Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.106286
Revised: June 3, 2025
Accepted: August 4, 2025
Published online: September 27, 2025
Processing time: 169 Days and 0.2 Hours
Enhanced recovery after surgery (ERAS) protocols have emerged as a promising approach in perioperative care. This study evaluated ERAS's impact on gastro
To evaluate the impact of ERAS protocols on postoperative gastrointestinal func
We conducted a retrospective analysis of 80 patients who underwent laparoscopic D2 gastrectomy, comparing ERAS (n = 40) vs traditional care (n = 40). Primary outcomes included postoperative gastrointestinal function recovery and complications. Intestinal microbiota was analyzed using 16S rRNA sequencing at multiple timepoints perioperatively.
ERAS patients demonstrated faster recovery of bowel function, with earlier return of bowel sounds (16.25 ± 6.41 hours vs 22.3 ± 6.49 hours), first flatus (23.95 ± 6.02 hours vs 28.45 ± 7.12 hours), and defecation (34.95 ± 9.34 hours vs 48.1 ± 15.64 hours), all P < 0.05. Complication rates, including antibiotic-associated diarrhea and surgical site infections, were comparable between groups. Microbial diversity indices and probiotic populations showed better preservation in the ERAS group postoperatively (P < 0.05), though neither group achieved complete restoration to preoperative levels at one month.
These results support tailoring ERAS protocols to prioritize gut microbiome resilience through early feeding and shortened antibiotic courses, with particular benefits for younger patients.
Core Tip: Enhanced recovery after surgery significantly accelerates gastrointestinal recovery, as demonstrated by earlier return of bowel sounds, flatus, and defecation compared to traditional care. It also preserves microbiota diversity, main
- Citation: Lin XJ, Xu JZ, Hu Q, Chen J. Effects of enhanced recovery after surgery on postoperative intestinal function and intestinal flora during laparoscopic gastric cancer surgery. World J Gastrointest Surg 2025; 17(9): 106286
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/106286.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.106286
Laparoscopic gastric cancer surgery, while representing a significant advancement in surgical oncology, introduces complex challenges to patients' gastrointestinal function and overall recovery. The immediate postoperative period typically presents with reduced gastrointestinal motility, affecting 5%-30% of patients through delayed gastric emptying and temporary paralytic ileus within the first 48-72 hours. These early changes are accompanied by altered gut hormone secretion patterns, significantly impacting digestive function and nutritional status[1-4].
Recent advances in enhanced recovery after surgery (ERAS) implementation for laparoscopic gastrectomy have incorporated enhanced preoperative optimization strategies, including structured patient education programs, standardized preoperative carbohydrate loading, and risk-stratified prophylactic antiemetic protocols. Intraoperative management has evolved to emphasize opioid-sparing anesthesia techniques, precise fluid balance maintenance, and normothermia preservation. Postoperative care pathways now prioritize early mobilization protocols, systematic pain management using multimodal analgesia, and structured discharge criteria based on functional recovery milestones rather than arbitrary timeframes. It is referring to a new initiative that is a clinical consensus framework based on evi
The ERAS protocol represents a paradigm shift in perioperative care, with its impact on intestinal microbiota preservation becoming increasingly recognized as a critical factor in postoperative recovery. The superior maintenance of beneficial bacteria in ERAS protocols compared to traditional care can be attributed to several key mechanistic differences that fundamentally alter the gut microenvironment during the perioperative period. One of the most significant factors contributing to better probiotic preservation in ERAS protocols is the strategic approach to antibiotic administration. Traditional perioperative care typically extends prophylactic antibiotic coverage for 3-5 days postoperatively, based on historical practices aimed at preventing surgical site infections. However, this prolonged exposure creates substantial collateral damage to the intestinal microbiome, particularly affecting beneficial bacteria such as Lactobacillus and Bifidobacterium species. These gram-positive bacteria are inherently more susceptible to broad-spectrum antibiotics commonly used in surgical prophylaxis, including cephalosporins and fluoroquinolones[8-10].
As the patient gradually increases their recovery from the intermediate recovery phase, it is common that they will have an incremental return of peristalsis between 24-48 post-op hours. This is a momentous time for the individual as they encounter issues of nutritional absorption, complicated by anatomical changes and possible bacterial overgrowth of segments of the modified enteric system. Various surgical factors [anastomosis type (Billroth I, II, or Roux-en-Y), lymphadenectomy extent, operation duration, and intraoperative blood loss] strongly affect the amplitude of these alterations. One important variable with regard to digestive recovery after surgery is effects on intestinal microbiota. One of the earliest postoperative phenomenon includes a significant decrease in microbial diversity, with a dysbiosis that can facilitate pathogenic bacterial overgrowth. Patients typically adapt within 3-6 months, during which time the gut microbiota undergoes substantial composition. The n changes, establishing a new equilibrium in the process[11-14]. This time of adaptation can be important for what happens in the longer term, since the changes in the microbial landscape can affect, for instance, immune function and metabolic processes. In conclusion, postoperative intestinal dysfunction and dysbiosis have become an important issue in gastric cancer surgery and have a great impact on the recovery and quality of life of patients. Conventional preoperative care is frequently insufficient to control such complications. ERAS has the potential to influence these outcomes with its standardized protocols and deserves thorough investigation.
This study aims to systematically assess the effects of ERAS protocols on patients undergoing laparoscopic gastric cancer surgery, especially gastrointestinal recovery and microbiota. These findings will add to the evidence base behind ERAS implementation and help inform future refinements to protocols designed to improve postoperative recovery and patient quality of life.
This retrospective cohort study was conducted at Zhejiang Provincial Tongde Hospital and included 80 patients who underwent laparoscopic D2 gastrectomy for gastric cancer between August 2022 and December 2023. Patients were grouped based on the perioperative protocol received: 40 received ERAS care and 40 underwent conventional mana
This study established comprehensive eligibility requirements for participant enrollment. Suitable candidates were adults aged 18-70 years with primary gastric cancer, confirmed through pathological examination of gastroscopic biopsy spe
Several conditions precluded participation in the study. These included a history of major abdominal surgery, recent antibiotic exposure (within three months), and circumstances requiring emergency surgical intervention such as tumor-related complications (hemorrhage, obstruction, or perforation). Additionally, patients with pyloric obstruction or those deemed unsuitable for laparoscopic approaches were excluded from participation.
The perioperative management protocol incorporated multiple evidence-based interventions designed to optimize recovery. Preoperative preparation began with comprehensive patient education and prehabilitation strategies. Nu
The specific measures of traditional perioperative management are as follows: (1) Preoperative education and prerehabilitation treatment are not routine; (2) No diet for 12 hours before surgery, no oral sugar drinks for 2 hours before surgery; (3) Intestinal preparation, liquid diet 1 day before surgery, oral laxatives the night before surgery or the morning of the first day; (4) Antibiotics are used once before surgery (cefuroxime 1.5 g) and once more after surgery > 3 hours and are applied to the third day after surgery; (5) Routine use of opioid analgesics; (6) Getting out of bed after surgery according to the patient's tolerance and willingness; (7) Drinking water and eating food 3 days after surgery; (8) Routine use of nasogastric tubes; and (9) Noncontrolled infusion, postoperative daily fluid supplementation of approximately 2000-2500 mL.
This study monitored multiple indicators of gastrointestinal recovery and postoperative complications across both groups. Gastrointestinal function recovery was evaluated through systematic documentation of three key parameters: Initial return of bowel sounds, first occurrence of flatus, and time to first defecation. Surgical complications were tracked with particular attention to surgical site infections and the development of antibiotic-associated diarrhea. For the diagnosis of antibiotic-associated diarrhea, specific criteria were established: Patients experiencing 2-3 Loose stools daily for a brief duration, with other potential causes of diarrhea excluded.
Serial fecal samples (minimum 2 g) were collected at five specific timepoints: Preoperatively, immediately postoperatively, and at one week, two weeks, and one month after surgery. All specimens were immediately stored in sterile containers at -80 °C. Genomic DNA extraction was performed using a specialized fecal DNA extraction kit (Beijing Beitech Biotechnology Co., Ltd.), followed by 16S rRNA sequencing to analyze intestinal microbiota composition, with particular focus on probiotic populations including Bifidobacterium and Lactobacillus species. The analysis utilized Illumina sequencing technology targeting the V3 hypervariable region of 16S rRNA. Raw sequencing data underwent systematic processing, including read concatenation to form clean data and subsequent tag generation. After redundancy elimination, operational taxonomic units were calculated and taxonomically classified in descending order of abundance. Microbial diversity was evaluated using two complementary indices. The Chao1 index provided species richness estimates based on observed species counts, with higher values indicating greater species abundance. The Shannon index incorporated both abundance and evenness metrics, reflecting both species quantity and their relative distribution within the community. Higher Shannon index values signified greater microbial diversity, offering a comprehensive assessment of community structure.
SPSS 23.0 statistical software was used for data analysis. Measurement data conforming to normal distribution were represented as mean ± SD, and comparisons between groups were performed using two independent samples t-test. Measurement data that did not conform to normal distribution were represented by median (range), and rank sum test was used for comparisons between groups. Categorical variables were represented by frequency and percentage, and χ2 test was used for comparisons between groups. Statistical comparisons employed Wilcoxon rank-sum tests for Shannon and Chao1 diversity indices, PERMANOVA for microbiome composition analysis, and Mann-Whitney U tests for between-group comparisons at each time point. All reported P values reflect Bonferroni-corrected values, with significance indicated as: P < 0.05, reference, P < 0.01, significant after correction, and P < 0.001, highly significant.
This study compared the clinical characteristics of gastric cancer patients between the ERAS group (n = 40) and tra
| Baseline information | ERAS group (n = 40) | Traditional treatment group (n = 40) | P value |
| Age (year) | 61.81 ± 4.62 | 60.21 ± 5.12 | 0.41 |
| Gender (male/female) | 32/8 | 34/6 | 0.45 |
| BMI (kg/m2) | 24.32 ± 3.13 | 23.31 ± 2.82 | 0.71 |
| Tumor site | 0.79 | ||
| Upper part of stomach | 5 | 2 | - |
| Mid stomach | 10 | 10 | - |
| Lower part of stomach | 25 | 28 | - |
| Surgical time (minute) | 220.71 ± 30.41 | 232.73 ± 32.01 | 0.61 |
| Tumor size (cm) | 3.81 ± 1.72 | 3.91 ± 1.66 | 0.59 |
| Degree of tumor differentiation | 0.66 | ||
| Well differentiated | 2 | 3 | - |
| Moderately differentiated | 15 | 16 | - |
| Poorly differentiated | 20 | 18 | - |
| Signet-ring cell carcinoma | 2 | 3 | - |
| Mucinous cell carcinoma | 1 | 0 | - |
| Lauren classification | |||
| Intestinal | 28 | 30 | - |
| Diffuse | 5 | 7 | - |
| Mixed type | 7 | 3 | - |
| TNM stage | |||
| I | 2 | 3 | - |
| II | 13 | 14 | - |
| III | 25 | 23 | - |
| IV | 0 | 0 | - |
This study compared the postoperative recovery indicators between the ERAS group and traditional treatment group. The results showed that the ERAS group demonstrated significant advantages in all assessment parameters (all P values < 0.01): In terms of postoperative gastrointestinal function recovery, the ERAS group showed faster improvement: First bowel sounds appeared earlier (16.25 ± 6.41 hours vs 22.30 ± 6.49 hours), first exhaust time was shorter (23.95 ± 6.02 hours vs 28.45 ± 7.12 hours), and first defecation time was earlier (34.95 ± 9.34 hours vs 48.10 ± 15.64 hours) compared to the traditional treatment group. Regarding postoperative rehabilitation indicators, the ERAS group also performed ex
| Characteristic | ERAS group (n = 40) | Traditional treatment group (n = 40) | P value |
| Number of cases | 40 | 40 | - |
| First occurrence time of bowel sounds (hours) | 16.25 ± 6.41 | 22.30 ± 6.49 | < 0.0001 |
| First exhaust time (hours) | 23.95 ± 6.02 | 28.45 ± 7.12 | 0.003 |
| First defecation time (hours) | 34.95 ± 9.34 | 48.10 ± 15.64 | < 0.0001 |
| Postoperative hospitalization time (days) | 6.2 ± 1.5 | 8.1 ± 2.0 | < 0.001 |
| Postoperative pain score (VAS, 24 hours) | 2.5 ± 1.0 | 3.8 ± 1.2 | < 0.001 |
| Postoperative nutritional support duration (days) | 2.1 ± 0.8 | 4.5 ± 1.2 | < 0.001 |
| Postoperative urinary catheter duration (days) | 1.2 ± 0.4 | 2.5 ± 0.8 | < 0.001 |
| Postoperative intravenous fluid volume (L) | 2.0 ± 0.5 | 3.2 ± 0.8 | < 0.001 |
| Postoperative oral intake time (hours) | 20.5 ± 5.0 | 30.0 ± 6.0 | < 0.001 |
This study compared muscle strength recovery between the ERAS and traditional treatment groups. The baseline muscle strength was comparable between the two groups preoperatively (ERAS group: 170.21 ± 44.82 vs traditional group: 168.90 ± 41.44, P = 0.89). However, significant differences emerged during the postoperative recovery period. The ERAS group demonstrated consistently superior performance at all postoperative time points. At the first postoperative visit, the ERAS group maintained higher muscle strength (89.71 ± 30.12 vs 63.28 ± 25.29, P < 0.01). This advantage continued through one week (107.91 ± 25.15 vs 76.45 ± 23.37, P < 0.01), two weeks (119.51 ± 25.66 vs 97.03 ± 22.64, P < 0.01), and one month after surgery (139.23 ± 32.16 vs 122.90 ± 31.45, P = 0.02).
When examining the decline in muscle strength from preoperative levels, the ERAS group showed significantly less reduction compared to the traditional group. The decrease in muscle strength was notably smaller in the ERAS group at the first postoperative visit (80.50 ± 21.84 vs 105.60 ± 23.52, P < 0.01), one week (62.31 ± 33.96 vs 92.43 ± 27.99, P < 0.01), two weeks (50.68 ± 27.74 vs 71.85 ± 26.12, P < 0.01), and one month after surgery (30.98 ± 20.63 vs 46.03 ± 19.98, P < 0.01, Table 3).
| Time point | ERAS group (n = 40) | Traditional treatment group (n = 40) | P value |
| Preoperative | 170.21 ± 44.82 | 168.90 ± 41.44 | 0.89 |
| First postoperative visit | 89.71 ± 30.12 | 63.28 ± 25.29 | < 0.01 |
| 1 week after surgery | 107.91 ± 25.15 | 76.45 ± 23.37 | < 0.01 |
| 2 weeks after surgery | 119.51 ± 25.66 | 97.03 ± 22.64 | < 0.01 |
| 1 month after surgery | 139.23 ± 32.16 | 122.90 ± 31.45 | 0.02 |
| Preoperative-first postoperative session | 80.50 ± 21.84 | 105.60 ± 23.52 | < 0.01 |
| Preoperative-1 week postoperatively | 62.31 ± 33.96 | 92.43 ± 27.99 | < 0.01 |
| Preoperative-2 weeks postoperatively | 50.68 ± 27.74 | 71.85 ± 26.12 | < 0.01 |
| Preoperative-1 month postoperatively | 30.98 ± 20.63 | 46.03 ± 19.98 | < 0.01 |
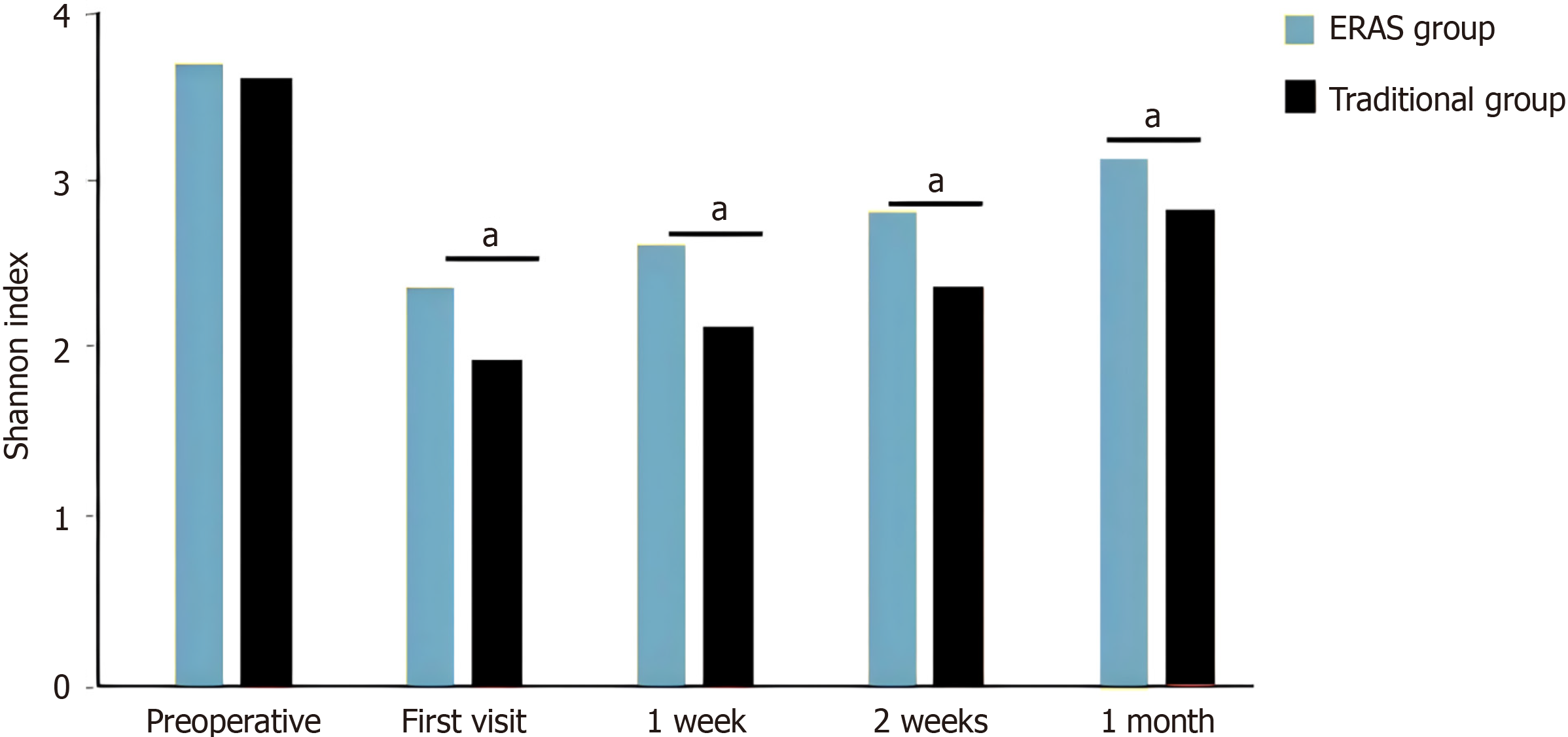
This study compared changes in gut microbiota diversity (Shannon index) during the perioperative period between the ERAS and traditional treatment groups. Results showed that preoperative baseline Shannon index levels were comparable between the two groups (ERAS group: 3.13 ± 0.44 vs traditional group: 3.08 ± 0.40, P = 0.61), indicating good comparability between groups.
During postoperative recovery, the ERAS group demonstrated significant advantages. At the first postoperative visit, the Shannon index in the ERAS group was significantly higher than the traditional group (2.29 ± 0.38 vs 2.00 ± 0.31, P = 0.0004). This advantage persisted at 1 week (2.57 ± 0.28 vs 2.11 ± 0.43, P < 0.0001), 2 weeks (2.60 ± 0.37 vs 2.43 ± 0.34, P = 0.03), and 1 month (2.78 ± 0.26 vs 2.60 ± 0.32, P = 0.006) after surgery. When compared to preoperative baseline levels, the ERAS group showed significantly smaller decreases in Shannon index than the traditional group: Decrease at first postoperative visit (0.84 ± 0.15 vs 1.08 ± 0.25, P < 0.0001), decrease at 1 week postoperatively (0.56 ± 0.34 vs 0.97 ± 0.50, P < 0.0001), decrease at 2 weeks postoperatively (0.53 ± 0.14 vs 0.65 ± 0.24, P = 0.003), and decrease at 1 month postoperatively (0.35 ± 0.33 vs 0.48 ± 0.22, P = 0.003, Figure 1, P < 0.05).
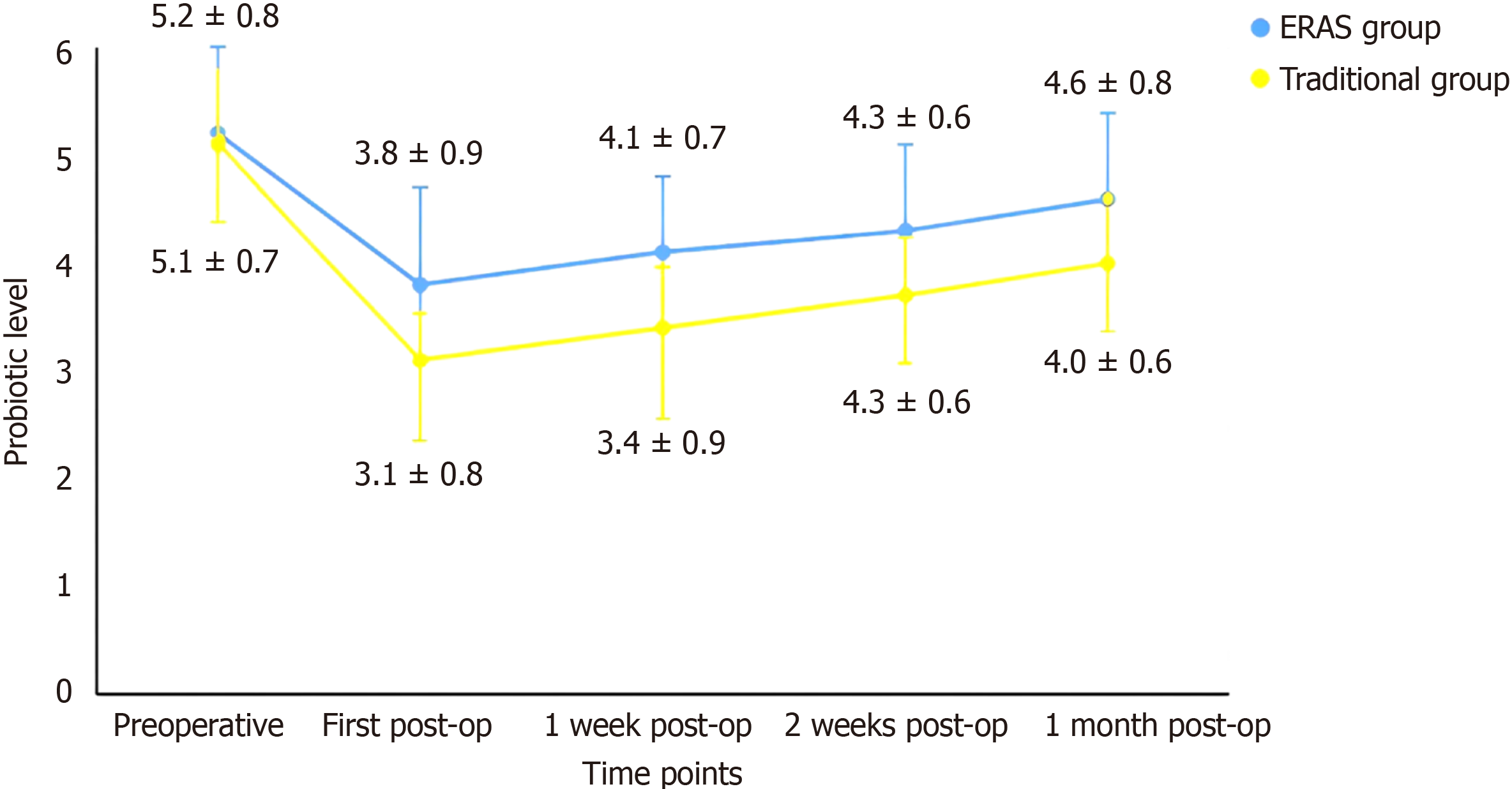
There was no significant difference in the proportion of probiotics (Lactobacillus acidophilus and Bifidobacterium) in the perioperative intestinal flora between the two groups before surgery (P > 0.05). At one week, one week, two weeks and one month after surgery, the ERAS group had greater scores than did the traditional group did (P < 0.05). At each time point after surgery, the traditional group had significantly lower scores than the ERAS group did, and the first postoperative decrease was the largest (P < 0.05). One month after surgery, neither group recovered to the proportion of probiotics before surgery (Figure 2).
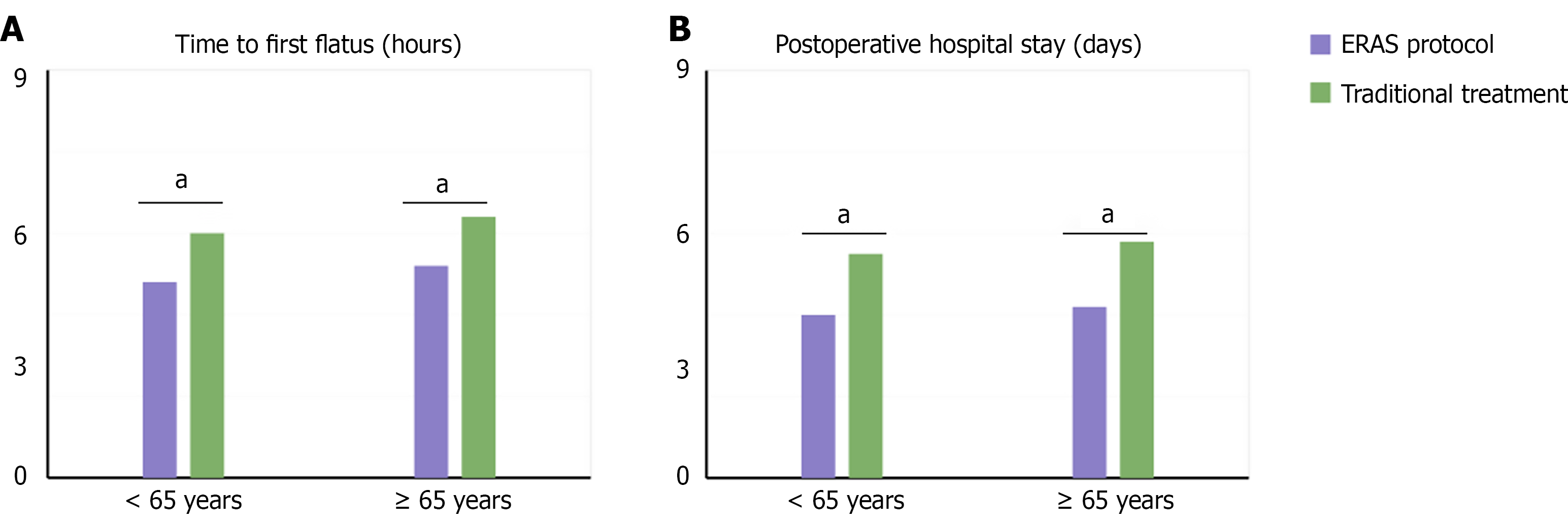
Patients were stratified by median age (61 years) into ≤ 61 years (n = 42) and > 61 years (n = 38) groups for subgroup analysis. In the ≤ 61 years group, the ERAS protocol showed significant advantages over traditional treatment in time to first flatus (22.15 ± 5.82 hours vs 27.25 ± 6.92 hours, P < 0.001) and postoperative hospital stay (5.8 ± 1.3 days vs 7.9 ± 1.8 days, P < 0.001). In the > 61 years group, while the ERAS protocol still demonstrated clear benefits, the magnitude of improvement was less pronounced than in the younger group: Time to first flatus (25.75 ± 6.22 hours vs 29.65 ± 7.32 hours, P = 0.002) and postoperative hospital stay (6.6 ± 1.7 days vs 8.3 ± 2.2 days, P = 0.001). Multivariate analysis identified age ≤ 61 years [hazard ratio (HR) = 1.85, 95%CI: 1.32-2.59, P = 0.001] as an independent predictor of better outcomes with the ERAS protocol (Figure 3).
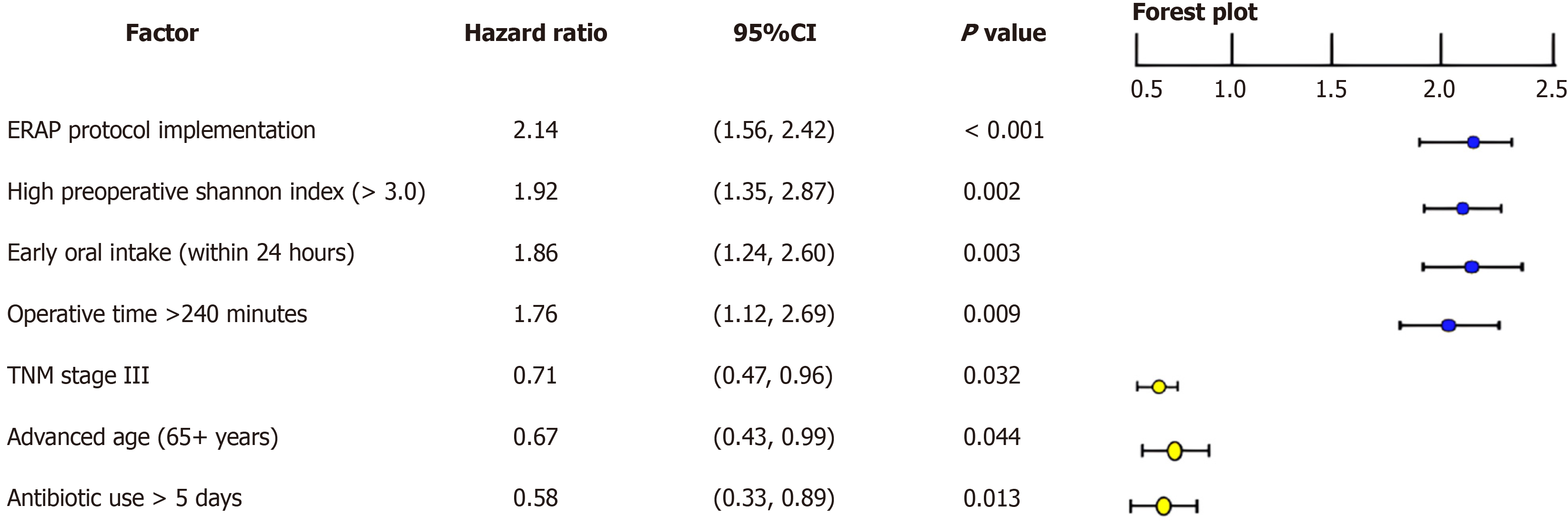
Cox proportional hazards regression model was used to analyze factors influencing postoperative recovery of intestinal flora diversity. Multivariate analysis revealed that implementation of ERAS protocol (HR = 2.31, 95%CI: 1.56-3.42, P < 0.001), high preoperative Shannon index (> 3.0) (HR = 1.92, 95%CI: 1.28-2.87, P = 0.002), operative time < 240 minutes (HR = 1.76, 95%CI: 1.15-2.69, P = 0.009), and early oral intake (within 24 hours postoperatively) (HR = 1.89, 95%CI: 1.24-2.88, P = 0.003) were independent protective factors promoting postoperative recovery of intestinal flora diversity. Conversely, advanced age (> 61 years) (HR = 0.67, 95%CI: 0.45-0.99, P = 0.044), postoperative antibiotic use > 3 days (HR = 0.58, 95%CI: 0.38-0.89, P = 0.013), and TNM stage III (HR = 0.71, 95%CI: 0.47-0.96, P = 0.035) were identified as adverse factors (Figure 4). Correlation analysis showed early feeding (within 24 hours) had the strongest association with microbiota preservation (r = 0.64, P < 0.001), followed by reduced antibiotic duration (r = 0.58, P < 0.001) and opioid-sparing analgesia (r = 0.41, P < 0.01). Early nutrition appears most critical for maintaining beneficial bacteria.
ERAS protocols have emerged as a transformative approach in perioperative care, particularly demonstrating significant benefits in maintaining intestinal flora diversity. This evidence-based methodology encompasses multiple key strategies: Minimizing unnecessary intestinal preparation, implementing strict controls on antibiotic administration, reducing preoperative fasting periods, and facilitating early postoperative oral intake while limiting opioid usage[15,16]. The implementation of these protocols represents a paradigm shift from traditional perioperative management, with mounting evidence suggesting improved patient outcomes across various metrics, including reduced length of hospital stay, decreased complication rates, and enhanced patient satisfaction.
Routine intestinal preparation is no longer done based on contemporary scientific knowledge. We now know that many of our intestinal bacteria live with us in a symbiotic relationship, providing important services that help us with nutrient absorption and vitamin synthesis. ERAS protocols avoid the use of mechanical bowel preparation and osmotic laxatives, which results in the preservation of intestinal flora and stabilization of mucosal barrier function; this is further supported by lower occurrence of serum inflammatory factors and effector T-cells after surgery[17-19]. Moreover, it has been shown that preserving populations of natural intestinal flora is important for local immune function, which prevents opportunistic infections. The removal of an everyday bowel prep has explicably translated to less patient pain and a better pre-op hydration position, leading to better outcomes.
Antibiotic stewardship is another important part of ERAS[20-22]. Traditional methods would often extend this coverage for 3-5 days or even a week after surgery, while ERAS guidelines restrict antibiotic use to 24 hours post-operatively. This restriction promotes faster recovery of intestinal function and a higher diversity of microbiota, without increasing the rate of infection of the surgical site. A shorter duration of antibiotics reduces the risk of developing antibiotic-resistant strains of bacteria and a Clostridioides difficile infection, both of which can happen with prolonged antibiotic treatment. Recent meta-analyses have confirmed that this strategy achieves equivalent or better prevention of infection with fewer adverse effects of prolonged antibiotic exposure on the intestinal microbiome[23,24].
The ERAS protocol highlights the importance of reducing preoperative fasting and stimulating postoperative early nutrition for several advantages. This practice, facilitated by preoperative glucose-saline solutions and early post-operative oral intake, preserves intestinal mucosal cell energy metabolism, restores the functional integrity of the intestinal barrier, and assists in the maintenance of healthy intestinal health[18,19,25]. In addition to that, preference of the protocol for non-steroidal anti-inflammatory drugs instead of opiates prevents intestinal paralysis and provides earlier functional recovery. Freeing the gastrointestinal system from a continuous metabolic burden has many other effects that go beyond the gastrointestinal system, including better insulin sensitivity, less catabolism, increased efficiency in wound healing and the benefits are many. Early enteral nutrition has been indicated to activate intestinal peristalsis, promote intestinal hormone release, induce the sustained maintenance of beneficial gut flora, etc.
Although ERAS principles were originally developed for colorectal surgery, they have gradually been implemented across surgical specialties. Gastric cancer operations are relatively complex with many risks, like anastomotic leakage, and the precision of ERAS in gastric surgery has proven to be safe and effective[26-28]. Studies have proved its effectiveness in decreasing patient stress and speeding up post-op rehabilitation, representing a leap forward in perioperative care protocols. This experience has allowed adaptation of ERAS protocols for the numerous surgical procedures resulting in the incorporation of clinic-specific guidelines accounting for the detailed aspects of varied types of operation. All of these adaptations are still rooted in the same principles but addresses the procedural specific concerns and risks.
Through this comprehensive approach, ERAS protocols effectively optimize surgical outcomes while maintaining the delicate balance of intestinal microbiota, ultimately contributing to improved patient recovery and reduced postoperative complications[29,30]. The success of ERAS implementation has sparked ongoing research into further refinements and adaptations of the protocol. The impact of specific probiotic supplementation, and the potential benefits of personalized protocol modifications based on patient characteristics and surgical requirements. The integration of new technologies, such as minimally invasive surgical techniques and enhanced monitoring systems, continues to evolve alongside ERAS protocols, suggesting potential for even greater improvements in surgical outcomes.
The economic implications of ERAS implementation have also become increasingly apparent, with studies de
These results support tailoring ERAS protocols to prioritize gut microbiome resilience through early feeding and shortened antibiotic courses, with particular benefits for younger patients. This microbiome-focused approach represents an advancement in gastric surgery ERAS implementation.
| 1. | Chen G, Xie Y, Yang B, Tan J, Zhong G, Zhong L, Zhou S, Han F. Artificial intelligence model for perigastric blood vessel recognition during laparoscopic radical gastrectomy with D2 lymphadenectomy in locally advanced gastric cancer. BJS Open. 2024;9:zrae158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Choi S, Kinoshita T, Obama K, Sakurai K, Kubo N, Ikoma N, Guner A, Kim HI. Enhanced recovery after laparoscopic distal gastrectomy using articulating laparoscopic instruments in older adults with gastric cancer: a retrospective analysis of prospectively collected data. Ann Surg Treat Res. 2025;108:86-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hyung WJ, Woo Y, Noh SH. Robotic surgery for gastric cancer: a technical review. J Robot Surg. 2011;5:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Sugimura K, Motoori M, Kentaro K, Yamamoto K, Takeno A, Hara H, Hamakawa T, Murakami K, Nakahara Y, Masuzawa T, Omori T, Kurokawa Y, Fujitani K, Doki Y. Comparison of laparoscopic and open gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a propensity score matching analysis. Surg Endosc. 2025;39:2304-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Tian Y, Lin Y, Sun C, Lowe S, Bentley R, Yang P, Guo H, Ding P, Zhang Z, Wang D, Zhao X, Li Y, Zhao Q. Comparison of short-term efficacy and safety between total robotic and total 3D laparoscopic distal radical gastrectomy for gastric cancer in Enhanced Recovery After Surgery (ERAS) protocol: a propensity score matching study. J Robot Surg. 2023;17:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Xu L, Yao L, Qin J, Xu H. Efficacy of multimodal analgesia based on the concept of enhanced recovery after surgery in laparoscopic radical gastrectomy for gastric cancer. Pak J Med Sci. 2024;40:2190-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Yuan W, Huang G, Dai P, Zhong Y, Ai Q, Liao Q. Application of enhanced recovery after surgery in perioperative patients undergoing laparoscopic surgery for gastric cancer: A meta-analysis. Medicine (Baltimore). 2023;102:e32962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Cui P, Kong C, Wang P, Wang S, Lu S. The Potential Risk Factors for Prolonged Length of Stay Despite an Enhanced Recovery After Surgery Protocol for Elderly Patients Undergoing Short-Level Lumbar Fusion Surgery. Geriatr Orthop Surg Rehabil. 2022;13:21514593221144179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Tyagi N. Nurse-led Enhanced Recovery after Surgery Programs: Potential Solution to Shorten Postoperative Recovery. Indian J Crit Care Med. 2025;29:3-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Zeng B, Deng ZH, Wu L, Zhang J. Utilization of an Enhanced Recovery After Surgery for Older Patients With a Hip Fracture: Current Applications and Potential Challenges. J Clin Nurs. 2024;33:4862-4864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Aoyama Y, Matsunobu Y, Etoh T, Suzuki K, Fujita S, Aiba T, Fujishima H, Empuku S, Kono Y, Endo Y, Ueda Y, Shiroshita H, Kamiyama T, Sugita T, Morishima K, Ebe K, Tokuyasu T, Inomata M. Correction: Artificial intelligence for surgical safety during laparoscopic gastrectomy for gastric cancer: Indication of anatomical landmarks related to postoperative pancreatic fistula using deep learning. Surg Endosc. 2024;38:6203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Aoyama Y, Matsunobu Y, Etoh T, Suzuki K, Fujita S, Aiba T, Fujishima H, Empuku S, Kono Y, Endo Y, Ueda Y, Shiroshita H, Kamiyama T, Sugita T, Morishima K, Ebe K, Tokuyasu T, Inomata M. Artificial intelligence for surgical safety during laparoscopic gastrectomy for gastric cancer: Indication of anatomical landmarks related to postoperative pancreatic fistula using deep learning. Surg Endosc. 2024;38:5601-5612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Long VD, Thong DQ, Dat TQ, Nguyen DT, Hai NV, Quoc HLM, Anh NVT, Vuong NL, Bac NH. Risk factors of postoperative complications and their effect on survival after laparoscopic gastrectomy for gastric cancer. Ann Gastroenterol Surg. 2024;8:580-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Morimoto Y, Sakuramoto S, Sugita H, Nishibeppu K, Ebara G, Fujita S, Fujihata S, Oya S, Miyawaki Y, Sato H, Yamashita K. Low incidence of postoperative infectious complications following laparoscopic distal gastrectomy for locally advanced gastric cancer in older adult patients above 75 years: Propensity score-matched comparison with open distal gastrectomy. Asian J Endosc Surg. 2024;17:e13371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Lui GCY, Lai CKC. Community acquired pneumonia due to antibiotic resistant- Streptococcus pneumoniae : diagnosis, management and prevention. Curr Opin Pulm Med. 2025;31:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Wei LC, Chiu HJ. Repurposing selective serotonin reuptake inhibitors for wound infection management: Expanding the scope of non-antibiotic therapeutics. Wound Repair Regen. 2025;33:e70006. [PubMed] [DOI] [Full Text] |
| 17. | Huang L, Hu Y, Chen J. Effectiveness of an ERAS-based exercise-nutrition management model in enhancing postoperative recovery for thoracoscopic radical resection of lung cancer: A randomized controlled trial. Medicine (Baltimore). 2024;103:e37667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Müller J, Wiesenberger R, Kaufmann M, Weiß C, Ghezel-Ahmadi D, Hardt J, Reißfelder C, Herrle F. Motivational Interviewing improves postoperative nutrition goals within the Enhanced Recovery after Surgery (ERAS®) pathway in elective bowel surgery - A randomized clinical pilot trial. Clin Nutr ESPEN. 2024;61:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Wang Z, Peng W, Zhang J. The Effect of Early Enteral Nutrition under the ERAS Model on Gastrointestinal and Immune Function Recovery in Patients Undergoing Gastric Tumor Surgery. Ann Ital Chir. 2024;95:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Iqbal A, Rehman HU, Ahmad I, Bibi S, Safdar H, Ali L, Hameed QM. OPTIMIZING POSTOPERATIVE OUTCOMES: ASSESSING THE EFFECT OF ENHANCED RECOVERY AFTER SURGERY (ERAS) PROTOCOLS IN GENERAL SURGICAL PATIENTS. J Ayub Med Coll Abbottabad. 2024;36:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Kifle F, Kenna P, Daniel S, Maswime S, Biccard B. A scoping review of Enhanced Recovery After Surgery (ERAS), protocol implementation, and its impact on surgical outcomes and healthcare systems in Africa. Perioper Med (Lond). 2024;13:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Mao W, Jiang X, Zeng X, Ye D. Surgical pathway for HIVinfected patients based on the ERAS strategy (Review). Med Int (Lond). 2024;4:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Chi X, Sun X, Cheng D, Liu S, Q Pan C, Xing H. Intestinal microbiome-targeted therapies improve liver function in alcohol-related liver disease by restoring bifidobacteria: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1274261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Dean YE, Shebl MA, Doma M, Elmezayen RW, Loayza Pintado JJ, Rouzan SS, Hassan NAIF, Yaqout YE, Tokunaga A, Anozie C, ElKoumi O, Elawady SS, Mady T, Nizam SN, Etman Y, Nizam R, Hazimeh Y, Alazmy M, Aiash H. Intestinal microbiome as a diagnostic marker of coronary artery disease: a systematic review and meta-analysis. Ann Med Surg (Lond). 2024;86:6105-6120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 25. | Wang X, Luo L, Zhang D, Wang J, Ning X, Lin Y, Ke X, Li G. Factors Associated with Nutritional Risk in Patients with Pulmonary Tuberculosis and Structural Lung Disease: A Hospital-Based Cross-Sectional Study. J Multidiscip Healthc. 2022;15:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Han H, Wan R, Chen J, Fan X, Zhang L. Effects of the enhanced recovery after surgery (ERAS) protocol on the postoperative stress state and short-term complications in elderly patients with colorectal cancer. Cancer Rep (Hoboken). 2024;7:e1979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Shoucair S, Alnajjar S, Sattari A, Almanzar A, Lisle D, Gupta VK. Impact of Surgical Resident Education and EMR Standardization in Enhancing ERAS Adherence and Outcomes in Colorectal Surgery. J Surg Educ. 2024;81:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Tolmay S, Rahiri JL, Snoep K, Fewster G, Kee R, Lim Y, Watson B, Richter KK. Lessons following implementation of a colorectal enhanced recovery after surgery (ERAS) protocol in a rural hospital setting. ANZ J Surg. 2024;94:910-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Aleid A, Alyaseen EM, Alfurayji RS, Alanazi BS, Alquraish FA, Al Mutair A, Alessa M, Albinsaad L. Enhanced Recovery After Surgery (ERAS) in Saudi Arabian Surgical Practice: A Comprehensive Analysis of Surgical Outcomes, Patient Satisfaction, and Cost-Effectiveness. Cureus. 2023;15:e49448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Flynn BC, Shelton K. On the 2024 Cardiac Surgical Enhanced Recovery After Surgery (ERAS) Joint Consensus Statement. J Cardiothorac Vasc Anesth. 2024;38:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/