Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.105134
Revised: April 3, 2025
Accepted: July 30, 2025
Published online: September 27, 2025
Processing time: 254 Days and 19 Hours
Percutaneous transhepatic biliary drainage (PTBD) is one of the primary clinical treatment options for patients with obstructive jaundice. In recent years, PTBD assisted by three-dimensional (3D) reconstruction technology has been widely implemented, but its advantages over traditional methods remains inconclusive. Thus, a discussion is warranted.
To explore the safety and efficacy of 3D reconstruction technology-assisted PTBD.
We systematically searched the databases including the Cochrane Library, PubMed, EMBASE, Web of Science and China National Knowledge Infras
A total of 15 studies were included, involving 1434 patients. The results of the meta-analysis showed that compared with the traditional group, the overall post-operative complications rate in the 3D reconstruction technology group was significantly lower [odds ratio = 0.25; 95% confidence interval (CI): 0.17-0.36, P < 0.00001]. The overall puncture success rate in the 3D reconstruction group was better than those in the traditional group (odds ratio = 3.61; 95%CI: 1.98-6.55, P < 0.0001). However, there was no significant difference between the two groups in the reduction levels of postoperative total bilirubin (mean difference = -1.38; 95%CI: -3.29 to 0.53, P = 0.16). Subgroup analysis were conducted on the surgery time according to guidance stages of the 3D recon
3D reconstruction technology significantly improves the puncture success rate and safety of PTBD. However, it has no significant advantage in bile drainage effectiveness. Continued research is warranted to further explore its clinical value and optimize its application.
Core Tip: This meta-analysis of 15 studies demonstrates that three-dimensional reconstruction technology significantly improves the safety and puncture success rate of percutaneous transhepatic biliary drainage compared to traditional methods. Although no significant advantage in bile drainage efficacy was observed, the technology exhibits critical clinical value by reducing complication risks through precise preoperative planning and real-time intraoperative navigation. Future large-scale studies, particularly stratified studies based on biliary anatomy and disease subtypes, are required to clarify its applicability across diverse clinical scenarios, thereby advancing standardized protocols and widespread clinical adoption of this in
- Citation: Chen ZH, Zhang LJ, Lin ZX, Lin SX, Song ZF, Wu ZJ, Lin W. Safety and efficacy of three-dimensional reconstruction technology-assisted percutaneous transhepatic biliary drainage: A meta-analysis. World J Gastrointest Surg 2025; 17(9): 105134
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/105134.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.105134
The accumulation of bile not only increases the pressure of intrahepatic and extrahepatic biliary tract, causing liver function damage, but also excessive bilirubin entering the bloodstream can affect the normal function of other organs, and even cause organ failure[1]. Research has shown that actively reducing jaundice and drainage is of great significance in improving the prognosis of patients with obstructive jaundice[2].
Since Glenn et al[3] first reported percutaneous transhepatic biliary drainage (PTBD) in 1962, this procedure has continued to mature with the advancement of modern technology. Due to its low anesthesia requirements, convenient operation, and flat learning curve, it has become the preferred clinical treatment, especially for end-stage patients in poor general condition who cannot tolerate general anesthesia[4]. Accurate positioning, precise guidance, and effective drainage are crucial for PTBD. However, traditional methods are limited in these aspects[5]. Studies have indicated that the risk of postoperative complications is higher with traditional methods, particularly in terms of postoperative bleeding and biliary fistula, etc[6].
Three-dimensional (3D) reconstruction technology involves reprocessing traditional imaging data to create individualized models. Based on these models, it enables preoperative localization and simulation, as well as real-time intraoperative monitoring and navigation, etc[7,8]. In recent years, 3D reconstruction technology has been widely applied in PTBD due to its advantages of multi-angle, intuitive, scientific, and precise imaging. However, whether 3D reconstruction technology is significantly superior to traditional methods in clinical practice remains unclear. Current controversies primarily focus on three key aspects: (1) Whether it can significantly improve puncture success rate; (2) Whether it can effectively reduce post-operative complication rates; and (3) Whether it can enhance biliary drainage efficacy. Notably, existing studies demonstrate marked heterogeneity in aspects such as inclusion criteria, statistical methods, and sample sizes. Additionally, there is a lack of high-quality evidence derived from meta-analysis. Therefore, this study aims to synthesize the existing evidence through a meta-analysis, provide a reference framework for clinical decision-making, and offer guidance for future research directions.
Two independent researchers conducted a systematic literature search in PubMed, EMBASE, Cochrane Library and China National Knowledge Infrastructure. The search period ranged from the inception of each database to November 7, 2024. A hybrid strategy combining subject headings and free-text terms was employed. Search terms mainly included “three-dimensional reconstruction”, “three-dimensional visualization”, and “percutaneous transhepatic biliary drainage (PTBD)”. The search strategy was dynamically adjusted according to the indexing systems of each database, using synonym expansions. The search was limited to human studies, with no language restrictions, and reference lists of literature were also manually examined for eligible studies to ensure the recall.
The study inclusion criteria are as follows: (1) Study participants were patients diagnosed with obstructive jaundice; (2) The study compared the use of 3D reconstruction technology-assisted PTBD with traditional methods; and (3) The literature provided extractable or calculable data on at least one of the following: Surgery time, number of punctures, incidence of specific postoperative complications, overall post-operative complications rate, or reduction levels of postoperative total bilirubin, aspartate transaminase, and alanine transaminase. The exclusion criteria are as follows: (1) Articles published as abstracts, reviews, case reports, meta-analysis, letters, or conference proceedings; (2) Studies with unavailable or incomplete data, or lacking surgical parameters, postoperative complications, and follow-up results; (3) Non-human studies; (4) Uncontrolled single-group analyses; and (5) Duplicate publications.
Two researchers independently screened the literature according to the inclusion and exclusion criteria, extracted data, and conducted data verification and quality assessment. if necessary, adjudicated by a third researcher. Extracted data includes: First author, year of publication, patient demographics (e.g., race, age), sample size, group allocation ratio (3D reconstruction vs traditional), surgery time, number of punctures, incidences of postoperative bleeding, biliary fistula, infection, overall post-operative complication rate, and reduction levels of postoperative total bilirubin, aspartate transaminase and alanine transaminase, along with other relevant outcomes.
The quality of included randomized controlled trials was conducted using the Cochrane Risk of Bias Assessment Tool. For cohort studies, the Newcastle-Ottawa Scale was applied[9], with a total score of 9 points. A score > 6 was considered indicative of high-quality research. Two independent reviewers assessed study quality, resolving any disagreements through discussion or third-party adjudication.
Meta-analysis was performed using Review Manager 5.4.1. For continuous variables, such as surgery time, number of punctures, reduction levels of postoperative total bilirubin, aspartate transaminase and alanine transaminase, the mean difference (MD) and 95% confidence interval (CI) were calculated. For dichotomous variables such as incidence rate of postoperative complications and overall complications rate, odds ratios (ORs) with 95%CIs were used. Heterogeneity among studies was measured using the χ2 test and quantified by the I2 statistic. If I2 ≥ 50% or P < 0.10, significant heterogeneity was assumed, and a random-effects model was applied. Otherwise, a fixed-effect model was used. Funnel plots were employed to assess publication bias. A P < 0.05 was considered statistically significant.
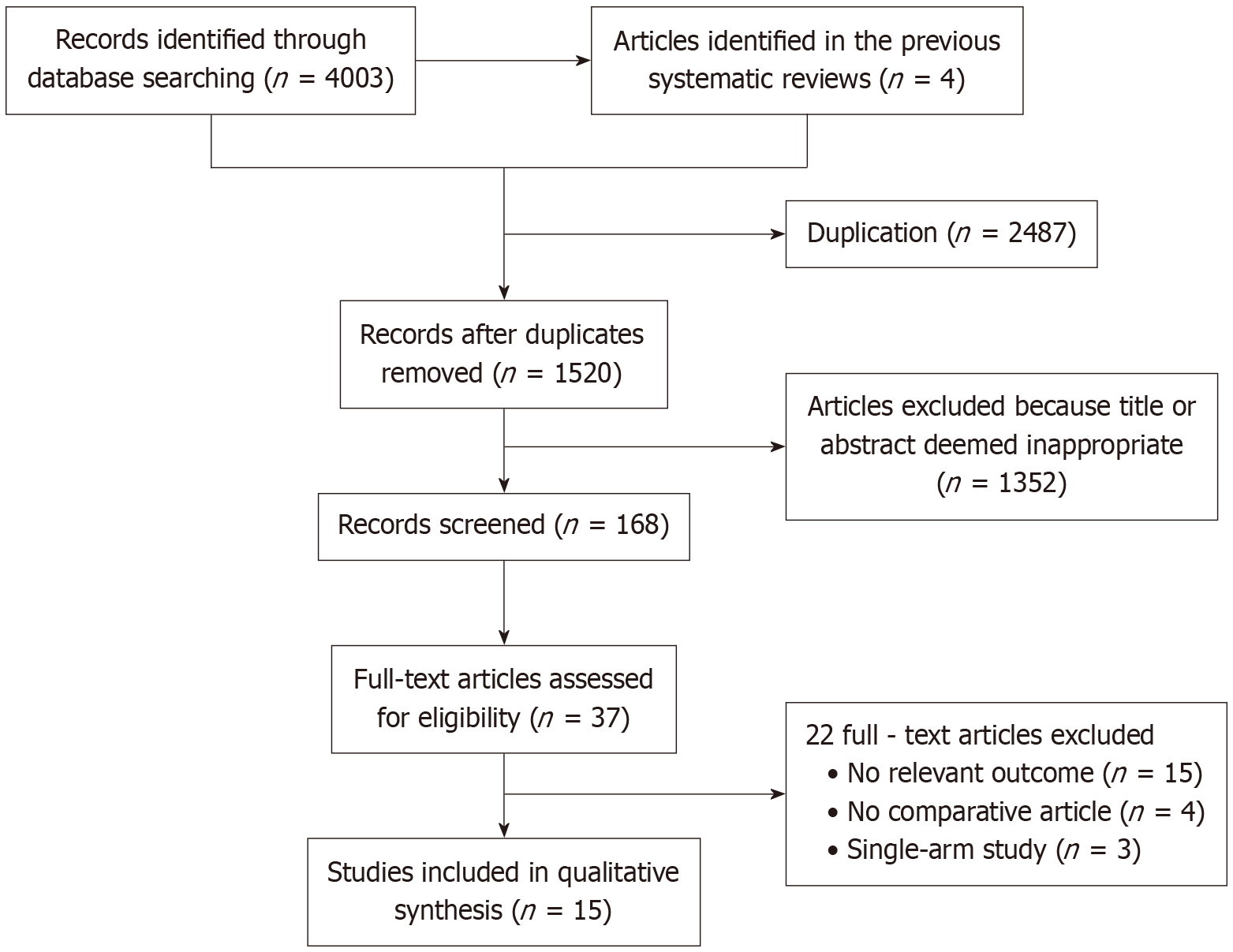
As shown in Figure 1, a total of 4003 studies were identified through the initial search strategy, and an additional 4 studies were determined by screening the references of relevant studies. After removing duplicates, 1520 studies remained. Of these, 1352 were excluded based on title and abstract screening. Following full-text review of 168 articles, 153 were excluded, a total of 15 studies were finally included in the meta-analysis.
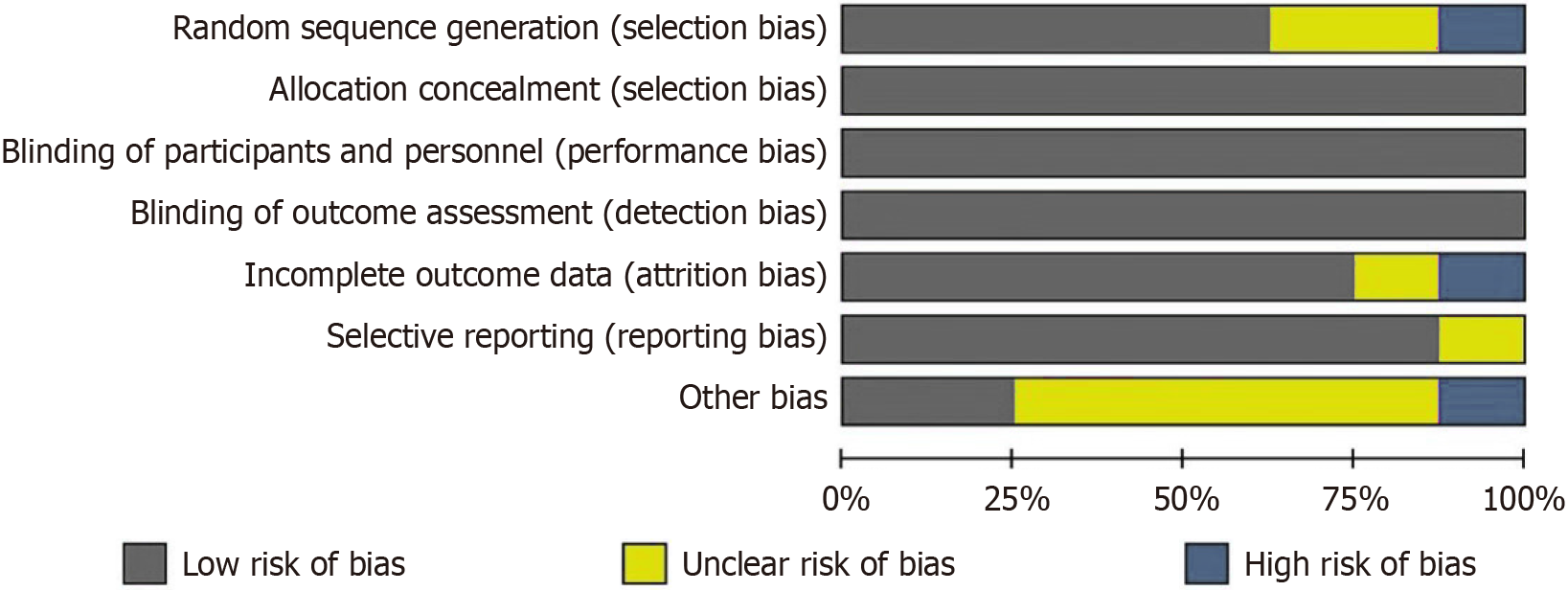
As shown in Table 1, the final analysis included 15 studies involving 1434 patients. These comprised 8 randomized controlled trials and 7 cohort studies, all published between 2016 and 2024. Three studies were obtained from English-language databases, while twelve were from Chinese databases. Among the total patients, 742 patients received PTBD assisted by 3D reconstruction technology, and 692 received traditional methods. The quality of randomized controlled trials was assessed using the Cochrane Collaboration's risk of bias assessment tool (Figure 2), and cohort studies were evaluated with the Newcastle-Ottawa Scale (Table 2).
| Ref. | Sample size 3D/tradition | Age 3D/tradition, mean ± SD | Gender (male/female) 3D tradition | Timing of 3D | Imaging modality | Design |
| Liu et al[22], 2023 | 81/73 | 66.5 ± 10.6/67.9 ± 11.4 | 46/35, 38/35 | Pre-operation and intraoperation | 3D of CT and MR | Cohort study |
| Li et al[10], 2020 | 42/45 | NR | NR | Intraoperation | 3D of CT | RCT |
| Zhou et al[11], 2021 | 30/30 | 61.1 ± 5.3/60.3 ± 5.5 | 20/10, 18/12 | Pre-operation | 3D of MR | Cohort study |
| Li et al[23], 2017 | 63/64 | 38.73 ± 11.34/35.16 ± 10.65 | 34/29, 32/32 | Intraoperation | 3D of ultrasound | Cohort study |
| Duan et al[12], 2017 | 50/50 | 47.6 ± 6.7/46.5 ± 5.4 | 28/22, 27/23 | Intraoperation | 3D of ultrasound | RCT |
| Zhuo et al[13], 2024 | 32/32 | NR | 22/10, 21/11 | Intraoperation | 3D of ultrasound | RCT |
| Zhou et al[24], 2022 | 120/60 | NR | NR | Pre-operation | 3D of CT and MR | Cohort study |
| Liu et al[14], 2022 | 30/30 | NR | NR | Intraoperation | 3D of CT | RCT |
| Kinoshita et al[15], 2017 | 12/10 | NR | NR | Pre-operation | 3D of CT | Cohort study |
| Wang[16], 2019 | 52/52 | 47.34 ± 4.08/47.15 ± 4.16 | 27/25, 26/26 | Pre-operation and intraoperation | 3D of ultrasound and CT | Cohort study |
| Huang et al[17], 2016 | 60/60 | 48.6 ± 5.2/47.5 ± 6.8 | 28/32, 31/29 | Pre-operation and intraoperation | 3D of ultrasound and CT | RCT |
| Huang et al[18], 2016 | 40/40 | 63.45 ± 3.62/62.97 ± 3.59 | 21/19, 22/18 | Intraoperation | 3D of ultrasound | RCT |
| Huang et al[19], 2020 | 30/30 | 51.95 ± 17.58/51.67 ± 17.35 | 22/8, 21/9 | Intraoperation | 3D of ultrasound | RCT |
| Chen et al[20], 2020 | 80/80 | NR | 46/34, 45/35 | Pre-operation and intraoperation | 3D of ultrasound and CT | RCT |
| Yang et al[21], 2023 | 20/36 | 66.5 ± 12.4/62.4 ± 10.9 | 9/11, 23/13 | Pre-operation and intraoperation | 3D of ultrasound and CT | Cohort study |
| Ref. | Representativeness of the exposed cohort (1) | Selection of the non-exposed cohort (1) | Ascertainment of exposure (1) | Demonstration that outcome of interest was not present at start of study | Compare ability of cohorts on the basis of the design or analysis (2) | Assessment of outcome (1) | Was follow up long enough for outcomes to occur (1) | Adequacy of follow up of cohorts (1) | Total |
| Liu et al[22], 2023 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhou et al[11], 2021 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Li et al[10], 2020 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zhou et al[24], 2022 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Wang[16], 2019 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Yang et al[21], 2023 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Kinoshita et al[15], 2017 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
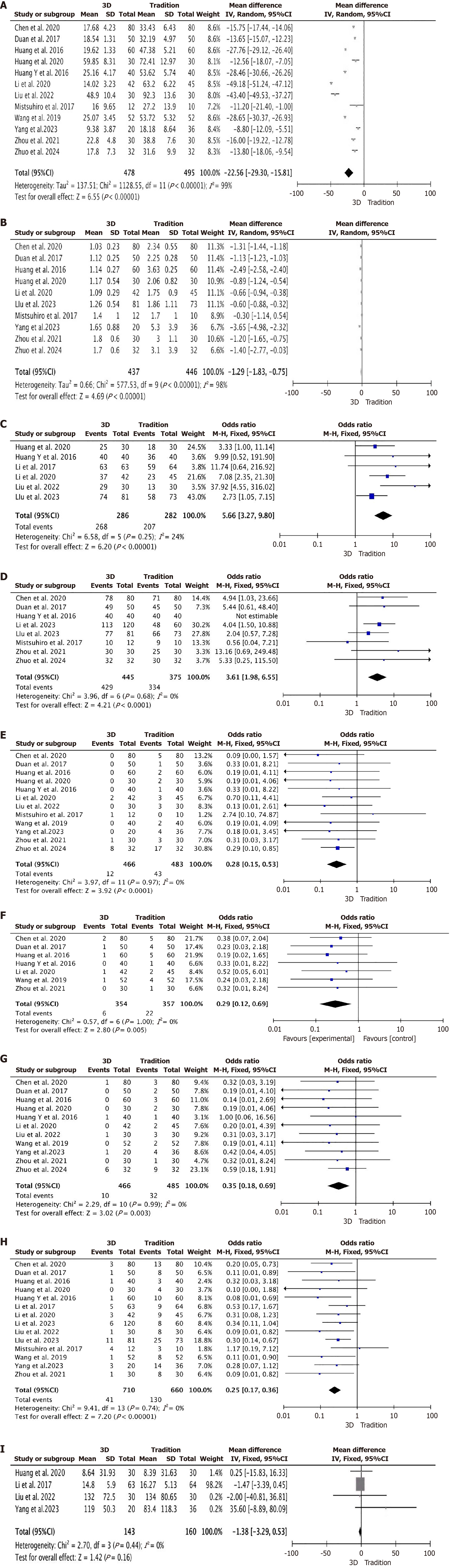
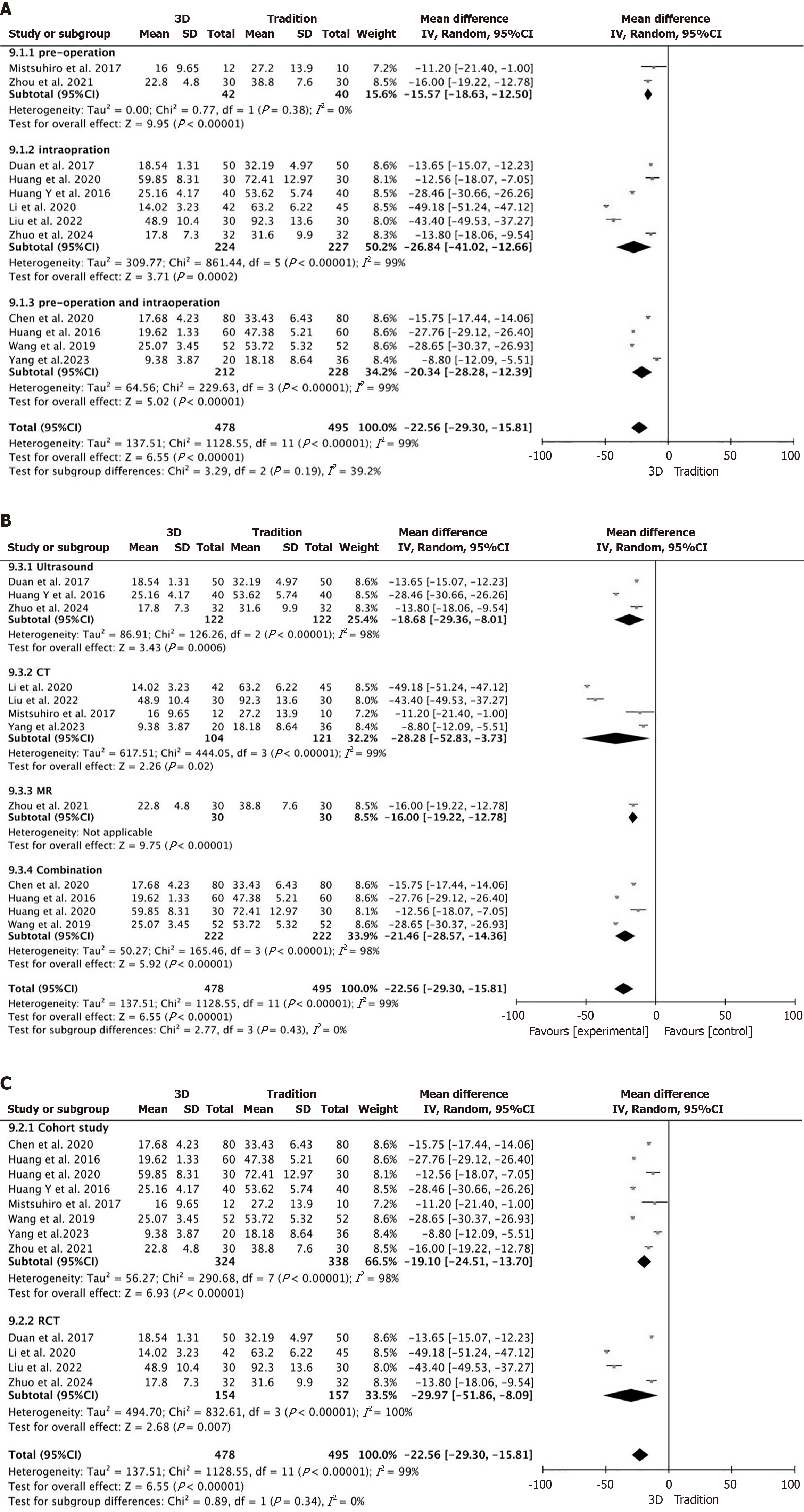
Surgery time: A total of 12 studies were included[10-21], all of which provided detailed reports on surgery time (Figure 3A), with 478 participants in the 3D group and 495 in the traditional group. The results showed considerable heterogeneity across the studies (P < 0.00001, I2 = 99%), therefore, a random effects model was applied. The meta-analysis results indicated: MD = -22.56; 95%CI: -29.30 to -15.81, P < 0.00001, the surgery time in the 3D group was significantly shorter than that in the traditional group.
Number of punctures: A total of 10 studies were included[10-15,17,19-21], all of which reported the number of punctures in detail (Figure 3B), with 437 participants in the 3D group and 446 in the traditional group. The results showed notable heterogeneity among the studies (P < 0.00001, I2 = 98%), consequently, a random effects model was utilized. The findings from the meta-analysis indicated: MD = -1.29; 95%CI: -1.83 to -0.75, P < 0.00001, the number of punctures in the 3D group was significantly lower than that in the traditional group.
First puncture success rate: A total of 6 studies were included[10,14,18,19,22,23], all of which reported in detail the first puncture success rate (Figure 3C), with 286 people in the 3D group and 282 in the traditional group. There was no considerable variation among the studies (P = 0.25, I2 = 24%), a fixed-effect model was employed. The outcomes of the meta-analysis demonstrated: OR = 5.66; 95%CI: 3.27-9.80, P < 0.00001, the first puncture success rate in the 3D group was significantly higher than that in the traditional group.
Overall puncture success rate: A total of 8 studies were included[11-13,15,18,20,22,24], all of which reported detailed data on the overall puncture success rate (Figure 3D), with 445 people in the 3D group and 375 people in the traditional group. The results showed no notable heterogeneity in the studies (P = 0.68, I2 = 0%), thus, a fixed-effect Model was implemented. The meta-analysis findings suggested: OR = 3.61; 95%CI: 1.98-6.55, P < 0.0001, the overall puncture success rate in the 3D group was significantly higher than that in the traditional group.
Post-operative bleeding: A total of 12 studies were included[10-21], all of which reported post-operative bleeding in detail (Figure 3E), with 466 patients in the 3D group and 483 patients in the traditional group. The studies showed no notable differences (P = 0.97, I2 = 0%), a fixed-effect model was adopted. The results derived from the meta-analysis revealed: OR = 0.28; 95%CI: 0.15-0.53, P < 0.0001, post-operative bleeding occurred significantly less in the 3D group than that in the traditional group.
Biliary fistula: A total of 7 studies were included[10-12,16-18,20], all of which reported in detail on biliary fistula (Figure 3F), with 354 people in the 3D group and 357 in the traditional group. There was no considerable variation among the studies (P = 1.00, I2 = 0%), hence, a fixed-effect model was implemented. According to the meta-analysis results: OR = 0.29; 95%CI: 0.12-0.69, P = 0.005, the incidence of postoperative biliary fistula was significantly lower in the 3D group than that in the traditional group.
Postoperative infection: A total of 11 studies were included[10-14,16-21], all of which provided detailed reports on postoperative infections (Figure 3G), with 466 people in the 3D group and 485 people in the traditional group. The studies showed no notable differences (P = 0.99, I2 = 0%), a fixed-effect model was applied. The findings from the meta-analysis indicated: OR = 0.35; 95%CI: 0.18-0.69, P = 0.003, the incidence of postoperative infections in the 3D group was significantly lower than that in the traditional group.
Overall post-operative complications rate: A total of 14 studies were included[10-12,14-24], all of which reported the overall post-operative complications rate in detail (Figure 3H), with 710 people in the 3D group and 660 in the traditional group. There was no considerable heterogeneity across the studies (P = 0.74, I2 = 0%), consequently, a fixed-effect model was implemented. The outcomes of the meta-analysis demonstrated: OR = 0.25; 95%CI: 0.17-0.36, P < 0.00001, the overall post-operative complications rate in the 3D group was significantly lower than that in the traditional group.
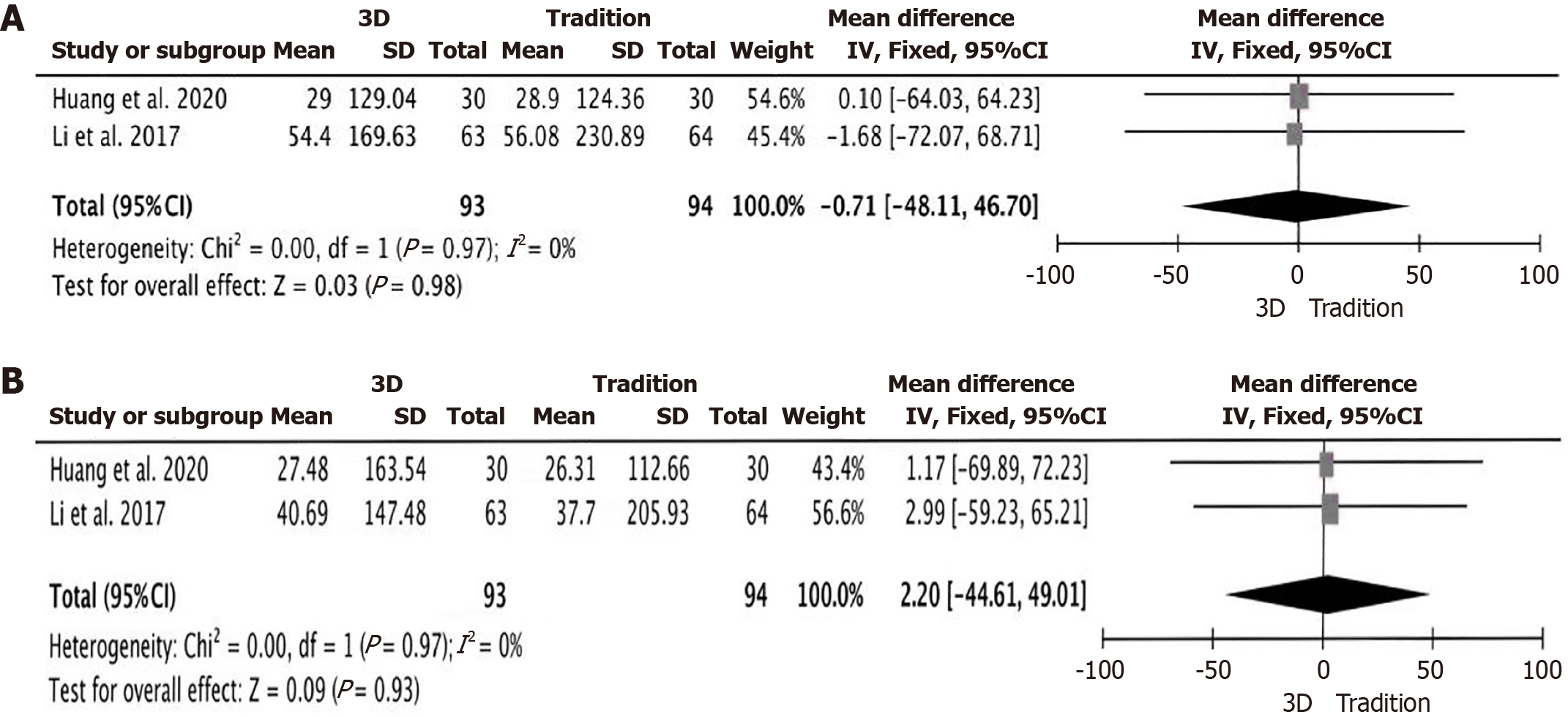
Bile duct drainage effect: A total of 4 studies were included[14,19,21,23], all of which reported in detail the reduction levels of postoperative total bilirubin (Figure 3I), with 143 people in the 3D group and 160 people in the traditional group. The results showed no marked heterogeneity in the studies (P = 0.44, I2 = 0%), a fixed-effect model was employed. The results of the meta-analysis demonstrated: MD = -1.38; 95%CI: -3.29 to 0.53, P = 0.16, there was no significant difference in the reduction levels of postoperative total bilirubin between the 3D group and the traditional group. A total of 2 studies were included[19,23], both of which reported in detail the reduction levels of aspartate transaminase and alanine transaminase (Figure 4A and B), with 93 patients in the 3D group and 94 patients in the traditional group. The results showed no significant heterogeneity among the studies (P = 0.97, I2 = 0%) and (P = 0.97, I2 = 0%), consequently, a fixed-effect model was implemented. The findings from the meta-analysis indicated: MD = -0.71; 95%CI: -48.11 to 46.70, P = 0.98 and MD = 2.20; 95%CI: -44.61 to 49.01, P = 0.93, indicating that there was no significant difference in the reduction levels of aspartate transaminase and alanine transaminase between the 3D group and the traditional group.
Subgroup analysis were conducted on the surgery time according to guidance stages of the 3D reconstruction, 3D reconstruction imaging modalities, and types of studies. The results showed no significant reduction in the heterogeneity across subgroups. The overall meta-analysis outcomes remained stable and consistent (Figure 5A-C).
For the high heterogeneity of the meta-analysis, sensitivity analysis was conducted by excluding one study at a time to assess the impact of study quality on the stability of the meta-analysis. The results showed that no single study had a significant impact on the research findings. This indicates that our meta-analysis results are reliable.
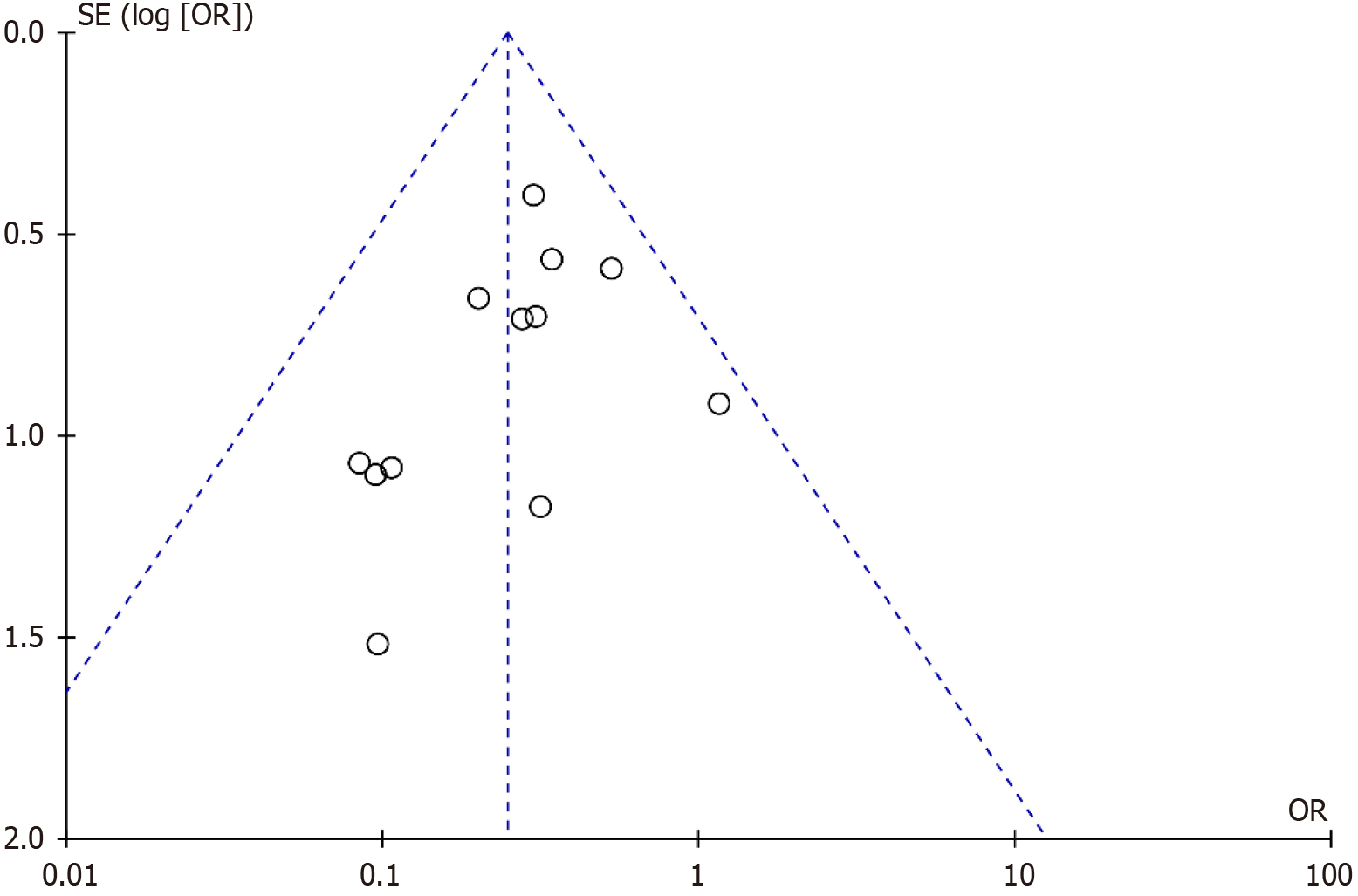
Publication bias was assessed using a funnel plot based on the overall post-operative complications rate (Figure 6). The plot showed a generally symmetrical distribution, with all studies falling within the area bordered by the two diagonal lines, suggesting no significant publication bias.
In traditional PTBD, the surgeon mainly locates the puncture point by interpreting two-dimensional imaging data, and then uses X-ray or ultrasound for guidance during the operation. The entire surgery mainly relies on the surgeon’s anatomical knowledge and subjective judgment[25-27]. However, in patients with obstructive jaundice, liver morphology and vascular anatomy often vary significantly. In such cases, blindly performing programmed puncture operations will bring unpredictable risks to the patient[28].
With the continuous development of modern information technology in the medical field, 3D reconstruction technology has gradually become an important technical means to guide PTBD. It mainly reprocesses traditional two-dimensional imaging data, using advanced segmentation algorithms to accurately extract contour information of key anatomical structures, such as the liver and bile ducts. Additional image processing techniques, including denoising and enhancement, and artifact removal, help to restore the true morphology of tissues. Through multi-modal image fusion, this process generates a detailed, individualized 3D model, offering surgeons a comprehensive view of the patient’s liver anatomy[29]. This personalized model enables surgeons to visualize lesion locations and bile duct pathways, simulate surgical procedures in advance, evaluate different puncture paths, and optimize surgical plans. Consequently, it enhances both the precision and safety of PTBD procedures[7,8].
The success of PTBD depends critically on puncture point selection and real-time needle trajectory adjustment[12,30]. 3D reconstruction offers substantial advantages in both areas. Preoperatively, it allows for a thorough understanding of biliary anatomy - including branching patterns, directions, and vascular relationships - facilitating accurate puncture planning while minimizing vascular injury[10,11,13,22]. Intraoperatively, the real-time 3D model provides dynamic navigation, enabling the surgeon to fine-tune the needle’s angle and depth to ensure successful cannulation and reduce the risks associated with repeated blind punctures[10,13]. The result of this study fully confirms this point. The application of 3D reconstruction technology significantly improves the puncture success rate of PTBD, effectively shortens the operation time and reduces the number of punctures, which has obvious advantages compared with the traditional methods. Previous studies have shown that prolonged operation time and increased number of punctures are important factors leading to a high incidence of postoperative complications[21,22,31]. The results of this study are consistent with this. The incidence of postoperative bleeding, biliary fistula and infection in the traditional group is significantly higher than that in the 3D reconstruction group. In some cases, even death occurs due to serious complications, highlighting the important value of 3D reconstruction technology in improving surgical safety[10].
Effective drainage is the top priority of PTBD, and the accurate selection of the target bile duct is essential to achieving it[32]. In theory, the high-resolution anatomical visualization provided by 3D reconstruction should enhance target duct identification, supporting better drainage outcomes[10]. Some studies have confirmed this theoretical advantage[24]. However, in this meta-analysis, 3D reconstruction technology did not show a significant advantage in the effect of bile drainage. This result may be related to the following factors. First is lacking of real-time adaptability. Preoperative 3D models do not account for dynamic anatomical changes caused by respiratory motion or patient positioning during surgery, which may reduce puncture accuracy[13]. Then, the absence of stratification could be the other reason. Most included studies did not stratify patients by bile duct dilation degree or obstruction site, potentially masking the subgroups most likely to benefit. Finally, the limited number of studies assessing bile drainage introduces risk of bias and limits statistical power. Despite this, the observed trend in favor of 3D reconstruction indicates potential clinical value. Future large-scale, multicenter randomized controlled trials - stratified by anatomical classification and obstruction grading - are needed to clarify the technology’s impact on drainage efficacy.
This study included 15 studies, 13 of which were randomized controlled trials or prospective cohorts with high methodological quality. However, significant heterogeneity was detected in several outcomes, particularly surgery time (I2 = 99%). Subgroup analyses based on guidance stages of 3D reconstruction, imaging modalities, and study types did not significantly reduce heterogeneity. Nonetheless, the direction of the effect sizes across all subgroups consistently favored the 3D group, indicating robustness of the findings.
The residual heterogeneity may be attributed to uncontrolled primary heterogeneity sources, and limited subgroup sample sizes. Differences in surgeon experience, puncture techniques, disease types, study designs, and reconstruction software may have had a greater impact than the subgroup variables. Insufficient numbers of studies in each subgroup reduced the sensitivity of the I2 statistic, limiting the detection of meaningful heterogeneity changes. Despite the unresolved heterogeneity, the consistency in effect direction strengthens the reliability of our overall conclusions.
In conclusion, 3D reconstruction technology offers significant benefits in PTBD for patients with obstructive jaundice, especially in improving puncture success rate and safety. Although its impact on bile drainage effectiveness has not been fully demonstrated, preliminary evidence suggests positive potential. Therefore, 3D-assisted PTBD has broad clinical application. Future studies should focus on large-scale, multicenter, and high-quality trial designs, ideally stratified by anatomical and clinical factors, to refine its application strategies and further improve the treatment outcomes for obstructive jaundice.
3D reconstruction technology significantly improves the puncture success rate and safety of PTBD. However, it has no significant advantage in bile drainage effectiveness. Continued research is warranted to further explore its clinical value and optimize its application.
We would like to thank all of the study participants.
| 1. | Jin H, Pang Q, Liu H, Li Z, Wang Y, Lu Y, Zhou L, Pan H, Huang W. Prognostic value of inflammation-based markers in patients with recurrent malignant obstructive jaundice treated by reimplantation of biliary metal stents: A retrospective observational study. Medicine (Baltimore). 2017;96:e5895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Pavlidis ET, Pavlidis TE. Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int. 2018;17:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Glenn F, Evans JA, Mujahed Z, Thorbjarnarson B. Percutaneous transhepatic cholangiography. Ann Surg. 1962;156:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Yang F, Jin C, Zou C, Di Y, Hao S, Huang H, Warshaw AL, Fu D. Delaying surgery after preoperative biliary drainage does not increase surgical morbidity after pancreaticoduodenectomy. Surgery. 2019;166:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Cao J, Wang Z, Cai H, Zhang J, Yue Y, Liu X, Zhang D. Effect of PTCD-based biliary stent placement combined with 125I particle intracavitary irradiation in treating pancreatic head cancer. J BUON. 2020;25:1056-1062. [PubMed] |
| 6. | Kim GH, Ryoo SK, Park JK, Park JK, Lee KH, Lee KT, Lee JK. Risk Factors for Pancreatitis and Cholecystitis after Endoscopic Biliary Stenting in Patients with Malignant Extrahepatic Bile Duct Obstruction. Clin Endosc. 2019;52:598-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Pedicelli A, Verdolotti T, Pompucci A, Desiderio F, D'Argento F, Colosimo C, Bonomo L. Interventional spinal procedures guided and controlled by a 3D rotational angiographic unit. Skeletal Radiol. 2011;40:1595-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Tam AL, Mohamed A, Pfister M, Chinndurai P, Rohm E, Hall AF, Wallace MJ. C-arm cone beam computed tomography needle path overlay for fluoroscopic guided vertebroplasty. Spine (Phila Pa 1976). 2010;35:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Moreau P, Robillard N, Jégo G, Pellat C, Le Gouill S, Thoumi S, Avet-Loiseau H, Harousseau JL, Bataille R. Lack of CD27 in myeloma delineates different presentation and outcome. Br J Haematol. 2006;132:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Li SK, Hu XK. [Clinical application of XperCT-assisted PTCD for precise treatment of malignant obstructive jaundice]. Zhonghua Jieru Fanshexue Dianzi Zazhi. 2020;8:170-174. [DOI] [Full Text] |
| 11. | Zhou SL, Ji TJ, Xu L, Jiang LL. [Application of MRI three-dimensional reconstruction in PTCD localization]. Yixue Yingxiangxue Zazhi. 2021;31:1354-1358. |
| 12. | Duan XJ, Huang YB, Hu PX, Long S, Zhu YF. [Clinical value of ultrasound-guided percutaneous transhepatic cholangiocath placement for treating obstructive jaundice]. Zhongguo Liaoyang Yixue. 2017;26:501-502. [DOI] [Full Text] |
| 13. | Zhuo H, Wu C, Tan ZM, Tang WW, Zhu DM, Xu Y, Zhao J, Gu JP, Wang XH, Song JH. [Preliminary clinical application of novel magnetic navigation and ultrasound-guided percutaneous transhepatic cholangiography drainage through the right liver duct for malignant obstructive jaundice]. Zhonghua Nei Ke Za Zhi. 2024;63:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Liu H, Jiang P, Fu W. [CT-guided percutaneous transhepatic cholangiography for the treatment of obstructive jaundice: A clinical study]. Shiyong Ganzangbing Zazhi. 2022;25:136-139. [DOI] [Full Text] |
| 15. | Kinoshita M, Shirono R, Takechi K, Yonekura H, Iwamoto S, Shinya T, Takao S, Harada M. The Usefulness of Virtual Fluoroscopic Preprocedural Planning During Percutaneous Transhepatic Biliary Drainage. Cardiovasc Intervent Radiol. 2017;40:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wang F. [Effect of ultrasound navigation-assisted percutaneous biliary drainage in PTCD]. Zhongguo Weisheng Biaozhun Guanli. 2019;10:24-26. |
| 17. | Huang YB, Duan XJ, Hu PX, Xiao M. [Effect of ultrasound navigation-assisted percutaneous transhepatic cholangiocatheter placement in treating intrahepatic bile duct stone obstruction]. Zhonghua Shiyong Zhenduan Yu Zhiliao Zazhi. 2016;30:287-289. [DOI] [Full Text] |
| 18. | Huang YB, Duan XJ, Long S, Hu PX, Xiao XP. [Clinical value of ultrasound navigation in percutaneous transhepatic cholangiocatheter placement]. Zhongguo Minzu Minjian Yiyao. 2016;25:84-86. |
| 19. | Huang YB, Duan XJ, Hu PX, Wu J, Zhu YF. [Ultrasound navigation-assisted percutaneous transhepatic cholangiocatheter placement: A clinical study]. Zhongguo Wulixue Zazhi. 2020;37:54-58. |
| 20. | Chen Y, Zhou S, Wang CR, Zhang J. [Analysis of the clinical value of ultrasound navigation assisted percutaneous intrahepatic bile duct drainage in the treatment of obstructive jaundice]. Zhongguo Shequ Yishi. 2020;36:20-21. [DOI] [Full Text] |
| 21. | Yang T, Gao DZ, Xu TT, Shi DH, Liu L, Chen JH, Xu J. [Technical exploration of electromagnetic navigation-assisted percutaneous transhepatic biliary drainage]. Yixue Yanjiu Yu Zhanchangshang Jiuzhi. 2023;36:503-508. [DOI] [Full Text] |
| 22. | Liu J, Zhou SL, Wei N, Cao G, Zhang GS, Ji TJ. [Application value of CT and MR three-dimensional reconstruction in puncture localization for malignant obstructive jaundice caused by malignant tumors]. Jieru Fangshexue Zazhi. 2023;32:792-795. |
| 23. | Li PY, Chen XP, Lao W. [Effect of three-dimensional ultrasound-guided percutaneous transhepatic cholangiocatheter placement in treating malignant obstructive jaundice]. Shiyong Linchuang Yiyao Zazhi. 2017;21:127-128. |
| 24. | Zhou SL, Ji TJ, Zhou XY, Xu L, Xin Y, Huang QJ. Application value of computed tomography and magnetic resonance imaging three-dimensional reconstruction and digital subtraction angiography in percutaneous transhepatic cholangial drainage. Front Surg. 2022;9:932901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Gouma DJ, Coelho JC, Fisher JD, Schlegel JF, Li YF, Moody FG. Endotoxemia after relief of biliary obstruction by internal and external drainage in rats. Am J Surg. 1986;151:476-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | O'Brien S, Bhutiani N, Egger ME, Brown AN, Weaver KH, Kline D, Kelly LR, Scoggins CR, Martin RCG 2nd, Vitale GC. Comparing the efficacy of initial percutaneous transhepatic biliary drainage and endoscopic retrograde cholangiopancreatography with stenting for relief of biliary obstruction in unresectable cholangiocarcinoma. Surg Endosc. 2020;34:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Bokemeyer A, Müller F, Niesert H, Brückner M, Bettenworth D, Nowacki T, Beyna T, Ullerich H, Lenze F. Percutaneous-transhepatic-endoscopic rendezvous procedures are effective and safe in patients with refractory bile duct obstruction. United European Gastroenterol J. 2019;7:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Wu XW, Zhou SJ, Zhang SJ, Heng MD. [Common complications and preventive measures of percutaneous transhepatic cholangiography drainage]. Nongken Yixue. 2001;1:166-167. |
| 29. | Liu K, Yu H, Liu SY, Dong S. [The significance of image processing techniques in medical imaging teaching]. Linchuang He Shiyan Yixue Zazhi. 2010;9:1437. [DOI] [Full Text] |
| 30. | Wu LL, Yuan SF, Zheng RQ, Li K. [Application of real-time virtual navigation in liver cancer where conventional ultrasound is difficult to display]. Zhongguo Ganzangbing Zazhi. 2013;5:62-63. |
| 31. | Gu HT, Zhang JY, Wang ZW, Chen GQ, Xu JM. [Efficacy of preoperative biliary drainage for hilar cholangiocarcinoma complicated with obstructive jaundice]. Zhonghua Ganzang Waike Shoushuxue Dianzi Zazhi. 2021;10:29-32. [DOI] [Full Text] |
| 32. | Boulay BR, Birg A. Malignant biliary obstruction: From palliation to treatment. World J Gastrointest Oncol. 2016;8:498-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/