Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.109030
Revised: May 27, 2025
Accepted: July 31, 2025
Published online: September 27, 2025
Processing time: 149 Days and 13.5 Hours
Proximal gastrectomy (PG) with double tract reconstruction (DTR) has recently emerged as a function-preserving alternative to total gastrectomy (TG) with Roux-en-Y (RNY) reconstruction in patients with proximally located gastric cancer.
To evaluate the current evidence comparing PG-DTR with TG-RNY in terms of perioperative outcomes, long-term survival, complication rates, nutritional status and reflux esophagitis.
A systematic literature search was conducted using PubMed, MEDLINE, Web of Science and the Cochrane Library for studies published between 2010 and January 2025. Search terms included gastric cancer, DTR and TG. Trials comparing PG-DTR with TG-RNY or PG-esophagogastrostomy (EG) were included. Data on ope
PG-DTR was found to have comparable long-term oncological outcomes to TG-RNY, despite a lower extent of lymph node dissection. Operative time and in
Its superiority over TG-RNY in postoperative nutrition and reflux prevention, together with comparable com
Core Tip: Proximal gastrectomy (PG) with double tract reconstruction (DTR) has gained attention as a function-preserving surgical technique for proximal gastric cancer. This systematic review demonstrates that PG-DTR achieves oncological outcomes comparable to total gastrectomy with Roux-en-Y reconstruction, while offering significant advantages in postoperative nutritional status. Preservation of the residual stomach and duodenum appears to play a critical role in minimizing weight loss and maintaining better haematological parameters. These findings suggest that PG-DTR may be a superior surgical option for selected patients without compromising oncological safety.
- Citation: Ilhan E, Okut G. Double tract reconstruction in proximal gastric tumors: A systematic review of clinical and functional outcomes. World J Gastrointest Surg 2025; 17(9): 109030
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/109030.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.109030
The global incidence of gastric cancer, especially proximal stomach tumors, has shown a consistent upward trend[1]. Total gastrectomy (TG) has long been regarded the standard surgical procedure for proximal gastric cancer. However, recent treatment guidelines from East Asian countries have introduced proximal gastrectomy (PG) as a viable alternative surgical approach[2]. Compared with TG, PG offers potential advantages in preserving gastric function and improving patients’ quality of life. The Japanese Gastric Cancer Association recommends three primary reconstruction options after PG: Esophagogastrostomy (EG), double tract reconstruction (DTR), and jejunal interposition (JI)[2]. Historically, reconstruction after PG has been performed using a gastric tube in combination with either EG or JI[3]. However, these methods are linked to increased rates of anastomotic stricture and reflux esophagitis caused by severe gastroesophageal reflux[4].
DTR has been developed to address these functional complications. It involves PG with distal stomach preservation and creating a side-to-side anastomosis between the digestive tract limb of a standard Roux-en-Y (RNY) configuration and the remnant stomach. Compared to TG-RNY, PG-DTR may be better adapted to the physiological and anatomical requirements of the digestive tract. The preservation of normal digestive processes and the reservoir function of the distal stomach are thought to contribute to superior nutritional outcomes[5]. In addition to these potential nutritional benefits, several studies have shown that PG-DTR provides oncologically acceptable results[6]. In recent years, both single- and multicenter studies comparing PG-DTR with TG-RNY and PG-EG have highlighted the perioperative, oncological, and nutritional advantages of PG-DTR (Table 1)[7-25]. This systematic review aims to compare PG-DTR and TG-RNY in terms of operative characteristics, postoperative complications, long-term survival, nutritional outcomes, and rates of reflux esophagitis based on the most recent data available in the literature.
| Ref. | Coun | Year | Study period | Study design | Group | Sample size | Pati | Age | Sex (male/ | Pre | Access (% | Opera | Bleed | Retri | Early comp | Lenght of stay hospi | TNM stage (I/II/III) | Reflux eso | Mean BMI diffe | Follow up (mon | Survi |
| Kim and Kim[7] | Korea | 2016 | 2009-2014 | Retro | DTR | 34 | 17 | 64.7 ± 9.9 | 14/3 | 24.2 ± 3.8 | Laparoscopic | 268.2 ± 40.9 | 33.4 ± 17.3 | 3 | 9.4 ± 4.1 | 16/1/0 | 2 | 12 | |||
| TG-RNY | 17 | 60.9 ± 12.9 | 10/7 | 23.4 ± 5 | 270 ± 43.4 | 39.3 ± 20.4 | 3 | 10 ± 3.6 | 15/2/0 | 1 | 12 | ||||||||||
| Jung et al[8] | Korea | 2017 | 2008-2016 | Retro | DTR | 248 | 92 | 59.8 ± 11.4 | 77/15 | 23.5 ± 2.7 | Laparoscopic | 198.3 ± 38.8 | 84.7 ± 81.7 | 46.1 ± 19.6 | 8.5 ± 11.3 | 1 | DTR < TGRY | 26.6 ± 10.3 | The 5-year OS rate; 96.1% and 95.9% for the PG-DTR and TG groups, respecti | ||
| TG-RNY | 156 | 58.7 ± 10.8 | 120/36 | 23.9 ± 3.3 | 225.4 ± 51.6 | 128.3 ± 112.5 | 60.0 ± 25.7 | 8.6 ± 5.7 | 3 | 43.5 ± 23.2 | |||||||||||
| Ko et al[9] | Korea | 2020 | 2008-2016 | Retro | DTR | 104 | 52 | 61.5 ± 12.3 | 35/17 | 23.7 ± 3.1 | Laparoscopic | 206.1 ± 38.2 | 133.6 ± 138.6 | 26.5 ± 11.1 | 4 | 13.3 ± 8.6 | 45/5/2 | 1 | 22.7 ± 15.4 | The PG-DTR group had a signifi | |
| TG-RNY | 52 | 63.0 ± 9.2 | 35/17 | 23.4 ± 2.9 | 204.2 ± 47.5 | 167.3 ± 94.4 | 36.2 ± 16.5 | 7 | 18.0 ± 15.5 | 40/7/5 | 0 | 36.3 ± 23.1 | |||||||||
| Li et al[10] | China | 2019 | 2015-2017 | RCT | DTR | 300 | 103 | 90/13 | 66.10 ± 3.618 (kg) | Open | 143.62 ± 2.98 | 140.22 ± 7.93 | 23 ± 3 | 0 | 10.52 ± 1.18 | 87/16/0 | 9 | 2.02 kg | 18 | ||
| JI | 98 | 88/10 | 66.33 ± 3.116 (kg) | 144.37 ± 2.51 | 139.09 ± 8.85 | 22 ± 3 | 1 | 10.53 ± 1.31 | 81/17/0 | 4 | 1.78 kg | 18 | |||||||||
| TG-RNY | 99 | 82/17 | 66.36 ± 3.824 (kg) | 143.29 ± 4.51 | 139.78 ± 8.52 | 37 ± 4 | 1 | 10.68 ± 1.35 | 78/21/0 | 11 | 8.11 kg | 18 | |||||||||
| Ji et al[11] | China | 2021 | 2014-2019 | Retro | DTR | 64 | 25 | 64.5 ± 8.9 | 24/1 | 24.5 ± 2.6 | Open | 240 (210-270) | 50 (50-100) | 19 (16.3-23) | 0 | 13 (11-18) | 23/2/0 | 2 | 39.8 | The 3-year OS rates of the EG and PG-DTR groups were 79.9% and 90.9%, respecti | |
| EG | 39 | 62.1 ± 9.1 | 31/8 | 24.5 ± 3.7 | 195 (170-240) | 100 (50-100) | 22.5 (17-31) | 1 | 11 (10-14) | 21/12/6 | 12 | N | 39.8 | ||||||||
| Ma et al[12] | China | 2020 | 2010-2018 | PSM | DTR | 192 | 86 | 60.5 ± 8.2 | 79/7 | Laparoscopic/ | 177 ± 30 | 91 ± 32 | 23 ± 6 | 2 | 11.3 ± 3.4 | 31/27/ | 8.8 kg | 48 | 5 year OS rates in patholo | ||
| TG-RNY | 86 | 62,7 ± 10,5 | 76/10 | Laparoscopic/ | 164 ± 17 | 106 ± 62 | 25 ± 7 | 3 | 10.5 ± 2.1 | 35/22/ | 12.7 kg | 48 | 5-year OS rates in patholo | ||||||||
| Wang et al[13] | China | 2020 | 2016-2017 | Retro | DTR | 36 | 12 | 55.8 ± 4.1 | 6/6 | 23.29 ± 0.76 | Laparoscopic | 215.8 ± 14.69 | 104.2 ± 14.38 | 28.50 ± 3.76 | 0 | 10.8 ± 1.19 | 10/2/0 | 1 | DTR < TGRY | 12 | |
| TG-RNY | 24 | 58.38 ± 1.82 | 15/9 | 23.5 ± 0.68 | 209.3 ± 12.87 | 111.7 ± 12.2 | 36.29 ± 1.35 | 0 | 13.0 ± 2.1 | 22/2/0 | 11 | 12 | |||||||||
| Sato et al[14] | Japan | 2021 | 2013-2019 | Retro | DTR | 150 | 75 | 68.8 ± 10.2 | 59/16 | 22.4 (15.8-32.2) | Laparoscopic/ | 312 (199-552) | 23 (4-192) | 9 | 9 (7-58) | 60/11/4 | 6 | 36.3 (22.2-51.0) | |||
| TG-RNY | 75 | 66.2 ± 11.8 | 51/24 | 22.8 (16.0-34.8) | Laparoscopic/ | 311 (206-495) | 25 (3-182) | 4 | 9 (7-49) | 44/16/ | 4 | 36.3 (22.2-51.0) | |||||||||
| Kimuro et al[15] | Japan | 2021 | 2011-2018 | Retro | DTR | 16 | 8 | 70.4 (55-77) | 8/0 | 22.9 (19.3-24.9) | Laparoscopic | 294.1 ± 33.9 | 8.2 ± 4.3 | 2 | 15.4 ± 3.3 | 8/0/0 | 92 ± 5.7 (weight 24th month) | 24 | |||
| TG-RNY | 8 | 68.3 (45-83) | 8/0 | 23.7 (20.8-29.8) | 383.4 ± 20.7 | 48.8 ± 16.8 | 1 | 14.6 ± 2.2 | 8/0/0 | 82.4 ± 3.7 (weight 24th month) | 24 | ||||||||||
| Eom et al[16] | Korea | 2021 | 2013-2017 | Retro | DTR | 103 | 58 | 65.5 (52.8-72.0) | 47/11 | 23.5 ± 3.1 | Laparoscopic | 34.0 (24.6-40.3) | 2 | There is no signifi | 12 | ||||||
| EG | 45 | 63.0 (53.5-72.5) | 13/12 | 25.0 ± 3.3 | 26.0 (20.5-36.5) | 8 | 12 | ||||||||||||||
| Hwang et al[17] | Korea | 2022 | 2016-2018 | RCT | DTR | 128 | 63 | 58.6 ± 10.2 | 35/28 | 24.5 ± 2.9 | Laparoscopic | 217.1 ± 67.5 | 75.5 ± 74.7 | 40.8 ± 18.3 | 3 | 7.4 ± 3.2 | 60/3/0 | 9 | 0.5 | ||
| TG-RNY | 65 | 61.3 ± 11.5 | 47/18 | 24.5 ± 2.9 | 200.8 ± 51.9 | 65.5 ± 62.7 | 55.1 ± 24.9 | 2 | 7.3 ± 2.9 | 58/7/0 | 9 | 0.5 | |||||||||
| Li et al[18] | China | 2023 | 2012-2015 | Retro | DTR | 100 | 50 | 65.8 ± 4.5 | 40/10 | 24.1 ± 1.8 | Laparoscopic | 192.1 ± 7.7 | 122.4 ± 45.8 | 25.7 ± 4.6 | 3 | 14.1 ± 4.4 | 50/0/0 | 1 | 22.9 ± 2.0 (BMI: 12nd month) | 60 | 5-year OS rates in the PG-DTR and TG-RNY were 98% and 90%, respecti |
| TG-RNY | 50 | 66.9 ± 3.6 | 40/10 | 23.8 ± 2.0 | 187.4 ± 6.7 | 117.0 ± 51.3 | 34.4 ± 5.2 | 2 | 14.6 ± 6.0 | 50/0/0 | 2 | 20.6 ± 1.3 | 60 | ||||||||
| Ma et al[19] | China | 2022 | 2013-2018 | Retro | DTR | 66 | 33 | 23 patients (> 60 years) | 28/5 | Laparoscopic | 204 ± 37 | 171 ± 71 | 21.5 ± 9.5 | 3 | 15.4 ± 2.3 | 14/9/10 | 8 | DTR < TGRY | 54 | 5-year OS rates in the PG-DTR and TG groups were 61% and 60%, respecti | |
| TG-RNY | 33 | 21 patients (> 60 years) | 28/5 | 205 ± 50 | 176 ± 96 | 29.0 ± 10.9 | 3 | 17.2 ± 3.8 | 10/12/ | 11 | 61 | ||||||||||
| Park et al[20] | Korea | 2023 | 2016-2020 | RCT | DTR | 128 | 63 | 58.6 ± 10.3 | 35/28 | 24.5 ± 2.9 | Laparoscopic | 217.1 ± 67.5 | 75.5 ± 74.6 | 40.8 ± 18.3 | 7.4 ± 3.2 | 60/3/0 | 1 | 24 | 2-year OS rates; PG-DTR and TG-RNY were 98.5% and 100%, respecti | ||
| TG-RNY | 65 | 61.3 ± 11.5 | 47/18 | 24.5 ± 2.9 | 200.8 ± 51.9 | 65.5 ± 62.7 | 55.1 ± 24.9 | 7.3 ± 2.9 | 58/7/0 | 2 | 24 | ||||||||||
| Hasegawa et al[21] | Japan | 2024 | 2011-2022 | Retro | DTR | 73 | 39 | 66.6 ± 14.0 | 27/12 | 22.3 ± 3.0 | Laparoscopic/ | 404.5 ± 88.8 | 114.8 ± 143.9 | 23.6 ± 12.5 | 6 | 16.1 ± 10.9 | 30/6/3 | 0 | There is no signifi | 36 | |
| EG | 34 | 69.4 ± 9.9 | 27/7 | 23.0 ± 3.6 | Laparoscopic/ | 365.1 ± 99.5 | 122.3 ± 195.5 | 23.9 ± 13.9 | 2 | 15.8 ± 12.6 | 29/4/1 | 5 | 36 | ||||||||
| Shoda et al[22] | Japan | 2024 | 2020-2022 | Prospec | DTR | 20 | 10 | 72.3 ± 6.3 | 9/1 | 24.0 ± 2.9 | Laparoscopic | 554.6 ± 163.2 | 123.8 ± 118.3 | 0 | 14.6 ± 3.8 | 5/0/5 | 0.17 ± 0.08 | 12 | |||
| EG | 10 | 68.8 ± 8.9 | 6/4 | 23.0 ± 4.2 | 413.5 ± 91.2 | 78.7 ± 75.8 | 0 | 12.0 ± 6.7 | 8/1/1 | 0.14 ± 0.08 | 12 | ||||||||||
| Wang et al[23] | China | 2024 | 2020-2022 | Retro | DTR | 295 | 87 | 64.8 ± 6.67 | 69/18 | 24.24 ± 3.27 | Laparoscopic | 230 (210-255) | 85 (50-100) | 22 (19-31) | 2 | 7 (7-9) | 30/22/ | 8 | 2.51 ± 1.38 | 12 | |
| EG | 96 | 64.8 ± 6.62 | 74/22 | 23.79 ± 2.79 | 195 (180-250) | 100 (50-100) | 21 (18-26) | 1 | 8 (7-9) | 26/21/ | 42 | 4.37 ± 1.81 | 12 | ||||||||
| TG-RNY | 112 | 63.15 ± 9.64 | 81/31 | 24.75 ± 3.36 | 200 (180-240) | 100 (50-100) | 28 (22-35) | 4 | 8 (7-9) | 23/26/ | 26 | 3.96 ± 1.59 | 12 | ||||||||
| Sun et al[24] | China | 2024 | 2019-2023 | Retro | DTR | 156 | 93 | 63.16 ± 9.75 | 74/19 | 23.08 ± 3.64 | Laparoscopic | 166.38 | 113.23 | 18.10 | 5 | 13.83 | 33 | 1.40 ± 0.655 | 12 | ||
| EG | 63 | 61.70 ± 8.30 | 47/16 | 23.20 ± 3.39 | 174.06 | 150.16 | 16.81 | 5 | 13.11 | 49 | 1.81 ± 0.759 | 12 | |||||||||
| Wu et al[25] | China | 2024 | 2020-2022 | Retro | DTR | 43 | 23 | 70 (66-76) | 19/4 | 22.1 ± 2.8 | Laparoscopic | 187 (177-198) | 21.2 ± 4.1 | 20.4 ± 10.3 | 0 | 8 (8-9) | 15/8/0 | 9 | 22.3 ± 2.6 (BMI: 12nd month) | 12 | |
| SOA | 20 | 65.5 (60.8-71.8) | 17/3 | 21.9 ± 3.3 | 197.5 (190-207) | 21.9 ± 3.0 | 24.8 ± 11.0 | 0 | 8.0 (7.0-8.8) | 16/4/0 | 2 | 22.0 ± 2.5 | 12 |
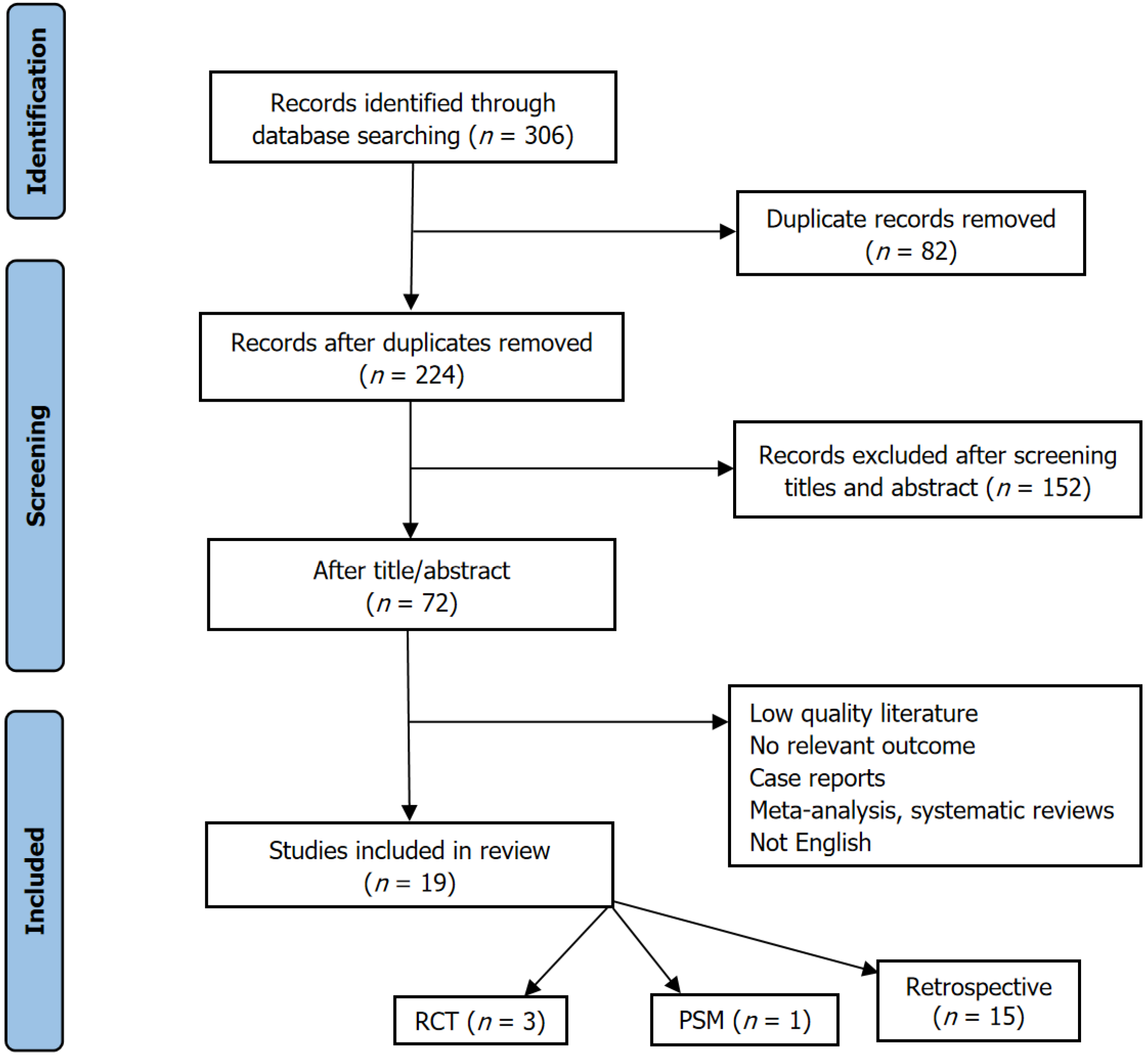
A systematic literature search of international databases (PubMed, Web of Science, MEDLINE, and Cochrane Library) was conducted for trials published between 2010 and January 2025. The search terms included (“stomach” and “gastric”) and (“neoplasm” and “cancer” and “tumor” and “carcinoma”) and (“double tract reconstruction” and “double tract anastomosis”) and (“total gastrectomy”). A total of 1106 articles were initially identified based on these criteria. The analysis focused on studies comparing various reconstruction methods following PG, including DTR. Case reports and letters to the editor were excluded from analysis. Ultimately, 19 studies were included. Data extracted from these articles encompassed demographic characteristics, surgery type, operative time, intraoperative blood loss, number of lymph nodes dissected, tumor staging, postoperative complications of Clavien-Dindo grade III or higher, body mass index (BMI), and incidence of reflux esophagitis. All included studies were reported as PRISMA complain (Figure 1)[26].
The methodological quality of the included studies was assessed using both the Cochrane Risk of Bias 2.0 (RoB 2.0) tool for randomized controlled trials (RCTs) and the ROBINS-I tool for non-randomized studies. Among the 19 included studies, four were RCTs, one was a prospective cohort study with a defined intervention protocol, one was a propensity score-matched study, and 13 were retrospective observational studies.
RCTs (RoB 2.0): According to the RoB 2.0 assessment, two of the four RCTs (Hwang et al[17]; Li et al[10]) were judged to have a low overall risk of bias across all five domains, including the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. The study by Park et al[20] raised some concerns due to limitations in outcome measurement reporting, while Shoda et al[22], although prospective, exhibited some concerns in randomization transparency and selective reporting. A detailed summary is presented in the supplementary risk of bias table (Supplementary Table 1).
Non-randomized studies (ROBINS-I): Among the 15 non-randomized studies, the overall risk of bias was high in 14 studies, and moderate in 1 (Ma et al[12]; propensity score-matched; Supplementary Table 2). The predominant sources of bias include: (1) Confounding and selection bias: Most retrospective studies lacked sufficient control for confounding factors, such as baseline nutritional status, tumor stage, and institutional surgical expertise; (2) Classification of interventions: Given the surgical nature of the interventions, the consistency in defining PG-DTR vs TG-RNY or PG-EG was not always clear, especially in multicenter data or studies spanning long recruitment periods; (3) Outcome as
Impact of study design heterogeneity: Significant methodological and clinical heterogeneity was observed among the included studies, particularly in: (1) Study design and sample size: Study designs ranged from single-center retrospective cohorts with small sample sizes to multicenter trials with larger populations; (2) Surgical techniques and access: Laparoscopic vs open vs robotic approaches were variably used across and even within studies; and (3) Outcome definitions and follow-up durations varied considerably (from 0.5 to 60 months), affecting comparability. This heterogeneity limited the feasibility and reliability of quantitative synthesis (meta-analysis) for specific endpoints, especially functional and nutritional outcomes. Although pooled estimates were calculated for a subset of comparable studies (n = 13), the certainty of evidence remained moderate to low, primarily due to the risk of bias and inconsistency across studies.
The included studies generally compared PG-DTR, performed either by open or laparoscopic approach, with TG-RNY or PG-EG.
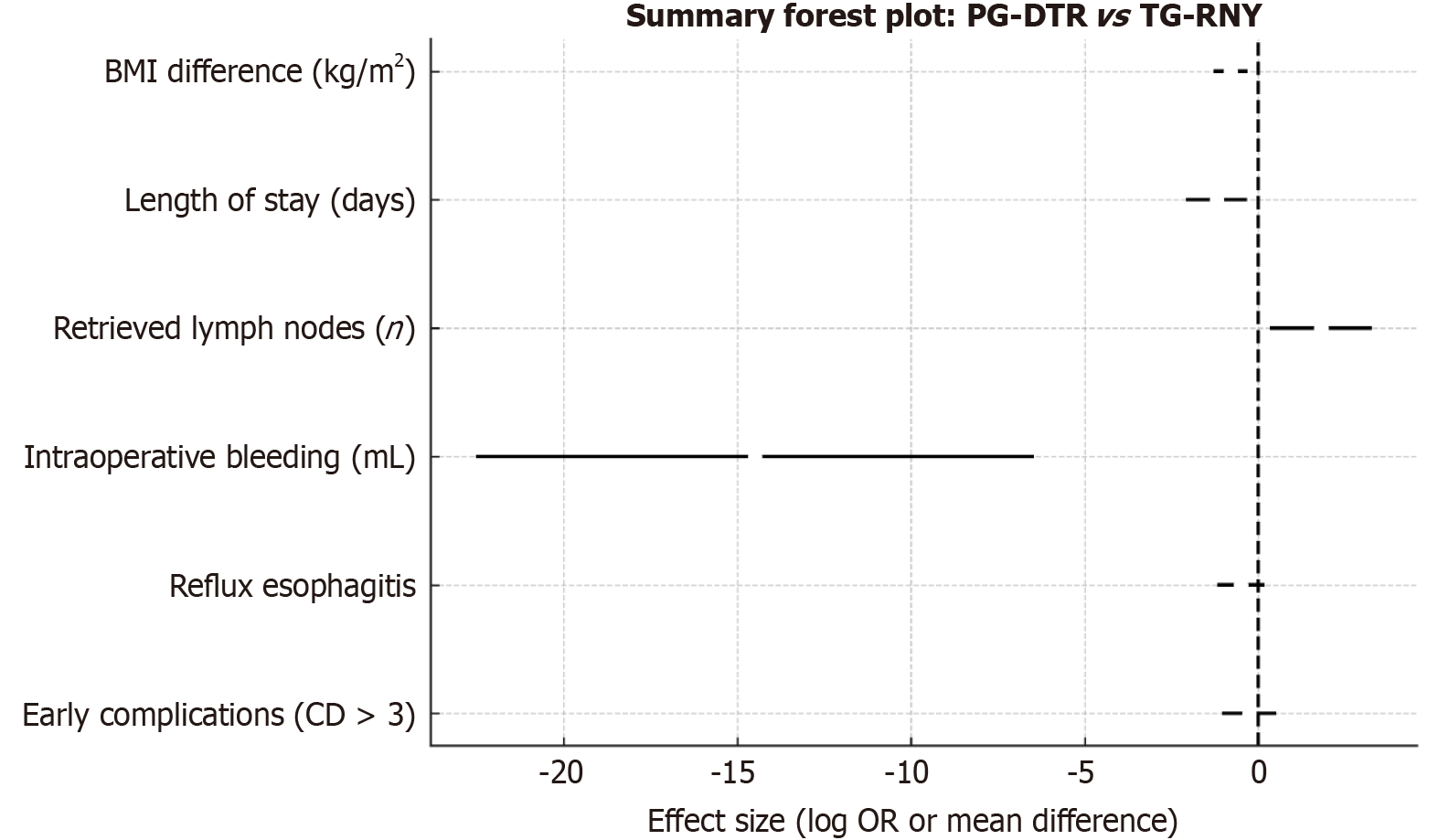
Standard D2 Lymphadenectomy is typically performed during TG-RNY, whereas PG-DTR and PG-EG are usually accompanied by D1+ lymphadenectomy involving stations 1-2-3a-4sa-4sb-7-8a-9-11p. Regarding the number of lymph nodes retrieved, TG is superior to both PG-DTR and PG-EG[8,9,13,17,19,20,23]. No significant difference was found between PG-DTR and PG-EG[11,16,21,22,24]. The advantage of TG in lymph node yield is attributed to the broader range of lymph node stations resected. For Siewert type II and III adenocarcinoma, studies comparing PG followed by DTR with TG-RNY have shown comparable results in terms of tumor recurrence, metastasis, and long-term survival[8,11,12,18-21]. Ko et al[9] reported that DTR resulted in superior overall survival compared to TG-RNY. This difference in overall survival has been attributed to the older age of patients who experienced mortality in the TG-RNY group and the nutritional disadvantages associated with TG-RNY than PG-DTR.
Several studies have reported no statistically significant difference in operative time and intraoperative bleeding between PG-DTR and TG-RNY[7,9-11,13,14,17-20,23,25]. However, studies by Kimura et al[15] and Jung et al[8] found that PG-DTR was associated with shorter operative times and less intraoperative blood loss than TG-RNY. These results were thought to be influenced by anastomotic technique and surgeon experience. EG, another reconstruction method following PG involves only a single anastomosis and is considered technically simpler than DTR. Several studies have shown that EG can be performed with a shorter operative time and less blood loss than DTR[11,21,24].
Compared to TG-RNY and EG, DTR has shown similar rates of early postoperative complications[7-15,17-25]. Reflux esophagitis remains one of the most discussed long-term complications. Evaluation for reflux was made according to the Visick scoring or the Los Angeles classification. Several studies have shown that PG-DTR has comparable or even lower rates of reflux esophagitis than TG-RNY[7,9,12,13,17-20], with some suggesting a slight advantage[10,23]. Given that one of the traditional justifications for TG over PG is a reduced risk of reflux, the comparable results achieved with PG-DTR are noteworthy. A greater distance between the esophagojejunostomy and gastrojejunostomy appears to correlate with a reduced incidence of reflux, although a distance greater than 30 cm may compromise endoscopic surveillance and delay the detection of secondary malignancies[20].
Among PG reconstruction techniques, EG has consistently been associated with higher rates of reflux esophagitis. Modifications such as fixation of the remnant stomach to the diaphragm to create a neo-His angle or pseudo-fundus[16,23], fundoplication with conventional anastomosis[21,22], and the side overlap with fundoplication by Yamashita te
Body weight is a surrogate marker of nutritional status. Changes in patient body weight are given in mean BMI or kilograms. Studies have shown that PG-DTR is more effective in maintaining postoperative body weight than TG-RNY[8,10,12,18,19]. This advantage is likely due to the preservation of the gastric reservoir and maintaining the duodenal passage, which supports enzyme flow and nutrient absorption. EG is also associated with better weight maintenance than TG-RNY, but is not significantly different from DTR. Although some studies have reported better weight maintenance with DTR after the first postoperative year[24], the overall consensus is that weight outcomes are comparable between DTR and EG[16,21-23]. The preserved reservoir function of the remnant stomach and the intact duodenopancreatic axis is thought to mitigate excessive weight loss.
The stomach plays a central role in vitamin B12 metabolism. While evidence on long-term postoperative B12 and iron levels is inconsistent, patients who undergo PG-DTR appear to require less supplementation than those who undergo TG-RNY[7,8,10,18-20]. PG-DTR has also been associated with more favorable changes in laboratory parameters such as hemoglobin, total protein, and albumin[10,13,15,18,19]. No significant differences were observed between PG-DTR and PG-EG in postoperative levels of B12, total protein, albumin, or haemoglobin[11,21,24], probably due to the preserved functional capacity of the remnant stomach.
However, these observations should be interpreted with caution, as most of the supporting studies are retrospective and subject to selection bias, inconsistent outcome reporting, and short-to-intermediate follow-up durations. Con
While accumulating evidence supports the functional and oncological safety of PG with DTR, limited guidance exists regarding the clinical criteria for its optimal implementation. The included studies in this study provide a heterogeneous mix of patient populations, surgical approaches, and institutional protocols, reflecting the current variability in adopting this technique. PG-DTR appears to be particularly suitable for patients with early to locally advanced proximal gastric cancer (cT1-T3, N0-N1) in whom the tumor is located in the upper third of the stomach but without esophageal invasion or need for TG due to margin concerns[8,12,13,20]. Patients with relatively preserved performance status and adequate nutritional reserves may benefit more from function-preserving strategies, such as PG-DTR. Additionally, carefully selected elderly patients may experience better postoperative quality of life and nutritional outcomes than those un
Despite its potential benefits, PG-DTR faces persistent real-world barriers to widespread adoption. First, PG-DTR demands considerable technical expertise, especially in constructing a tension-free esophagojejunostomy and accurately positioning the jejunal limbs to ensure uninterrupted food passage through the remnant stomach and jejunum. Studies, such as those by Wang et al[13], Ma et al[12], and Li et al[10], were conducted in high-volume centers with extensive experience in laparoscopic or open gastric cancer surgery, suggesting that institutional learning curves may considerably impact outcomes.
Second, anatomical limitations, including patient BMI, remnant stomach size, and mesenteric length, may limit the feasibility of DTR. Sato et al[14] and Hwang et al[17] noted the importance of sufficient jejunal mobility to prevent anastomotic tension and maintain adequate blood supply. In patients with short mesentery or intra-abdominal adhesions, alternative reconstruction techniques such as EG or JI may be preferred. Third, the reconstruction is more complex and time-consuming than standard RNY or EG anastomoses, which may limit its application in minimally invasive surgery, especially in centers with limited access to robotic platforms. However, some included studies (e.g., Sato et al[14], Eom et al[16], and Shoda et al[22]) have demonstrated the feasibility of PG-DTR in laparoscopic and robotic settings, provided that surgical teams have sufficient expertise and institutional support.
Finally, there is a lack of uniform consensus or guidelines on when PG-DTR should be favored over other techniques. Although it shows promise in preserving postoperative function, the decision to employ PG-DTR should be individualized, considering tumor characteristics, patient comorbidities, anatomical feasibility, and surgical expertise. In summary, while PG-DTR is a function-preserving and oncologically acceptable alternative to TG-RNY in selected patients with proximal gastric cancer, its broader adoption in routine clinical practice requires addressing technical challenges, standardizing surgical protocols, and training surgeons in both open and minimally invasive techniques.
In recent years, PG-DTR has emerged as a promising surgical technique for proximal gastric tumors. Although PG-DTR may involve a slightly longer operative time than TG-RNY and PG-EG, it yields comparable outcomes regarding major postoperative complications (Clavien-Dindo grade ≥ III), hospital stay, and overall survival. Its nutritional superiority over TG-RNY and the lower incidence of reflux esophagitis compared to PG-EG further support its clinical utility. PG-DTR is widely regarded as a rational and effective surgical approach for patients with proximal gastric cancer.
| 1. | Zhu G, Jiao X, Zhou S, Zhu Q, Yu L, Sun Q, Li B, Fu H, Huang J, Lang W, Lang X, Zhai S, Xiong J, Fu Y, Liu C, Qu J. Can proximal gastrectomy with double-tract reconstruction replace total gastrectomy? a meta-analysis of randomized controlled trials and propensity score-matched studies. BMC Gastroenterol. 2024;24:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 847] [Article Influence: 282.3] [Reference Citation Analysis (2)] |
| 3. | Zapletal Ch, Heesen Ch, Origer J, Pauthner M, Pech O, Ell Ch, Lorenz D. Quality of life after surgical treatment of early Barrett's cancer: a prospective comparison of the Ivor-Lewis resection versus the modified Merendino resection. World J Surg. 2014;38:1444-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Rosa F, Quero G, Fiorillo C, Bissolati M, Cipollari C, Rausei S, Chiari D, Ruspi L, de Manzoni G, Costamagna G, Doglietto GB, Alfieri S. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG). Gastric Cancer. 2018;21:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Hölscher A, Berlth F, Hemmerich M, Minot S, Schmidt H. [Double Tract Reconstruction Following Limited Oesophagogastric Resection of AEG Types II and III Adenocarcinomas]. Zentralbl Chir. 2020;145:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Hong J, Wang SY, Hao HK. A Comparative Study of Double-Tract Reconstruction and Roux-en-Y After Gastrectomy for Gastric Cancer. Surg Laparosc Endosc Percutan Tech. 2019;29:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Kim DJ, Kim W. Laparoscopy-assisted Proximal Gastrectomy with Double Tract Anastomosis Is Beneficial for Vitamin B12 and Iron Absorption. Anticancer Res. 2016;36:4753-4758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Jung DH, Lee Y, Kim DW, Park YS, Ahn SH, Park DJ, Kim HH. Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc. 2017;31:3961-3969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Ko HJ, Kim KH, Lee SH, Choi CW, Kim SJ, In Choi C, Kim DH, Kim DH, Hwang SH. Can Proximal Gastrectomy with Double-Tract Reconstruction Replace Total Gastrectomy? A Propensity Score Matching Analysis. J Gastrointest Surg. 2020;24:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Li Z, Dong J, Huang Q, Zhang W, Tao K. Comparison of three digestive tract reconstruction methods for the treatment of Siewert II and III adenocarcinoma of esophagogastric junction: a prospective, randomized controlled study. World J Surg Oncol. 2019;17:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Ji X, Jin C, Ji K, Zhang J, Wu X, Jia Z, Bu Z, Ji J. Double Tract Reconstruction Reduces Reflux Esophagitis and Improves Quality of Life after Radical Proximal Gastrectomy for Patients with Upper Gastric or Esophagogastric Adenocarcinoma. Cancer Res Treat. 2021;53:784-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Ma F, Guo D, Zhang B, Zhang Y, Peng L, Ma Q, Ji S, Chai J, Hua Y, Chen X, Wang H, Xu S, Luo S. Short and long-term outcomes after proximal gastrectomy with double tract reconstruction for Siewert type III adenocarcinoma of the esophagogastric junction: a propensity score matching study from a 10-year experience in a high-volume hospital. J Gastrointest Oncol. 2020;11:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Wang L, Xia Y, Jiang T, Li F, Wang W, Zhang D, Xu H, Yang L, Xu Z. Short-Term Surgical Outcomes of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction Versus Laparoscopic Total Gastrectomy for Adenocarcinoma of Esophagogastric Junction: A Matched-Cohort Study. J Surg Res. 2020;246:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Sato R, Kinoshita T, Akimoto E, Yoshida M, Nishiguchi Y, Harada J. Feasibility and quality of life assessment of laparoscopic proximal gastrectomy using double-tract reconstruction. Langenbecks Arch Surg. 2021;406:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Kimura K, Ebihara Y, Tanaka K, Nakanishi Y, Asano T, Noji T, Kurashima Y, Murakami S, Nakamura T, Tsuchikawa T, Okamura K, Shichinohe T, Hirano S. Initial Results of Laparoscopic Proximal Gastrectomy With Double-tract Reconstruction Using Oblique Jejunogastrostomy Method on the Long-term Outcome of Postoperative Nutritional Status: A Propensity Score-matched Study. Surg Laparosc Endosc Percutan Tech. 2021;31:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Eom BW, Park JY, Park KB, Yoon HM, Kwon OK, Ryu KW, Kim YW. Comparison of nutrition and quality of life of esophagogastrostomy and the double-tract reconstruction after laparoscopic proximal gastrectomy. Medicine (Baltimore). 2021;100:e25453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Hwang SH, Park DJ, Kim HH, Hyung WJ, Hur H, Yang HK, Lee HJ, Kim HI, Kong SH, Kim YW, Lee HH, Kim BS, Park YK, Lee YJ, Ahn SH, Lee IS, Suh YS, Park JH, Ahn S, Han SU. Short-Term Outcomes of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction Versus Laparoscopic Total Gastrectomy for Upper Early Gastric Cancer: A KLASS 05 Randomized Clinical Trial. J Gastric Cancer. 2022;22:94-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Li Z, Dong J, Huang Q. Feasibility of laparoscopic proximal gastrectomy with piggyback jejunal interposition double-tract reconstruction for proximal gastric cancer: A propensity score-matching analysis. J Minim Access Surg. 2023;19:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Ma X, Zhao M, Wang J, Pan H, Wu J, Xing C. Clinical Comparison of Proximal Gastrectomy With Double-Tract Reconstruction Versus Total Gastrectomy With Roux-en-Y Anastomosis for Siewert Type II/III Adenocarcinoma of the Esophagogastric Junction. J Gastric Cancer. 2022;22:220-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Park DJ, Han SU, Hyung WJ, Hwang SH, Hur H, Yang HK, Lee HJ, Kim HI, Kong SH, Kim YW, Lee HH, Kim BS, Park YK, Lee YJ, Ahn SH, Lee I, Suh YS, Park JH, Ahn S, Park YS, Kim HH. Effect of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction vs Total Gastrectomy on Hemoglobin Level and Vitamin B12 Supplementation in Upper-Third Early Gastric Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2023;6:e2256004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Hasegawa T, Kubo N, Sakurai K, Nishimura J, Iseki Y, Nishii T, Shimizu S, Inoue T, Nishiguchi Y, Maeda K. Study of Short-Term and Long-Term Outcomes Between Esophagogastrostomy and Double-Tract Reconstruction After Proximal Gastrectomy. J Gastrointest Cancer. 2024;55:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Shoda K, Kubota T, Kawaguchi Y, Akaike H, Maruyama S, Higuchi Y, Nakayama T, Saito R, Takiguchi K, Furuya S, Shiraishi K, Amemiya H, Kawaida H, Ichikawa D. Differences in glycemic trends due to reconstruction methods after proximal gastrectomy from the perspective of continuous glucose-monitoring. Surg Today. 2024;54:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Wang ZJ, Xu ZY, Huang ZJ, Li L, Guan D, Gao YH, Wang XX. Double tract reconstruction improves the quality of life and better maintain the BMI of patients with proximal gastric cancer. BMC Surg. 2024;24:171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Sun Y, Chen C, Hou L, Zhao E. Short-term outcomes and quality of life of esophagogastrostomy versus the double-tract reconstruction after laparoscopic proximal gastrectomy. BMC Cancer. 2024;24:1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Wu CY, Zhong WJ, Ye K. Comparison of the efficacy, safety and postoperative quality of life between modified side overlap anastomosis and double-tract anastomosis after laparoscopic proximal gastrectomy. Updates Surg. 2024;76:2255-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51894] [Article Influence: 10378.8] [Reference Citation Analysis (2)] |
| 27. | Yamashita Y, Yamamoto A, Tamamori Y, Yoshii M, Nishiguchi Y. Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric Cancer. 2017;20:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/