Published online Jan 27, 2026. doi: 10.4240/wjgs.v18.i1.114262
Revised: October 24, 2025
Accepted: November 21, 2025
Published online: January 27, 2026
Processing time: 123 Days and 3.8 Hours
Hepatocellular carcinoma (HCC) commonly arises in cirrhotic livers. Laparoscopic hepatectomy (LH) has shown promising outcomes, but its safety in mode
To compare short-term and long-term outcomes of LH and OH in patients with HCC LDGS grade B or C.
The 97 patients with HCC and LDGS grade B or C who underwent hepatectomy (26 LH; 71 OH) between 2010 and 2022 at Tokyo Women’s Medical University Hospital were retrospectively analyzed. Propensity score matching (1:1) was applied. Baseline biochemical and tumor characteristics were compared. Short-term and long-term outcomes were assessed.
Before matching patients who underwent LH had smaller tumors (2.7 cm vs 4.5 cm, P = 0.004) and lower surgical difficulty scores (P < 0.001). After matching LH was associated with lower intraoperative blood loss (242 mL vs 941 mL; P = 0.049), reduced postoperative ascites (0% vs 21.2%; P = 0.035), and shorter hospital stay with no conversion to OH. The 5-year overall survival rate was significantly higher in the LH group (91% vs 36%; P = 0.021) while recurrence-free survival was comparable.
LDGS provides a comprehensive assessment of surgical candidates with moderate cirrhosis. In patients with HCC and grade B or C liver damage, LH appears to have better long-term outcomes than OH due to reduced morbidity and preservation of liver function.
Core Tip: Laparoscopic hepatectomy (LH) is a feasible and safe procedure for patients with hepatocellular carcinoma and moderate cirrhosis, particularly Liver Damage Grading System grade B or C. In this single-center retrospective study with propensity score matching, LH significantly reduced intraoperative blood loss and postoperative ascites, shortened hospital stay, and improved 5-year overall survival compared with open hepatectomy. The Liver Damage Grading System provides a practical tool for selecting surgical candidates. These findings support LH as a preferred approach in patients with hepatocellular carcinoma and grade B liver damage, optimizing long-term outcomes while preserving liver function.
- Citation: Kijpongpans K, Ariizumi S, Ome Y, Kawamoto Y, Matsunaga Y, Honda G. Laparoscopic hepatectomy is feasible for patients diagnosed with hepatocellular carcinoma and cirrhotic liver. World J Gastrointest Surg 2026; 18(1): 114262
- URL: https://www.wjgnet.com/1948-9366/full/v18/i1/114262.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v18.i1.114262
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, and its management is often complicated by underlying cirrhosis[1,2]. Liver resection, or hepatectomy, has evolved into a safe procedure with a low operative mortality rate and remains the most promising curative treatment for HCC[1-3].
Over the past decade laparoscopic hepatectomy (LH) has become increasingly adopted as a minimally invasive alternative to open hepatectomy (OH)[4]. Several studies have demonstrated that LH offers advantages such as reduced blood loss, decreased morbidity, less postoperative liver decompensation, and shorter hospital stay while providing oncological outcomes comparable with OH in selected patients[5-10]. Nevertheless, in patients with moderate cirrhosis (Child-Pugh class B) or impaired liver function, the safety and applicability of LH remain controversial. According to current guidelines, the surgical indications for LH and OH are not entirely identical, and LH is usually limited to patients with sufficient functional liver reserve and technically accessible tumors[1,3].
Traditionally, the Child-Pugh classification has been used to evaluate patients with chronic liver disease and determine the indications for hepatectomy or other treatment(s)[11,12]. It is the best-known indicator of functional liver reserve. However, patients with a low functional liver reserve may still be included in the Child-Pugh class A cohort even though one of the important factors for safely performing hepatectomy in patients with cirrhotic liver disease is the preoperative estimation of the functional liver reserve.
In Japan the incorporation of indocyanine green retention at 15 min (ICG R15) is widely used to more precisely estimate functional liver reserve(s)[13]. ICG R15 helps to select patients suitable for hepatectomy and determines the safe extent of resection. Building on this, an alternative system, the Liver Damage Grading System (LDGS), proposed by the Liver Cancer Study Group of Japan, uses the ICG R15 value instead of encephalopathy as a safer indicator for deter
Several studies have reported favorable outcomes of LH in patients with compensated cirrhosis. However, surgical outcomes in relation to LDGS remained limited, and data for patients with moderate liver damage (LDGS grade B or C) are especially scarce. Therefore, this study aimed to compare short-term and long-term outcomes after LH and OH in patients diagnosed with HCC and grade B or C liver damage using propensity score matching (PSM)[17,18]. The findings may help clarify the potential role and limitations of LH in patients with moderate cirrhosis.
This study was approved by the Ethics Committee of Tokyo Women’s Medical University (Approval No. 2024-0133). The study protocol adhered to the principles of the Declaration of Helsinki, and informed consent was waived due to the retrospective nature of the study.
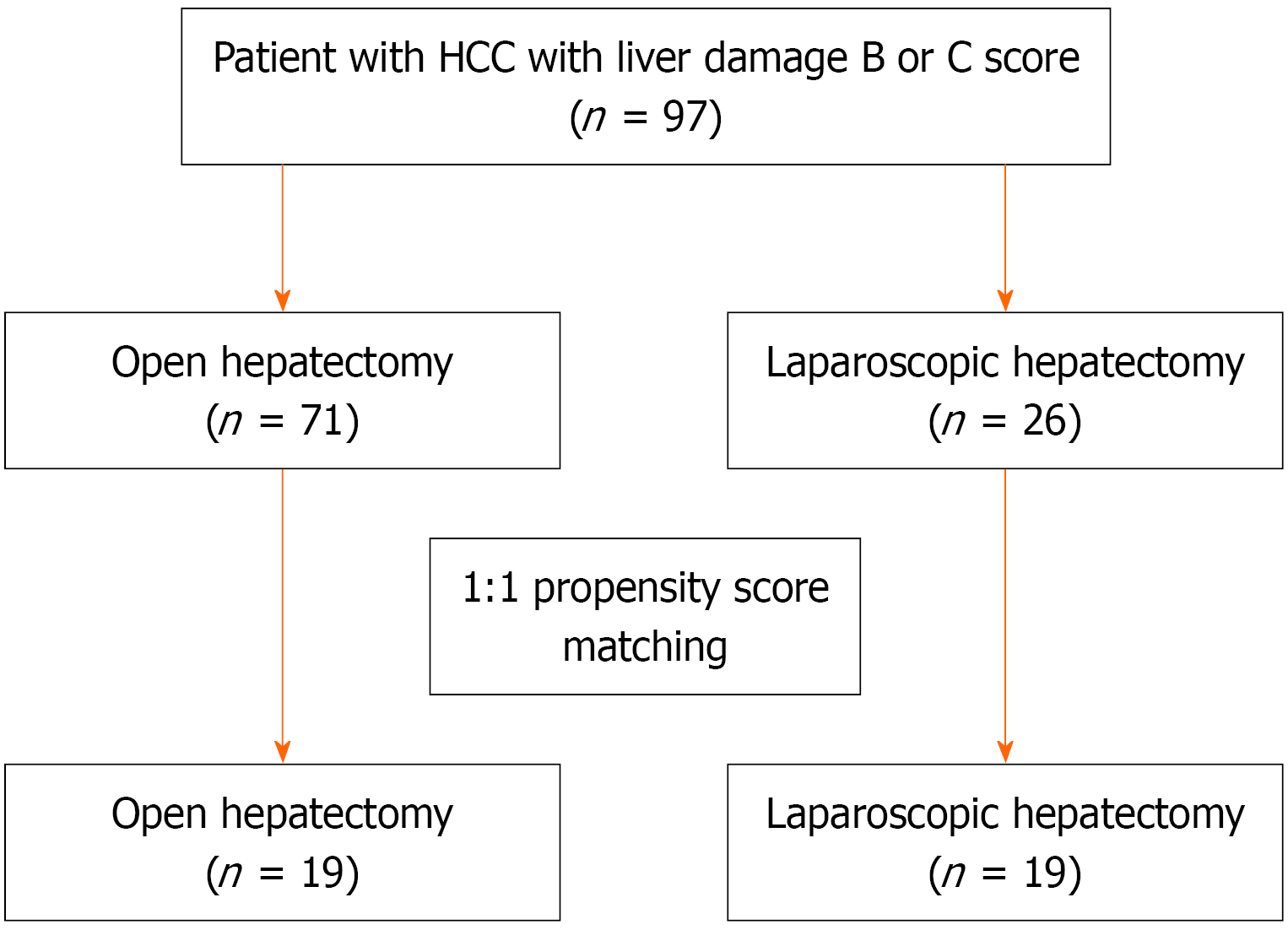
Between 2010 and 2022, we retrospectively analyzed a total of 848 patients with histopathologically confirmed HCC who underwent either LH or OH at the Tokyo Women’s Medical University Hospital (Tokyo, Japan). Histopathological diagnosis of HCC was made in accordance with the Japan Society of Hepatology guidelines[16]. All patients included in this study had liver damage graded B or C according to LDGS, underwent either LH or OH, and presented without distant metastasis. Patients with previous liver transplantation or combined resections of other organs were excluded. After applying these criteria 97 patients were eligible for analysis, including 26 who underwent LH and 71 who underwent OH as shown in Figure 1.
Liver damage was graded using the Child-Pugh classification system and the LDGS[16]. The LDGS incorporates five parameters: Ascites; serum total bilirubin; serum albumin; prothrombin activity; and ICG R15 (Table 1). Patients were classified into three grades of liver damage: (1) Grade A (mild): No ascites, bilirubin < 2.0 mg/dL, albumin > 3.5 g/dL, prothrombin activity > 80%, ICG R15 < 15%; (2) Grade B (moderate): Controllable ascites, bilirubin 2.0-3.0 mg/dL, albumin 3.0-3.5 g/dL, prothrombin activity 50%-80%, ICG R15 15%-40%; and (3) Grade C (severe): Intractable ascites, bilirubin > 3.0 mg/dL, albumin < 3.0 g/dL, prothrombin activity < 50%, ICG R15 > 40%. The severity of liver damage was determined when more than two criteria were met for a higher grade.
| A | B | C | |
| Ascites | Absent | Curative | Minimal therapeutic effect |
| Serum bilirubin (mg/dL) | < 2.0 | 2.0-3.0 | > 3.0 |
| Serum albumin (g/dL) | > 3.5 | 3.0-3.5 | < 3.0 |
| ICG R15 (%) | < 15 | 15-40 | > 40 |
| Prothrombin activity (%) | > 80 | 50-80 | < 50 |
Preoperative laboratory data, including total bilirubin, albumin, prothrombin activity, platelet count, and ICG R15 values, were obtained from hospital laboratory tests. Body mass index was calculated as weight (kg)/height (m2). Demographic data such as age, sex, and viral hepatitis markers were recorded from medical charts.
Tumor-related variables included tumor size, number, location (anterolateral or posterosuperior segments), and pathological findings (differentiation, microscopic portal vein invasion, and intrahepatic metastasis)[16] were recorded. Tumor location was classified according to Couinaud’s segments[12].
The choice of hepatectomy procedure (LH or OH) was based on tumor location, size, gross type, ICG R15 value, and the period when hepatectomy was performed. In addition, the surgical difficulty was assessed using the Ban Difficulty Scoring System (BAN score), which incorporates tumor size, location, proximity to major vessels, extent of liver resection, and liver function[19].
Since LH was introduced in 2010 at Tokyo Women’s Medical University Hospital, patients with small simple nodular-type HCC in the anterolateral and posterosuperior sections underwent laparoscopic non-anatomical partial resection, and open anatomical segmentectomy or non-anatomical partial resection, depending on functional liver reserve, respectively. In patients with extranodular or multinodular-type HCC in the anterolateral and posterosuperior sections, laparoscopic and open sectionectomies or segmentectomies were preferred and performed, respectively, depending on the functional liver reserve. Various anatomical hepatectomies are performed using the Glissonean pedicle approach in which a section or segment is confirmed by clamping and then resecting the Glissonean pedicle. Liver parenchymal dissection was performed using a Cavitron ultrasonic surgical aspirator. An intermittent Pringle maneuver involving occlusion periods of 10-15 min alternating with 5 min reperfusion intervals was initiated if the operative field was not dry.
Post-hepatectomy liver failure (PHLF) and biliary leakage were graded on or after postoperative day 5 in accordance with definitions from the International Study Group of Liver Surgery[20,21]. The severity of all complications was classified in accordance with definitions based on the Clavien-Dindo classification[22]. The calculation of all survival periods commenced from the hepatectomy date.
The primary endpoint was overall survival (OS) as a long-term surgical outcome. The secondary endpoints were short-term surgical outcomes, including mortality at 30 days and 90 days, morbidity classified as Clavien-Dindo class IIIa or higher, and ascites classified as Clavien-Dindo class I or II.
Baseline characteristics were expressed as mean ± SD for continuous data, and comparisons between the two groups were performed using Student’s t-test or Mann-Whitney U test. Categorical data were expressed as n (%), and comparisons were performed using the χ2 test. A survival analysis model was used to evaluate factors associated with OS and recurrence-free survival (RFS). Patient survival was calculated using the Product-Limit Survival Fit method of Kaplan-Meier[23], and differences in survival between the groups were assessed using the log-rank test.
To reduce selection bias PSM was performed in a 1:1 ratio using the nearest-neighbor matching method[17,18]. Matching variables included sex, platelet count, ICG R15, tumor size, BAN score, tumor location, cirrhosis, and surgical procedure. Baseline characteristics data and survival analyses after PSM were performed using the same methods as described above. All analyses were performed using JMP version 17.0.0 (SAS Institute, Cary, NC, United States), and a two-tailed P < 0.05 was considered statistically significant.
A total of 97 patients with HCC and LDGS grade B or C liver damage were included in this study with 26 in the LH group and 71 in the OH group. Before PSM the proportion of patients with Child-Pugh class B was relatively low and did not significantly differ between the OH and LH groups (12.7% vs 7.7%; P = 0.49), whereas the mean ICG R15 value was significantly higher in the LH group than in the OH group (32.5% vs 26.8%; P = 0.02) as shown in Table 2. Patients in the LH group had significantly smaller tumor size (2.7 cm vs 4.5 cm; P = 0.004) than those in the OH group. Only 1 patient (3.9%) underwent major hepatectomy (hemi-hepatectomy or sectionectomy) in the LH group and 17 patients (23.9%) in the OH group, and the surgical procedure was significantly different between the groups (P = 0.003). In addition, LH was more frequently performed in patients with anterolateral tumors (80.8% vs 46.5%; P = 0.003). After PSM (1:1) yielded 19 patient-matched pairs, balanced baseline characteristics were achieved between the two groups as shown in Table 2.
| Before propensity score matching | After propensity score matching | |||||
| Open group, n = 71 | Laparoscopic group, n = 26 | P value | Open group, n = 19 | Laparoscopic group, n = 19 | P value | |
| Age (years) | 67.7 ± 13.8 | 71.3 ± 6.8 | 0.21 | 69.4 ± 7.0 | 70.7 ± 6.3 | 0.56 |
| Male | 54 (76.1) | 13 (50) | 0.014 | 13 (68.4) | 11 (57.9) | 0.5 |
| BMI (kg/m2) | 24.0 ± 4.2 | 25.2 ± 3.1 | 0.19 | 24.8 ± 4.2 | 25.0 ± 3.2 | 0.89 |
| Etiology | ||||||
| HBsAg-positive | 5 (7.1) | 1 (3.9) | 0.56 | 1 (5.3) | 1 (5.3) | 1 |
| Anti-HCV-positive | 29 (40.9) | 13 (50.0) | 0.42 | 7 (36.8) | 8 (42.1) | 0.74 |
| Preoperative assessment | ||||||
| Total bilirubin (mg/dL) | 1.1 ± 0.6 | 1.1 ± 0.4 | 0.54 | 1.2 ± 0.5 | 1.1 ± 0.5 | 0.59 |
| Albumin (g/dL) | 3.6 ± 0.5 | 3.6 ± 0.3 | 0.91 | 3.6 ± 0.1 | 3.6 ± 0.1 | 0.91 |
| ICG R15 (%) | 26.8 ± 10.3 | 32.5 ± 11.7 | 0.02 | 31.3 ± 11.7 | 30.1 ± 12.0 | 0.75 |
| PT (%) | 73.7 ± 11.4 | 70.4 ± 9.9 | 0.19 | 69.0 ± 9.7 | 70.6 ± 10.8 | 0.63 |
| Platelet count (× 104/µL) | 14.0 ± 8.9 | 9.7 ± 3.8 | 0.027 | 11.7 ± 9.6 | 10.0 ± 4.2 | 0.51 |
| Ascites-present | 8 (11.3) | 1 (3.9) | 0.26 | 2 (13.3) | 0 (0) | 0.11 |
| Child-Pugh class A | 62 (87.3) | 24 (92.3) | 0.49 | 16 (84.2) | 18 (94.7) | 0.29 |
| Child-Pugh class B | 9 (12.7) | 2 (7.7) | 3 (15.8) | 1 (5.3) | ||
| Tumor characteristics | ||||||
| α-fetoprotein (ng/mL) | 4099 ± 16987 | 85 ± 346 | 0.25 | 646 ± 2483 | 116 ± 411 | 0.39 |
| PIVKA-2 (mAU/mL) | 11877 ± 72439 | 671 ± 2326 | 0.45 | 1326 ± 4100 | 909 ± 2750 | 0.73 |
| Tumor size (cm) | 4.5 ± 3.1 | 2.7 ± 1.2 | 0.004 | 2.9 ± 1.3 | 2.9 ± 1.3 | 1 |
| Tumor number-multiple | 24 (33.8) | 5 (19.2) | 0.17 | 5 (26.3) | 4 (21.1) | 0.7 |
| Tumor location | ||||||
| Anterolateral segment | 33 (46.5) | 21 (80.8) | 0.003 | 9 (47.4) | 14 (73.7) | 0.1 |
| Posterosuperior segment | 38 (53.5) | 5 (19.2) | 10 (52.6) | 5 (26.3) | ||
The LH group had significantly lower surgical difficulty as indicated by the lower BAN score (P < 0.001) as shown in Table 3. No patients in the LH group required conversion from LH to OH. After PSM the mean blood loss was significantly lower in the LH group than in the OH group (242 mL vs 941 mL; P = 0.049). Surgical margin status did not differ between the groups. The proportion of patients with histologically confirmed liver cirrhosis was significantly higher in the LH group than in the OH group (92.3% vs 66.2%, respectively; P = 0.01).
| Before propensity score matching | After propensity score matching | |||||
| Open group, | Laparoscopic group, | P value | Open group, | Laparoscopic group, | P value | |
| BAN score | ||||||
| Low | 14 (19.7) | 15 (57.7) | < 0.001 | 10 (52.6) | 8 (42.1) | 0.780 |
| Intermediate | 20 (28.2) | 8 (30.8) | 7 (36.8) | 8 (42.1) | ||
| High | 37 (52.1) | 3 (11.5) | 2 (10.5) | 3 (15.8) | ||
| Surgical procedure | ||||||
| Hemihepatectomy | 2 (2.8) | 0 (0) | 0.003 | 0 (0) | 0 (0) | 1.000 |
| Sectionectomy | 15 (21.1) | 1 (3.9) | 1 (5.3) | 1 (5.3) | ||
| Segmentectomy | 21 (29.6) | 3 (11.5) | 3 (15.8) | 3 (15.8) | ||
| Partial hepatectomy | 33 (46.5) | 22 (84.6) | 15 (79.0) | 15 (79.0) | ||
| Operative time (minute) | 250 ± 114 | 214 ± 90 | 0.140 | 225 ± 114 | 216 ± 89 | 0.770 |
| Blood loss (18) | 1169 ± 1994 | 199 ± 374 | 0.016 | 941 ± 1436 | 242 ± 427 | 0.049 |
| Pathological findings | ||||||
| High differentiation | 8 (11.3) | 5 (19.2) | 0.220 | 2 (10.5) | 3 (15.8) | 0.690 |
| Moderately differentiation | 37 (52.1) | 16 (61.6) | 12 (63.2) | 13 (68.4) | ||
| Poorly differentiation | 26 (36.6) | 5 (19.2) | 5 (26.3) | 3 (15.8) | ||
| Microscopic portal vein invasion | 22 (31.0) | 3 (11.5) | 0.050 | 3 (15.8) | 2 (10.5) | 0.630 |
| Intrahepatic metastasis | 17 (23.9) | 4 (15.4) | 0.360 | 3 (15.8) | 3 (15.8) | 1.000 |
| Surgical margin-positive | 7 (9.9) | 1 (3.9) | 0.340 | 1 (5.3) | 0 (0) | 0.310 |
| Cirrhosis | 47 (66.2) | 24 (92.3) | 0.010 | 16 (84.2) | 17 (89.5) | 0.630 |
After PSM the frequency of ascites classified as Clavien-Dindo class I or II was significantly lower in the LH group than in the OH group (0% vs 21.2%; P = 0.035), whereas that classified as Clavien-Dindo class III or IV was 0% in both groups as shown in Table 4. Although the mean length of hospital stay was shorter in the LH group than in the OH group, the difference was not significant. Morbidity (Clavien-Dindo class III or IV) was not significantly different between the two groups. Despite the three cases of 90-day mortality in the OH group before PSM, there were no statistically significant differences in the 30-day and 90-day mortality rates between the two groups before and after PSM.
| Before propensity score matching | After propensity score matching | |||||
| Open group, | Laparoscopic group, | P value | Open group, | Laparoscopic group, | P value | |
| Short-term outcome | ||||||
| Length of stay (day) | 27 ± 17 | 17 ± 14 | 0.010 | 24 ± 10 | 19 ± 16 | 0.260 |
| Ascites (C-D I or II) | 13 (18.3) | 0 (0) | 0.019 | 4 (21.1) | 0 (0) | 0.035 |
| Ascites (C-D III or IV) | 3 (4.2) | 0 (0) | 0.290 | 0 (0) | 0 (0) | NA |
| Bile leakage (C-D III or IV) | 6 (8.5) | 1 (3.9) | 0.440 | 1 (5.3) | 1 (5.3) | 1.000 |
| Liver failure (C-D III or IV) | 9 (12.7) | 3 (11.5) | 0.880 | 2 (10.5) | 2 (10.5) | 1.000 |
| All morbidity (C-D III or IV) | 14 (19.7) | 3 (11.5) | 0.350 | 3 (15.8) | 2 (10.5) | 0.630 |
| Mortality at 30 days | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Mortality at 90 days | 3 (4.2) | 0 (0) | 0.290 | 0 (0) | 0 (0) | NA |
| Long-term outcome | ||||||
| Recurrence | 47 (66.2) | 14 (53.9) | 0.260 | 11 (57.9) | 9 (47.4) | 0.520 |
| Death | 38 (53.5) | 4 (15.4) | 0.001 | 10 (52.6) | 2 (10.5) | 0.005 |
| Cancer | 27 (38.0) | 4 (15.4) | 0.018 | 7 (36.8) | 2 (10.5) | 0.080 |
| Liver failure due to cirrhosis | 5 (7.0) | 0 (0) | 0.160 | 1 (5.3) | 0 (0) | 0.310 |
| Infection | 4 (5.6) | 0 (0) | 0.220 | 1 (5.3) | 0 (0) | 0.310 |
| Other cancer | 2 (2.8) | 0 (0) | 0.390 | 1 (5.3) | 0 (0) | 0.310 |
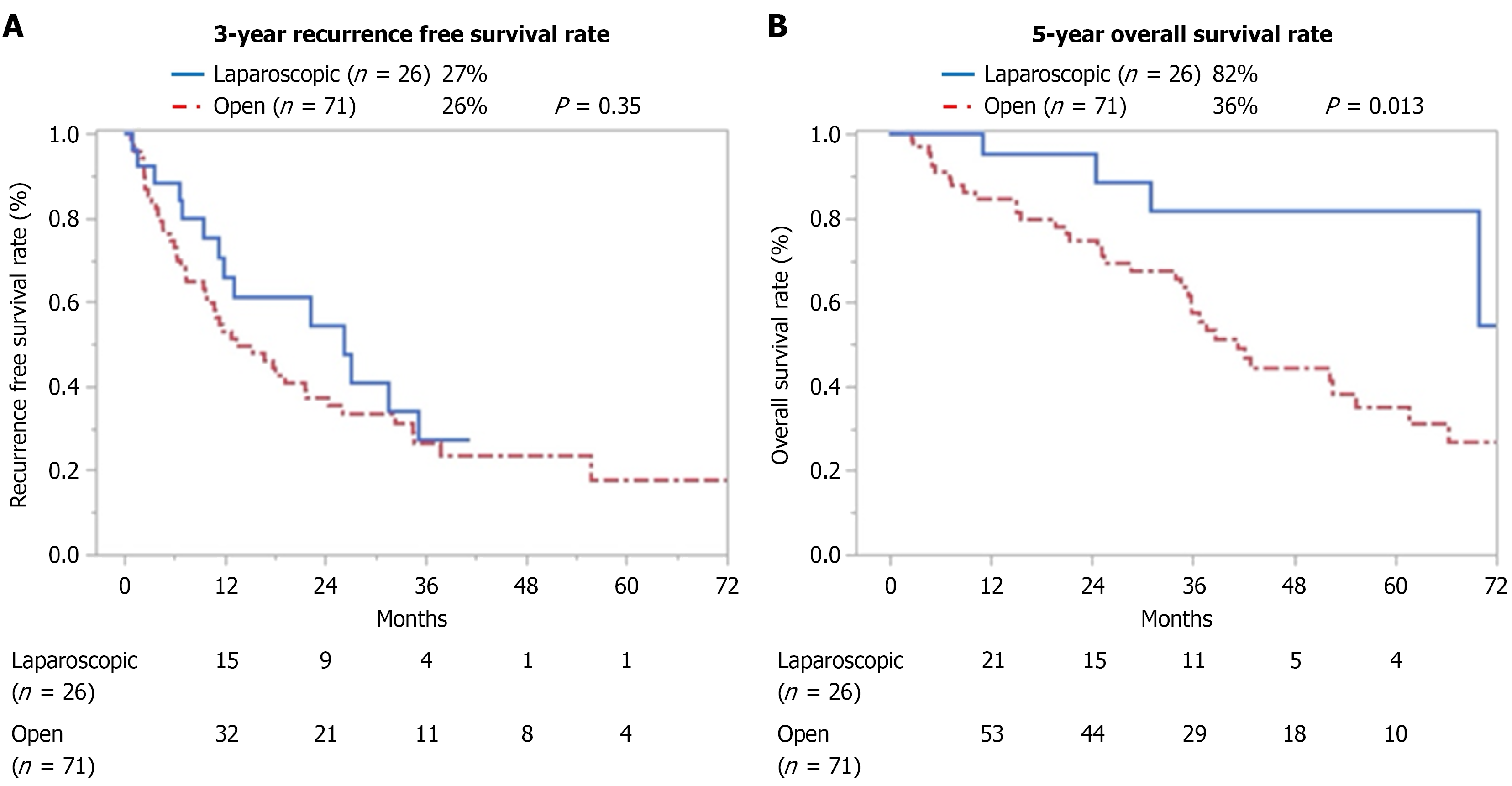
Before PSM the 3-year RFS rate did not differ between the LH and OH groups (27% vs 26%; P = 0.35) as shown in Figure 2A; however, the 5-year OS rate was significantly higher in the LH group than in the OH group (82% vs 36%; P = 0.013) as shown in Figure 2B.
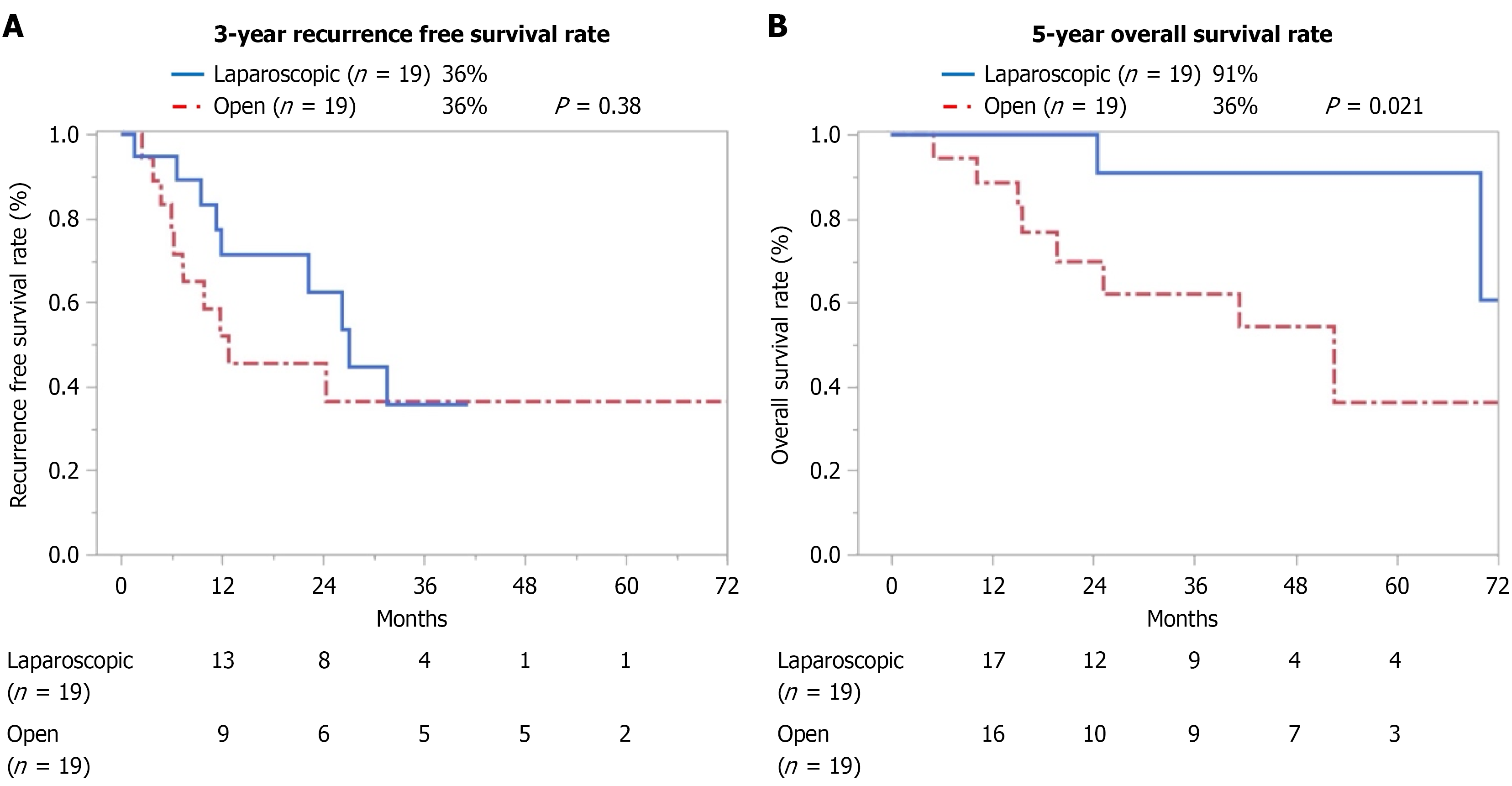
After PSM the 3-year RFS rates did not differ between the LH and OH groups (36% vs 36%; P = 0.38) as shown in Figure 3A; however, the 5-year OS rate was significantly higher in the LH group than in the OH group (91% vs 36%; P = 0.021) as shown in Figure 3B.
Hepatectomy is the standard treatment for HCC in patients with normal or compensated liver function(s)[2,3]. Many studies have investigated short-term and long-term outcomes of hepatectomy in patients with moderate cirrhosis[6,8]. In these patients PHLF is a serious complication that can easily develop and affect survival. The long-term outcomes after hepatectomy, including PHLF, have been associated with reduced blood loss and morbidity[24,25]. Furthermore, in our previous studies OS and RFS after anatomical OH were significantly lower in patients with morbidity than in those without, and morbidity was identified as an independent prognostic factor for OS and RFS[10,26].
PHLF, a frequent complication after hepatectomy, is caused by a combination of several factors, such as preoperative liver function, extent of hepatectomy, and intraoperative blood loss[27]. Assessment of preoperative liver function is crucial to ensure safe hepatectomy during the planning process. Although several scoring systems and imaging-based or scintigraphy-based assessments have been proposed as tools to evaluate preoperative liver function[28,29], LDGS has been widely used to identify patients at risk of PHLF in Japan[14,15,30].
In this study 18 (18.6%) of the 97 patients underwent major hepatectomy (hemi-hepatectomy, n = 2; sectionectomy, n = 16). Of these 18 patients 2 patients were classified as Child-Pugh class B. These patients had large tumors, and the functional liver parenchymal volume of the resected liver was limited; accordingly, right hemihepatectomy and right posterior sectionectomy were performed. Both patients experienced prolonged postoperative ascites (Clavien-Dindo class II); however, they ultimately recovered with medication alone. As shown in these two cases, even if the Child-Pugh classification is class B, major hepatectomy can be considered by precisely estimating the functional liver reserve according to the ICG R15 value.
In this study patients classified as having grade B or C liver damage exhibited elevated mean ICG R15 values (OH, 26.8%; LH, 32.5%); however, most patients were classified as Child-Pugh class A (OH, 88.7%; LH, 92.3%). This indicates that our study targeting patients classified as having grade B or C liver damage evaluated the benefits of hepatectomy in moderate cirrhosis more comprehensively than previous studies that focused solely on patients with Child-Pugh class B, who predominantly had compensated liver cirrhosis.
Our study demonstrated that LH significantly reduced intraoperative blood loss as previously reported[4,6,31]. These results are consistent with those of previous systematic reviews reporting that LH reduces intraoperative blood loss and transfusion rates in normal and compensated cirrhotic livers[32,33]. Although patients with liver cirrhosis are at risk of bleeding due to thrombocytopenia and hypercoagulopathy[31,34,35], LH reduces bleeding from the hepatic vein during parenchymal transection due to the effect of pneumoperitoneum pressure[33,36,37]. Pneumoperitoneum often reduces bleeding from the portal vein and makes the time of inflow occlusion (Pringle maneuver) during the parenchymal transection phase shorter[34,38]. This is expected to reduce ischemic and reperfusion injuries in the remnant liver, resulting in a lower PHLF rate. In addition, image magnification, advanced laparoscopic instruments that assist meticulous dissection and hemostasis[39-42], and preservation of portosystemic shunts and lymphatic flow around the liver by minimal incision are other advantages of LH that reduce blood loss[6,43].
Regarding postoperative ascites related to PHLF, no patient in the LH group developed excess postoperative ascites, allowing early drain removal within 5 days postoperatively without any subsequent events related to ascites and earlier discharge compared with OH as previously reported[8,37]. Reduced postoperative ascites can be attributed to several factors inherent to LH, such as the preservation of collateral circulation and lymphatic flow caused by smaller abdominal incisions and decreased liver mobilization. Minimizing liver mobilization using the intercostal trocar and flex scope, especially for tumors in the posterosuperior segments (5 cases in this study), likely contributed to these favorable outcomes[44,45]. Additionally, the hermetic pneumoperitoneum environment and reduced blood loss may lessen the local immune response and lead to hyperaldosteronism, further decreasing third-space fluid accumulation[33]. These factors can make LH beneficial, especially for patients with grade B liver damage who are at greater risk of complications such as postoperative ascites and PHLF.
After PSM baseline characteristics between the two groups were well balanced, particularly regarding surgical difficulty as reflected by the BAN score[19]. This adjustment minimized potential bias arising from differences in operative complexity, allowing a more reliable comparison of long-term outcomes. Regarding long-term outcomes, the 5-year OS rate was significantly higher in the LH group after PSM; however, the 3-year RFS rate did not differ between the two groups. Generally, the prognosis of patients with HCC and liver cirrhosis, such as grade B liver damage, depends not only on HCC progression but also on deteriorating liver function[46]. In fact, the cause of death in 4 patients (15.4%) in the LH group was not postoperative complications or liver failure but recurrent HCC. The causes of death in the OH group were recurrent HCC [n = 27 (38.0%)], liver failure [n = 5 (7.0%)], infection [n = 4 (5.6%)], and other malignancies [n = 2 (2.8%)]. Other malignancies refer to secondary cancers (colon, pancreas, and prostate) diagnosed during long-term follow-up, independent of the primary HCC. LH can preserve functional liver reserves, general conditions, and immune function because it is a minimally invasive approach.
The study had several limitations. The first of these was a retrospective, single-center design and a small sample size may limit generalizability. Notably, outcomes in the LH group were affected by technological advances as laparoscopic procedures were developed and standardized during the study period (between 2010 and 2022), whereas those for OH were completely standardized at the beginning of the study (2010) at Tokyo Women’s Medical University Hospital. Additionally, long-term survival was affected by recurrent disease and non-cancer causes, including liver failure and other malignancies. Despite these limitations future multicenter prospective studies with larger sample sizes and standardized surgical criteria are needed to confirm the safety and long-term benefits of LH in this population.
LDGS can be a more useful framework for surgical risk assessment, evaluating the benefits of hepatectomy in patients with moderate cirrhosis compared with the Child-Pugh classification. In patients with HCC and grade B or C liver damage, LH appears feasible and safe, offering better long-term outcomes than OH. Minimizing complications such as PHLF further highlights the advantages of minimally invasive approaches.
| 1. | Kudo M. Surveillance, Diagnosis, and Treatment Outcomes of Hepatocellular Carcinoma in Japan: 2021 Update. Liver Cancer. 2021;10:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1718] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 3. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3096] [Article Influence: 774.0] [Reference Citation Analysis (61)] |
| 4. | Troisi RI, Rompianesi G, D'Hondt M, Vanlander A, Bertrand C, Hubert C, Detry O, Van den Bossche B, Malvaux P, Weerts J, Sablon T, Vermeiren K, Biglari M, Gryspeerdt F, De Meyere C, Dili A, Boterbergh K, Lucidi V. Multicenter Belgian prospective registry on minimally invasive and open liver surgery (BReLLS): experience from 1342 consecutive cases. Langenbecks Arch Surg. 2025;410:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Fuji H, Seo S, Toda R, Yoh T, Ikeno Y, Fukumitsu K, Ishii T, Taura K, Hatano E, Kaido T, Uemoto S. Optimal introduction of laparoscopic liver resection for Child-Pugh B. Asian J Endosc Surg. 2019;12:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Troisi RI, Berardi G, Morise Z, Cipriani F, Ariizumi S, Sposito C, Panetta V, Simonelli I, Kim S, Goh BKP, Kubo S, Tanaka S, Takeda Y, Ettorre GM, Russolillo N, Wilson GC, Cimino M, Montalti R, Giglio MC, Igarashi K, Chan CY, Torzilli G, Cheung TT, Mazzaferro V, Kaneko H, Ferrero A, Geller DA, Han HS, Kanazawa A, Wakabayashi G, Aldrighetti L, Yamamoto M. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg. 2021;108:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 7. | Di Sandro S, Bagnardi V, Najjar M, Buscemi V, Lauterio A, De Carlis R, Danieli M, Pinotti E, Benuzzi L, De Carlis L. Minor laparoscopic liver resection for Hepatocellular Carcinoma is safer than minor open resection, especially for less compensated cirrhotic patients: Propensity score analysis. Surg Oncol. 2018;27:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, Kim S, Goh BKP, Kubo S, Tanaka S, Takeda Y, Ettorre GM, Wilson GC, Cimino M, Chan CY, Torzilli G, Cheung TT, Kaneko H, Mazzaferro V, Geller DA, Han HS, Kanazawa A, Wakabayashi G, Troisi RI. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol. 2020;72:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | He Y, Fang D, Liang T, Mo S, Meng Y, Chen Z, Zhao S, Liao Y, Huang K, Nong S, Zhou W, Han C, Peng T. Laparoscopic versus open hepatectomy for hepatocellular carcinoma with cirrhosis: a single-center propensity score matching analysis. Ann Transl Med. 2021;9:1733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Yang S, Ni H, Zhang A, Zhang J, Zang H, Ming Z. Impact of postoperative morbidity on the prognosis of patients with hepatocellular carcinoma after laparoscopic liver resection: a multicenter observational study. Sci Rep. 2025;15:1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5817] [Article Influence: 109.8] [Reference Citation Analysis (2)] |
| 12. | Ger R. Surgical anatomy of the liver. Surg Clin North Am. 1989;69:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 601] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 14. | Omagari K, Ohba K, Kadokawa Y, Hazama H, Masuda J, Kinoshita H, Matsuo I, Ohnita K, Mizuta Y, Hayashida K, Kohno S. Comparison of the grade evaluated by "Liver damage" of Liver Cancer Study Group of Japan and Child-Pugh classification in patients with hepatocellular carcinoma. Hepatol Res. 2006;34:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Nanashima A, Sumida Y, Morino S, Yamaguchi H, Tanaka K, Shibasaki S, Ide N, Sawai T, Yasutake T, Nakagoe T, Nagayasu T. The Japanese integrated staging score using liver damage grade for hepatocellular carcinoma in patients after hepatectomy. Eur J Surg Oncol. 2004;30:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 17. | Liang J, Hu Z, Zhan C, Wang Q. Using Propensity Score Matching to Balance the Baseline Characteristics. J Thorac Oncol. 2021;16:e45-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Propensity Score Methods for Bias Reduction in Observational Studies of Treatment Effect. Rheum Dis Clin North Am. 2018;44:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 20. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1819] [Article Influence: 121.3] [Reference Citation Analysis (1)] |
| 21. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1527] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 22. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9184] [Article Influence: 540.2] [Reference Citation Analysis (1)] |
| 23. | Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457-481. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32610] [Cited by in RCA: 31456] [Article Influence: 462.6] [Reference Citation Analysis (0)] |
| 24. | Chok KSH, Chan MMY, Dai WC, Chan ACY, Cheung TT, Wong TCL, She WH, Lo CM. Survival outcomes of hepatocellular carcinoma resection with postoperative complications - a propensity-score-matched analysis. Medicine (Baltimore). 2017;96:e6430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Yang T, Liu K, Liu CF, Zhong Q, Zhang J, Yu JJ, Liang L, Li C, Wang MD, Li ZL, Wu H, Xing H, Han J, Lau WY, Zeng YY, Zhou YH, Gu WM, Wang H, Chen TH, Zhang YM, Zhang WG, Pawlik TM, Wu MC, Shen F. Impact of postoperative infective complications on long-term survival after liver resection for hepatocellular carcinoma. Br J Surg. 2019;106:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Ariizumi SI, Katagiri S, Kotera Y, Yamashita S, Omori A, Kato T, Shibuya G, Egawa H, Takasaki K, Yamamoto M. Improved Mortality, Morbidity, and Long-Term Outcome After Anatomical Hepatectomy With the Glissonean Pedicle Approach in Patients With Hepatocellular Carcinoma: 30 Years' Experience at a Single Institute. Ann Surg. 2022;275:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 27. | Schwarz C, Plass I, Fitschek F, Punzengruber A, Mittlböck M, Kampf S, Asenbaum U, Starlinger P, Stremitzer S, Bodingbauer M, Kaczirek K. The value of indocyanine green clearance assessment to predict postoperative liver dysfunction in patients undergoing liver resection. Sci Rep. 2019;9:8421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Morris-Stiff G, Gomez D, Prasad R. Quantitative assessment of hepatic function and its relevance to the liver surgeon. J Gastrointest Surg. 2009;13:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Morandi A, Risaliti M, Montori M, Buccianti S, Bartolini I, Moraldi L. Predicting Post-Hepatectomy Liver Failure in HCC Patients: A Review of Liver Function Assessment Based on Laboratory Tests Scores. Medicina (Kaunas). 2023;59:1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 30. | Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, Makuuchi M, Kojiro M, Ichida T, Arii S, Yamaoka Y; Liver Cancer Study Group of Japan. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Yamamoto M, Kobayashi T, Oshita A, Abe T, Kohashi T, Onoe T, Fukuda S, Omori I, Imaoka Y, Honmyo N, Ohdan H. Laparoscopic versus open limited liver resection for hepatocellular carcinoma with liver cirrhosis: a propensity score matching study with the Hiroshima Surgical study group of Clinical Oncology (HiSCO). Surg Endosc. 2020;34:5055-5061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Chen J, Bai T, Zhang Y, Xie ZB, Wang XB, Wu FX, Li LQ. The safety and efficacy of laparoscopic and open hepatectomy in hepatocellular carcinoma patients with liver cirrhosis: a systematic review. Int J Clin Exp Med. 2015;8:20679-20689. [PubMed] |
| 33. | Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, Sutton R, Liu XB, Hu WM. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6657-6668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Wu X, Huang Z, Lau WY, Li W, Lin P, Zhang L, Chen Y. Perioperative and long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with well-preserved liver function and cirrhotic background: a propensity score matching study. Surg Endosc. 2019;33:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Pan Y, Xia S, Cai J, Chen K, Cai X. Efficacy of Laparoscopic Hepatectomy versus Open Surgery for Hepatocellular Carcinoma With Cirrhosis: A Meta-analysis of Case-Matched Studies. Front Oncol. 2021;11:652272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Peng Y, Chen K, Li B, Xu H, Wei Y, Liu F. Laparoscopic versus open liver resection for resectable HCC with BCLC stage B: a propensity score-matched analysis. Updates Surg. 2022;74:1291-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls M, Scuderi V, Barkhatov L, Edwin B, Troisi RI, Dagher I, Reggiani P, Belli G, Aldrighetti L, Abu Hilal M. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc. 2018;32:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Molina V, Sampson-Dávila J, Ferrer J, Fondevila C, Díaz Del Gobbo R, Calatayud D, Bruix J, García-Valdecasas JC, Fuster J. Benefits of laparoscopic liver resection in patients with hepatocellular carcinoma and portal hypertension: a case-matched study. Surg Endosc. 2018;32:2345-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Honda G, Kurata M, Okuda Y, Kobayashi S, Tadano S, Yamaguchi T, Matsumoto H, Nakano D, Takahashi K. Totally laparoscopic hepatectomy exposing the major vessels. J Hepatobiliary Pancreat Sci. 2013;20:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Yoon YI, Kim KH, Cho HD, Kwon JH, Jung DH, Park GC, Song GW, Ha TY, Lee SG. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: a retrospective study. Surg Endosc. 2020;34:796-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Xu HW, Liu F, Li HY, Wei YG, Li B. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surg Endosc. 2018;32:712-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (61)] |
| 42. | Honda G, Ome Y, Yoshida N, Kawamoto Y. How to dissect the liver parenchyma: Excavation with cavitron ultrasonic surgical aspirator. J Hepatobiliary Pancreat Sci. 2020;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Belli G, Fantini C, D'Agostino A, Cioffi L, Langella S, Russolillo N, Belli A. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Ome Y, Honda G, Doi M, Muto J, Seyama Y. Laparoscopic Anatomic Liver Resection of Segment 8 Using Intrahepatic Glissonean Approach. J Am Coll Surg. 2020;230:e13-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Okuda Y, Honda G, Kurata M, Kobayashi S, Sakamoto K, Takahashi K. A safe and valid procedure for pure laparoscopic partial hepatectomy of the most posterosuperior area: the top of segment 7. J Am Coll Surg. 2015;220:e17-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6627] [Article Influence: 441.8] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/