Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.107356
Revised: July 9, 2025
Accepted: July 31, 2025
Published online: September 27, 2025
Processing time: 119 Days and 0.7 Hours
Limited evidence exists regarding the role of enhanced recovery after surgery (ERAS) protocols in optimizing pain management and functional recovery after colorectal cancer (CRC) surgery.
To evaluate the impact of ERAS protocols on postoperative pain management and functional recovery in patients undergoing CRC surgery.
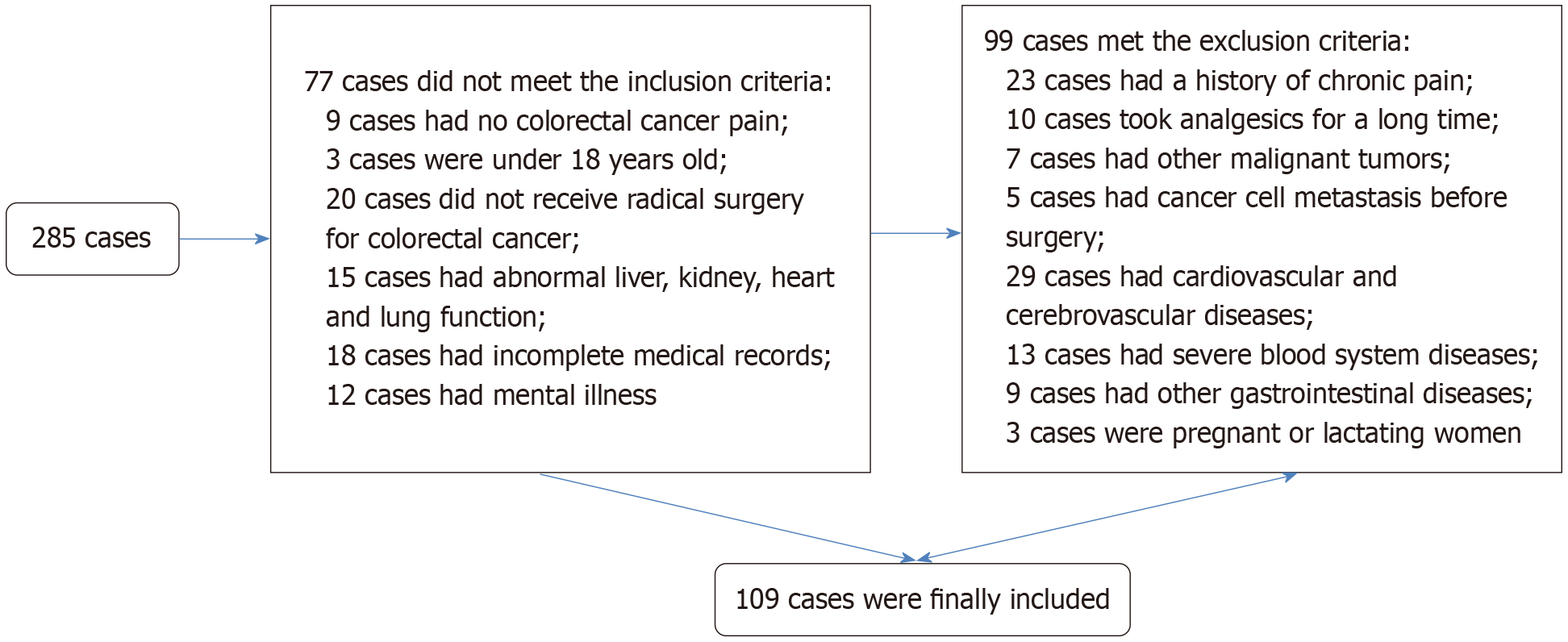
A total of 109 patients with CRC admitted to The Third Affiliated Hospital of Jinzhou Medical University between June 2021 and June 2024 were enrolled in this study. They were divided into two groups: A control group (n = 50) receiving standard perioperative care and an observation group (n = 59) managed under an ERAS protocol. Clinical outcomes, including postoperative pain intensity [as
The observation group exhibited significantly lower VAS scores at 72 hours postoperatively, shorter durations of maximum VAS scores, earlier recovery of functional indicators (time to first ambulation, bowel sound recovery, first anal gas discharge, and first defecation), and shorter hospitalization compared with the control group. Additionally, average sleep duration on postoperative day 3 was significantly longer in the observation group. Furthermore, the observation group demonstrated significantly improved sleep quality (lower Pittsburgh Sleep Quality Index scores) and higher quality of life (higher Short Form 36 Health Survey scores across all domains) than both the baseline and control groups. The incidence of total postoperative complications was also significantly lower in the observation group than in the control group.
ERAS protocols are highly effective in relieving postoperative pain, accelerating functional recovery, and improving overall clinical outcomes in patients with CRC undergoing surgery, supporting their broader clinical application.
Core Tip: Colorectal cancer has a high incidence and mortality. This study evaluated the effectiveness of enhanced recovery after surgery protocols on postoperative pain management and functional recovery in patients undergoing colorectal cancer surgery, and found significant improvements in pain control, functional recovery, quality of life, length of hospitalization, and complication rates.
- Citation: Wu D, Wang J. Impact of enhanced recovery after surgery on postoperative pain management and functional recovery in patients with colorectal cancer. World J Gastrointest Surg 2025; 17(9): 107356
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/107356.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.107356
Colorectal cancer (CRC) is a major global malignancy linked to genetic susceptibility and environmental risk factors, including obesity, alcohol use, tobacco use, and sedentary behavior[1,2]. Its multifactorial pathogenesis involves chronic inflammation, oxidative stress, impaired cell proliferation and differentiation, resistance to apoptosis, and metastases[3,4]. In 2020, CRC accounted for more than 1.9 million new cases and approximately 1 million deaths worldwide, un
Inclusion criteria: Histologically confirmed CRC[14]; presence of CRC-induced pain; age ≥ 18 years; candidate for radical oncological resection; preserved major organ function (hepatic, renal, and cardiopulmonary); complete medical records; and intact neurological and psychiatric status with normal communication ability. Exclusion criteria: History of chronic pain syndrome; long-term analgesic use; concurrent diagnosis of other malignancies; preoperative distant metastasis or local invasion of adjacent organs/tissues; comorbid cardio-cerebrovascular disease; severe hematological disorder; concurrent gastrointestinal disease; pregnancy or lactation. A total of 109 eligible patients, admitted to the Third Affiliated Hospital of Jinzhou Medical University between June 2021 and June 2024, were therefore enrolled in this study. They were assigned to a control group (n = 50) receiving standard perioperative care and an observation group (n = 59) managed under an ERAS protocol.
The control group received standard perioperative care: (1) Upon admission, the nursing staff assisted patients in completing routine preoperative assessments, as well as receiving verbal education regarding the disease, surgical procedure, and perioperative expectations. The patients also received psychological support to alleviate preoperative anxiety and emotional distress; (2) Bowel preparation was initiated 1 day before surgery; fasting for 12 hours and fluid restriction for 4 hours before surgery. An enema was administered on the evening before and on the morning of surgery. Nasogastric tube, urinary catheter, and peritoneal drain were inserted preoperatively and removed following pos
The observation group received a comprehensive ERAS intervention with the following components: (1) Perioperative care: A multidisciplinary team of physicians and nurses, educated patients on surgical precautions, perioperative expectations, and potential outcomes. Patients observed a 6-hour fast and a 2-hour fluid restriction before surgery. To maintain metabolic stability, a carbohydrate-rich glucose solution was administered orally 2 hours before the fasting period. Routine mechanical bowel preparation was avoided to reduce discomfort. Intraoperatively, individualized anesthesia and active warming were used, drainage tube usage was minimized, and continuous reassurance was provided to alleviate patient anxiety. Postoperatively, oral intake began promptly after consciousness returned, starting with sips of warm water and progressing to early enteral nutrition as tolerated; (2) Rehabilitation training: A patient-specific rehabilitation plan encouraged ambulation at least four times daily, with a minimum walking distance of 60 m per session, while avoiding excessive exertion; (3) Psychological support and pain management: Ongoing communication addressed the patients’ psychological needs and provided tailored emotional support, fostering a positive recovery mindset. Preoperatively, a multimodal pain management approach was implemented: For mild pain, non-pharmacological interventions such as distraction techniques were utilized. For moderate to severe pain, analgesic medications, including oxycodone and sufentanil, were administered according to a predefined protocol, with dosages adjusted based on pain severity and patient response. On postoperative days 0-1, an epidural patient-controlled analgesia pump (bupivacaine 75 mg + morphine 2 mg in 150 mL saline) was used. Thereafter, non-steroidal anti-inflammatory drugs or opioids were admi
(1) Pain intensity: Postoperative pain was assessed 72 hours after surgery using the Visual Analogue Scale (VAS)[15], ranging from 0 (pain-free) to 10 (most intense pain), with higher scores indicating greater pain severity. The duration of peak VAS score was also recorded to evaluate sustained maximal pain; (2) Functional recovery: Postoperative recovery indicators included time to first ambulation, bowel sound recovery, first anal gas discharge, and first defecation; (3) Sleep quality and hospitalization: The average sleep duration on postoperative day 3 was recorded. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI)[16] before and 3 days post-intervention. The total length of hospitalization was also documented; (4) Quality of life: Quality of life was assessed before and after the intervention using the Short Form 36 Health Survey (SF-36)[17], which covers eight domains: General health, health status, mental health, physical pain, physical function, vitality, social function, and role limitations due to emotional problems. Scores range from 0 to 100, with higher scores reflecting a better quality of life; and (5) Complications: Postoperative complications, including surgical site infections, pulmonary infections, abdominal distension/pain, and ileus, were recorded in both groups.
Continuous variables are expressed as mean ± SD. Between-group comparisons were analyzed using independent samples t-tests, whereas within-group (pre-intervention vs post-intervention) comparisons were assessed using paired t-tests. Categorical variables are expressed as frequencies and percentages and compared using the χ2 test. All statistical analyses were performed using SPSS version 22.0 or GraphPad Prism 6. A P value < 0.05 was considered statistically significant.
A detailed flowchart for patient selection can be found in Figure 1. Comprehensive comparisons were made between the control and observation groups in terms of age, body mass index, gender, pathological classification, tumor-node-metastasis staging, and type of surgery. Statistical analysis revealed no significant inter-group differences in these parameters (P > 0.05, Table 1).
| Indicators | Control group (n = 50) | Observation group (n = 59) | χ2/t | P value |
| Age (years) | 58.50 ± 6.87 | 55.64 ± 8.75 | 1.873 | 0.064 |
| BMI (kg/m2) | 22.52 ± 2.90 | 23.05 ± 2.85 | 0.960 | 0.339 |
| Gender | 0.005 | 0.943 | ||
| Male | 30 (60.00) | 35 (59.32) | ||
| Female | 20 (40.00) | 24 (40.68) | ||
| Pathological classification | 0.801 | 0.371 | ||
| Adenocarcinoma | 28 (56.00) | 38 (64.41) | ||
| Mucinous adenocarcinoma | 22 (44.00) | 21 (35.59) | ||
| TNM staging | 1.169 | 0.557 | ||
| I | 18 (36.00) | 19 (32.20) | ||
| II | 25 (50.00) | 27 (45.76) | ||
| III | 7 (14.00) | 13 (22.03) | ||
| Type of surgery | 1.312 | 0.859 | ||
| Right hemicolectomy | 8 (16.00) | 6 (10.17) | ||
| Left hemicolectomy | 7 (14.00) | 7 (11.86) | ||
| Transverse colectomy | 7 (14.00) | 10 (16.95) | ||
| Sigmoid colectomy | 6 (12.00) | 6 (10.17) | ||
| Radical resection of rectal cancer | 22 (44.00) | 30 (50.85) |
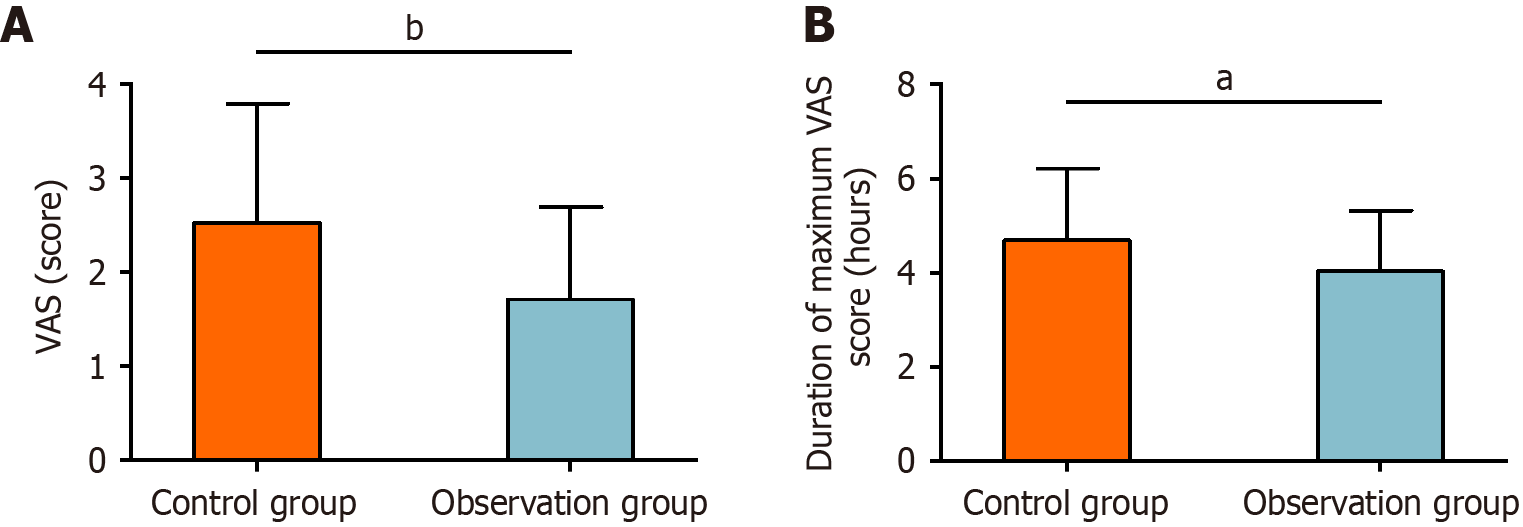
Postoperative pain levels were assessed using the VAS. The VAS in the control group was 2.52 ± 1.27 points, compared with 1.71 ± 0.98 points in the observation group. The duration of the maximum VAS score was 4.69 ± 1.53 hours in the control group and 4.03 ± 1.29 hours in the observation group. Statistical analysis showed significantly lower VAS points and shorter maximum VAS durations in the observation group than in the control group (P < 0.05, Figure 2).
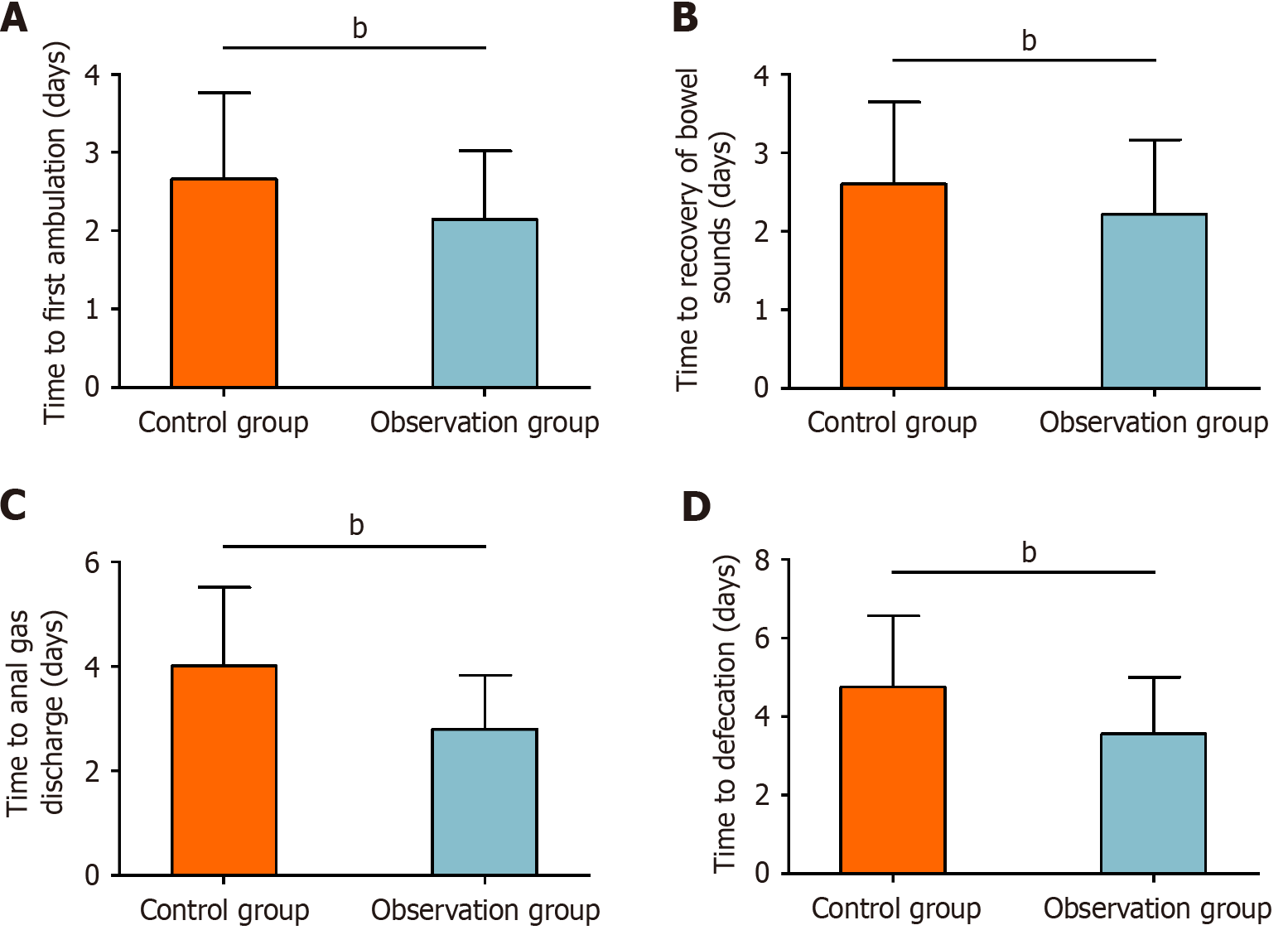
Functional recovery was evaluated by recording the time to first ambulation, bowel sound recovery, anal gas discharge, and defecation. The time to first ambulation was 2.66 ± 1.10 days in the control group and 2.15 ± 0.87 days in the observation group. The time to bowel sound recovery was 2.60 ± 1.05 days in the control group and 2.22 ± 0.95 days in the observation group. The time to anal gas discharge was 4.02 ± 1.49 days in the control group and 2.80 ± 1.03 days in the observation group. The time to first defecation was 4.76 ± 1.81 days in the control group and 3.56 ± 1.45 days in the observation group. All indicators of functional recovery were significantly improved in the observation group compared with the control group (P < 0.01, Figure 3).
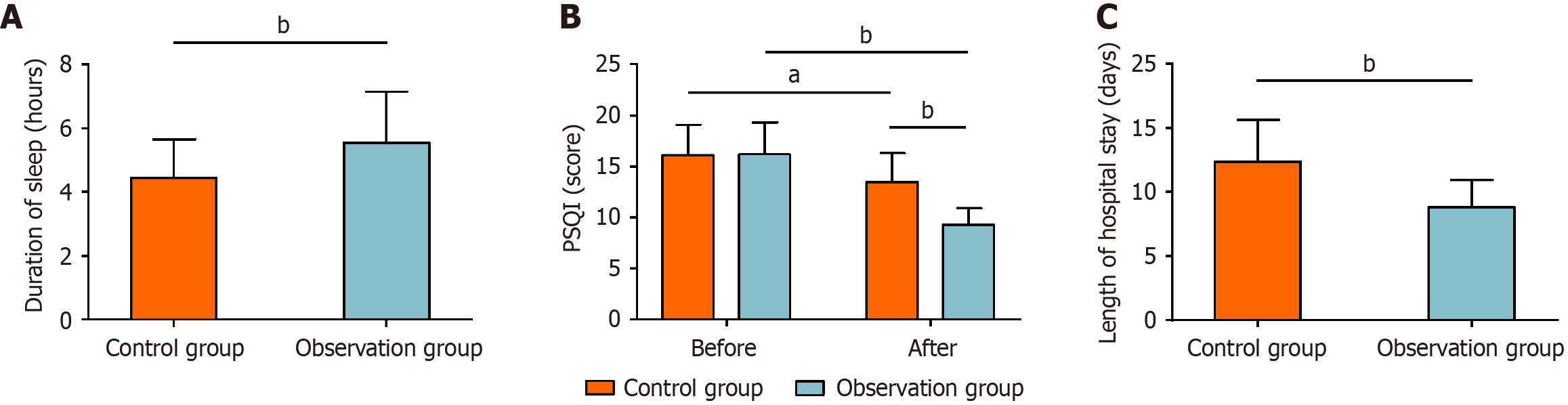
On postoperative day 3, the average sleep duration was 4.44 ± 1.21 hours in control group and 5.56 ± 1.59 hours in the observation group. Regarding PSQI scores, the pre-intervention values were 16.08 ± 3.02 points in the control group and 16.17 ± 3.18 points in the observation group. Post-intervention, PSQI scores decreased to 13.44 ± 2.87 points in the control group and 9.25 ± 1.64 points in the observation group. The average length of hospitalization was 12.38 ± 3.25 days in the control group and 8.81 ± 2.15 days in the observation group. Statistical analysis indicated that the observation group had significantly longer sleep duration on postoperative day 3 compared with the control group (P < 0.01), significantly lower PSQI scores post-intervention compared with both its baseline and the control group (P < 0.01), and significantly shorter hospitalization (P < 0.01). Detailed data are graphically presented in Figure 4.
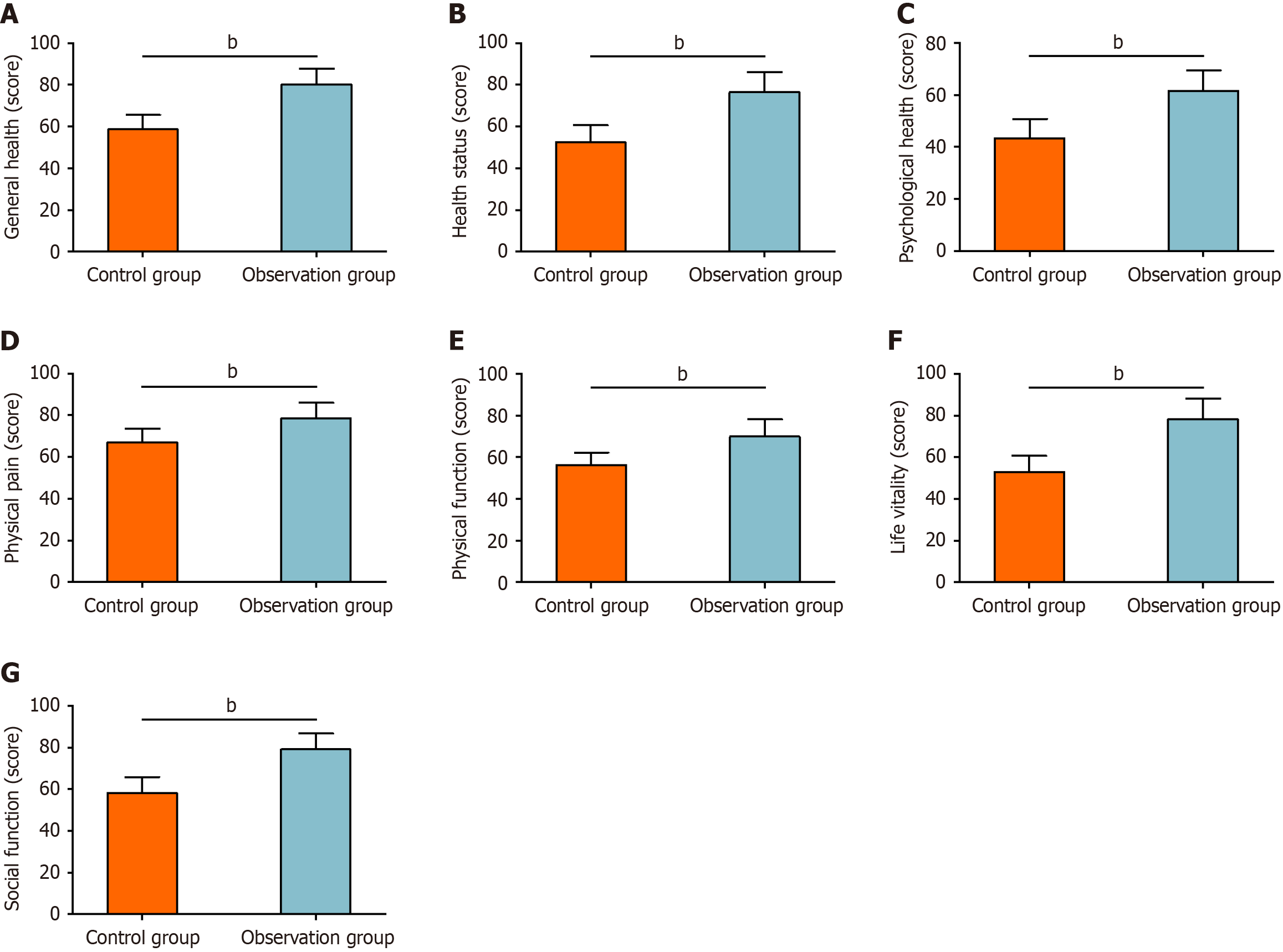
The quality of life was assessed using the SF-36. The general health scores were 58.62 ± 6.89 points in the control group and 80.22 ± 7.46 points in the observation group. The health status scores were 52.50 ± 8.11 points in the control group and 76.49 ± 9.58 points in the observation group. The mental health scores were 43.40 ± 7.34 points in the control group and 61.49 ± 7.78 points in the observation group. The physical pain scores were 66.96 ± 6.69 points in the control group and 78.49 ± 7.58 points in the observation group. The physical function scores were 56.34 ± 5.92 points in the control group and 69.95 ± 8.27 points in the observation group. The vitality scores were 53.00 ± 7.70 points in the control group and 78.34 ± 9.75 points in the observation group. Social functioning scores were 58.12 ± 7.74 points in the control group and 79.14 ± 7.60 points in the observation group. Statistical analysis demonstrated that SF-36 scores in all domains were significantly higher in the observation group than in the control group (P < 0.05, Figure 5).
The overall incidence of complications, including surgical site infection, pulmonary infection, abdominal distension/pain, and ileus, was significantly lower in the observation group than in the control group (P < 0.01, Table 2).
| Complications | Control group (n = 50) | Observation group (n = 59) | χ2 | P value |
| Surgical site infections | 1 (2.00) | 0 (0.00) | ||
| Pulmonary infections | 2 (4.00) | 0 (0.00) | ||
| Abdominal distension/pain | 3 (6.00) | 1 (1.69) | ||
| Ileus | 5 (10.00) | 2 (3.39) | ||
| Total | 11 (22.00) | 3 (5.08) | 6.918 | 0.009 |
Inadequate postoperative pain control in patients with CRC can impede functional recovery, foster chronic pain syndromes, and prolong hospitalization. Implementing ERAS protocols, which optimize analgesia and prevent postoperative complications, can significantly reduce pain levels and minimize the risk of adverse events, thereby facilitating patient recovery[18]. In this study, the observation group demonstrated significantly lower VAS scores and shorter maximum VAS than the control group, indicating superior analgesic efficacy with ERAS for patients undergoing radical resection for CRC compared with standard care. This benefit likely reflects comprehensive ERAS pain control: Rigorous monitoring, pain severity-based individualized analgesic dosing, and strong psychological support, which mitigates pain while enhancing patient adherence. Similarly, Cao et al[19] reported that ERAS intervention significantly reduced VAS scores in patients with CRC after laparoscopic radical resection and attenuated early postoperative inflammation, consistent with our findings.
Regarding functional recovery, ERAS patients achieved significantly earlier ambulation, faster bowel sound recovery, and accelerated anal gas discharge and defecation, suggesting accelerated postoperative recovery. Wang et al[20] similarly observed earlier first oral intake, anal exhaust, defecation, and bowel sound recovery after radical gastrectomy under ERAS protocols, along with significant pain alleviation and better quality of life. Additionally, our study demonstrated that the ERAS protocols increased the average sleep duration on postoperative day 3, lowered PSQI scores, and shortened hospitalization duration. These benefits may be attributed to standardized perioperative management and individualized care, rehabilitation training, psychological support, multimodal analgesia, dietary and medication guidance, and proactive complication prevention, which collectively enhance care quality while expediting recovery[21]. In a report on ERAS applications in patients with esophageal cancer, the ERAS protocol substantially improved patients’ sleep and quality of life while alleviating anxiety[22], supporting our findings. Improved sleep quality after CRC surgery accelerates recovery and benefits psychological well-being and overall quality of life[23].
Furthermore, the observation group scored significantly higher across all SF-36 domains, indicating that ERAS improves the quality of life after radical CRC resection. Consistent with our findings, Wu et al[24] reported that ERAS in elderly patients undergoing colon cancer surgery significantly improved Gastrointestinal Quality of Life Index scores and treatment satisfaction. Our study also showed a lower overall incidence of complications, including surgical site infection, pulmonary infection, abdominal distension/pain, and ileus, in patients under ERAS intervention, underscoring its superior clinical safety profile. In a study on the application of ERAS in patients undergoing colorectal surgery by Song et al[25], ERAS was found to be more advantageous in reducing postoperative complications, such as anastomotic leakage, wound infection, and intestinal obstruction, compared with conventional nursing, findings that align with our current research.
Several limitations warrant attention. First, this single-center study may limit generalizability; multicenter investigations would improve external validity. Second, the retrospective design introduces inherent selection bias due to non-randomized allocation, suggesting the need for randomized controlled trials. Third, the absence of long-term outcome assessments (e.g., chronic pain and recurrence rates) limits the assessment of sustained ERAS benefits; extended follow-up is required for deeper insights. Future work will be directed toward the development of these identified areas.
In summary, ERAS interventions optimize postoperative pain control in patients with CRC, significantly alleviate pain, accelerate functional recovery, improve sleep quality and overall quality of life, shorten hospitalization, and reduce the incidence of total postoperative complications.
| 1. | Jung KU, Kim HO, Kim H. Epidemiology, Risk Factors, and Prevention of Colorectal Cancer-An English Version. J Anus Rectum Colon. 2022;6:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Matsuda T, Fujimoto A, Igarashi Y. Colorectal Cancer: Epidemiology, Risk Factors, and Public Health Strategies. Digestion. 2025;106:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 3. | Bardelčíková A, Šoltys J, Mojžiš J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants (Basel). 2023;12:901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 132] [Reference Citation Analysis (0)] |
| 4. | Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina (Kaunas). 2023;59:1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 5. | Roshandel G, Ghasemi-Kebria F, Malekzadeh R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers (Basel). 2024;16:1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 184] [Reference Citation Analysis (1)] |
| 6. | Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y, Yoshida H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J Nippon Med Sch. 2022;89:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 145] [Reference Citation Analysis (2)] |
| 7. | Yue TM, Sun BJ, Xu N, Ohkuma R, Fowler C, Lee B. Improved Postoperative Pain Management Outcomes After Implementation of Enhanced Recovery After Surgery (ERAS) Protocol for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC). Ann Surg Oncol. 2024;31:3769-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Montroni I, Ugolini G, Saur NM, Rostoft S, Spinelli A, Van Leeuwen BL, De Liguori Carino N, Ghignone F, Jaklitsch MT, Kenig J, Garutti A, Zingaretti C, Foca F, Vertogen B, Nanni O, Wexner SD, Audisio RA; SIOG Surgical Task Force/ESSO GOSAFE Study Group. Predicting Functional Recovery and Quality of Life in Older Patients Undergoing Colorectal Cancer Surgery: Real-World Data From the International GOSAFE Study. J Clin Oncol. 2023;41:5247-5262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 9. | Tian Y, Li Q, Pan Y. Prospective study of the effect of ERAS on postoperative recovery and complications in patients with gastric cancer. Cancer Biol Med. 2021;19:1274-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Rosa F, Longo F, Pozzo C, Strippoli A, Quero G, Fiorillo C, Mele MC, Alfieri S. Enhanced recovery after surgery (ERAS) versus standard recovery for gastric cancer patients: The evidences and the issues. Surg Oncol. 2022;41:101727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Du J, Wu C, Li A, Chen J, Li Q, Wu X. Effectiveness of Enhanced Recovery After Surgery-Based Respiratory Function Exercise in Elderly Patients with Lung Cancer and its Effect on Postoperative Functional Recovery. Altern Ther Health Med. 2023;29:56-61. [PubMed] |
| 12. | Qiu J, Wang Y. Effect of accelerated rehabilitation combined with enteral nutrition on gastrointestinal function recovery after hepatectomy. Support Care Cancer. 2022;30:8927-8933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Sun XJ, Feng TC, Wang YM, Wang F, Zhao JB, Liu X, Li FL. The effect of the enhanced recovery after surgery protocol and the reduced use of opioids on postoperative outcomes in elderly patients with colorectal cancer. Eur Rev Med Pharmacol Sci. 2023;27:10053-10060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ, Lightner AL, Feingold DL, Paquette IM. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis Colon Rectum. 2022;65:148-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 15. | Åström M, Thet Lwin ZM, Teni FS, Burström K, Berg J. Use of the visual analogue scale for health state valuation: a scoping review. Qual Life Res. 2023;32:2719-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 16. | Gao X, Qiao Y, Chen Q, Wang C, Zhang P. Effects of different types of exercise on sleep quality based on Pittsburgh Sleep Quality Index in middle-aged and older adults: a network meta-analysis. J Clin Sleep Med. 2024;20:1193-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Wu Q, Chen Y, Zhou Y, Zhang X, Huang Y, Liu R. Reliability, validity, and sensitivity of short-form 36 health survey (SF-36) in patients with sick sinus syndrome. Medicine (Baltimore). 2023;102:e33979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 18. | Huang L, Zhang T, Wang K, Chang B, Fu D, Chen X. Postoperative Multimodal Analgesia Strategy for Enhanced Recovery After Surgery in Elderly Colorectal Cancer Patients. Pain Ther. 2024;13:745-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Cao L, Zhang L, Chen B, Yan L, Shi X, Tian L. Application of multimodal standardized analgesia under the concept of enhanced recovery after surgery in laparoscopic radical colorectal cancer surgery. Front Oncol. 2024;14:1381809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Wang X, Li Y, Yang W. Rapid Rehabilitation Program Can Promote the Recovery of Gastrointestinal Function, Speed Up the Postoperative Rehabilitation Process, and Reduce the Incidence of Complications in Patients Undergoing Radical Gastrectomy. J Oncol. 2022;2022:1386382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Shi C, Cai B, Huang X, Hou J. Effect of accelerated rehabilitation surgery nursing on laparoscopic radical surgery for elderly patients with colorectal cancer. Rev Assoc Med Bras (1992). 2022;68:958-962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Chen HM, Lin YY, Wu YC, Huang CS, Hsu PK, Chien LI, Lin YJ, Huang HL. Effects of Rehabilitation Program on Quality of Life, Sleep, Rest-Activity Rhythms, Anxiety, and Depression of Patients With Esophageal Cancer: A Pilot Randomized Controlled Trial. Cancer Nurs. 2022;45:E582-E593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Wang G, Pan S. The impact of sleep interventions combined with enhanced nutritional support on sleep quality, nutritional status, pain management, psychological well-being, and quality of life in postoperative colon cancer patients. J Cancer Res Clin Oncol. 2025;151:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Wu J, Wei C, Li F, Wang X, Sun F. The effect of comprehensive nursing on the recovery speed and prognosis of elderly colon cancer patients. Am J Transl Res. 2021;13:5491-5497. [PubMed] |
| 25. | Song JY, Cao J, Mao J, Wang JL. Effect of rapid rehabilitation nursing on improving clinical outcomes in postoperative patients with colorectal cancer. World J Gastrointest Surg. 2024;16:2119-2126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (12)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/