Published online Jan 27, 2026. doi: 10.4240/wjgs.v18.i1.114022
Revised: September 30, 2025
Accepted: November 24, 2025
Published online: January 27, 2026
Processing time: 133 Days and 20.8 Hours
Meckel’s diverticulum (MD) is most commonly encountered during emergency interventions for complications or as an incidental finding during an unrelated procedure. Definitive pre-operative diagnosis of MD-associated adenocarcinoma, permitting radical resection, is very rare in clinical practice.
A 36-year-old male presented with recurrent dark-red hematochezia. Initial gas
For recurrent obscure gastrointestinal hemorrhage, combined Meckel scan and double-balloon enteroscopy is critical for diagnosing MD-associated, enabling curative resection.
Core Tip: This case report describes the pre-operative diagnosis of Meckel’s-diverticulum-associated adenocarcinoma identified by technetium-99m pertechnetate scanning and double-balloon enteroscopy. The patient underwent laparoscopic radical small-bowel resection (R0, 19 lymph nodes), based on the National Comprehensive Cancer Network guidelines, followed by adjuvant chemotherapy. Despite these high-risk features, 36 months of disease-free survival was achieved, highlighting a potential new diagnostic and therapeutic approach for this rare malignancy.
- Citation: Yu ZH, Ling CR, Yu JY, Zhang QH, Wei SS. Long-term disease-free survival following preoperative diagnosis and laparoscopic radical resection for Meckel’s diverticulum adenocarcinoma: A case report. World J Gastrointest Surg 2026; 18(1): 114022
- URL: https://www.wjgnet.com/1948-9366/full/v18/i1/114022.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v18.i1.114022
Meckel’s diverticulum (MD) arises from the vitelline duct during embryogenesis, and it is the most prevalent congenital gastrointestinal anomaly in adults, with an estimated incidence of 2%[1]. Approximately 90% of MDs occur in the ileum, within 100 cm of the ileocecal valve, usually 3 cm in length[2]. Meckel’s-diverticulum-associated carcinoma (MDC) is very rare, affecting 0.5% to 3.2% of symptomatic patients[3,4]. Adenocarcinomas are particularly uncommon among these malignancies, accounting for only 11.04% of reported MDCs[5].
For asymptomatic MD or those with benign complications, such as hemorrhage, obstruction, or perforation, surgical approaches typically include linear stapled resection, wedge resection of the diverticulum, or limited segmental bowel resection[6]. MDC is a distinct and uncommon subtype that is often diagnosed incidentally, post-operatively. Currently, there are no standardized treatment guidelines for this condition. Most of the available literature is limited to case reports, and there is a lack of consensus regarding the extent of surgical resection and lymphadenectomy. Furthermore, the prognosis is generally poor due to frequent presentation with extensive local invasion and/or lymphatic or distant metastasis[5,7,8].
This case establishes the feasibility of obtaining a pre-operative diagnosis of MD-associated adenocarcinoma (MDA) using a combination of a technetium-99m pertechnetate Meckel scan (Meckel scan) and double-balloon enteroscopy (DBE). To our knowledge, this is the first reported case of adenocarcinoma arising in an MD that was pre-operatively diagnosed and successfully managed with laparoscopic radical resection, based on the National Comprehensive Cancer Network (NCCN) guidelines (Available from: https://www.nccn.org/). Curative-intent surgery with guideline-compliant adjuvant chemotherapy resulted in a 36-month disease-free survival. This successful strategy provides valuable insights into the guiding and standardizing of the management of this rare malignancy (Table 1)[9-11].
| Ref. | Sex | Age, years | Symptom | Examination | Location | Treatment | Resection margin | Lymph node dissection | Pathology | Stage | Prognosis |
| Principe et al[9], 2022 | Male | 62 | Melena | CE, DBE | Distal jejunum/proximal ileum | Exploratory laparotomy, small bowel resection | NA | NA | Moderately to poorly differentiated adenocarcinoma | T4N0M0 | NA |
| Sakio et al[10], 2021 | Female | 45 | Recurrent abdominal pain | DBE with contrast | 160 cm from ileocecal valve | Bowel resection + lymphadenectomy | NA | NA | Well-differentiated adenocarcinoma | pT4aN0M0 | Recurrence 2 years post-op |
| Sato et al[11], 2009 | Male | 58 | Elevated tumor marker | CE, SBE | 90 cm from ileocecal valve | Ileal segmental resection + regional lymphadenectomy | NA | NA | Moderately differentiated adenocarcinoma | T3 | NA |
| Our case | Male | 36 | Melena | Meckel’s scan, DBE | 60 cm from ileocecal valve | Radical resection of small bowel adenocarcinoma + lymphadenectomy | Negative, 20 cm proximal and distal margins | 0/19 | Moderately differentiated adenocarcinoma | pT3N0M0 | Disease-free at 3 years |
A 36-year-old male had recurrent hematochezia for 10 months, with a new episode lasting two days.
Over the past 10 months, the patient has had three episodes of intermittent dark-red bloody stools. Diagnostic investigations conducted at other hospitals revealed unremarkable findings. Following symptom resolution, the patient self-discharged, on each occasion, with no definitive cause identified. The patient was admitted to our hospital for a definitive diagnosis.
The patient had no relevant past illnesses.
The patient had no specific medical history, and there was no relevant family history.
The patient was 170 cm tall and weighed 100 kg, with a body mass index of 34.6 kg/m2. Physical examination revealed mild periumbilical tenderness. The remainder of the examination was unremarkable.
Positive fecal occult blood test and a hemoglobin level of 107 g/L. Other laboratory results were within normal ranges.
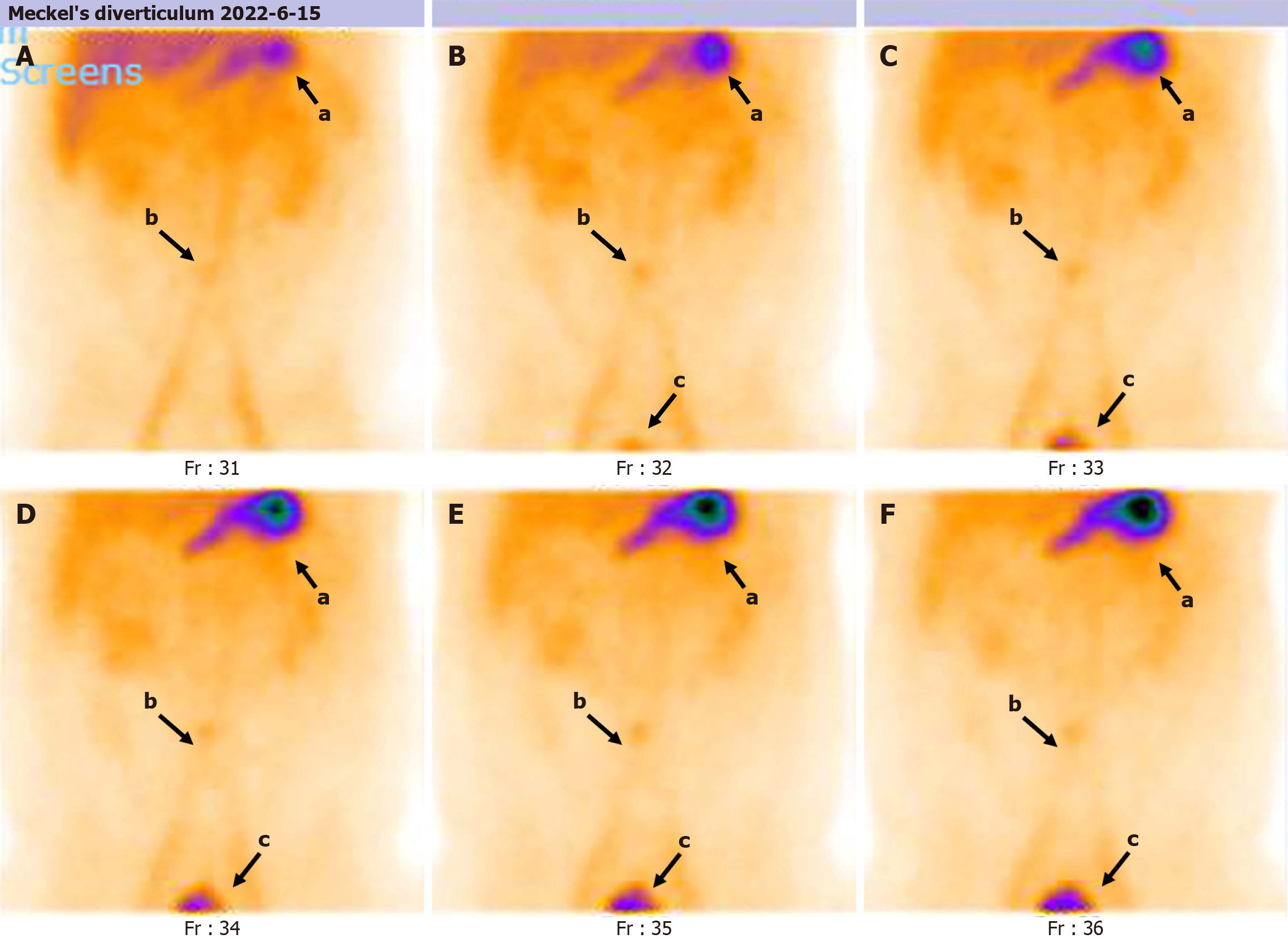
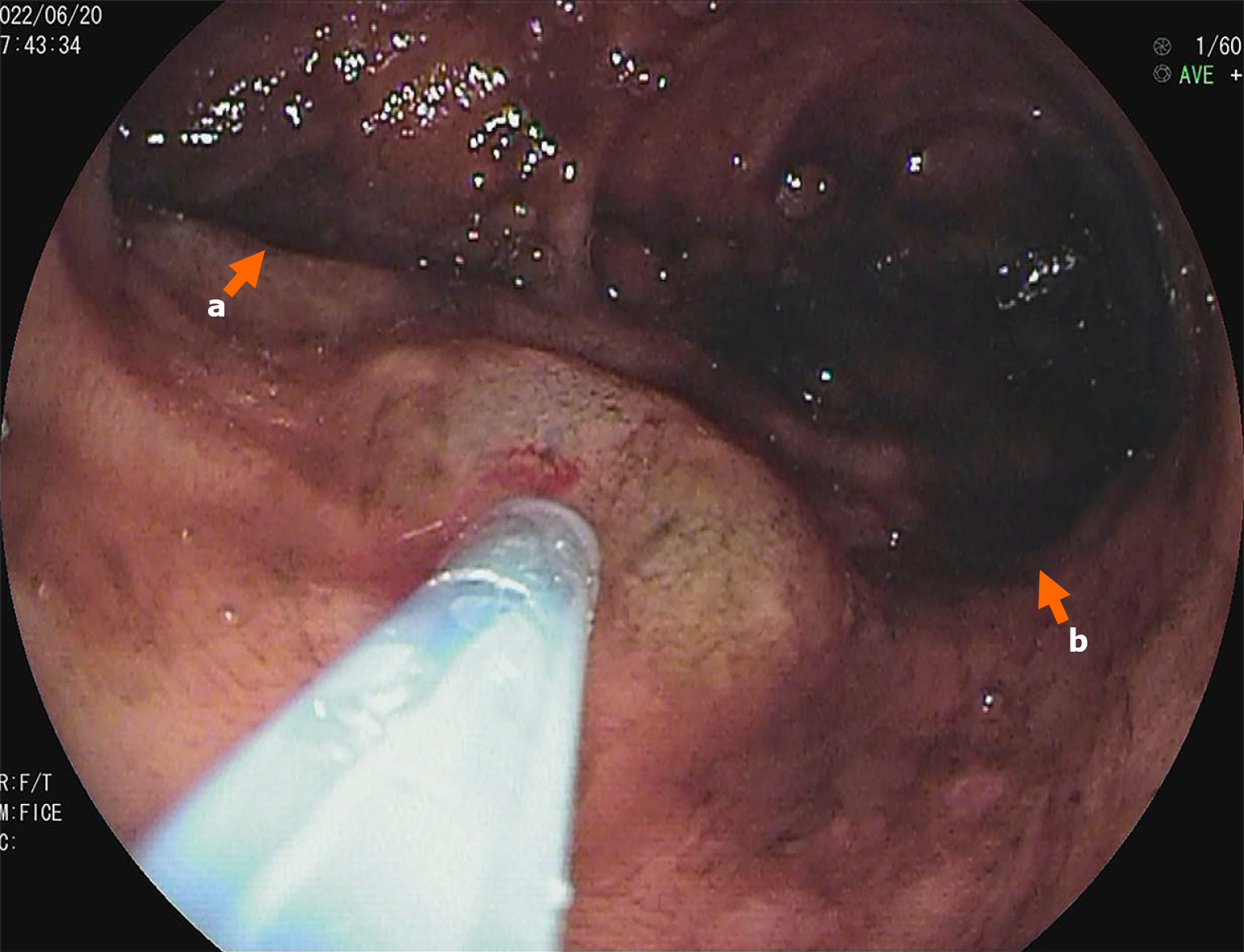
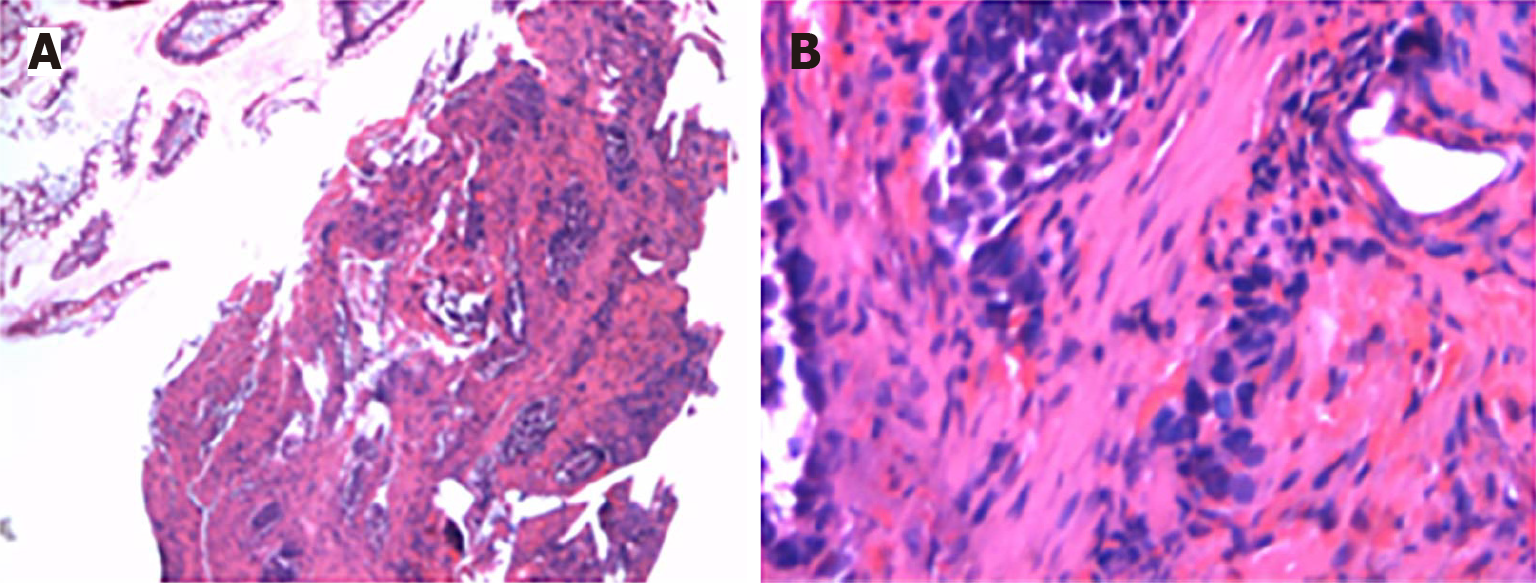
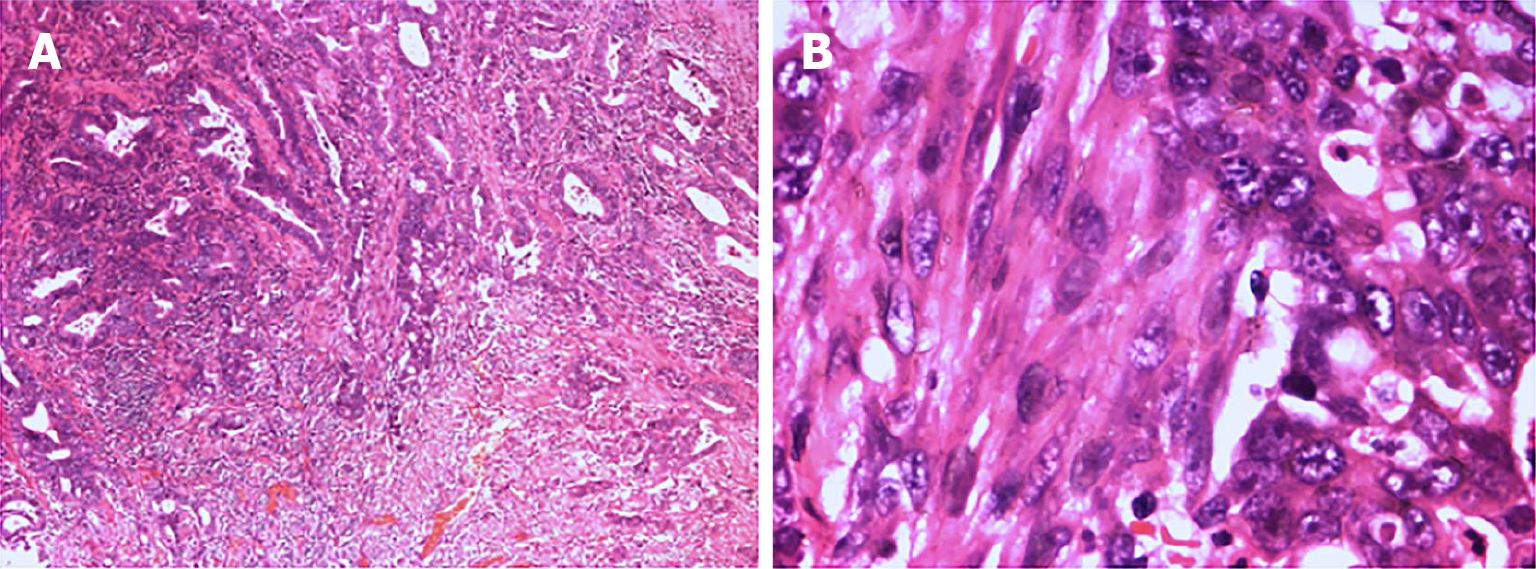
The Meckel scan: After injection of the radiotracer (99mTcO4-), gastric imaging was performed. An abnormal tracer concentration was observed in the mid-abdomen, which appeared as a round lesion. Over time, the tracer concentration increased slightly; however, there were no significant changes in shape or location (Figure 1). Examination conclusion: Positive imaging of ectopic gastric mucosa. Based on this, DBE was performed, and a mucosal protrusion was identified 60 cm proximal to the ileocecal valve, presenting with a “double lumen sign” (Figure 2). The distal lumen had normal ileal mucosa, whereas the proximal lumen had a circumferential ulcerated stricture occupying 75% of the bowel circumference, with exudate. The ulcer bed had raised bordering mucosa, contact hemorrhage, and a firm texture. After biopsy, the lesion was marked with India ink. Histopathological examination confirmed ileal adenocarcinoma (Figure 3) with mismatch repair proficiency (MutL homolog 1+, MutS homolog 2+, MutS homolog 6+, and postmeiotic segregation increased 2+).
The final pathological diagnosis after surgical resection was MDA, pT3N0M0, Stage IIA [American Joint Committee on Cancer (AJCC) 8th edition].
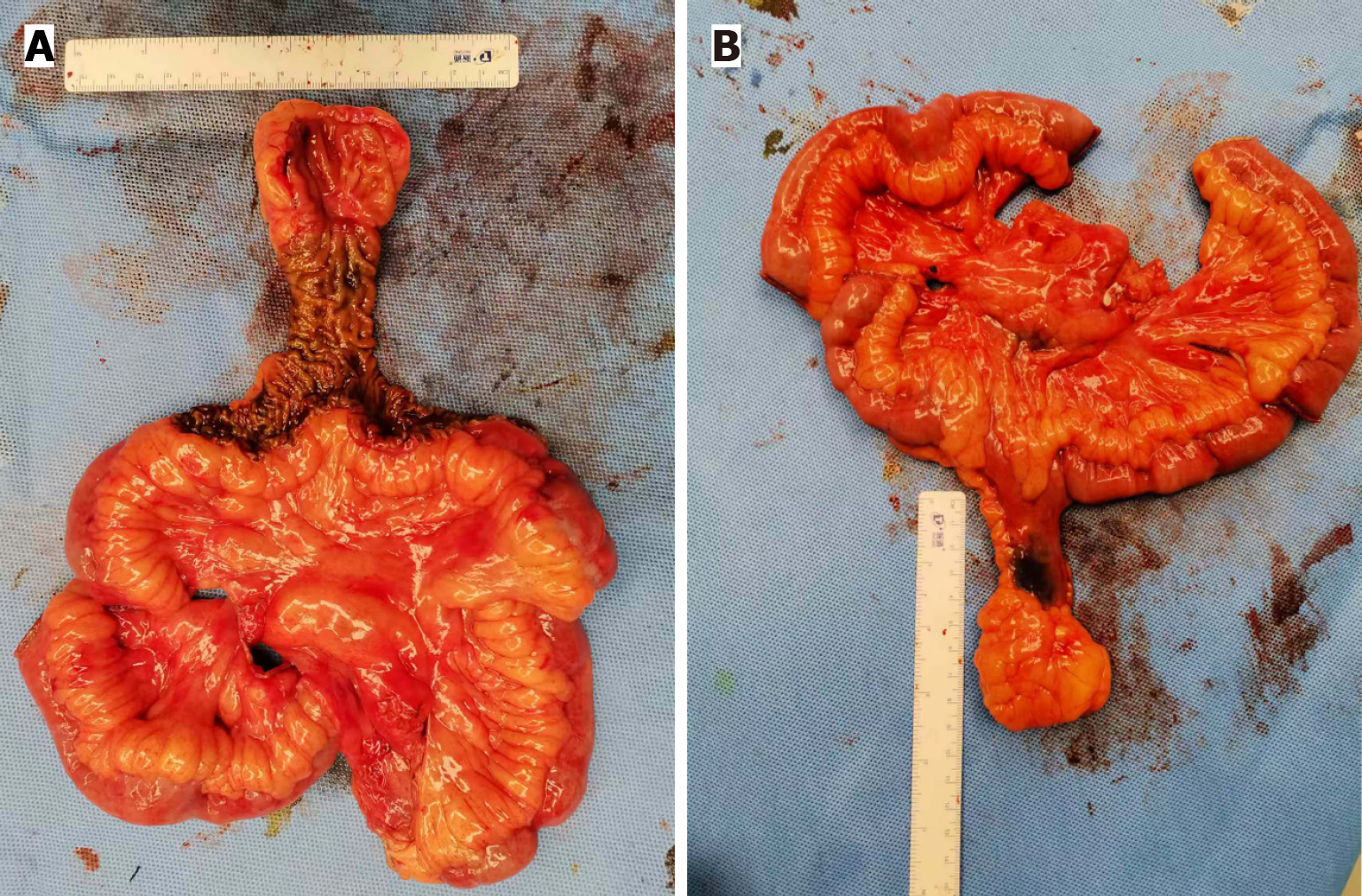
After informed consent was obtained, a laparoscopic exploratory procedure was performed. A 4 cm × 8 cm diverticulum was identified approximately 60 cm from the ileocecal valve, arising from the mesenteric border of the ileum, and localized by Indian ink marking (Figure 4). Subsequently, an enlarged mesenteric lymph node, suspected to be a metastasis, was identified adjacent to the diverticulum. To achieve complete lymphadenectomy, a 5-cm midline incision was made in the upper abdomen and extended towards the root of the superior mesenteric artery. Key vascular branches were selectively ligated to minimize intraoperative blood loss. After lymph node dissection, compromised perfusion was observed in the bowel segments proximal and distal to the lesion. To secure well-vascularized anastomotic margins and to reduce the risk of post-operative leakage, the resection was extended to include only segments with a confirmed normal blood supply, resulting in final margins of approximately 20 cm on both sides. The bowel was transected using a linear stapler, and the specimen was subsequently submitted for histopathological examination. A side-to-side anastomosis of the proximal and distal ileum was performed using microsutures. The muscular layer was reinforced with purse-string sutures to invert the residual ends, and the mesenteric defect was closed. Final histopathological exa
The patient recovered uneventfully and was discharged on post-operative day 8. No tumor recurrence or metastasis was detected during 36 months of follow-up. The surveillance protocol was as follows: In the first post-operative year, hematological tests, including tumor markers, were performed every three months, abdominal contrast-enhanced computed tomography or total gastrointestinal imaging was performed every six months, and DBE was performed annually. From the second year onwards, the frequency of hematological tests was reduced to every six months, while the schedules for imaging and DBE remained unchanged. In addition, the patient was instructed to return immediately for reassessment if there were symptoms such as melena or hematochezia.
MDCs are frequently diagnosed incidentally during surgery because of their insidious presentation and the absence of specific diagnostic tools. At diagnosis, the disease is often advanced, resulting in a poor prognosis[8]. Carcinoid tumors represent the most common MDC, followed by gastrointestinal stromal tumors, leiomyosarcomas, and rarer adenocarcinomas of gastric/intestinal origin[12-14]. Despite their lower incidence, adenocarcinomas occur at younger ages (mean 55.9 years) and have a poorer prognosis, with a median overall survival of 13 months[5]. Surveillance, Epidemiology, and End Results database analysis revealed an increasing incidence of MDA malignancies, predominantly in males, but increasing more rapidly in females. MD confers a 70-fold higher cancer risk than the adjacent ileum, with the incidence increasing with age[5].
The current pre-operative diagnostic modalities for MDC are limited. While contrast-enhanced abdominal computed tomography/magnetic resonance imaging may detect an MD, uncomplicated MDs are frequently overlooked and misidentified as bowel loops[15]. Typically, esophagogastroduodenoscopy and colonoscopy cannot access the MDs. The Meckel scan effectively detects the gastric mucosa in an MD but fails to identify malignant lesions[16]. Capsule endoscopy visualizes the MD orifice, but it carries risks of entrapment within the diverticulum or stenotic segments, with an inability to obtain biopsies or exclude missed lesions[10,17]. Small-bowel enteroscopy offers superior diagnostic value, enabling direct visualization, targeted biopsies, and minimizing procedure-related risk of obstruction, which are particularly advantageous for the diagnosis of MDC[18]. Similar to our case, DBE is critical for pre-operative confirmation of MDC[9,10]. Furthermore, India ink marking was performed to enable identification of the lesion to facilitate intra-operative localization.
MDCs typically present with complications such as gastrointestinal hemorrhage, perforation, or bowel obstruction[19], requiring emergency admission. Clinical deterioration often necessitates urgent surgical intervention. As pre-operative diagnosis of MDC remains elusive, surgical interventions are frequently limited to diverticulectomy or segmental resection without oncological principles, such as adequate margins or lymphadenectomy[5]. Contemporary case reports describe negative margins and lymph node sampling, but fail to quantify resection margin distances[9,10]. Although Hendrickx et al[7] reported a case of metastasis-free lymph nodes (0/5), inadequate lymph node dissection still poses a risk, because lymph node metastasis is a strong predictor of prognosis[20]. For MDCs, failure to accurately locate the tumor or incomplete resection of the tumor, resulting in residual lesions post-operatively, leads to a poorer prognosis. Re-operation is often ineffective, and adjuvant chemotherapy does not provide significant benefits[21]. Intraoperative frozen-section histopathology may also delay decision-making. In this case, early presentation allowed for a pre-operative diagnosis of MDC. Despite the lack of specific guidelines, the NCCN small-bowel cancer principles of R0 resection with
The anatomical location and ligation of vessels close to the mesenteric root are crucial for complete lymph node dissection. Although radical dissection can improve survival, the division of vessels at their roots may compromise blood flow, increasing the risk of anastomotic ischemia and fistulas. To reduce this risk, surgeons may extend resection to obtain a well-perfused segment for anastomosis[22-24]. Extensive resection can cause malabsorption of nutrients and electrolyte imbalance[25]; however, most nutrients are absorbed by the proximal small intestine, which can adapt to partially offset the reduced absorption area. Resection of ≤ 50% of the small intestine is generally tolerated, particularly if the ileocecal valve is preserved[26]. Surgeons must balance oncological radicality with bowel preservation. In this case, although the resection exceeded the guideline-recommended margin (> 20 cm vs NCCN recommendation of 10 cm), it yielded a higher lymph node count (19 vs NCCN recommendation of ≥ 8). At the three-year post-operative follow-up, the patient had no electrolyte disturbances, chronic diarrhea, or weight loss (body mass index = 28.7 kg/m2). To the best of our knowledge, this is the first reported case of MDA definitively diagnosed pre-operatively and treated with laparoscopic radical surgery. Post-operative histopathological examination revealed an intravascular cancer thrombus, which is a risk factor for recurrence and metastasis. The patient subsequently received adjuvant chemotherapy and remained free of recurrence or metastasis during the three-year follow-up. For post-operative surveillance of MDA, whenever feasible, adherence to the NCCN guidelines is recommended: Clinical evaluation every three months during the first two years with imaging as indicated, every six months from years three to five, and annually thereafter. If patient compliance is suboptimal, the follow-up schedule should be appropriately adjusted.
In conclusion, the successful 36-month disease-free survival in this case underscores that pre-operative diagnosis of MDA is achievable using a combination of a Meckel scan and DBE, enabling radical resection based on oncological principles. We recommend that this diagnostic approach be considered for patients with obscure gastrointestinal hemorrhage after initial negative routine evaluations. This strategy will inform our practice by prioritizing small bowel assessment and structured lymphadenectomy in similar presentations. Further multicenter studies are required to establish standardized pre-operative diagnostic pathways and surgical guidelines for this rare malignancy.
| 1. | Hu S, Du H, Wen J, Wu M, Huang B, Zhong J, Shi C, Liu C. Diagnosis of Inverted Meckel's diverticulum by double-balloon enteroscopy: a case report. AME Case Rep. 2024;8:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Yahchouchy EK, Marano AF, Etienne JC, Fingerhut AL. Meckel's diverticulum. J Am Coll Surg. 2001;192:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Lorusso R, Forte A, Urbano V, Soda G, D'Urso A, Bosco MR, Mancini P, Bezzi M. [Small bowel stromal tumors in a "meckelian" location. About a clinical observation]. Ann Ital Chir. 2003;74:707-711. [PubMed] |
| 4. | Morcillo Rodenas MA, Planells Roig M, García Espinosa R, Moliner Quiles C, Prieto Rodríguez M, López Andújar R, Rodero Rodero D. [Neoplasms of the Meckel diverticulum. Apropos of 2 new cases]. Rev Esp Enferm Dig. 1990;77:143-146. [PubMed] |
| 5. | Thirunavukarasu P, Sathaiah M, Sukumar S, Bartels CJ, Zeh H 3rd, Lee KK, Bartlett DL. Meckel's diverticulum--a high-risk region for malignancy in the ileum. Insights from a population-based epidemiological study and implications in surgical management. Ann Surg. 2011;253:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Ji K, Crispin M, Goonawardena J, Ravindra R. Diverting opinions: surgical debate around method of Meckel's resection. BMJ Case Rep. 2025;18:e265448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Hendrickx T, Van Herpe F, D'Hoore A, Dresen RC, Sabino J. An unusual hidden secret of a Meckel's diverticulum: a rare case of small bowel adenocarcinoma. Acta Gastroenterol Belg. 2025;88:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zhao Y, Yang X, Ye Y. Adenocarcinoma located at a Meckel's Diverticulum: A case report and literature review. J Cancer Res Ther. 2017;13:878-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Principe DR, Nesper P, Metropulos AE, Rubin J, Marinov MN. Intestinal adenocarcinoma originating from an undiagnosed Meckel's diverticulum. J Surg Case Rep. 2022;2022:rjac128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Sakio R, Ito H, Ota G, Tahara M, Yano T, Koinuma K, Horie H, Lefor AK, Yamamoto H, Sata N. Metachronous Krukenberg tumor from adenocarcinoma in a Meckel's diverticulum: a case report. J Surg Case Rep. 2021;2021:rjab374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Sato Y, Tanaka S, Ko Y, Okuda T, Tamura F, Fujimi A, Doi T, Kanisawa Y, Ohta H. Adenocarcinoma of Meckel's diverticulum diagnosed by capsule endoscopy and single-balloon enteroscopy. Clin J Gastroenterol. 2009;2:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Nies C, Zielke A, Hasse C, Rüschoff J, Rothmund M. Carcinoid tumors of Meckel's diverticula. Report of two cases and review of the literature. Dis Colon Rectum. 1992;35:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Moyana TN. Carcinoid tumors arising from Meckel's diverticulum. A clinical, morphologic, and immunohistochemical study. Am J Clin Pathol. 1989;91:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Anderson DJ. Carcinoid tumor in Meckel's diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100:432-434. [PubMed] |
| 15. | Elsayes KM, Menias CO, Harvin HJ, Francis IR. Imaging manifestations of Meckel's diverticulum. AJR Am J Roentgenol. 2007;189:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Lin S, Suhocki PV, Ludwig KA, Shetzline MA. Gastrointestinal bleeding in adult patients with Meckel's diverticulum: the role of technetium 99m pertechnetate scan. South Med J. 2002;95:1338-1341. [PubMed] |
| 17. | Ling CR, Wang MJ, Zhuang W. Capsule retention for 7.5 years in Meckel's diverticulum. Dig Endosc. 2017;29:386-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Wang L, Xie M, Hong L, Zhang C, Zhang T, Fan R, Zhong J, Wang Z. The Diagnostic Yields and Safety of Double-Balloon Enteroscopy in Obscure Gastrointestinal Bleeding and Incomplete Small Bowel Obstruction: Comparison between the Adults and Elderly. Gastroenterol Res Pract. 2020;2020:8121625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | van Malderen K, Vijayvargiya P, Camilleri M, Larson DW, Cima R. Malignancy and Meckel's diverticulum: A systematic literature review and 14-year experience at a tertiary referral center. United European Gastroenterol J. 2018;6:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374-5382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Kabir SA, Raza SA, Kabir SI. Malignant neoplasms of Meckel's diverticulum; an evidence based review. Ann Med Surg (Lond). 2019;43:75-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Negoi I, Beuran M, Hostiuc S, Negoi RI, Inoue Y. Surgical Anatomy of the Superior Mesenteric Vessels Related to Pancreaticoduodenectomy: a Systematic Review and Meta-Analysis. J Gastrointest Surg. 2018;22:802-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Crane J, Hamed M, Borucki JP, El-Hadi A, Shaikh I, Stearns AT. Complete mesocolic excision versus conventional surgery for colon cancer: A systematic review and meta-analysis. Colorectal Dis. 2021;23:1670-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Brown KGM, Ng KS, Solomon MJ, Chapuis PH, Koh CE, Ahmadi N, Austin KKS. Complete mesocolic excision for colon cancer: current status and controversies. ANZ J Surg. 2024;94:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Andersson H, Bosaeus I, Brummer RJ, Fasth S, Hultén L, Magnusson O, Strauss B. Nutritional and metabolic consequences of extensive bowel resection. Dig Dis. 1986;4:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Seetharam P, Rodrigues G. Short bowel syndrome: a review of management options. Saudi J Gastroenterol. 2011;17:229-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/