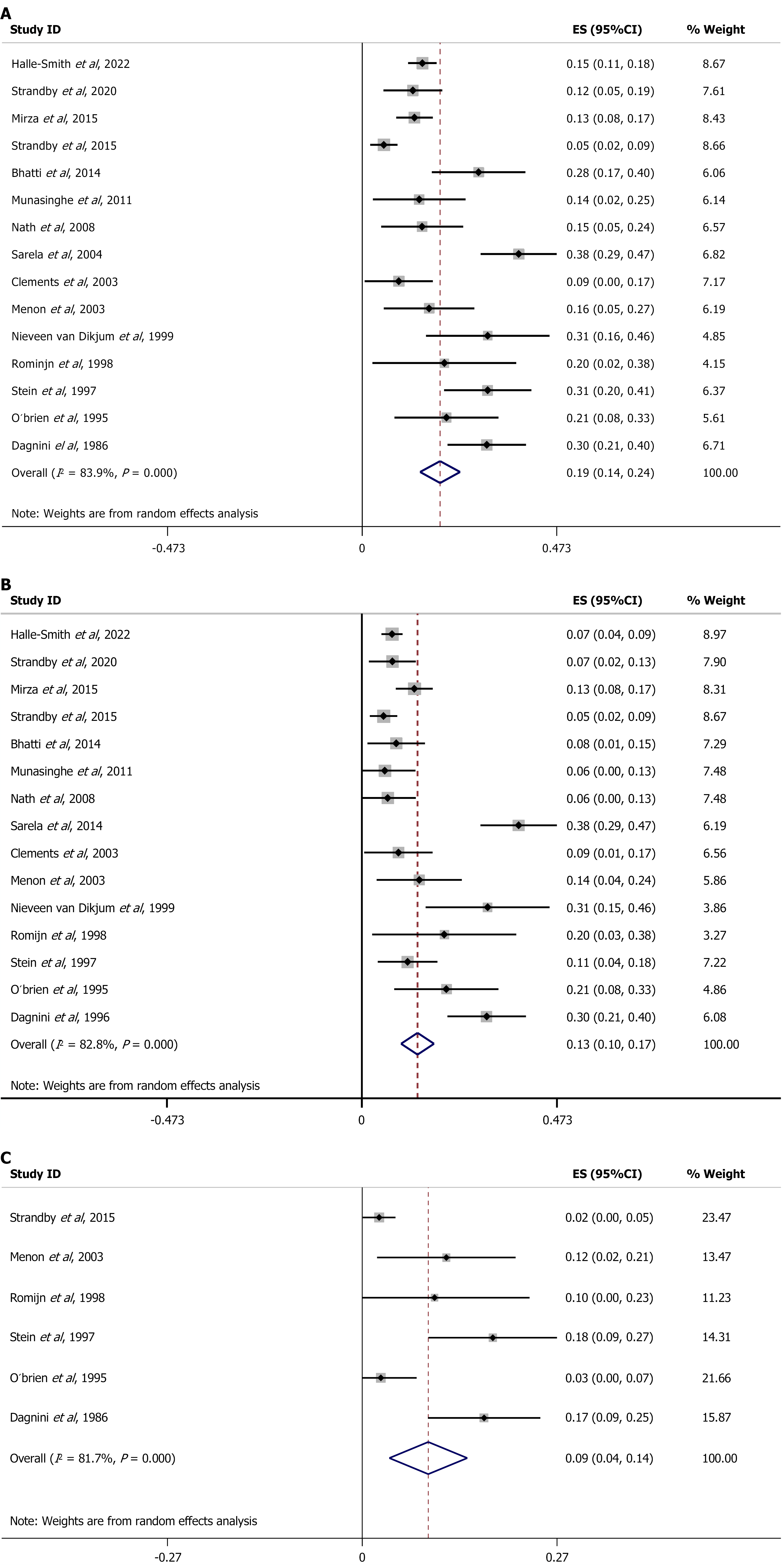
The meta-analysis recorded the following results. Regarding the number of patients who tested positive for PPM, the overall effect size (ES) suggested that 19% of patients had PPC (ES = 0.19, 95%CI: 0.14-0.24), with high heterogeneity (I2 = 83.6%, P < 0.001, Figure 3A). As for PPC, 13% of patients tested positive (ES = 0.13, 95%CI: 0.10-0.17), again with high heterogeneity (I2 = 82.8%, P < 0.001, Figure 3B). Nine per cent of patients tested positive for HMD (ES = 0.09, 95%CI: 0.04-0.14), with high heterogeneity (I2 = 81.7%, P < 0.001, Figure 3C).
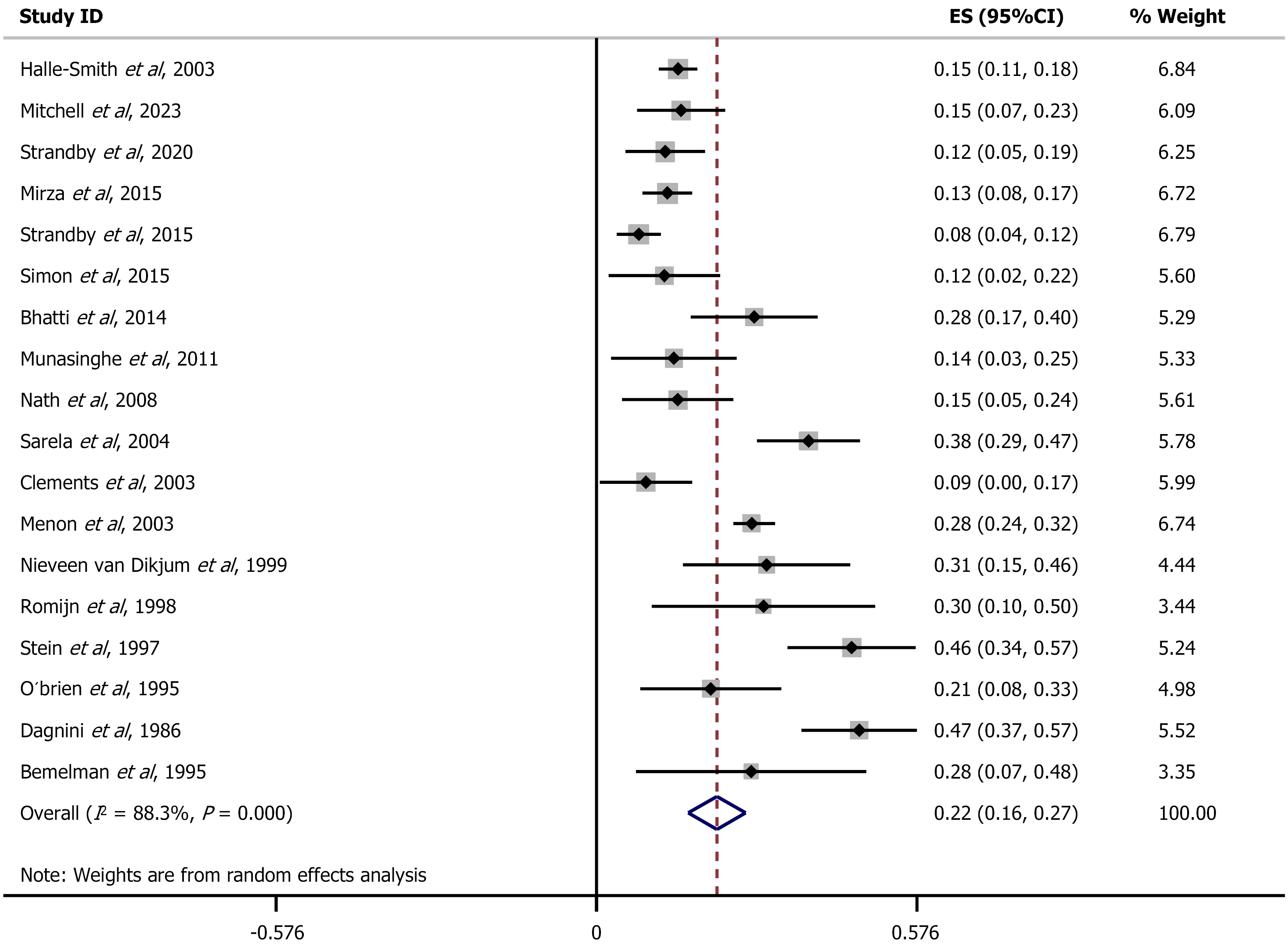
Finally, with regard to the DP, the overall ES suggested that 22% of patients were upstaged (ES = 0.22, 95%CI: 0.17-0.27), with high heterogeneity (I2 = 88.3%, P < 0.00, Figure 4).
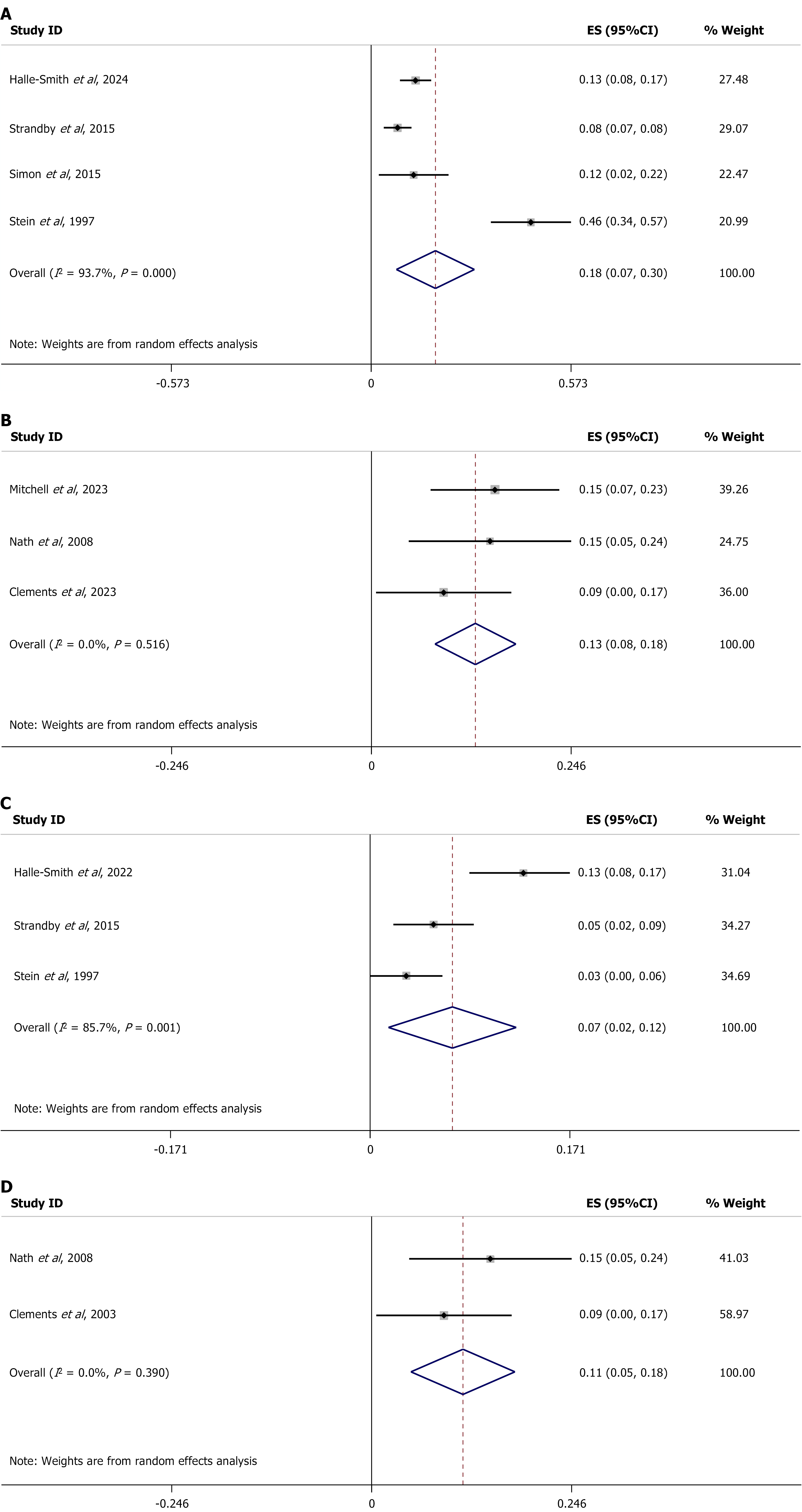
For the sensitivity analysis, a stratified study was performed according to Siewert type for PPM and for DP. For DP, in Siewert I GEJ cancers, 18% of patients experience a stage change (ES = 0.18, 95%CI: 0.08-0.28), with high heterogeneity (I2 = 93.7%, P < 0.001, Figure 5A). In Siewert II GEJ cancers, 13% of patients experience a stage change (ES = 0.13, 95%CI: 0.08-0.18), with no significant heterogeneity (I2 = 0.00%, P = 0.512, Figure 5B).
The same sensitivity analysis was performed for PPM. Seven per cent of patients with Siewert I GEJ cancers were upstaged (ES = 0.07, 95%CI: 0.01-0.12), with high heterogeneity (I2 = 84.4%, P < 0.002, Figure 5C), as were 11% of patients with Siewert II GEJ cancers, (ES = 0.11, 95%CI: 0.05-0.18), with no significant heterogeneity (I2 = 84.4%, P = 0.389, Figure 5D). Table 12 summarizes the findings of the meta-analysis and sensitivity analysis of the studies.
| Variable | Number of studies | Global effect size (%) | Heterogeneity (I2) |
| Overall diagnostic performance of the staging laparoscopy | 18 | 22 (95%CI: 17-27) | 88.3% |
| Positive peritoneal malignancy | 15 | 19 (95%CI: 14-24) | 83.6% |
| Positive peritoneal carcinomatosis | 18 | 13 (95%CI: 10-17) | 82.8% |
| Hepatic metastatic disease | 6 | 9 (95%CI: 4-14) | 81.7% |
| Diagnostic performance - Siewert I | 4 | 18 (95%CI: 8-28) | 93.7% |
| Diagnostic performance - Siewert II | 3 | 13 (95%CI: 8-18) | 0% |
| Positive peritoneal malignancy - Siewert I | 3 | 7 (95%CI: 1-12) | 84.4% |
| Positive peritoneal malignancy - Siewert II | 2 | 11 (95%CI: 5-18) | 0% |
Using the GRADE methodology, the primary outcomes (PPC, HMD, PPM, DP, and stratified analysis according to Siewert subtype) presented low certainty in most cases, due to high heterogeneity and the predominance of observational studies with moderate risk of bias. Only the DP and the PPM in in Siewert II inabsence of heterogeneity (Table 13). A summary of the tumor classification systems (Siewert and/or AJCC) used in the studies is presented in Table 14, highlighting notable heterogeneity in reporting.
| Outcome | Number of studies | Patients | Estimated effect | Certainty of evidence (GRADE) | Comments |
| Positive peritoneal carcinomatosis | 18 | 1591 | 13% (95%CI: 10-17) | Low | High heterogeneity (I2 > 80%), moderate risk of bias |
| Hepatic metastatic disease | 6 | 434 | 9% (95%CI: 4-14) | Low | High heterogeneity, small number of studies |
| Positive peritoneal malignancy | 15 | 1453 | 19% (95%CI: 14-24) | Low | Variable results, wide confidence intervals, potential publication bias |
| Overall diagnostic performance of staging laparoscopy | 18 | 1591 | 22% (95%CI: 17-27) | Low to moderate | High I2, majority observational studies, moderate risk of bias |
| Diagnostic performance - Siewert I | 4 | - | 18% (95%CI: 8-28) | Low | High heterogeneity, one outlier study |
| Diagnostic performance - Siewert II | 3 | - | 13% (95%CI: 8-18) | Moderate | Consistent results across studies, no heterogeneity |
| Positive peritoneal malignancy Siewert I | 3 | - | 7% (95%CI: 1-12) | Low | Very heterogeneous, low frequency of events, wide CI |
| Positive peritoneal malignancy Siewert II | 2 | - | 11% (95%CI: 5-18) | Moderate | Few studies, but consistent and homogeneous results |
| Ref. | Siewert classification | AJCC classification |
| Halle-Smith et al[13], 2024 | No | No |
| Mitchell et al[14], 2023 | Yes | Yes |
| Strandby et al[15], 2020 | No | Yes |
| Mirza et al[16], 2016 | yes | Yes |
| Strandby et al[17], 2016 | Yes | Yes |
| Simon et al[18], 2016 | Yes | No |
| Bhatti et al[19], 2014 | No | Yes |
| Munasinghe et al[20], 2013 | No | No |
| Nath et al[21], 2008 | Yes | No |
| Sarela et al[22], 2006 | No | No |
| Clements et al[23], 2004 | Yes | No |
| Menon and Dehn[24], 2003 | No | No |
| Nieveen van Dikjum et al[25], 1999 | No | No |
| Romijn et al[26], 1998 | No | No |
| Stein et al[27], 1997 | Yes | yes |
| O´brien et al[28], 1995 | No | No |
| Dagnini et al[29], 1986 | No | No |
| Bemelman et al[30], 1995 | No | No |
In this study, the results of the SL were similar to those obtained in previous reviews of gastric cancer, which have reported a greater efficiency in detecting peritoneal metastases than other diagnostic techniques habitually considered as the gold-standard[31].
In the descriptive analysis, the results of the studies showed that up to 19.8% of patients defined as resectable by diagnostic tests such as CT or positron emission tomography-CT were shown by SL to have metastatic disease and were upstaged to stage IV, thus ruling out the possibility of surgery.
Although data can be extracted for specific variables of macroscopic metastasis, peritoneal lavage, or HMD, these were only specified in some of the articles, and so the results are less robust. A third of the articles specified that they did not perform peritoneal lavage[23,24,26,28,29], and therefore may not have been able to diagnose existing peritoneal metastases. The statistical analysis did not establish any significant relationships between the variables studied (DP, PPM, PPC, HMD) and the Siewert type, the number of areas examined, the performance of peritoneal lavage, the lavage volume, or the performance of intraoperative ultrasound (Tables 6, 7, 8, 9, 10, and 11). This is largely due to the small number of studies that specify these variables for the subgroup of GEJ cancers.
In the meta-analysis, the results show that SL is useful for detecting cases of metastatic disease in GEJ cancers, since the rates of detection were statistically significant for PPC, HMD and PPM, and for staging changes after laparoscopy. The meta-analysis also revealed significant heterogeneity in the results. As a consequence, sensitivity analyses were performed, which showed homogeneity in the SL results for Siewert type II with regard to PPM (11%) and DP (13%). However, these results must be considered with caution, given that only seven studies specified the Siewert type. A more exhaustive analysis is needed to examine this heterogeneity, and, if confirmed, to establish its possible causes.
In the assessment of our secondary objectives, we were surprised to find that most studies did not specify complications related to the procedure or delays in initiation of oncological medical therapy.
Regarding technical aspects of the SL, although some areas such as the liver were examined systematically during laparoscopy, there was considerable heterogeneity in the remaining areas. This finding indicates that there is still no established common methodology for macroscopic examination. There seems to be greater consensus about other aspects of laparoscopy: For instance, the use of saline solution for peritoneal lavage, which was specified in all the studies that mentioned it. However, the amount applied varied widely between 150 mL and 1000 mL, with a preference for 500 mL.
Regarding the limitations of the evidence included in this review, using the GRADE rating[10] most outcomes presented low to moderate certainty due to the heterogeneity of the studies and the risk of bias identified, indicating that the estimated effects might change if studies of higher methodological quality were available. The most robust outcome was the DP in Siewert II tumors, which showed moderate certainty. The high heterogeneity in the overall results and the retrospective design of many of the studies limit the reliability of the conclusions and draw attention to the need for multicenter prospective studies that apply standardized methodologies.
The results of the QUADAS-2 tool[12] in this article should be interpreted with caution, as not all the studies included met its requirements for diagnostic test accuracy. Nevertheless, it provided a structured framework for identifying highlight domains with a high risk of bias, complementing ROBINS-I[11] and GRADE assessments.
Certain issues highlighted by the study deserve mention. Some of the variables were not specified in any of the articles (Table 2); others were not specified in a large number of articles, making it difficult to obtain robust results. Many articles did not report these variables according to group, a circumstance that prevented the use of some of these data because they were not specified for the GEJ subgroup (Table 3). In this situation, it is difficult to carry out an in-depth study of the characteristics associated with the SL that goes any further than recording the number of patients who were upstaged as a result. Therefore, the evidence, especially for secondary endpoints, may be limited.
As the SL was performed by different surgeons, the results may be influenced by individual experience and skill. However, this is not a major limitation; in all likelihood, the use of an acceptable number of articles with different surgeons means that the results reflect a level of surgical skill that is quite close to the average.
Determining the association between the patients’ stage prior to the intervention and the results obtained might allow us to define the situations in which SL is indicated. Unfortunately, few studies specify the T and N values taken as a threshold for performing SL: They only stated that the tumors were resectable and that there was no metastasis. Commonly used guidelines such as European Society for Medical Oncology[6] and National Comprehensive Cancer Network[7] apply the indications of T3 or higher, N+, or stages IB-III, but these data were not specified in the studies included here. As for the site of the tumor, only seven articles specified which Siewert type is taken as the basis for defining a GEJ tumor, and none of them considered Siewert type 3 as GEJ; many included type 3 tumors under the heading of gastric cancers.
Other limitations of the study include the fact that only eight articles specify the sources of information and the use of a single database (PubMed) for the literature search, even though the search was conducted without any language or publication status restrictions. While this may reduce the overall comprehensiveness of the search, PubMed offers high sensitivity and relevance in gastrointestinal oncology literature. In addition, our search strategy was broad and included manual screening of reference lists to capture potentially missing studies.
A further limitation of this systematic review is that it was not prospectively registered in any public registry, such as the international prospective register of systematic reviews[32]. Although the review adhered to PRISMA 2020 guidelines and followed a predefined protocol, the absence of registration may limit transparency and reproducibility.
Finally, substantial heterogeneity observed in several pooled estimates (I2 > 80%). While high heterogeneity typically warrants subgroup analyses, we found that key study-level characteristics - such as tumor classification, use of peritoneal cytology, or preoperative imaging techniques - were inconsistently reported across the included studies. This lack of uniform data prevented the reliable definition of clinically meaningful subgroups. Nevertheless, the direction of the effect was consistent across studies, supporting the robustness of the main findings.
This study highlights the need for future studies of GEJ tumors that properly disaggregate all the data for this subgroup. A Delphi study would be desirable to determine the key variables. For the moment, the results obtained corroborate the usefulness of SL in GEJ cancer for diagnosing metastatic disease in patients in whom it has not been previously detected. They also demonstrate the value of peritoneal lavage when performing SL, given that it increases the number of positive results by up to 9% in comparison with the macroscopic examination performed previously.
However, due to the lack of key data provided in the studies - for instance, the T and N grades on which the indication for laparoscopy is based, and the results stratified according to pre-SL stage or Siewert type - it is difficult to offer guidance regarding the situations in which SL is indicated or would be most effective. It is also difficult to determine the specific morbidity and mortality associated with this technique, as most studies did not report relevant data. Another factor that was not specified is the time to initiate cancer treatment, an issue that is of particular importance since a delay could impact survival.
Although there is consensus on the usefulness of SL in cases of gastric cancer, further studies are needed to assess its value in the subgroup of GEJ tumors, considering not only its performance in detecting disseminated disease based on Siewert type, T, and N, but also its surgical outcomes, such as possible associated morbidity, extended length of stay, and its effect on oncological outcomes. Finally, future studies should include the different aspects of patient experience among their objectives, something that this study has not addressed.
The findings confirm the usefulness of SL in the diagnosis of metastatic disease (about 22%) in GEJ tumors previously considered resectable, particularly in Siewert type II. However, the strength of the evidence is limited by the high proportion of observational studies in the sample and the notable methodological heterogeneity. Significant heterogeneity was also observed in the meta-analysis, particularly among Siewert type I. Future multicenter prospective studies should incorporate standardized reporting of technical details and should present results according to Siewert type, indication of SL based on Siewert type, and a formal assessment of complications and oncological outcomes.
| 1. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 2. | Escrig Sos J, Gómez Quiles L, Maiocchi K. The 8th edition of the AJCC-TNM classification: New contributions to the staging of esophagogastric junction cancer. Cir Esp (Engl Ed). 2019;97:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4672] [Article Influence: 519.1] [Reference Citation Analysis (4)] |
| 4. | Liu K, Chen XZ, Zhang WH, Zhang DY, Luo Y, Yu Y, Yang K, Yang SJ, Chen XL, Sun LF, Zhao LY, Zhou ZG, Hu JK. "Four-Step Procedure" of laparoscopic exploration for gastric cancer in West China Hospital: a retrospective observational analysis from a high-volume institution in China. Surg Endosc. 2019;33:1674-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 896] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 6. | Obermannová R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, van Grieken NCT, Vogel A, Smyth EC; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 422] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 7. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1159] [Article Influence: 289.8] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 5178] [Article Influence: 1035.6] [Reference Citation Analysis (1)] |
| 9. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 5598] [Article Influence: 1119.6] [Reference Citation Analysis (0)] |
| 10. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 16181] [Article Influence: 898.9] [Reference Citation Analysis (4)] |
| 11. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12449] [Article Influence: 1244.9] [Reference Citation Analysis (2)] |
| 12. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10338] [Article Influence: 689.2] [Reference Citation Analysis (3)] |
| 13. | Halle-Smith JM, Bage T, Kamarajah SK, Siddaiah-Subramanya M, Pande R, Whiting JL, Griffiths EA. A preoperative predictive tool to assess the need for staging laparoscopy in oesophagogastric cancer patients. Ann R Coll Surg Engl. 2024;106:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Mitchell KG, Bayley EM, Ikoma N, Antonoff MB, Mehran RJ, Rajaram R, Rice DC, Roth JA, Sepesi B, Swisher SG, Vaporciyan AA, Walsh GL, Maru DM, Erasmus JJ, Weston BR, Ajani JA, Badgwell BD, Hofstetter WL. Gastric Extent of Tumor Predicts Peritoneal Metastasis in Siewert II Adenocarcinoma. Ann Thorac Surg. 2024;117:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Strandby RB, Svendsen LB, Ambrus R, Rostved AA, Hasselby JP, Achiam MP. The Incidence of Free Peritoneal Tumor Cells before and after Neoadjuvant Chemotherapy in Gastroesophageal Junction Cancer. J Cytol. 2020;37:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Mirza A, Galloway S. Laparoscopy, computerised tomography and fluorodeoxyglucose positron emission tomography in the management of gastric and gastro-oesophageal junction cancers. Surg Endosc. 2016;30:2690-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Strandby RB, Svendsen LB, Fallentin E, Egeland C, Achiam MP. The Multidisciplinary Team Conference's Decision on M-Staging in Patients with Gastric- and Gastroesophageal Cancer is not Accurate without Staging Laparoscopy. Scand J Surg. 2016;105:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Simon M, Mal F, Perniceni T, Ferraz JM, Strauss C, Levard H, Louvet C, Fuks D, Gayet B. Accuracy of staging laparoscopy in detecting peritoneal dissemination in patients with gastroesophageal adenocarcinoma. Dis Esophagus. 2016;29:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Bhatti AB, Haider S, Khattak S, Syed AA. Staging laparoscopy in gastroesophageal and gastric adenocarcinoma: first experience from Pakistan. Indian J Cancer. 2014;51:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Munasinghe A, Kazi W, Taniere P, Hallissey MT, Alderson D, Tucker O. The incremental benefit of two quadrant lavage for peritoneal cytology at staging laparoscopy for oesophagogastric adenocarcinoma. Surg Endosc. 2013;27:4049-4053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Nath J, Moorthy K, Taniere P, Hallissey M, Alderson D. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. Br J Surg. 2008;95:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Sarela AI, Lefkowitz R, Brennan MF, Karpeh MS. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg. 2006;191:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Clements DM, Bowrey DJ, Havard TJ. The role of staging investigations for oesophago-gastric carcinoma. Eur J Surg Oncol. 2004;30:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Menon KV, Dehn TC. Multiport staging laparoscopy in esophageal and cardiac carcinoma. Dis Esophagus. 2003;16:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Nieveen van Dijkum EJ, de Wit LT, van Delden OM, Kruyt PM, van Lanschot JJ, Rauws EA, Obertop H, Gouma DJ. Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg. 1999;189:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Romijn MG, van Overhagen H, Spillenaar Bilgen EJ, Ijzermans JN, Tilanus HW, Laméris JS. Laparoscopy and laparoscopic ultrasonography in staging of oesophageal and cardial carcinoma. Br J Surg. 1998;85:1010-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Stein HJ, Kraemer SJ, Feussner H, Fink U, Siewert JR. Clinical value of diagnostic laparoscopy with laparoscopic ultrasound in patients with cancer of the esophagus or cardia. J Gastrointest Surg. 1997;1:167-72; discussion 72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | O'Brien MG, Fitzgerald EF, Lee G, Crowley M, Shanahan F, O'Sullivan GC. A prospective comparison of laparoscopy and imaging in the staging of esophagogastric cancer before surgery. Am J Gastroenterol. 1995;90:2191-2194. [PubMed] |
| 29. | Dagnini G, Caldironi MW, Marin G, Buzzaccarini O, Tremolada C, Ruol A. Laparoscopy in abdominal staging of esophageal carcinoma. Report of 369 cases. Gastrointest Endosc. 1986;32:400-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Bemelman WA, van Delden OM, van Lanschot JJ, de Wit LT, Smits NJ, Fockens P, Gouma DJ, Obertop H. Laparoscopy and laparoscopic ultrasonography in staging of carcinoma of the esophagus and gastric cardia. J Am Coll Surg. 1995;181:421-425. [PubMed] |
| 31. | Schena CA, Laterza V, De Sio D, Quero G, Fiorillo C, Gunawardena G, Strippoli A, Tondolo V, de'Angelis N, Alfieri S, Rosa F. The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives. Cancers (Basel). 2023;15:3425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 32. | National Institute for Health and Care Research. International prospective register of systematic reviews (PROSPERO). [cited 12 October 2025]. Available from: https://www.crd.york.ac.uk/prospero/. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/