Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.110347
Revised: July 8, 2025
Accepted: July 30, 2025
Published online: September 27, 2025
Processing time: 103 Days and 1 Hours
Colorectal polypectomy is fundamental to the prevention of colorectal cancer, utilizing several endoscopic techniques. Robust comparative data regarding the efficacy and safety of these modalities in clinical practice are limited.
To evaluate and compare the efficacy and safety of three endoscopic polypectomy techniques, namely, high-frequency electroresection (HFE), cold snare polypec
This single-center retrospective cohort study included adults who underwent endoscopic resection of pathologically confirmed colorectal polyps at Central Hospital Affiliated to Shandong First Medical University between January 2015 and December 2023. Patients were grouped by technique: HFE (n = 107), CSP (n = 106), and EMR (n = 108). Standardized preoperative, intraoperative, and post
Baseline demographics and polyp characteristics, except for polyp diameter, were comparable among groups. CSP achieved the highest en bloc resection rate, whereas HFE had a higher R0 resection rate. Polyp diameter was largest in the EMR group. Procedure duration was shortest with HFE. Adverse reactions were more frequent with HFE, particularly post-polypectomy bleeding and delayed perforation, whereas CSP demonstrated a superior safety profile and the lowest incidence of complications. Postoperative pain diminished in all groups over time but was consistently low for CSP and EMR. Recurrence rates were significantly higher in the EMR group vs CSP group, with HFE showing intermediate recurrence.
CSP offers the best safety profile and lowest recurrence rate among patients undergoing endoscopic resection of colorectal polyps, whereas HFE confers a high R0 resection rate but increased risk of adverse events. EMR remains essential for large polyps despite elevated recurrence. Technique selection should be tailored according to polyp characteristics and patient risk factors to optimize outcomes.
Core Tip: This study evaluates the efficacy and safety of three endoscopic polypectomy techniques, high-frequency electroresection, cold snare polypectomy, and endoscopic mucosal resection, for treating colonic polyps. Key findings indicate that cold snare polypectomy offers the best safety profile with the lowest complication rates and recurrence, while high-frequency electroresection achieves a higher R0 resection rate but is associated with more adverse events. Endoscopic mucosal resection remains essential for larger polyps despite higher recurrence rates. These results highlight the importance of selecting the appropriate technique based on polyp characteristics and patient risk factors to optimize outcomes in colorectal cancer prevention.
- Citation: Ji DH, Guan ZA. Evaluation of efficacy and safety of different endoscopic polypectomy techniques for colonic polyps. World J Gastrointest Surg 2025; 17(9): 110347
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/110347.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.110347
Colorectal polyps are among the most common findings during colonoscopy examinations and serve as a critical precursor to colorectal cancer, a primary cause of cancer-related morbidity and mortality worldwide[1]. Early detection and removal of neoplastic polyps are essential strategies for colorectal cancer prevention, reducing incidence and mortality through interruption of the adenoma-carcinoma sequence[2]. Over recent decades, the widespread adoption and evolution of endoscopic techniques have significantly enhanced the effectiveness, safety, and tolerability of colorectal polypectomy[3].
Several endoscopic resection techniques have been developed and refined to optimize outcomes for patients with colonic polyps, each offering distinct advantages and potential limitations depending on polyp characteristics such as size, morphology, and location[4]. High-frequency electroresection (HFE), commonly referred to as hot snare po
Cold snare polypectomy (CSP) has emerged as an alternative technique, especially for tiny and small polyps[6]. It eliminates the need for electrocautery, relying instead on mechanical transection of the polyp, which reduces the risk of thermal injury and associated complications[7]. Multiple studies and systematic reviews have reported that CSP is highly effective for complete resection and possesses a favorable safety profile, particularly in patients at increased risk for electrocautery-related complications[8]. Nevertheless, concerns remain regarding the efficacy of CSP for large lesions or polyps with complex morphology, for which en bloc and R0 (complete) resection rates may be lower than those achieved with thermal techniques[8]. Ongoing research aims to optimize CSP protocols for large polyps and complex lesions, where their efficacy remains under investigation.
Endoscopic mucosal resection (EMR) is widely employed for the removal of large, sessile, or flat adenomas[9]. This technique involves submucosal injection to lift the lesion from the muscularis propria, facilitating safe and complete resection, often with enhanced assessment of margins[10]. EMR has been shown to increase successful en bloc resection rates for sizeable polyps but may need piecemeal removal in lesions greater than 20 mm, which elevates the risk of local recurrence[11]. Recent advances in EMR include the use of novel submucosal injection solutions and advanced imaging techniques to improve resection completeness and reduce recurrence rates. Although EMR is associated with increased rates of delayed bleeding and recurrence compared with other modalities, its role remains essential for specific polyp populations[11].
Despite the wide integration of these techniques, the comparative efficacy and safety profile of HFE, CSP, and EMR in routine clinical practice, particularly regarding variables such as resection completeness, complication rates, postope
This study is a single-center retrospective cohort study, with data sourced from the electronic medical record system of Central Hospital Affiliated to Shandong First Medical University, including patients who underwent endoscopic co
The inclusion criteria were as follows: Age ≥ 18 years; patients who satisfied the diagnostic guidelines for colorectal polyps[13]; underwent endoscopic polypectomy (HFE, CSP, or mucosal resection); polyps confirmed by endoscopic macroscopic morphology assessment; and complete case records. The exclusion criteria were as follows: Known or suspected malignant polyps; recurrent polyps or subepithelial lesions that were not completely removed previously; inflammatory bowel disease (ulcerative colitis or Crohn’s disease); ongoing anticoagulant/antiplatelet therapy; co
This study adopted standardized surgical procedures and perioperative management protocols. All enrolled patients received a systematic preoperative evaluation, including a detailed medication history check, requiring the discontinuation of anticoagulant medications for at least 7 days. Bowel preparation protocol: Initiate a semi-liquid diet 24 hours before the procedure, administer 30 mL of castor oil orally 6 hours before the procedure, and consume polyethylene glycol electrolyte solution in divided doses starting 5 hours before the procedure (250 mL every 15 minutes, total volume 2000 mL), until clear watery stools were passed. Bowel cleanliness was objectively assessed using the Boston Bowel Preparation Scale.
The surgical procedures were performed using the Olympus CF-HQ290 series high-definition electronic colonoscope. Polyp characteristics were assessed strictly according to the Paris classification system, with a standardized 8 mm biopsy forceps opening used as a reference for size measurement. Preoperative laboratory tests included prothrombin time (PT)/international normalized ratio measured by the STA-R Evolution fully automated coagulation analyzer, with white blood cell count, platelet count, and hemoglobin levels detected by the Sysmex XN-9000 hematology analyzer.
Polyp removal techniques were selected based on polyp characteristics. For HFE, using the Olympus CF-HQ290 series high-definition electronic colonoscope, polyp characteristics were assessed strictly according to the Paris classification system, with a standardized 8 mm biopsy forceps opening used as a reference for size measurement. HFE was performed using the ERBE VIO 300D electrosurgical system with settings of Endocut Q mode, effect 3, and power of 50 W, which involved saline injection at the base of the polyp to lift the lesion, followed by complete or piecemeal resection using a hot snare. Post-resection, the site was flushed, air suctioned, and carefully observed for residual tumor tissue, with additional biopsies obtained using white light and narrow band imaging (NBI). Resected specimens were immediately placed in 10% neutral buffered formalin for histopathological examination. For CSP, the Olympus SnareMaster 15 mm cold snare was used without electrocautery; polyps were directly removed using the cold snare, followed by flushing the resection site, air suction, and careful observation for potential residual tumor tissue. Additional biopsies were performed at the most probable sites of residual tumor using white light and NBI, and the resected specimens were promptly immersed in 10% neutral buffered formalin for histopathological analysis. For EMR, the Olympus Captivator II 20 mm snare was employed after submucosal injection of saline containing indigo carmine dye via a 23G injection needle to elevate the lesion; polyps were excised either en bloc or piecemeal when en bloc resection was not feasible. Following resection, the site was flushed, air suctioned, and carefully examined for residual tumor tissue, with additional biopsies obtained using white light and NBI. All resected specimens were immediately placed in 10% neutral buffered formalin for histopathological examination. After flushing, air suction, and careful observation of the polypectomy site most likely to harbor residual tumor tissue, two additional biopsies were performed using white light and NBI. The resected specimens were immediately placed in 10% neutral buffered formalin and submitted for histopathological examination.
Immediate bleeding was defined as persistent bleeding exceeding 30 seconds, requiring endoscopic hemostasis with either a 1:10000 epinephrine injection or hemoclips. Delayed bleeding was defined as postoperative hemorrhage from the polypectomy site within 2 weeks after the procedure, requiring hospital admission or additional hemostatic inter
Postoperative management included a strict dietary regimen: Fasting for 12 hours post-procedure, a residue-free diet for 48 hours, and a semi-liquid diet for 7 days. Patients were instructed to avoid heavy lifting for 14 days after the procedure. During this period, abdominal conditions and stool status were closely monitored to assess for adverse events such as perforation and bleeding. Supplementary Table 1 provides detailed information on surgical equipment and consumables.
Resection status: En bloc resection was defined as the complete removal of a lesion, resulting in a single specimen. R0 resection indicated complete removal with negative lateral and basal margins. R1 resection denoted the presence of microscopic residual tumor without macroscopic remnants, whereas Rx resection indicated indeterminate margins.
Postoperative pain: Postoperative abdominal pain was self-assessed using the visual analog scale (VAS)[14] at 1 hour, 3 hours, and 5 hours post-surgery. The VAS scores were categorized as follows: 0 for absent, 1-3 for mild, 4-6 for moderate, and 7-10 for severe pain.
Recurrence rate: During the 12-month follow-up period, recurrence was evaluated by endoscopists through surveillance colonoscopy at 6 months and 12 months post-procedure, including local recurrence at the site of previous polypectomy and metachronous distant polyp occurrence. Specifically, polypectomy scars were first analyzed under white light, followed by NBI. To confirm any suspicion of residual adenoma within the scar, we conducted a histological biopsy.
Statistical analyses were performed using IBM SPSS Statistics software (version 23.0). Continuous variables, including age and VAS scores, were expressed as mean ± SD and analyzed using independent samples t-tests for normally distributed data or Mann-Whitney U tests for non-normally distributed data, as appropriate. Categorical variables, such as resection rates and adverse event incidence, were presented as n (%) and compared using χ² tests or Fisher’s exact tests, depending on expected cell frequencies. A two-tailed P < 0.05 with Bonferroni correction was considered statistically significant for all analyses.
Age, gender, smoking status, drinking status, course of disease, hypertension, diabetes, hyperlipidemia, coronary heart disease, indication for procedure, and bowel preparation quality (Boston Bowel Preparation Scale) showed no significant differences among the HFE, CSP, and EMR groups (P > 0.05; Table 1). These findings indicated that the study groups were well-matched for relevant baseline variables.
| Parameters | HFE (n = 107) | CSP (n = 106) | EMR (n = 108) | P1 value | P2 value | P3 value |
| Age (years) | 57.90 ± 13.20 | 56.80 ± 11.61 | 57.29 ± 12.35 | 1.551 | 2.289 | 2.172 |
| Gender | 2.847 | 2.658 | 2.505 | |||
| Male | 57 (53.27) | 56 (53.27) | 56 (51.85) | |||
| Female | 50 (46.73) | 50 (46.73) | 52 (48.15) | |||
| Smoking | 22 (20.56) | 22 (20.56) | 22 (20.37) | 2.916 | 2.835 | 2.916 |
| Drinking | 35 (79.44) | 38 (32.71) | 38 (35.18) | 1.887 | 2.757 | 2.106 |
| Course of disease, months | 14.24 ± 2.45 | 14.31 ± 2.25 | 14.08 ± 2.92 | 2.517 | 1.557 | 1.989 |
| Hypertension | 20 (18.69) | 21 (19.81) | 22 (20.37) | 2.508 | 2.757 | 2.268 |
| Diabetes | 11 (10.28) | 10 (9.43) | 13 (12.03) | 2.508 | 1.617 | 2.049 |
| Hyperlipidemia | 20 (18.69) | 22 (20.75) | 23 (21.29) | 2.115 | 2.769 | 1.899 |
| Coronary heart disease | 8 (7.48) | 6 (5.89) | 8 (7.15) | 1.779 | 1.815 | 2.955 |
| Indication | 2.796 | 2.832 | 2.904 | |||
| Screening | 33 (30.84) | 36 (33.96) | 35 (32.63) | |||
| Follow up | 19 (17.76) | 16 (15.09) | 21 (19.44) | |||
| For polypectomy | 22 (20.56) | 22 (20.75) | 22 (19.45) | |||
| Positive FOBT | 6 (5.61) | 8 (7.55) | 7 (6.48) | |||
| Symptoms | 27 (25.23) | 24 (22.64) | 23 (21.29) | |||
| Bowel preparation | 2.235 | 2.199 | 3.000 | |||
| BBPS < 6 | 5 (4.67) | 6 (5.66) | 5 (4.62) | |||
| BBPS ≥ 6 | 102 (95.33) | 100 (94.34) | 103 (95.38) |
Preoperative laboratory results, including PT, white blood cell count, platelet count, and hemoglobin concentration, showed no statistically significant differences among patients in the HFE, CSP, and EMR groups (P > 0.05; Table 2). The mean PT was 11.54 ± 1.02 seconds in the HFE group, 11.25 ± 1.73 seconds in the CSP group, and 11.73 ± 1.45 seconds in the EMR group. White blood cell counts, platelet counts, and hemoglobin levels were also similar across all groups, indicating that hematologic status was comparable among patients prior to polypectomy.
| Parameters | HFE (n = 107) | CSP (n = 106) | EMR (n = 108) | P1 value | P2 value | P3 value |
| Prothrombin time (seconds) | 11.54 ± 1.02 | 11.25 ± 1.73 | 11.73 ± 1.45 | 0.837 | 0.093 | 0.759 |
| White blood cell count (× 109/L) | 5.89 ± 1.12 | 5.74 ± 1.28 | 5.52 ± 1.34 | 1.104 | 0.633 | 0.090 |
| Platelet count (× 109/L) | 205.37 ± 54.99 | 208.37 ± 52.75 | 211.76 ± 51.84 | 2.055 | 1.905 | 1.146 |
| Hemoglobin (g/L) | 147.63 ± 16.93 | 145.72 ± 14.13 | 144.72 ± 16.21 | 1.113 | 1.887 | 0.597 |
Significant differences were observed in polyp diameter among the three groups, with mean sizes of 10.61 ± 2.25 mm in the HFE group, 7.89 ± 2.12 mm in the CSP group, and 14.20 ± 1.65 mm in the EMR group (P < 0.001 for all pairwise comparisons; Table 3). These results indicated that polyps treated with EMR were significantly larger compared with those treated with CSP and HFE, whereas polyps in the CSP group were the smallest. Polyp location, morphology, and histopathology demonstrated no statistically significant differences among groups (P > 0.05). Most polyps in each group were tubular adenomas with low-grade dysplasia, whereas tubular adenomas with high-grade dysplasia, tubulovillous adenomas, and sessile serrated adenomas/polyps were infrequent across all cohorts. Thus, aside from significant variation in polyp diameter, other clinical characteristics were comparable among the three groups.
| Parameters | HFE (n = 107) | CSP (n = 106) | EMR (n = 108) | P1 value | P2 value | P3 value |
| Polyp diameter (mm) | 10.61 ± 2.25 | 7.89 ± 2.12c | 14.20 ± 1.65c,e | < 0.001 | < 0.001 | < 0.001 |
| Location | 1.824 | 0.857 | 2.214 | |||
| Proximal colon | 65 (60.75) | 68 (64.15) | 68 (62.96) | |||
| Distal colon | 42 (39.25) | 38 (35.85) | 40 (37.04) | |||
| Morpholog | 1.068 | 0.919 | 1.227 | |||
| Flat | 32 (29.91) | 38 (35.85) | 38 (35.18) | |||
| Protruded | 75 (70.09) | 68 (64.15) | 70 (64.82) | |||
| Histopathology | 2.523 | 0.850 | 1.809 | |||
| TA with LGD | 104 (97.2) | 101 (95.28) | 101 (93.52) | |||
| TA with HGD | 1 (0.93) | 1 (0.94) | 3 (2.78) | |||
| TVA | 1 (0.93) | 1 (0.94) | 1 (0.92) | |||
| SSA/P | 1 (0.93) | 3 (2.83) | 3 (2.78) |
Significant differences were observed in total procedure time among the groups, with the HFE group requiring less time (29.38 ± 2.11) than the CSP group (32.58 ± 2.24) and the EMR group (31.34 ± 2.35, all P < 0.001; Table 4). Prophylactic clip use was more frequent in the HFE group compared (9.35%) with the CSP group (0.94%, P = 0.018), whereas no significant difference was found between CSP and EMR, or HFE and EMR (P > 0.05). We found no statistically significant differences among the three groups in operator experience, intraoperative bleeding volume, or length of hospital stay (P > 0.05). Overall, HFE was associated with a short procedure duration and high rate of prophylactic clip placement, whereas other surgical variables remained similar across groups.
| Parameters | HFE (n = 107) | CSP (n = 106) | EMR (n = 108) | P1 value | P2 value | P3 value |
| Total procedure time (minutes) | 29.38 ± 2.11 | 32.58 ± 2.24c | 31.34 ± 2.35c,e | < 0.001 | < 0.001 | < 0.001 |
| Prophylactic clip use | 10 (9.35) | 1 (0.94)b | 3 (2.77) | 0.018 | 0.627 | 0.129 |
| Operator experience | 0.207 | 0.530 | 0.654 | |||
| Experienced endoscopist | 106 (99.07) | 99 (93.4) | 103 (95.37) | |||
| Non-experienced endoscopist | 1 (0.93) | 7 (6.6) | 5 (4.63) | |||
| Intraoperative bleeding (mL) | 4.18 ± 0.25 | 4.16 ± 0.29 | 4.22 ± 0.13 | 1.806 | 0.162 | 0.264 |
| Hospital time (days) | 0.96 ± 0.58 | 0.82 ± 0.44 | 0.97 ± 0.56 | 0.114 | 0.090 | 2.703 |
Significant differences were observed in en bloc resection rates among the groups, with CSP achieving the highest en bloc rate (95, 89.62%), followed by HFE (91, 85.05%) and EMR [77, 71.30%, P = 0.948 (HFE vs CSP), P < 0.001 (CSP vs EMR), P = 0.045 (HFE vs EMR)]. The R0 resection rate was significantly higher in the HFE group than in the CSP group (P = 0.003), whereas EMR showed no significant difference vs either group (P > 0.05; Table 5). The rates of R1 and Rx resection were similar across all groups (P > 0.05). The mean ulcer base diameter 1 week after treatment was significantly greater for HFE (4.84 ± 1.02) than for CSP [3.19 ± 0.66, P < 0.001 (HFE vs CSP)] and also greater vs EMR [3.52 ± 0.85, P = 0.006 (CSP vs EMR), P < 0.001 (HFE vs EMR)]. Overall, CSP demonstrated the highest en bloc resection rate, whereas HFE was associated with a high R0 resection rate and large ulcer base diameter, with other resection outcomes comparable among groups.
| Parameters | HFE (n = 107) | CSP (n = 106) | EMR (n = 108) | P1 value | P2 value | P3 value |
| En bloc resection4 | 91 (85.05) | 95 (89.62) | 77 (71.30)a,e | 0.948 | < 0.001 | 0.045 |
| R0 resection5 | 87 (81.31) | 65 (61.32)b | 79 (73.14) | 0.003 | 0.195 | 0.462 |
| R1 resection6 | 20 (18.69) | 22 (20.75) | 30 (27.78) | 2.115 | 0.693 | 0.345 |
| Rx resection7 | 17 (15.89) | 16 (15.09) | 16 (14.81) | 2.619 | 2.862 | 2.481 |
| Ulcer base diameter one week after treatment (mm) | 4.84 ± 1.02 | 3.19 ± 0.66c | 3.52 ± 0.85c,d | < 0.001 | 0.006 | < 0.001 |
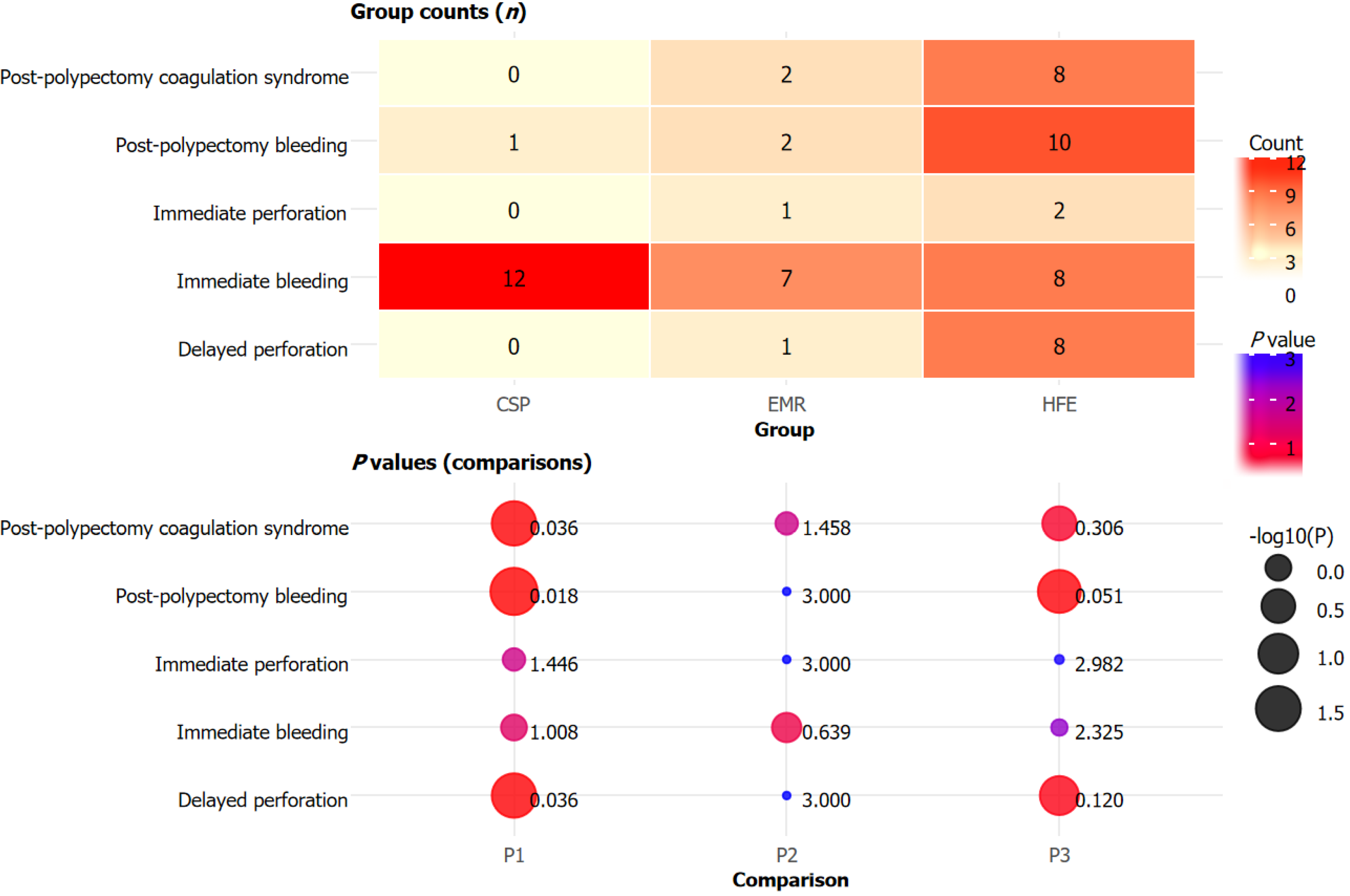
Adverse reaction profiles revealed significant differences among the groups. The incidence of post-polypectomy bleeding was significantly higher in the HFE group than in the CSP group (P = 0.018), with no significant difference between EMR and the other groups (P > 0.05; Figure 1). Delayed perforation and post-polypectomy coagulation syndrome were more frequently observed in the HFE group compared with the CSP group (all P = 0.036). Immediate bleeding and immediate perforation rates, as well as the corresponding rates for the EMR group, did not differ significantly among the groups (P > 0.05). Overall, CSP was associated with fewer adverse reactions compared with HFE, whereas the rates of most complications were similarly low across all groups.
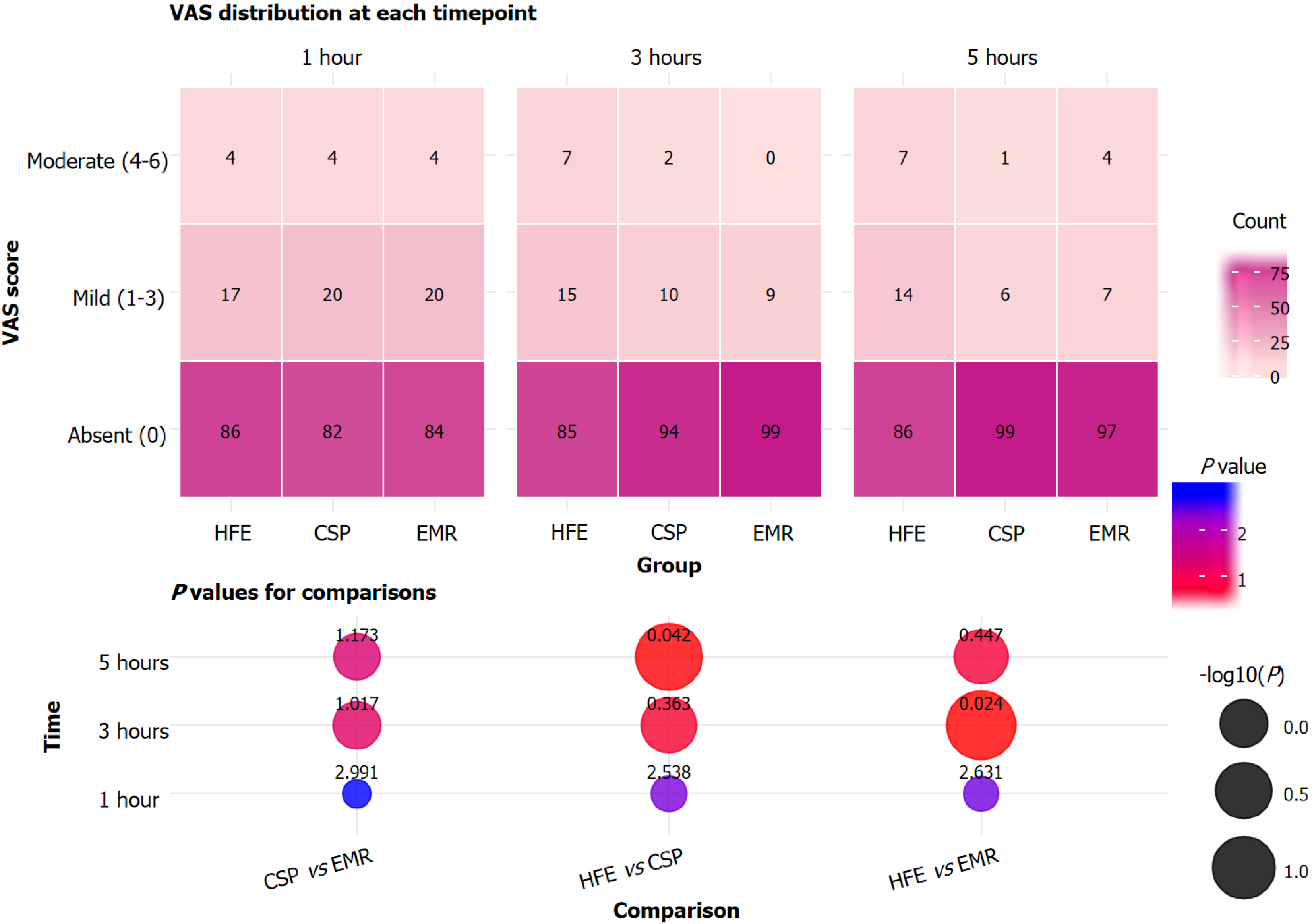
Postoperative pain, assessed by the VAS, showed no significant difference among groups at 1 hour after the procedure, with the majority of patients in all groups reporting no or only mild pain (P > 0.05; Figure 2). At 3 hours post-procedure, the proportion of patients reporting no pain was significantly higher in the EMR group than in the HFE group (P = 0.024), and moderate pain was absent in the EMR group at this time point. Similarly, at 5 hours post-procedure, a greater proportion of patients in the CSP group reported no pain compared with the HFE group. Mild and moderate pain rates declined over time and were lower in CSP and EMR than in HFE, with all differences reaching statistical significance where indicated (P = 0.042). Overall, postoperative pain diminished across all groups over time, with CSP and EMR associated with less pain than HFE in the later postoperative period.
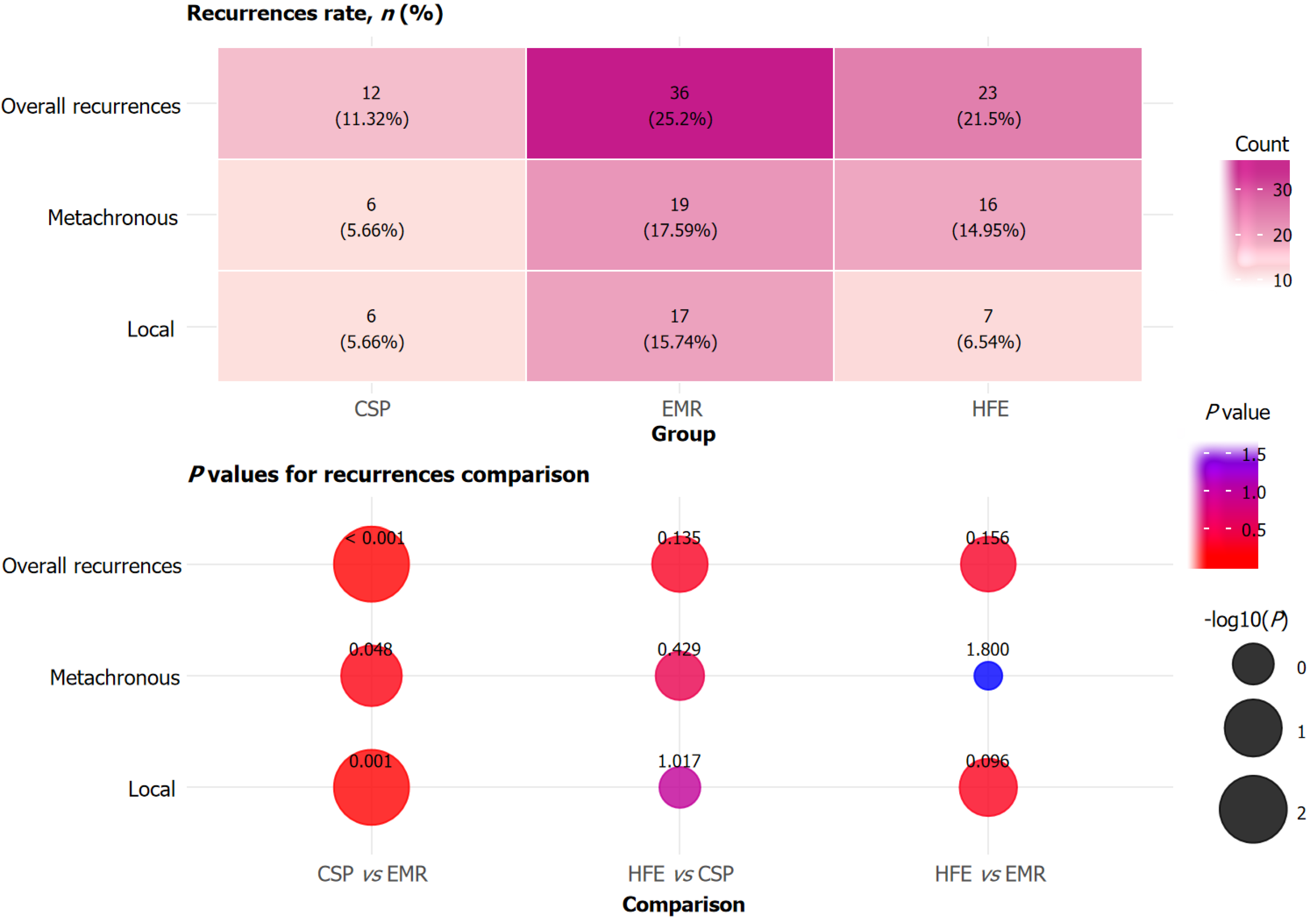
The overall recurrence rate was significantly higher in the EMR group than in the CSP group (P < 0.001), whereas no significant differences were observed between the HFE group and the other groups (P > 0.05; Figure 3). Metachronous recurrences occurred more frequently in the EMR group than in the CSP group (P = 0.048), with the HFE group showing an intermediate rate. Local recurrence was also significantly higher in the EMR group than in the CSP group (P = 0.001). No statistically significant differences were seen between HFE and CSP or between HFE and EMR for either overall, metachronous, or local recurrence rates (P > 0.05). Overall, CSP demonstrated the lowest recurrence rates, particularly compared with EMR.
This study provides a comprehensive evaluation of three commonly used endoscopic techniques for colonic polypectomy, namely, HFE, CSP, and EMR, focusing on their comparative efficacy and safety profiles within a substantial retrospective cohort. Our findings demonstrated significant differences among these modalities in terms of resection outcomes, procedure-related complications, recurrence rates, and postoperative pain. A thorough analysis of the underlying mechanisms and factors contributing to these results offers valuable insights for endoscopists involved in selecting polypectomy techniques.
At the biological and technical level, each polypectomy technique incorporates unique principles of tissue dissection and hemostasis, which inherently lead to differences in procedural outcomes and complication profiles[15]. CSP, which uses the mechanical transection of the polyp without thermal energy, maintains the integrity of submucosal tissue and vascular architecture[16]. The prevention of thermal injury likely accounts for the reduced incidence of immediate and delayed bleeding observed in CSP compared with HFE and EMR[17]. The hemostatic advantage of CSP may also be attributed to the reduced diameter of vessels typically found in the size range of polyps removed by this method, as well as the “clean-cut” effect of a sharp snare, which minimizes traumatic bleeding and facilitates prompt vascular constriction via natural tissue recoil[18].
By contrast, HFE and EMR involve application of electrosurgical current, resulting in tissue coagulation and incision[19]. The thermal effect facilitates immediate coagulation of small-caliber vessels; yet, it simultaneously increases the risk of delayed mucosal and submucosal necrosis, a principal mechanism for post-polypectomy syndrome, delayed bleeding, and, in rare scenarios, perforation. The elevated incidence of post-polypectomy bleeding and subsequent complications in the HFE group, as indicated by our data, are likely attributed to multiple factors: The combination of large average polyp size, deep resection planes, and thermal tissue injury increases the risk of delayed sloughing of eschar, leading to exposure and hemorrhage from underlying vessels. The EMR technique, while permitting the removal of large and complex polyps via submucosal lifting and en bloc excision, may exacerbate these risks, especially in lesions with increased vascular density or those located in thin-walled colonic segments[20].
From the perspective of completeness of resection, the mechanical precision of CSP facilitates high rates of en bloc resection for small to medium-sized polyps, which reduces the risk of residual neoplastic tissue and, consequently, local recurrence[21]. The superior en bloc resection rate of CSP in our study supports evidence from recent prospective trials[22], indicating that when technical parameters are strictly adhered to, CSP is appropriate for sessile lesions up to 10 mm in size. Although HFE and EMR can achieve broad margins and accommodate large polyp diameters, the complexity associated with piecemeal excision, particularly in EMR, may increase the risk of incomplete resection and margin uncertainty. This is reflected in the relatively elevated recurrence rates, especially local and metachronous recurrence, observed after EMR in our cohort. The physiological healing response following EMR, characterized by the formation of a large mucosal defect and ulceration, likely fosters conditions favorable for polyp regrowth at the resection margin or interval metachronous development, especially in genetically susceptible mucosa[23]. Notably, HFE demonstrated a higher R0 resection rate compared with CSP, suggesting that thermal cauterization may effectively eliminate microscopic lateral and basal margin involvement in certain cases[24]. However, this advantage may be offset by the increased tissue trauma and elevated incidence of adverse events, highlighting the trade-off between radicality and procedural safety[25].
Ulcer healing and tissue regeneration following polypectomy varies among procedures, largely influenced by the degree of mucosal and submucosal injury[26]. Larger ulcer base diameters observed for HFE align with deeper thermal penetration and more extensive tissue necrosis, prolonging the time required for the restoration of normal mucosal architecture[27]. By contrast, the small and clearly defined defects resulting from CSP generally heal more rapidly and completely, diminishing the risk of secondary infection and reducing the incidence of delayed complications and pain[28]. The interaction among ulcer size, depth, and local inflammatory response is likely a key factor in shaping postoperative pain trajectories, with the cold excision of CSP eliciting a restricted immediate inflammatory cascade[29]. This is consistent with the observed pattern of reduced postoperative pain and expedited symptom recovery in CSP and EMR compared with HFE, especially during the immediate and subacute postoperative periods.
The role of perioperative factors, such as prophylactic clip application and operator experience, warrants additional examination. Our findings suggested that increased clip use in HFE may reflect not only operator foresight regarding procedural risks associated with large polyp size and broad defects but also institutional tendencies or personal preferences influenced by previous adverse event experiences. Nonetheless, clip placement alone may not fully mitigate the intrinsic risk profile associated with each technique, as evidenced by consistently elevated bleeding rates in the HFE cohort despite proactive hemostatic measures.
Patient demographics and anatomic factors, while similar across all groups in our study, have been implicated in modulating polypectomy outcomes. Older age, right-sided polyp location, and coexistent comorbidities such as anticoagulant use (excluded in our analysis for clarity) are known predictors of adverse event risk and recurrence[30]. Our patient selection criteria, which excluded those with known confounding risks, provide a relatively “pure” assessment of technique-specific outcomes. However, in extensive clinical practice, these variables must continue to guide customized technique selection.
Although our study provides valuable insights into the efficacy and safety of HFE, CSP, and EMR, several limitations should be acknowledged. The retrospective design of the study introduces potential biases, including selection bias and information bias, which may affect the generalizability of our findings. The single-center nature of the study limits the external validity of our results, as practices and outcomes may vary across various institutions. The subjective nature of some outcome measures, such as postoperative pain, may lead to variability in reporting. Our patient population was relatively homogeneous in terms of baseline characteristics, but the exclusion of high-risk populations, such as those on anticoagulants or with coagulation disorders, restricts the applicability of our findings to a wide patient demographic. Future studies should aim to address these limitations by employing prospective, multicenter designs and including diverse patient populations.
The observed differences in recurrence, particularly the elevated rate after EMR, may be partly explained by the challenge in achieving complete resection in large, sessile, or laterally spreading lesions, which are areas where EMR is typically preferred. The limitations of histopathological margin assessment following piecemeal resection, along with a potentially underestimated field effect of neoplasia in adjacent mucosa, highlight the need for diligent follow-up and the prospective assessment of supplementary technologies such as endoscopic submucosal dissection, advanced imaging, and molecular margin analyses.
Overall, our findings support a tailored, lesion-specific approach to polypectomy. CSP emerges as the preferred strategy for small, non-pedunculated polyps because of its high efficacy, safety, and patient comfort. For large or complex polyps, EMR and HFE play a crucial role but require careful patient selection, precise procedural execution, and enhanced post-procedural surveillance. The risks of bleeding, perforation, and recurrence associated with thermal techniques highlight the importance of further enhancements in energy transmission, supplementary hemostatic tools, and operator training. Technological advances, including real-time histological assessment, artificial intelligence-assisted margin detection, and novel cold resection devices, offer potential for optimizing outcomes across all techniques[31]. Ultimately, interdisciplinary collaboration, continuous evaluation of safety and efficacy, and patient-centered decision-making are key principles in the advancement of endoscopic polypectomy.
In summary, the mechanisms underlying the differences and outcomes observed among HFE, CSP, and EMR reflect a complex interaction of resection method, polyp size and morphology, procedural trauma, and tissue healing dynamics. This study emphasizes the importance of individualized technique selection based on polyp characteristics and procedural risk and highlights potential directions for future research aimed at improving the efficacy and safety of endoscopic polypectomy.
| 1. | Kamal F, Khan MA, Lee-Smith W, Sharma S, Acharya A, Farooq U, Agarwal A, Aziz M, Chuang J, Kumar A, Schlachterman A, Loren D, Kowalski T, Adler D. Cold snare versus cold forceps polypectomy for endoscopic resection of diminutive polyps: meta-analysis of randomized controlled trials. Gastrointest Endosc. 2023;98:7-18.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Nakshabandi A, Rungta M, Othman MO. Polypectomy, Endoscopic Mucosal Resection, and Endoscopic Submucosal Dissection in the Cirrhotic Population. Clin Liver Dis. 2022;26:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Oh CK, Choi HS, Cho YS. Comparison of cold snare polypectomy and endoscopic mucosal resection for 3-10-mm colorectal polyps in end-stage renal disease patients. Saudi J Gastroenterol. 2022;28:67-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Liu W, Gong J, Gu L. The efficacy and safety of cold snare versus hot snare polypectomy for endoscopic removal of small colorectal polyps: a systematic review and meta-analysis of randomized controlled trials. Int J Colorectal Dis. 2023;38:136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Wang ST, Kong QZ, Li YQ, Ji R. Efficacy and Safety of Cold Snare Polypectomy versus Cold Endoscopic Mucosal Resection for Resecting 3-10 mm Colorectal Polyps: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Digestion. 2024;105:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Djinbachian R, von Renteln D. Endoscopic Polypectomy: How Should We Determine Complete Resection Status? Clin Gastroenterol Hepatol. 2022;20:242-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kwok K, Tran T, Lew D. Polypectomy for Large Polyps with Endoscopic Mucosal Resection. Gastrointest Endosc Clin N Am. 2022;32:259-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Anele CC, Xiang J, Martin I, Hawkins M, Man R, Clark SK, Faiz OD, Latchford A. Regular endoscopic surveillance and polypectomy is effective in managing rectal adenoma progression following colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Colorectal Dis. 2022;24:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Hayat M, Azeem N, Bilal M. Colon Polypectomy with Endoscopic Submucosal Dissection and Endoscopic Full-Thickness Resection. Gastrointest Endosc Clin N Am. 2022;32:277-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Sachdev R, Valori RM, Anderson JC. Improving outcomes in polypectomy. Gastrointest Endosc. 2022;96:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Copland AP, Kahi CJ, Ko CW, Ginsberg GG. AGA Clinical Practice Update on Appropriate and Tailored Polypectomy: Expert Review. Clin Gastroenterol Hepatol. 2024;22:470-479.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Arruda do Espirito Santo P, Meine GC, Baraldo S, Barbosa EC. Cold endoscopic mucosal resection versus cold snare polypectomy for colorectal lesions: a systematic review and meta-analysis of randomized controlled trials. Endoscopy. 2024;56:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Ferlitsch M, Hassan C, Bisschops R, Bhandari P, Dinis-Ribeiro M, Risio M, Paspatis GA, Moss A, Libânio D, Lorenzo-Zúñiga V, Voiosu AM, Rutter MD, Pellisé M, Moons LMG, Probst A, Awadie H, Amato A, Takeuchi Y, Repici A, Rahmi G, Koecklin HU, Albéniz E, Rockenbauer LM, Waldmann E, Messmann H, Triantafyllou K, Jover R, Gralnek IM, Dekker E, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2024. Endoscopy. 2024;56:516-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 149] [Article Influence: 74.5] [Reference Citation Analysis (1)] |
| 14. | Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. [Development and validation of the Visual Analogue Scale (VAS) Spine Score]. Unfallchirurg. 2001;104:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Mangira D, Raftopoulos S, Vogrin S, Hartley I, Mack A, Gazelakis K, Nalankilli K, Trinh A, Metz AJ, Appleyard M, Grimpen F, Elliott T, Brown G, Moss A. Effectiveness and safety of cold snare polypectomy and cold endoscopic mucosal resection for nonpedunculated colorectal polyps of 10-19 mm: a multicenter observational cohort study. Endoscopy. 2023;55:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 16. | Lin HY, Fu YW, Wang WH, Lu FT. Endoscopic polypectomy of a large juvenile polyp due to recurrent intussusception in a 3-year-old child with severe anemia. Pediatr Neonatol. 2022;63:659-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Uraoka T, Takizawa K, Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano HO, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Igarashi M, Toyonaga T, Ajioka Y, Fujimoto K, Inoue H. Guidelines for Colorectal Cold Polypectomy (supplement to "Guidelines for Colorectal Endoscopic Submucosal Dissection/Endoscopic Mucosal Resection"). Dig Endosc. 2022;34:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Boatman S, Mott SL, Shaukat A, Melton GB, Gaertner WB, Weiser M, Ikramuddin S, Madoff R, Hassan I, Goffredo P. Endoscopic polypectomy for malignant polyps: Should tumor location (right versus left side) guide clinical decisions? Surgery. 2023;173:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Esaki M, Mohapatra S, Fukami N. Advances in Endoscopic Resection. Gastroenterol Clin North Am. 2024;53:709-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Lv YC, Yao YH, Lei JJ, Tang T. Cold snare polypectomy compared to cold forceps polypectomy for endoscopic resection of guideline defined diminutive polyps: A systematic review and meta-analysis of randomized trials. Indian J Gastroenterol. 2023;42:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Jena A, Jain S, Sundaram S, Singh AK, Chandnani S, Rathi P. Electrosurgical unit in GI endoscopy: the proper settings for practice. Expert Rev Gastroenterol Hepatol. 2023;17:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Miyazaki K, Nakayama A, Sasaki M, Minezaki D, Morioka K, Iwata K, Masunaga T, Kubosawa Y, Mizutani M, Hayashi Y, Kiguchi Y, Akimoto T, Takatori Y, Kawasaki S, Matsuura N, Sujino T, Takabayashi K, Yamanoi K, Mori K, Kanai T, Yahagi N, Kato M. Resectability of Small Duodenal Tumors: A Randomized Controlled Trial Comparing Underwater Endoscopic Mucosal Resection and Cold Snare Polypectomy. Am J Gastroenterol. 2024;119:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Yoshii S, Hayashi Y, Nakamura T, Nishiyama O, Nagaike K, Nakamatsu D, Yamada T, Egawa S, Ogiyama H, Yamaguchi S, Inoue T, Uema R, Kato M, Inoue T, Tsujii Y, Shinzaki S, Iijima H, Michida T, Morii E, Takehara T. Endoscopic features and clinical course of colorectal carcinoma resected by cold snare polypectomy. J Gastroenterol Hepatol. 2023;38:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Hassan C, Spadaccini M, Mori Y, Foroutan F, Facciorusso A, Gkolfakis P, Tziatzios G, Triantafyllou K, Antonelli G, Khalaf K, Rizkala T, Vandvik PO, Fugazza A, Rondonotti E, Glissen-Brown JR, Kamba S, Maida M, Correale L, Bhandari P, Jover R, Sharma P, Rex DK, Repici A. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy : A Systematic Review and Meta-analysis. Ann Intern Med. 2023;176:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 25. | Wang KK, Kim K, Bancila L, Lew D, Larson BK, Kim S, Lee JY, Gaddam S, Lo SK. Novel Endoscopic Polypectomy Surveillance Technique for Fundic Gland Polyps in Familial Adenomatous Polyposis Can Improve Early Detection of Dysplasia and Gastric Cancer. Am J Gastroenterol. 2022;117:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Misirovs R, Chan R, Stewart K, Lipworth B. Phenotypic associations of medical polypectomy and revision surgery following endoscopic sinus surgery: a retrospective study of a single-centre experience in Scotland. J Laryngol Otol. 2023;137:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Ge PS, Aihara H. Advanced Endoscopic Resection Techniques: Endoscopic Submucosal Dissection and Endoscopic Full-Thickness Resection. Dig Dis Sci. 2022;67:1521-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 28. | O'Sullivan T, Cronin O, van Hattem WA, Mandarino FV, Gauci JL, Kerrison C, Whitfield A, Gupta S, Lee E, Williams SJ, Burgess N, Bourke MJ. Cold versus hot snare endoscopic mucosal resection for large (≥15 mm) flat non-pedunculated colorectal polyps: a randomised controlled trial. Gut. 2024;73:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (1)] |
| 29. | Yu Z, Albéniz E, Hu J, Li P, Li Q, Hu Y, Chen J, Wang J. Prevention of delayed post-polypectomy bleeding by prophylactic clipping after endoscopic colorectal polypectomy: a meta-analysis. Int J Colorectal Dis. 2022;37:2229-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Minakata N, Murano T, Wakabayashi M, Sasabe M, Watanabe T, Mitsui T, Yamashita H, Inaba A, Sunakawa H, Nakajo K, Kadota T, Shinmura K, Ikematsu H, Yano T. Hot snare polypectomy vs endoscopic mucosal resection using bipolar snare for intermediate size colorectal lesions: Propensity score matching. World J Gastroenterol. 2023;29:3668-3677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Kim MJ, Na SY, Kim JS, Choi HH, Kim DB, Ji JS, Kim BW, Choi H. Cold snare polypectomy versus cold endoscopic mucosal resection for small colorectal polyps: a multicenter randomized controlled trial. Surg Endosc. 2023;37:3789-3795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/