Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.104997
Revised: April 9, 2025
Accepted: July 28, 2025
Published online: September 27, 2025
Processing time: 259 Days and 5.9 Hours
Acute upper gastrointestinal bleeding (AUGIB) is a common emergency critical illness that requires prompt assessment upon admission to prevent disease deterioration. As a resuscitation mode, the fast track for emergency treatment increases the success rate and improves patient outcomes. However, misuse will consume resources. The Glasgow-Blatchford score (GBS) is considered to predict the cli
To validate the effectiveness of the GBS in establishing a fast track to reduce the time and cost of treatment for patients with AUGIB.
A retrospective analysis was performed using the data of 124 cases of AUGIB patients with GBS ≥ 6 treated at the University-Town Hospital of Chongqing Medical University from August 2020 to April 2023. Based on GBS risk stratification, patients were divided into moderate-risk (12 > GBS ≥ 6) and high-risk (GBS ≥ 12) groups. Furthermore, depending on whether a fast track was esta
In the comparison of the aforementioned indicators, the moderate-risk fast-track group did not show any sig
Establishing a fast track for emergency treatment based on GBS risk stratification has assessment value in reducing door-to-endoscopy time, decreasing total blood transfusion volume, and lowering hospitalization costs in patients with AUGIB. GBS ≥ 12 is recommended as the threshold for implementing the fast track for emergency treatment, and its clinical promotion is advised.
Core Tip: This study analyzed data from 124 patients with acute upper gastrointestinal bleeding with Glasgow-Blatchford score (GBS) ≥ 6. Based on GBS, patients were divided into moderate- and high-risk groups. Further categorization was done into control and fast track groups based on whether a fast track was established. Comparisons were made for stay times, door-to-endoscopy time, blood transfusions, hospitalization, and costs between groups at each risk level. Notably, the implementation of the emergency treatment fast track for patients with GBS ≥ 12 demonstrated significant advantages over traditional treatment models. Therefore, we identified GBS ≥ 12 as the threshold for implementing the fast track protocol.
- Citation: Zhang DQ, Zhou Q, Li YF, Jia XY, Li X, Chen S, Lin K. Evaluating Glasgow-Blatchford score for fast-track emergency management of patients with acute upper gastrointestinal bleeding. World J Gastrointest Surg 2025; 17(9): 104997
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/104997.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.104997
Acute upper gastrointestinal bleeding (AUGIB) is a common emergency critical illness with an annual incidence of approximately 100-150 cases per 100000 adults and an overall mortality rate ranging from 5% to 15%[1]. The disease is characterized by a sudden onset, rapid progression, and complex causes. Failure to assess the risk of the condition ac
The assessment methods for AUGIB patients in emergency departments include the Glasgow-Blatchford score (GBS), Rockall score, and AIMS65 score[4,5]. Of these, the GBS is considered to predict the need for clinical intervention mea
The fast track for emergency treatment is an important standardized treatment model for emergency resuscitation. It is based on emergency medical care and involves constructing a multi-disciplinary team (MDT) to rapidly concentrate advantageous medical resources for the assessment and timely treatment of critically ill patients[12]. The fast track can improve the success rate and quality of emergency resuscitation, improve patient outcomes, and reduce the likelihood of adverse events[13,14]. However, inaccurate assessment and frequent activation of the fast track may lead to the overconsumption of scarce emergency medical resources and increase the financial burden on patients[1]. In light of this, this study conducts a retrospective analysis of AUGIB patient data treated at the University-Town Hospital of Chongqing Medical University. It stratifies patients according to their GBS scores and compares the impact of establishing a fast-track pathway for emergency treatment on the efficiency of emergency operations and consumption of hospital resources under different GBS risk stratifications. The aim is to validate the effectiveness of the GBS in establishing a fast track to reduce the time and cost of treatment for patients with AUGIB.
This study retrospectively collected data on AUGIB patients treated at the University-Town Hospital of Chongqing Medical University from August 2020 to April 2023 who were selected according to preset inclusion and exclusion criteria. This study was approved by the Ethics Committee of the University-Town Hospital of Chongqing Medical University (Approval No. LL-202275). Informe consent was waived by the Ethics Committee of University-Town Hospital of Chongqing Medical University due to the retrospective design of the study.
Adult patients ( ≥ 18 years) of any gender presenting to the emergency department with clinical manifestations sug
Initial triage classification by emergency department nurses as requiring immediate resuscitation room care based on institutional protocols for suspected AUGIB.
Subsequent transfer to the inpatient department following emergency department stabilization.
Confirmation of AUGIB diagnosis by upper gastrointestinal endoscopy during the same hospitalization.
Complete documentation of all GBS parameters in the electronic medical record at presentation.
GBS ≥ 6 at initial presentation, classifying the patient as moderate or high risk according to validated risk stratification.
Complete medical records with documentation of all study outcome parameters, including timing of interventions, resource utilization metrics, and hospitalization details.
(1) Patients who bypassed the emergency department and were directly admitted to inpatient services through internal medicine, gastroenterology, or other specialty consultations; (2) Patients for whom upper endoscopy was not performed during the hospitalization or where the source of bleeding could not be definitively localized to the upper gastrointestinal tract; (3) Patients younger than 18 years of age due to different management protocols and risk profiles in pediatric populations; (4) Patients with GBS < 6 at presentation, classified as low-risk according to established guidelines; (5) Patients with substantial missing data in electronic medical records; (6) Patients transferred from other facilities with treatment already initiated, which would confound the assessment of our fast-track protocol; (7) Patients with traumatic causes of upper gastrointestinal bleeding (e.g., Mallory-Weiss tears from traumatic intubation) who followed different treatment pathways; and (8) Pregnant patients, due to physiological differences and specialized management considerations.
Patient-related information was retrospectively collected through the medical record system, including: (1) Basic information: Name, hospitalization number, admission date; (2) GBS-related indicators: Blood urea-nitrogen, hemoglobin, systolic blood pressure, pulse rate, presence of black stools or syncope, and comorbidities such as liver disease or heart failure; (3) Baseline indicators: Age, gender, GBS, time of onset, presence of underlying diseases such as cirrhosis, hypertension, diabetes, coronary heart disease, heart failure, respiratory failure, or renal failure and whether variceal bleeding was observed during gastroscopy; and (4) Comparative indicators: Duration of stay in the emergency re
This study stratified AUGIB patients according to GBS risk levels into moderate-risk (12 > GBS ≥ 6) and high-risk (GBS ≥ 12) groups[15]. Further categorizing the patients based on whether a fast track for treatment was established after they entered the emergency department resulted in four groups: A moderate-risk control group, a moderate-risk fast-track group, a high-risk control group, and a high-risk fast-track group.
Control group: Patients in the control group received routine emergency treatment, which included, but was not limited to, the following measures: Taking the patient’s medical history, performing a physical examination, monitoring vital signs, establishing intravenous access, completing necessary routine blood and biochemical tests as soon as possible, conducting volume resuscitation, transfusing blood if necessary, endoscopic therapy and applying emergency measures, such as pharmacological hemostasis. The endoscopic therapy mainly includes methods such as spraying, flushing, injecting hemostatic drugs, clamping, titanium clips, band ligation, thermal coagulation, electrocoagulation, etc.[16]. Once the patient’s condition stabilized, further diagnostic procedures were conducted to identify the cause, and systematic treatment was initiated.
Fast-track group: Patients in the fast-track group received treatment through the emergency fast-track pathway, with the following specific implementation process: (1) For patients presenting with symptoms such as vomiting blood, blood in the stools, black stools, dizziness, blurred vision, or fatigue, the medical history was taken, and a rapid physical examination was conducted to preliminarily diagnose whether AUGIB was present; (2) Patients suspected of having AUGIB were urgently assessed for the presence of consciousness disorders, collapsed airway, respiratory failure, circulatory failure, active bleeding, or a GBS score of > 1. Patients assessed as having high-risk upper gastrointestinal bleeding (i.e., possessing any of these risk factors) were immediately transferred to the resuscitation room, and in
The sample size of 124 patients was determined based on statistical power considerations for our primary analysis. Using G*Power software (version 3.1), we calculated that a minimum of 58 patients per comparison group (116 total) would be required to detect a moderate effect size (Cohen's d = 0.5) with 80% power and significance level of α = 0.05 for our primary outcomes (door-to-endoscopy time, transfusion volume, and hospitalization costs). Our final cohort of 124 patients exceeded this minimum requirement and was distributed into risk categories with 62 patients in the moderate-risk group (GBS 6-11) and 62 in the high-risk group (GBS ≥ 12). For multiple linear regression analysis, our sample maintained an observation-to-predictor ratio of approximately 17:1, exceeding the recommended minimum of 10:1 for stable coefficient estimates.
For each continuous variable, we performed the Shapiro-Wilk test using SPSS 24.0 statistical software. A significance level of P < 0.05 was established to reject the null hypothesis of normal distribution. If the data conformed to a normal distribution, they were expressed as mean ± SD, and the two-sample independent t-test was conducted. If the data did not conform to a normal distribution, they were expressed as the median (interquartile range) [M (Q1, Q3)], and the Mann-Whitney U test was performed. All count data were expressed as the number of cases (percentage) and analyzed using the χ2 test or Fisher’s exact test. Multiple linear regression analysis was performed using SAS9.2.
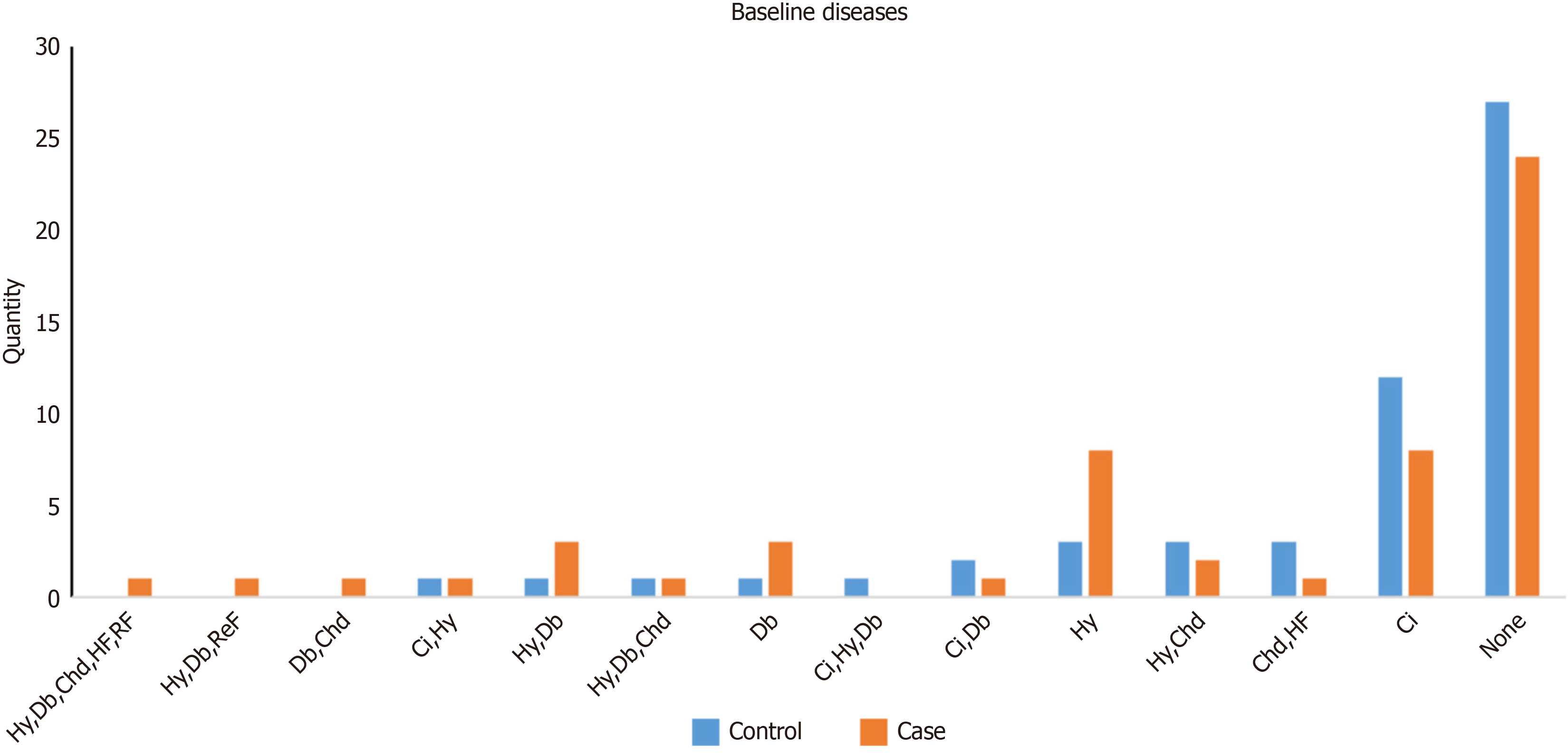
According to the inclusion and exclusion criteria of this study, a total of 124 patients were enrolled. The distribution of their underlying diseases is shown in Figure 1. There were 37 patients in the moderate-risk control group, 25 patients in the moderate-risk fast-track group, 26 patients in the high-risk control group, and 36 patients in the high-risk fast-track group. The Shapiro-Wilk test revealed that most of our quantitative variables did not follow normal distributions. Only age (P > 0.05) showed approximation to normal distribution. There were no significant differences in the baseline indicators between the control and fast-track groups (P > 0.05; Table 1), indicating comparability between the groups.
| Category of factors | Moderate-risk group | High-risk group | ||||
| Control | Fast track | P value | Control | Fast track | P value | |
| Population traits | ||||||
| Age (mean ± SD) | 56 ± 19 | 56 ± 22 | 0.899 | 58 ± 16 | 63 ± 12 | 0.201 |
| Gender (male) | 31 (83.8) | 20 (80.0) | 0.965 | 21 (80.8) | 29 (80.1) | 0.983 |
| GBS [M (P25, P75)] | 9 (8, 10) | 9 (9, 11) | 0.633 | 13 (12, 14) | 13 (12, 14) | 0.583 |
| Onset time [M (P25, P75), hour] | 13 (7, 48) | 12 (5, 48) | 0.604 | 24 (9, 72) | 24 (7, 78) | 0.937 |
| Underlying diseases (yes) | 15 (40.5) | 11 (44.0) | 0.787 | 17 (65.4) | 21 (58.3) | 0.574 |
| Gastroscopy diagnosis (esophageal and gastric varices) | 6 (18.1) | 3 (14.3) | 0.924 | 9 (34.6) | 5 (13.9) | 0.054 |
| Analysis parameters | ||||||
| Emergency resuscitation room stay time [M (P25, P75), minute] | 63 (37, 94) | 57 (43, 75) | 0.275 | 58.5 (42, 81) | 48 (33, 71) | 0.070 |
| Door-to-endoscopy time [M (P25, P75), minute] | 19 (12, 26) | 21 (14, 30) | 0.509 | 21.5 (11, 40) | 13.5 (9, 22) | 0.013a |
| Total blood transfusion, [M (P25, P75), unit] | 0 (0, 4) | 1 (0, 3) | 0.841 | 6.75 (4, 14.5) | 3 (2, 5) | 0.001a |
| Treatment outcome (death) | 0 (0.0) | 1 (4.8) | 0.403 | 0 (0.0) | 0 (0.0) | / |
| Hospitalization duration [M (P25, P75), day] | 6 (5, 7) | 7 (5, 8) | 0.552 | 7.5 (4, 10) | 7.5 (6, 10) | 0.226 |
| ICU length of stay [M (P25, P75), day] | 0 (0, 0) | 0 (0, 0) | 0.897 | 0 (0, 2) | 0 (0, 1) | 0.630 |
| Gastroenterology department length of stay [M (P25, P75), day] | 6 (5, 7) | 7 (5, 8) | 0.155 | 6 (4, 10) | 7 (6, 9) | 0.130 |
| Hospitalization costs [M (P25, P75), USD] | 1239.80 (829.11) | 1102.66 (815.27) | 0.460 | 2812.44 (1513.97) | 1570.26 (1159.86) | 0.0461 |
| Endoscopic therapy1 (yes) | 11 (29.7) | 9 (36.0) | 0.604 | 13 (50.0) | 14 (38.9) | 0.384 |
In contrast, the high-risk groups demonstrated significant differences in several key outcomes. The door-to-endoscopy time was significantly shorter in the fast-track group compared to the control group (median 13.5 minutes vs 21.5 minutes, P = 0.013). Similarly, total blood transfusion volume was significantly lower in the fast-track group (median 3 units vs 6.75 units, P = 0.001). Hospitalization costs were also significantly reduced in the fast-track group [median 1570.26 United States dollar (USD) vs 2812.44 USD, P = 0.046]. However, there were no significant differences in these metrics between the moderate-risk fast-track group and their corresponding control group (P > 0.05; Table 1).
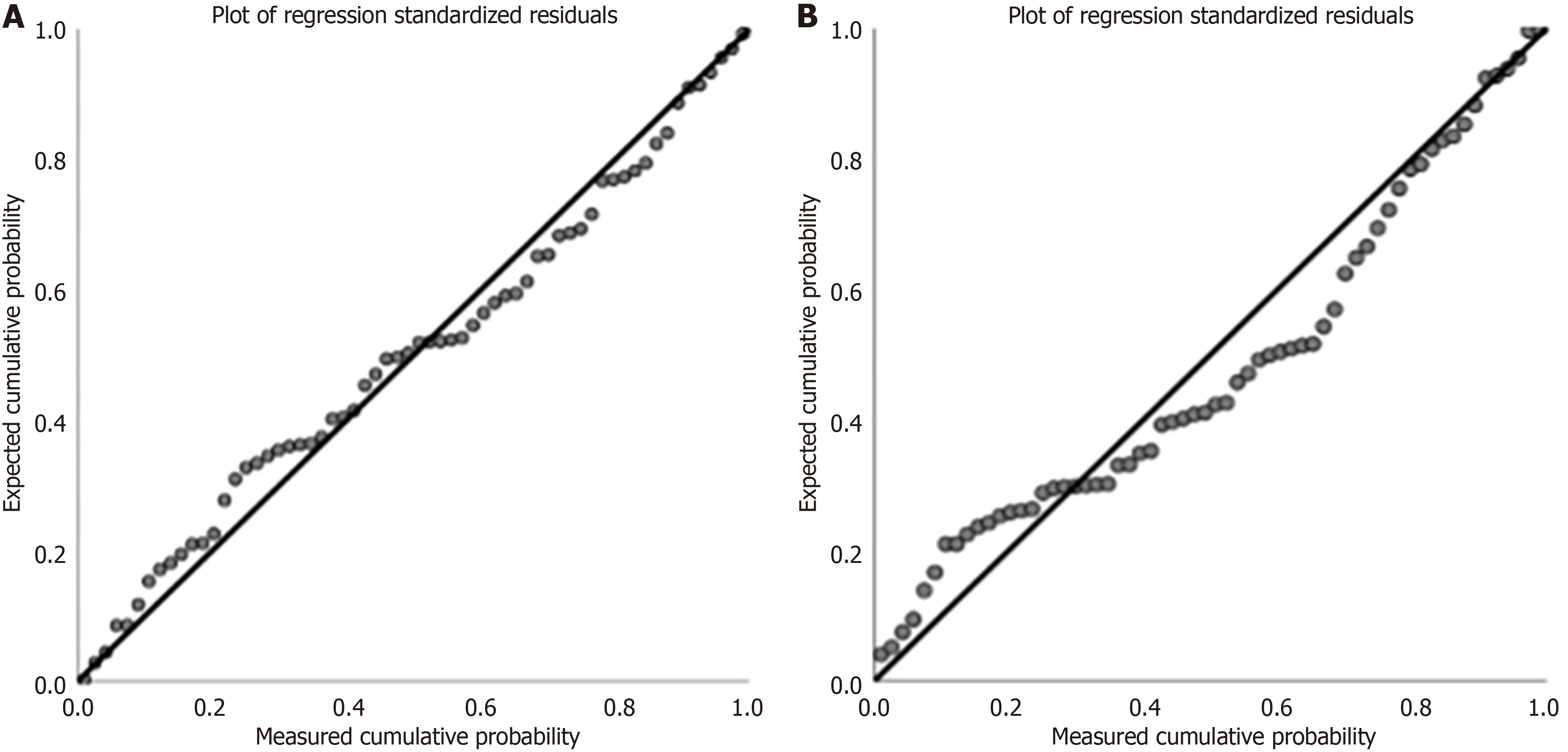
The study comprised a sample size of 124 cases, evenly distributed into two groups, namely, moderate-risk and high-risk groups, with 62 cases in each. The number of influencing factors to be analyzed was approximately seven, which meets the requirement for multiple linear regression analysis (the sample size must be about five to 10 times the number of independent variables). Multiple linear regression analysis was performed using SAS9.2, after which plots of the standardized residuals were drawn. The regression analysis tolerances were both greater than 0.2, and the variance inflation factors were less than 10, indicating that there was no serious collinearity problem (Table 2). The residuals distribution was approximately normal, showing that the model had a good fit with the data (Figure 2).
| Indicators | Standardized coefficient | Correlation test | Collinearity | ||||||
| Beta | T | Sig. | Zeroth | Partial | Semipartial | Tolerances | VIF | ||
| Moderate-risk | Const of function | 2.604 | 0.012 | ||||||
| Fast track grouping | -0.084 | -2.142 | 0.037 | -0.166 | -0.280 | -0.081 | 0.924 | 1.083 | |
| Age | 0.067 | 1.687 | 0.097 | 0.265 | 0.224 | 0.064 | 0.904 | 1.106 | |
| Gender | 0.009 | 0.218 | 0.828 | 0.200 | 0.030 | 0.008 | 0.786 | 1.272 | |
| Total blood transfusion | 0.471 | 8.037 | 0.000 | 0.859 | 0.738 | 0.303 | 0.413 | 2.422 | |
| Door-to-endoscopy time | 0.008 | 0.204 | 0.839 | 0.260 | 0.028 | 0.008 | 0.853 | 1.172 | |
| Emergency room stay time | -0.046 | -1.181 | 0.243 | 0.006 | -0.159 | -0.044 | 0.933 | 1.072 | |
| ICU length of stay | 0.546 | 10.383 | 0.000 | 0.886 | 0.816 | 0.391 | 0.513 | 1.950 | |
| High-risk | Const of function | 0.077 | 0.939 | ||||||
| Fast track grouping | -0.076 | 0.785 | 0.148 | -0.280 | 0.106 | 0.051 | 0.679 | 1.472 | |
| Age | -0.068 | -0.909 | 0.367 | 0.004 | -0.123 | -0.059 | 0.740 | 1.351 | |
| Gender | 0.101 | 1.412 | 0.164 | -0.037 | 0.189 | 0.091 | 0.814 | 1.228 | |
| Total blood transfusion | 0.008 | 0.110 | 0.912 | 0.009 | 0.015 | 0.007 | 0.889 | 1.125 | |
| Door-to-endoscopy time | 0.202 | 2.781 | 0.007 | 0.194 | 0.354 | 0.180 | 0.793 | 1.262 | |
| Emergency room stay time | 0.473 | 6.072 | 0.000 | 0.721 | 0.637 | 0.392 | 0.687 | 1.455 | |
| ICU length of stay | 0.562 | 7.256 | 0.000 | 0.740 | 0.703 | 0.469 | 0.695 | 1.438 | |
We selected hospitalization costs as the dependent variable because this parameter comprehensively evaluated various aspects related to the study subjects’ conditions, including treatment cost, duration, and prognosis. The independent variables included age, gender, GBS score, emergency room stay time, endoscopy time, total blood transfusion volume, ICU treatment time, etc. Due to the significant correlation between GBS score and grouping and the correlation between hospitalization duration and cost, we excluded factors above.
The chosen factors were analyzed with the regression method, and the results in the moderate-risk group showed significant correlations between grouping, total blood transfusion volume, ICU length of stay, and hospitalization expense (Table 2). In the moderate-risk group, the multiple linear regression model for hospitalization costs identified three significant predictors: Fast-track grouping (P = 0.037), total blood transfusion volume (P < 0.001), and ICU length of stay (P < 0.001). Non-significant predictors included age, gender, door-to-endoscopy time, and emergency room stay time. For the high-risk group, significant predictors of hospitalization costs included door-to-endoscopy time (P = 0.007), emergency room stay time (P < 0.001), and ICU length of stay (P < 0.001). Non-significant predictors were fast-track grouping, age, gender (standardized β = 0.101, t = 1.412, P = 0.164), and total blood transfusion volume.
Based on the fitted regression analysis, it can be calculated that the hospitalization costs for the fast-track group could be reduced by 8.4% compared to those of the control group. In the high-risk group, the portal endoscopy time, total blood transfusion volume, and ICU length of stay showed significant correlations with the dependent variables (Table 2).
Recent studies have confirmed that the GBS is highly valuable in urgently predicting the need for clinical intervention and assessing the risk of death, as it does not require an endoscopy but only uses patient history and laboratory data. The GBS is also increasingly used in emergency pre-examination triage, emergency nursing, and ICU monitoring assessments[17-19]. Although research has found that the Rockall score is superior to the GBS score in predicting mortality, the former requires endoscopy results, making it difficult to calculate quickly, and is thus more suitable for secondary assessment after the patient’s condition has stabilized[20]. The emergency fast track is a crucial mode in current emergency medicine. The Emergency Physicians Branch of the Chinese Medical Doctor Association, in collaboration with the China Emergency Medicine Specialist Alliance, has been promoting the standardization of the “Emergency Treatment Fast Track” project nationwide for AUGIB. This initiative aims to swiftly enhance the diagnostic and treatment level of emergency care for high-risk upper gastrointestinal bleeding, thereby reducing patient mortality rates. Promoting the standardization of the Emergency Treatment Fast Track helps address issues such as the inaccurate early-risk assessment of AUGIB patients, prolonged stay of hidden high-risk patients in resuscitation room, and lack of sufficient collaboration among various disciplines.
However, previous studies have shown that if the assessment is inaccurate, frequent activation of the Emergency Treatment Fast Track may lead to the excessive use of emergency medical supplies and increased costs for patients during diagnosis and treatment[1]. Given the good evaluative value of the GBS in the risk stratification of AUGIB patients, it is often used clinically to establish an Emergency Treatment Fast Track after assessment. Current research suggests that a score of 6 is the optimal threshold for predicting endoscopic intervention treatment and death in AUGIB patients[7,17,20]. Therefore, GBS ≥ 6 can be considered an important assessment tool for determining moderate-to-high-risk stratification in AUGIB patients and the active initiation of emergency resuscitation treatment. There is currently a lack of detailed comparisons of emergency operation efficiency, hospital resource consumption, and other indicators in moderate- to high-risk AUGIB patients under different GBS risk stratifications as well as recommendations for the ideal GBS threshold value based on these indicators.
In this study, the Shapiro-Wilk and Mann-Whitney U tests indicated that, compared to the control group, the high-risk fast-track group showed significant reductions in door-to-endoscopy time, total blood transfusion volume, and hospitalization costs (P < 0.05). This finding suggests that for patients with upper gastrointestinal bleeding at a risk level of GBS ≥ 12, the initiation of an emergency treatment fast track can further enhance the effectiveness of emergency endoscopic hemostasis, reduce patients’ hospitalization costs, and decrease the consumption of medical resources. However, for the moderate-risk group with 12 > GBS ≥ 6, the initiation of the fast track did not show significant differences in terms of rescue time efficiency, hospitalization duration, or medical resource consumption compared to the control group (P > 0.05). This finding indicates that the multidisciplinary emergency treatment fast track does not exhibit a clear advantage for moderate-risk AUGIB patients in terms of emergency efficacy and medical resource consumption. This may be due to the relatively slower progression of the condition in moderate-risk AUGIB patients and their lower dependence on the timing of emergency treatment. Further research may be required to explore optimal emergency treatment strategies for moderate-risk AUGIB patients. The Mann-Whitney U test also found that the fast-track grouping did not show significant improvements in the duration of the emergency resuscitation room stay, ICU length of stay, or total hospitalization time for patients at both risk levels of AUGIB. This finding suggests that the main effects of the fast track are to shorten the door-to-endoscopy time and reduce resource consumption.
After a regression model was used to analyze the original data, the moderate-risk fast-track grouping factor appeared significant. When a frequency test was used, in contrast, this factor did not appear to be significant. Hence, it appears that hospitalization costs are generated by multiple mixed effects. We built well-fitting regression models to identify multiple factors efficiently, as shown. The subsequent residual plot shows that the model for moderate-risk with a high efficiency level performs accurately. Previous separate comparisons between groups’ costs failed to identify the confounding effects of multiple factors. There is no significance in the high-risk groups. According to the residual plot, the reason may be related to the excessive variation of the sample data and the inability of the model to fit the data accurately. In the future, it is necessary to expand the sample size and reduce the variation of sample data to ensure the accuracy of analysis. In summary, both the comparison of components between groups and the regression results suggest that the impact of fast-track grouping is significant on hospitalization costs for both moderate- and high-risk patients. It also suggests that other factors, such as total blood transfusion volume, ICU length of stay, and special examinations, may lead to increased hospitalization costs.
This is the first study to demonstrate that GBS ≥ 12 can be recommended as the threshold for implementing the fast track for emergency treatment. Future studies could expand the sample size and seek multi-center research data to further confirm the findings. The evaluation results could also delve deeper into aspects such as the societal benefits of implementing the emergency treatment fast track, patient satisfaction, and mortality rates with the aim of finding the optimal threshold for establishing an emergency treatment fast track based on GBS risk stratification.
Our study provides evidence-based guidance for optimizing the emergency management of AUGIB: (1) The GBS threshold of ≥ 12 represents an optimal cutoff point for implementing fast-track protocols, with significant benefits observed exclusively in this high-risk patient group; (2) Implementation of the fast-track protocol for patients with GBS ≥ 12 yielded measurable clinical improvements, including reduced door-to-endoscopy time (median 13.5 minutes vs 21.5 minutes), decreased blood transfusion requirements (median 3 units vs 6.75 units), and lower hospitalization costs (median 1570.26 USD vs 2812.44 USD); and (3) The absence of significant benefits in moderate-risk patients (6 ≤ GBS < 12) indicates that selective application of fast-track protocols based on accurate risk stratification optimizes resource allocation while ensuring high-quality care for those with the greatest need. These findings have direct clinical implications for emergency departments and gastroenterology services. The GBS ≥ 12 threshold provides clinicians with a clear, objective criterion for rapid decision-making when triaging AUGIB patients, enabling more effective resource utilization and standardization of care pathways. By reserving intensive multidisciplinary interventions for patients who will derive demonstrable benefit, clinicians can improve outcomes for high-risk patients while preventing unnecessary resource utilization for those who can be effectively managed with standard protocols. This approach offers a practical balance between ensuring optimal care for the most critical patients and responsible stewardship of limited healthcare resources.
| 1. | Li Q, Cheng J, Zheng H, Yang H, Zeng FZ, Quan JC, Zhou SQ, Fan CQ, Zhou RJ. [Analysis of the curative effect of emergency fast track in the treatment of acute upper gastrointestinal bleeding based on propensity score matching analysis]. Zhongguo Jijiu Yixue. 2022;42:326-330. [DOI] [Full Text] |
| 2. | Paraoan MT. An important practical omission of SIGN guideline on management of acute upper and lower gastrointestinal bleeding. BMJ. 2021;377:a1832-a1839. |
| 3. | Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, Saluja V, Mohan Agarwal P, Bihari C, Shasthry SM, Jindal A, Bhardwaj A, Kumar G, Sarin SK. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology. 2020;71:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 4. | Redondo-Cerezo E, Vadillo-Calles F, Stanley AJ, Laursen S, Laine L, Dalton HR, Ngu JH, Schultz M, Jiménez-Rosales R. MAP(ASH): A new scoring system for the prediction of intervention and mortality in upper gastrointestinal bleeding. J Gastroenterol Hepatol. 2020;35:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Aquarius M, Smeets FG, Konijn HW, Stassen PM, Keulen ET, Van Deursen CT, Masclee AA, Keulemans YC. Prospective multicenter validation of the Glasgow Blatchford bleeding score in the management of patients with upper gastrointestinal hemorrhage presenting at an emergency department. Eur J Gastroenterol Hepatol. 2015;27:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Stanley AJ, Laine L, Dalton HR, Ngu JH, Schultz M, Abazi R, Zakko L, Thornton S, Wilkinson K, Khor CJ, Murray IA, Laursen SB; International Gastrointestinal Bleeding Consortium. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 7. | Sengupta N, Tapper EB, Patwardhan VR, Ketwaroo GA, Thaker AM, Leffler DA, Feuerstein JD. High Glasgow Blatchford Score at admission is associated with recurrent bleeding after discharge for patients hospitalized with upper gastrointestinal bleeding. Endoscopy. 2016;48:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Laursen SB, Oakland K, Laine L, Bieber V, Marmo R, Redondo-Cerezo E, Dalton HR, Ngu J, Schultz M, Soncini M, Gralnek I, Jairath V, Murray IA, Stanley AJ. ABC score: a new risk score that accurately predicts mortality in acute upper and lower gastrointestinal bleeding: an international multicentre study. Gut. 2021;70:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 9. | Martínez-Cara JG, Jiménez-Rosales R, Úbeda-Muñoz M, de Hierro ML, de Teresa J, Redondo-Cerezo E. Comparison of AIMS65, Glasgow-Blatchford score, and Rockall score in a European series of patients with upper gastrointestinal bleeding: performance when predicting in-hospital and delayed mortality. United European Gastroenterol J. 2016;4:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Laursen SB, Leontiadis GI, Stanley AJ, Møller MH, Hansen JM, Schaffalitzky de Muckadell OB. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc. 2017;85:936-944.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 11. | Ramaekers R, Mukarram M, Smith CA, Thiruganasambandamoorthy V. The Predictive Value of Preendoscopic Risk Scores to Predict Adverse Outcomes in Emergency Department Patients With Upper Gastrointestinal Bleeding: A Systematic Review. Acad Emerg Med. 2016;23:1218-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Dong L, Xu WG, Bian G, Wang WL. [A comparative study of Glasgow-Blatchford score early combined with multi-disciplinary team and traditional consultation mode in the treatment of upper gastrointestinal bleeding]. Lichuang Jizhen Zazhi. 2023;24:253-257. [DOI] [Full Text] |
| 13. | Xu PJ, Wu XL, Xu GS, Xu ZX, Wang P. [Effect of emergency fast-track treatment on dangerous upper gastrointestinal bleeding]. Huaxi Yixue. 2021;37:1636-1640. [DOI] [Full Text] |
| 14. | Luo Q, Zhou W, Xie FG, Chu YY, Pan ZH, Qian M, Zhang XZ. [Construction effect evaluation of fast-track emergency treatment mode for dangerous upper gastrointestinal hemorrhage]. Linchuang Jizhen Zazhi. 2023;24:73-77. [DOI] [Full Text] |
| 15. | Lim LG, Ho KY, Chan YH, Teoh PL, Khor CJ, Lim LL, Rajnakova A, Ong TZ, Yeoh KG. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy. 2011;43:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 17. | Schembre DB, Ely RE, Connolly JM, Padhya KT, Sharda R, Brandabur JJ. Semiautomated Glasgow-Blatchford Bleeding Score helps direct bed placement for patients with upper gastrointestinal bleeding. BMJ Open Gastroenterol. 2020;7:e000479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Lee HA, Jung HK, Kim TO, Byeon JR, Jeong ES, Cho HJ, Tae CH, Moon CM, Kim SE, Shim KN, Jung SA. Clinical outcomes of acute upper gastrointestinal bleeding according to the risk indicated by Glasgow-Blatchford risk score-computed tomography score in the emergency room. Korean J Intern Med. 2022;37:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Rivieri S, Carron PN, Schoepfer A, Ageron FX. External validation and comparison of the Glasgow-Blatchford score, modified Glasgow-Blatchford score, Rockall score and AIMS65 score in patients with upper gastrointestinal bleeding: a cross-sectional observational study in Western Switzerland. Eur J Emerg Med. 2023;30:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/