Published online Jan 27, 2026. doi: 10.4240/wjgs.v18.i1.114692
Revised: November 7, 2025
Accepted: November 28, 2025
Published online: January 27, 2026
Processing time: 103 Days and 1.5 Hours
Hepatocellular carcinoma (HCC) is a significant global health issue that is often diagnosed in advanced stages. Transcatheter arterial chemoembolization (TACE) is a standard intervention for unresectable HCC; however, it is frequently fol
To develop a comprehensive imaging strategy that can guide personalized treat
This retrospective study analyzed 124 patients with HCC who underwent TACE. Based on 1-year outcomes, the patients were stratified into good (n = 86) and poor (n = 38) prognostic groups. We compared the clinical and ultrasound data (CEUS and Doppler parameters) between the groups to identify prognostic factors. Mul
Significant differences were observed between the two groups in terms of TNM stage, number of lesions, tumor size, arrival time (AT), washout time (WT), hepatic artery peak systolic velocity (VPs), portal vein velocity, and blood flow grading (P < 0.05). Logistic regression analysis showed that TNM stage, tumor size, number of lesions, hepatic artery VPs, and blood flow grading were risk factors affecting the prognosis of patients with HCC following TACE, whereas AT, WT, and portal vein velocity were protective factors (P < 0.05). Receiver operating characteristic curve analysis demonstrated that the area under the curve values for predicting post-TACE pro
The combination of CEUS and Doppler ultrasound parameters, which reflect tumor vascularization and liver function, has high a predictive value for the prognosis of patients with HCC following TACE.
Core Tip: This study demonstrated the prognostic efficacy of combining contrast-enhanced ultrasound with Doppler ultrasound to predict postoperative outcomes in patients with hepatocellular carcinoma following transcatheter arterial chemoembolization (TACE). Patients with a favorable prognosis exhibited longer arterial transit and washout times, increased portal vein flow velocity, reduced peak systolic velocity in the hepatic artery, and lower blood flow grading than those with poor outcomes. The combination of these metrics demonstrates significant value in predicting prognosis fol
- Citation: Qian JJ, Xu M, Ji YS. Assessing predictive value of contrast-enhanced ultrasound combined with doppler ultrasound for post-transcatheter arterial chemoembolization prognosis in hepatocellular carcinoma. World J Gastrointest Surg 2026; 18(1): 114692
- URL: https://www.wjgnet.com/1948-9366/full/v18/i1/114692.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v18.i1.114692
Hepatocellular carcinoma (HCC) remains a major global health burden, characterized by its high incidence and tendency to remain asymptomatic until advanced stages. Consequently, many cases are diagnosed at an advanced disease phase, which significantly affects survival outcomes[1]. Transcatheter arterial chemoembolization (TACE) has emerged as a cornerstone intervention for patients with unresectable HCC, particularly with ongoing improvements in minimally invasive techniques[2]. However, due to the intricate vascular nature of liver tumors, TACE alone is often insufficient to completely eradicate tumors, resulting in considerable rates of residual disease and recurrence[3]. This underscores the need for reliable prognostic tools to improve follow-up management and extend patient survival after TACE.
In clinical practice, contrast-enhanced ultrasound (CEUS) and Doppler ultrasound have emerged as key non-invasive options for evaluating HCC. CEUS provides real-time visualization of tumor perfusion and microvascular patterns. Dop
This study aimed to evaluate the combined utility of CEUS and Doppler ultrasound in predicting clinical outcomes in patients with HCC who underwent TACE. Additionally, we sought to elucidate how this combined imaging strategy could support tailored treatment planning based on comprehensive hemodynamic and functional assessment.
A total of 124 patients with HCC who underwent TACE at our hospital between June 2021 and October 2023 were enrolled. The inclusion criteria were as follows: (1) Fulfillment of the diagnostic criteria for HCC[7]; (2) Patients receiving TACE; (3) Age > 18 years; (4) Child-Pugh class A or B liver function[8]; (5) Complete clinical data with follow-up exc
Preoperative preparation: All patients underwent a comprehensive medical history review and physical examination to exclude severe coagulation disorders, obvious hepatic impairment, or other contraindications. Preoperative imaging, including ultrasonography, computed tomography, and magnetic resonance imaging, was performed to evaluate the tumor location, size, vascular supply, and involvement of adjacent structures. Laboratory investigations, such as liver and renal function tests, tumor marker assays, and complete blood counts, were performed to assess the overall clinical status of patients.
Chemoembolization protocol: The chemotherapeutic regimen consisted of a mixture of cisplatin (50 mg) and doxo
The patients were placed in the supine position, and the bilateral groin regions were sterilized with iodine solution and draped under aseptic conditions. Following local anesthesia at the right femoral artery access site, a percutaneous puncture was performed using the Seldinger technique. A short introducer sheath was inserted into the right femoral artery. Under fluoroscopic guidance, the catheter was advanced through the sheath into the common hepatic artery via the celiac trunk. Hepatic artery angiography was performed in the anteroposterior and 30° right anterior oblique positions to confirm tumor location. A micro-guidewire and microcatheter were used to super-select the tumor-feeding artery. Angiography was performed in the arterial, parenchymal, and venous phases to accurately define tumor margins. Chemotherapeutic agents were administered through the catheter directly into the tumor-feeding arteries, followed by the injection of embolic agents. Real-time imaging using X-ray fluoroscopy, digital subtraction angiography, and ultrasonography ensured the precise deposition of chemotherapeutic and embolic materials and minimized non-target embolization of healthy liver tissue or adjacent critical vasculature. Continuous imaging feedback allows for dynamic adjustments in catheter positioning and therapeutic agent dosage to optimize targeting accuracy. Upon completion of the procedure, the catheter was withdrawn, and hemostasis at the puncture site was achieved through manual or mechanical compression. The number of TACE procedures was adjusted based on the postoperative liver function test results.
Clinical data, preoperative CEUS parameters, and Doppler ultrasound parameters were collected from the electronic medical records. The CEUS parameters included the following: (1) Arrival time (AT), the time for the contrast agent to reach the observation site; (2) Time to peak, the time from contrast injection to peak intensity (PKI); (3) Washout time (WT), the time from PKI to baseline intensity (BI); (4) BI, the signal intensity before contrast arrival; and (5) PKI. The Doppler ultrasound parameters included hepatic artery peak systolic velocity (VPs), portal vein velocity, resistance index (RI), and blood flow grading. RI was calculated as (hepatic artery VPs, hepatic artery end-diastolic velocity)/hepatic artery VPs[9]. Blood flow grading was assessed based on the blood flow within the lesion[10] (e.g., punctate blood flow, number of vessels, and blood flow volume) and classified as follows: (1) Grade 0: No blood supply; (2) Grade I: 1 and 2 punctate blood flows; (3) Grade II: 3 and 4 punctate blood flows; and (4) Grade III: Rich blood supply with > 4 punctate blood flows or > 2 vessels.
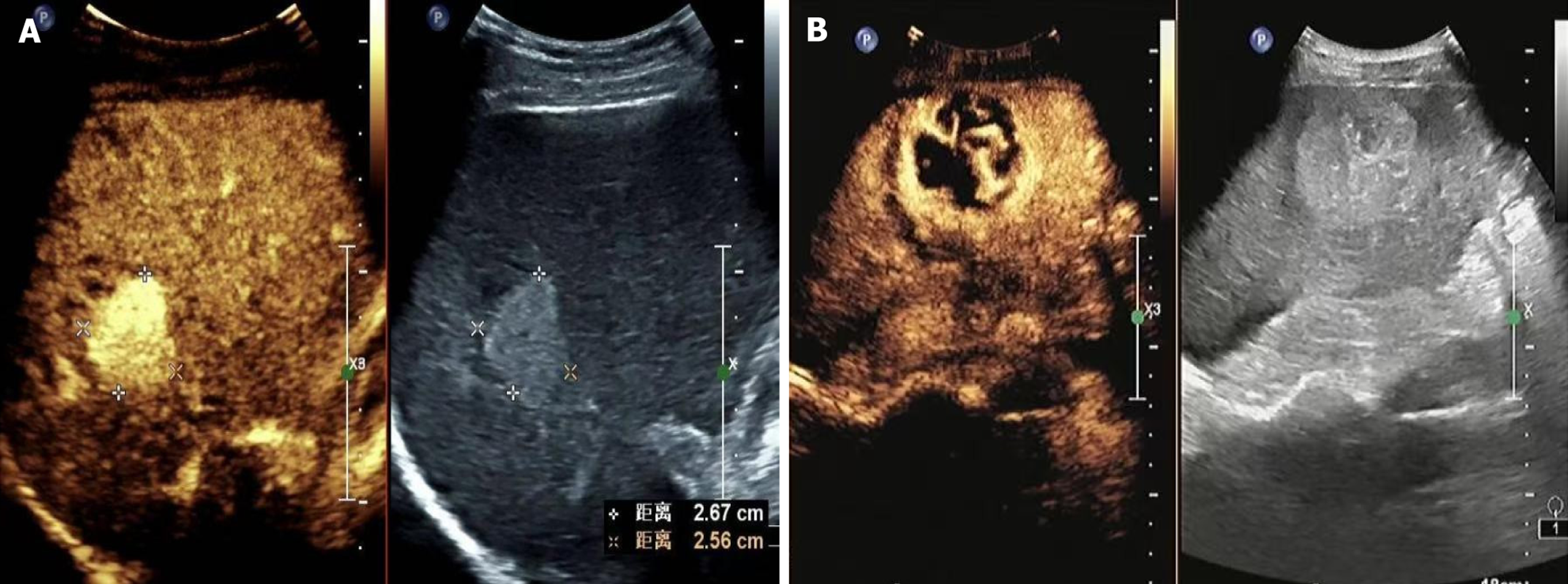
Patients were followed up for 1 year via telephone and outpatient visits. Follow-up began on the day of surgery, with computed tomography, ultrasonography, and liver function tests conducted 1 month and 2 months postoperatively, and every 3 months thereafter. The follow-up period ended in October 2024. According to the 1-year postoperative outcomes, patients were stratified into the poor-prognosis group (n = 38) if they died, experienced recurrence, or had metastasis, whereas the others were classified into the good-prognosis group (n = 86). Examples of good and poor prognoses are shown in Figure 1, respectively.
Statistical analyses were performed using Statistical Package for the Social Sciences 20.0 software. Measurement data were expressed as mean ± SD and compared using an independent samples t-test. Count data were expressed as n (%) and compared using the χ² test. Multivariate logistic regression analysis was used to explore factors influencing the prognosis of patients with HCC following TACE. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive efficacy of CEUS combined with Doppler ultrasound parameters for the prognosis of patients with HCC after TACE. Statistical significance was set at P < 0.05.
A comparison of the clinical data, CEUS parameters, and Doppler ultrasound parameters between the two groups is summarized in Table 1. No significant differences were noted between the two groups in terms of sex, age, body mass index, Child-Pugh classification, number of TACE procedures, time to peak, BI, PKI, or RI (P > 0.05). However, significant differences were observed between the two groups in terms of TNM stage, tumor size, number of lesions, AT, WT, he
| Clinical data | Good prognosis group (n = 86) | Poor prognosis group (n = 38) | t/χ2 | P value |
| Sex (male/female) | 57/29 | 22/16 | 0.801 | 0.371 |
| Age (years) | 62.52 ± 5.24 | 63.82 ± 6.63 | 1.165 | 0.246 |
| Body mass index (kg/m2) | 23.48 ± 2.37 | 23.39 ± 2.41 | 0.194 | 0.846 |
| TNM stage | 5.684 | 0.017 | ||
| I and II stage | 58 (67.44) | 17 (44.74) | ||
| III and IV stage | 28 (32.56) | 21 (5.26) | ||
| Child-Pugh classification | ||||
| Grade A | 53 (61.63) | 2 (52.63) | 0.881 | 0.348 |
| Grade B | 33 (38.37) | 18 (47.37) | ||
| Tumor size | 8.570 | 0.003 | ||
| > 5 cm | 30 (34.88) | 24 (63.16) | ||
| ≤ 5 cm | 56 (65.12) | 14 (36.84) | ||
| Number of lesions | 5.725 | 0.017 | ||
| Single | 64 (74.42) | 20 (52.63) | ||
| Multiple | 22 (25.58) | 18 (47.3) | ||
| Number of transcatheter arterial chemoembolization procedures (times) | 2.06 ± 0.67 | 2.29 ± 0.73 | 1.715 | 0.089 |
| Arrival time (seconds) | 12.47 ± 1.82 | 11.20 ± 1.62 | 3.699 | < 0.001 |
| Time to peak (seconds) | 25.29 ± 3.65 | 24.54 ± 3.78 | 1.042 | 0.299 |
| Washout time (seconds) | 79.44 ± 16.32 | 64.13 ± 14.58 | 4.971 | < 0.001 |
| Baseline intensity (dB) | 4.13 ± 1.27 | 4.36 ± 1.32 | 0.917 | 0.361 |
| Peak intensity (dB) | 33.29 ± 5.63 | 33.87 ± 5.76 | 0.525 | 0.601 |
| Hepatic artery peak systolic velocity (cm/second) | 113.54 ± 18.68 | 135.28 ± 19.53 | 5.892 | < 0.001 |
| Portal vein velocity (cm/second) | 14.38 ± 2.17 | 12.16 ± 1.67 | 5.612 | < 0.001 |
| Resistance index | 0.82 ± 0.24 | 0.86 ± 0.27 | 0.805 | 0.422 |
| Blood flow grading | 8.839 | 0.032 | ||
| Grade 0 | 6 (6.98) | 1 (2.63) | ||
| Grade I | 35 (40.70) | 7 (18.42) | ||
| Grade II | 26 (30.23) | 14 (36.84) | ||
| Grade III | 19 (22.09) | 16 (42.11) |
The multivariate logistic regression analysis of the factors associated with the prognosis of patients with HCC after TACE is shown in Table 2. Logistic regression analysis showed that tumor size, TNM stage, number of lesions, hepatic artery VPs, and blood flow grading were risk factors affecting the prognosis of HCC after TACE, whereas AT, WT, and portal vein velocity were protective factors (P < 0.05).
| Indicator | β | SE | Wald | P value | Odds ratio | 95%CI |
| TNM stage | 1.573 | 0.784 | 4.026 | 0.045 | 4.820 | 1.037-22.398 |
| Tumor size | 2.344 | 0.866 | 7.328 | 0.007 | 10.419 | 1.909-56.855 |
| Number of lesions | 1.522 | 0.764 | 3.971 | 0.046 | 4.582 | 1.025-20.473 |
| Arrival time | -0.564 | 0.231 | 5.959 | 0.015 | 0.569 | 0.361-0.895 |
| Washout time | -0.050 | 0.024 | 4.332 | 0.037 | 0.952 | 0.908-0.997 |
| Hepatic artery peak systolic velocity | 0.069 | 0.020 | 12.581 | < 0.001 | 1.072 | 1.031-1.113 |
| Portal vein velocity | -0.595 | 0.208 | 8.181 | 0.004 | 0.551 | 0.367-0.829 |
| Blood flow grading | 0.926 | 0.469 | 3.896 | 0.048 | 2.526 | 1.007-6.337 |
| Constant | -2.233 | 5.574 | 0.161 | 0.689 | - | - |
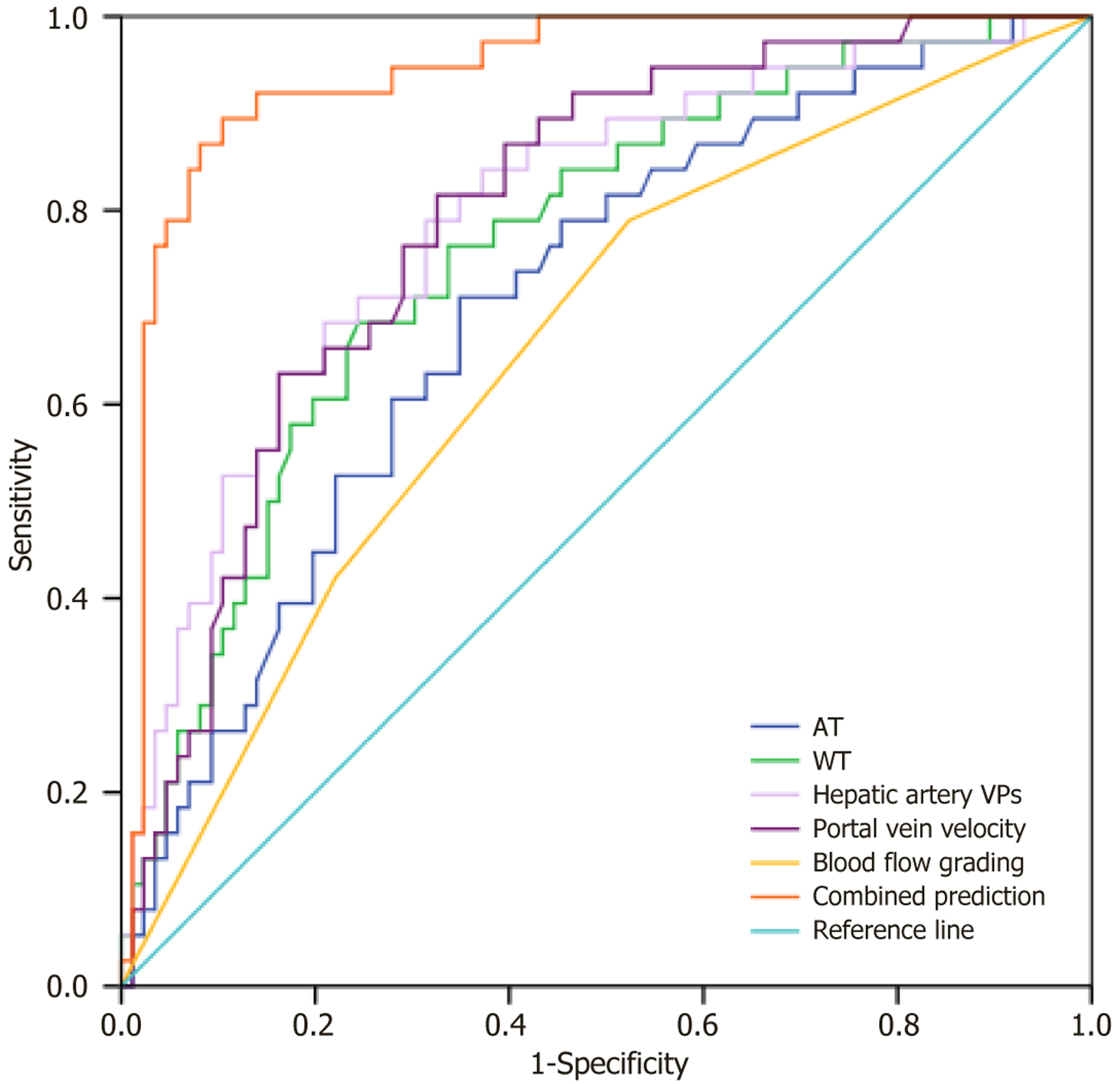
The ROC curve analysis of the predictive value of CEUS combined with Doppler ultrasound parameters for the prognosis of patients with HCC after TACE is summarized in Table 3 and Figure 2. ROC curve analysis showed that the area under the curve values for AT, WT, hepatic artery VPs, portal vein velocity, blood flow grading, and combined detection for predicting the prognosis of patients with HCC after TACE were 0.704, 0.762, 0.796, 0.796, 0.657, and 0.942, respectively.
| Indicator | Area under the curve | 95%CI | Optimal cutoff value | P value | Sensitivity (%) | Specificity (%) |
| Arrival time | 0.704 | 0.608-0.801 | 11.92 | < 0.001 | 71.11 | 65.06 |
| Washout time | 0.762 | 0.673-0.852 | 69.44 | < 0.001 | 68.40 | 75.63 |
| Hepatic artery peak systolic velocity | 0.796 | 0.710-0.882 | 120.70 | < 0.001 | 78.91 | 68.64 |
| Portal vein velocity | 0.796 | 0.715-0.876 | 13.59 | < 0.001 | 81.58 | 67.42 |
| Blood flow grading | 0.657 | 0.555-0.760 | - | 0.005 | 52.30 | 73.44 |
| Combined detection | 0.942 | 0.898-0.985 | - | < 0.001 | 89.45 | 89.45 |
HCC is the third leading cause of cancer-related deaths worldwide. Diverse treatments are available, including surgical resection, liver transplantation, ablation therapy, targeted therapy, and TACE[11]. TACE has been shown to improve survival by selectively blocking the blood supply to tumor vessels and delivering chemotherapeutic drugs directly to tumors. The evaluation of TACE efficacy and prognosis is crucial for clinical decision-making, as it is one of the primary treatment methods for intermediate-stage HCC[12]. However, the heterogeneity of HCC means that conventional prognostic models, such as the hepatoma arterial embolization prognostic score[13], which incorporates clinical variables such as tumor number, size, alpha-fetoprotein, bilirubin, and albumin, fail to fully capture the prognostic value offered by TACE. Therefore, the development of an individualized prognostic model for patients with HCC after TACE has clear clinical implications. We aimed to establish a novel approach for predicting treatment response and prognosis in patients with HCC following TACE by integrating CEUS with Doppler ultrasound.
The findings of this study indicate that TNM stage, tumor size, and number of lesions are significant prognostic factors. The underlying reasons for this finding can be explained as follows: TNM stage reflects the degree of tumor invasion and spread, with advanced stages correlating with poorer treatment response and prognosis. A larger tumor size often signifies more aggressive growth and increased invasive potential, which may reduce the effectiveness of TACE and adversely affect outcomes. Similarly, a greater number of lesions suggests more extensive intrahepatic dissemination, which can exacerbate liver dysfunction and further diminish the efficacy of TACE, thereby worsening prognosis[14,15].
CEUS enables real-time assessment of hepatic hemodynamics through the injection of microbubble-based contrast agents. It offers high sensitivity and non-invasiveness and has been widely adopted for the diagnosis and surveillance of liver tumors[16]. Doppler ultrasonography provides valuable information regarding tumor vascularity by evaluating blood flow patterns within the tumor region, offering critical insights into the malignancy and treatment response of HCC[17]. This study demonstrated that patients with a favorable prognosis exhibited longer AT and WT, lower VPs in the VPs, and a lower proportion of high blood flow grading (grades 0 and I) than those with a poor prognosis. These findings suggest that a poor prognosis is characterized by impaired blood perfusion and increased vascularization within the tumor. Multivariate analysis further identified AT, WT, and portal vein velocity as protective factors for post-TACE prognosis in patients with HCC, whereas hepatic artery VPs and higher blood flow grading were associated with an increased risk.
A prolonged AT may indicate a more stable or improved tumor blood supply, reflecting sufficient perfusion[18]. In TACE, adequate blood flow is essential for the effective delivery of chemotherapeutic or embolic agents to the target tumor. A longer AT allows more time for drug distribution and retention within the tumor, thereby enhancing the local drug concentration and tumor cell exposure, which, in turn, improves treatment efficacy. An extended WT typically suggests slower blood flow and contrast clearance, which may reflect favorable hemodynamics and preserved liver functions. This prolonged retention enhances the duration of chemotherapy within the tumor, contributing to a better treatment response. A higher portal vein velocity may facilitate more efficient drug delivery through the portal system, improving distribution throughout the liver, particularly to tumor-feeding arteries in HCC regions, and potentially enhancing drug clearance to reduce systemic toxicity[19].
Conversely, elevated hepatic artery VPs often indicate hypervascularization, which is frequently associated with aggressive tumor behavior[20]. Strong vascularization may reduce complete vascular occlusion, thereby limiting the efficacy of TACE. Moreover, high vascularity supports tumor growth and metastasis by improving access to oxygen and nutrients, thereby increasing the risk of recurrence and worsening the prognosis. Similarly, a higher blood flow grading reflects increased vascularization, which complicates complete embolization. Tumors with abundant blood supply are more likely to exhibit malignant phenotypes and metastatic potential, ultimately leading to poorer outcomes.
The integration of CEUS and Doppler ultrasound provides a synergistic advantage in assessing post-TACE prognosis by bridging the microvascular and macrovascular assessments. CEUS offers a real-time microvascular perspective by tracking the dynamic flow of microbubble contrast agents within the tumor microvasculature and capturing functional parameters, such as AT and WT, which are closely related to vascular density and perfusion patterns[21]. In contrast, Doppler ultrasound provides macrohemodynamic information, including hepatic artery VPs and portal vein velocity, which reflect the overall hepatic blood flow status and its regulatory impact on tumor perfusion[22]. This combined approach enables a comprehensive assessment of tumor biology and the liver function. For instance, a prolonged WT coupled with a higher portal vein velocity may indicate preserved liver metabolic function, facilitating efficient drug distribution and clearance. Conversely, elevated hepatic artery VPs and higher blood flow grading suggest intense tumor vascularization and aggressive behavior. Together, these parameters form a multifaceted imaging biomarker profile that robustly predicts TACE efficacy.
These hemodynamic parameters reflect the vascular characteristics and functional status of the liver in patients with HCC and provide significant insights into the efficacy of TACE and prognostic evaluation. This study demonstrated that the combination of AT, WT, hepatic artery VPs, portal vein velocity, and blood flow grading yielded an area under the curve of 0.942 for predicting post-TACE prognosis, indicating that CEUS combined with Doppler ultrasound parameters has a high predictive value for clinical outcomes in HCC patients after TACE.
However, this study did not explore the potential interactions between the identified ultrasound parameters and baseline liver function (e.g., alanine aminotransferase, aspartate aminotransferase) or serum tumor markers (e.g., alpha fetoprotein). Future studies that integrate these clinical and laboratory variables with multiparametric ultrasound data could potentially yield a more robust and comprehensive predictive model for post-TACE prognosis. The sample size of 124 patients, which is acceptable for a single-center retrospective study, remains relatively limited and may have introduced selection bias. Moreover, the single-center nature of the study, while providing a consistent procedural context, may further limit the generalizability of the findings owing to potential selection bias and specific institutional protocols. Future research should involve multi-center, prospective studies with larger, more diverse cohorts to validate and strengthen these promising results. Additionally, the 1-year follow-up period, while sufficient for initial prognostic assessment, may not fully capture the long-term recurrence patterns of HCC. Extending the observational timeline in future studies will provide a more comprehensive evaluation of the predictive power of the model for overall survival.
The combined application of CEUS and Doppler ultrasound offers a comprehensive assessment of tumor vascularization and liver function in patients with HCC. The integrated use of these modalities demonstrated strong predictive power for prognostic outcomes in patients with HCC following TACE. However, limitations such as the relatively small sample size, single-center design, and short follow-up period warrant further studies involving larger cohorts and extended observational timelines to validate our findings.
| 1. | Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun (Lond). 2022;42:1112-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 2. | Fite EL, Makary MS. Transarterial Chemoembolization Treatment Paradigms for Hepatocellular Carcinoma. Cancers (Basel). 2024;16:2430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 3. | Manjunatha N, Ganduri V, Rajasekaran K, Duraiyarasan S, Adefuye M. Transarterial Chemoembolization and Unresectable Hepatocellular Carcinoma: A Narrative Review. Cureus. 2022;14:e28439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | HonShideler C, Coffin B, Guez D. Imaging in Interventional Radiology: Applications of Contrast-Enhanced Ultrasound. Semin Intervent Radiol. 2024;41:241-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Squires JH, Fetzer DT, Dillman JR. Practical Contrast Enhanced Liver Ultrasound. Radiol Clin North Am. 2022;60:717-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Aziz MU, Eisenbrey JR, Deganello A, Zahid M, Sharbidre K, Sidhu P, Robbin ML. Microvascular Flow Imaging: A State-of-the-Art Review of Clinical Use and Promise. Radiology. 2022;305:250-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 7. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1561] [Article Influence: 390.3] [Reference Citation Analysis (41)] |
| 8. | Costa F, Wiedenmann B, Roderburg C, Mohr R, Abou-Alfa GK. Systemic treatment in patients with Child-Pugh B liver dysfunction and advanced hepatocellular carcinoma. Cancer Med. 2023;12:13978-13990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Lv T, Kong L, Yang J, Wu H, Wen T, Jiang L, Yang J. The postoperative hepatic artery resistance index after living donor liver transplantation can predict early allograft dysfunction. Medicine (Baltimore). 2020;99:e18677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zhang ZY, Lee JC, Yang W, Yan K, Wu W, Wang YJ, Chen MH. Percutaneous ablation of the tumor feeding artery for hypervascular hepatocellular carcinoma before tumor ablation. Int J Hyperthermia. 2018;35:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Maki H, Hasegawa K. Advances in the surgical treatment of liver cancer. Biosci Trends. 2022;16:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 12. | Hamaya S, Oura K, Morishita A, Masaki T. Cisplatin in Liver Cancer Therapy. Int J Mol Sci. 2023;24:10858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 13. | Tak KY, Jang B, Lee SK, Nam HC, Sung PS, Bae SH, Choi JY, Yoon SK, Jang JW. Use of M2BPGi in HCC patients with TACE. J Gastroenterol Hepatol. 2021;36:2917-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 294] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 15. | Wang Z, Qin H, Liu S, Sheng J, Zhang X. Precision diagnosis of hepatocellular carcinoma. Chin Med J (Engl). 2023;136:1155-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 16. | Chang GY, Fetzer DT, Porembka MR. Contrast-Enhanced Intraoperative Ultrasound of the Liver. Surg Oncol Clin N Am. 2022;31:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Okeke RI, Bettag J, Wells R, Wycoff M, Hallcox T, Lok J, Phocas A, Annakie DL, Shoela R, Nazzal M. Intraoperative Doppler Ultrasound for Detection of Early Postoperative Vascular Complications in Orthotopic Liver Transplants. Cureus. 2022;14:e26077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Hwang SY, Danpanichkul P, Agopian V, Mehta N, Parikh ND, Abou-Alfa GK, Singal AG, Yang JD. Hepatocellular carcinoma: updates on epidemiology, surveillance, diagnosis and treatment. Clin Mol Hepatol. 2025;31:S228-S254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 96] [Article Influence: 96.0] [Reference Citation Analysis (1)] |
| 19. | Feng F, Zhao Y. Hepatocellular Carcinoma: Prevention, Diagnosis, and Treatment. Med Princ Pract. 2024;33:414-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 20. | Ulu Öztürk F, Tezcan Ş, Ayvazoğlu Soy EH, Uslu N, Haberal M. Effect of meal intake for evaluating hepatic artery by Doppler ultrasonography in liver transplants: Does fasting matter for screening hepatic artery due to hemodynamic changes in splanchnic circulation? Clin Transplant. 2022;36:e14674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Machado P, Gillmore K, Tan A, Gonsalves C, Forsberg F. Contrast-Enhanced Ultrasound and High Sensitive Doppler for Monitoring Outcomes of Uterine Artery Embolization. Acad Radiol. 2023;30 Suppl 2:S211-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Jia WY, Gui Y, Chen XQ, Tan L, Zhang J, Xiao MS, Chang XY, Dai MH, Guo JC, Cheng YJ, Wang X, Zhang JH, Zhang XQ, Lv K. Efficacy of color Doppler ultrasound and contrast-enhanced ultrasound in identifying vascular invasion in pancreatic ductal adenocarcinoma. Insights Imaging. 2024;15:181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/