Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.107610
Revised: April 20, 2025
Accepted: June 6, 2025
Published online: July 27, 2025
Processing time: 118 Days and 3.9 Hours
Precancerous lesions of gastric cancer (PLGC) are crucial for the progression to gastric cancer, and early intervention in PLGC is pivotal in preventing its deve
Core Tip: Precancerous lesions of gastric cancer refer to a series of pathological changes in the gastric mucosa, including atrophic gastritis, intestinal metaplasia, and dysplasia. These lesions are significant risk factors for the development of gastric carcinoma. Genetically engineered mice, modified through techniques such as gene knockout, gene knock-in, transgenesis, and point mutation, are utilized to investigate gene function, disease mechanisms, and to develop novel therapeutic strategies. This review discusses the genetically engineered mouse models employed in the study of precancerous lesions of gastric cancer, with an emphasis on elucidating their pathological mechanisms.
- Citation: Quan Y, Jia YB, Wu CH, Jia QL, Chen YQ, Gu ZJ, Ling JH. Genetically engineered mouse models in gastric precancerous lesions research. World J Gastrointest Surg 2025; 17(7): 107610
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/107610.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.107610
In 2022, there were over 968000 new cases of gastric cancer (GC) and nearly 660000 deaths worldwide, ranking it fifth in both incidence (4.9%) and mortality (6.8%) globally[1]. Due to the lack of evident symptoms in the early stages, GC is often diagnosed at a late stage, leading to a high mortality rate[2]. However, precancerous lesions of GC (PLGC) present a unique opportunity for monitoring and intervening in the development of GC, paving the way for potential early intervention and treatment. PLGC comprise a series of pathological changes in the gastric mucosa, including atrophic gastritis, intestinal metaplasia (IM), and dysplasia. The pathological mechanisms involve chronic inflammation, DNA damage, cell proliferation, and apoptosis[3].
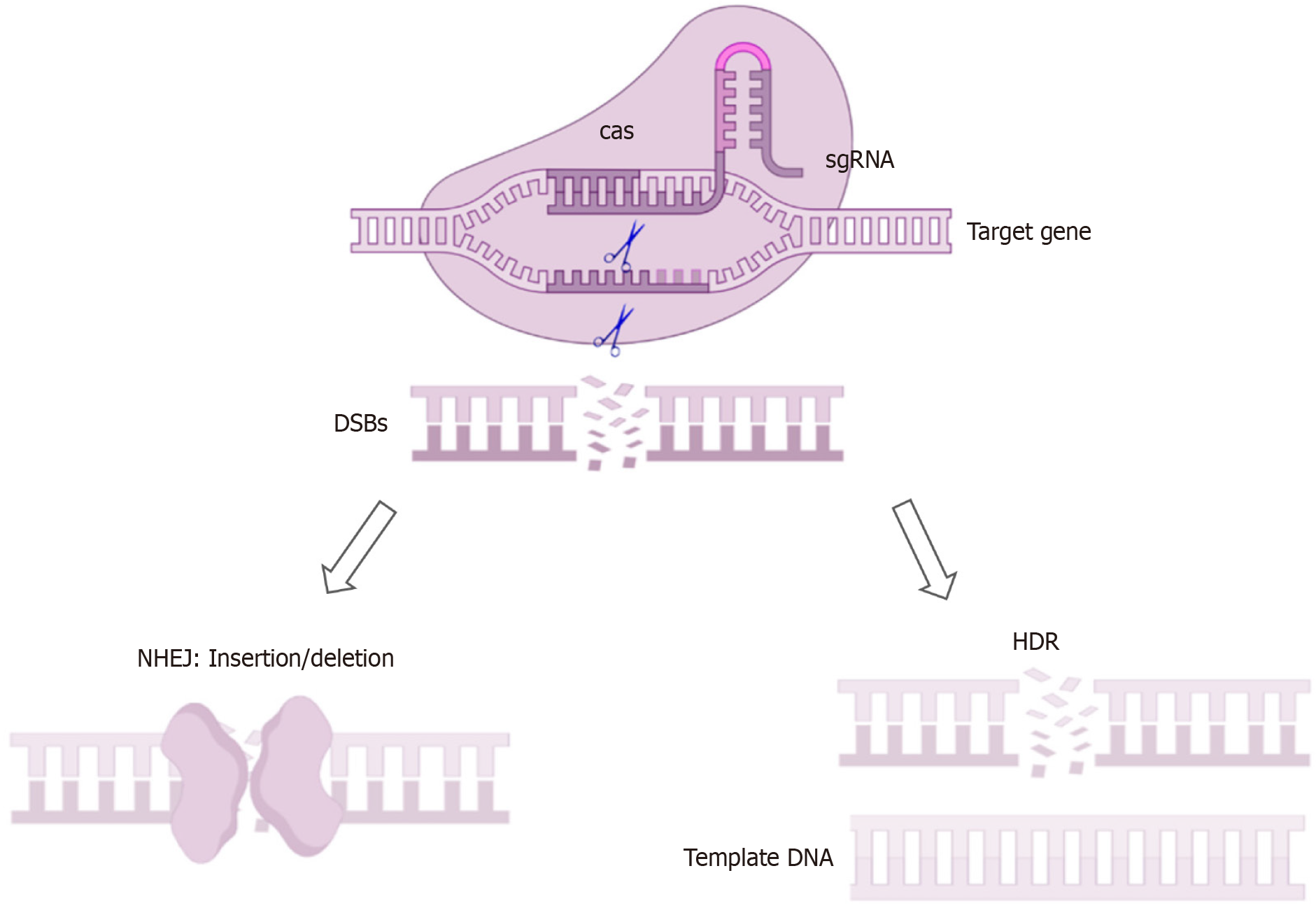
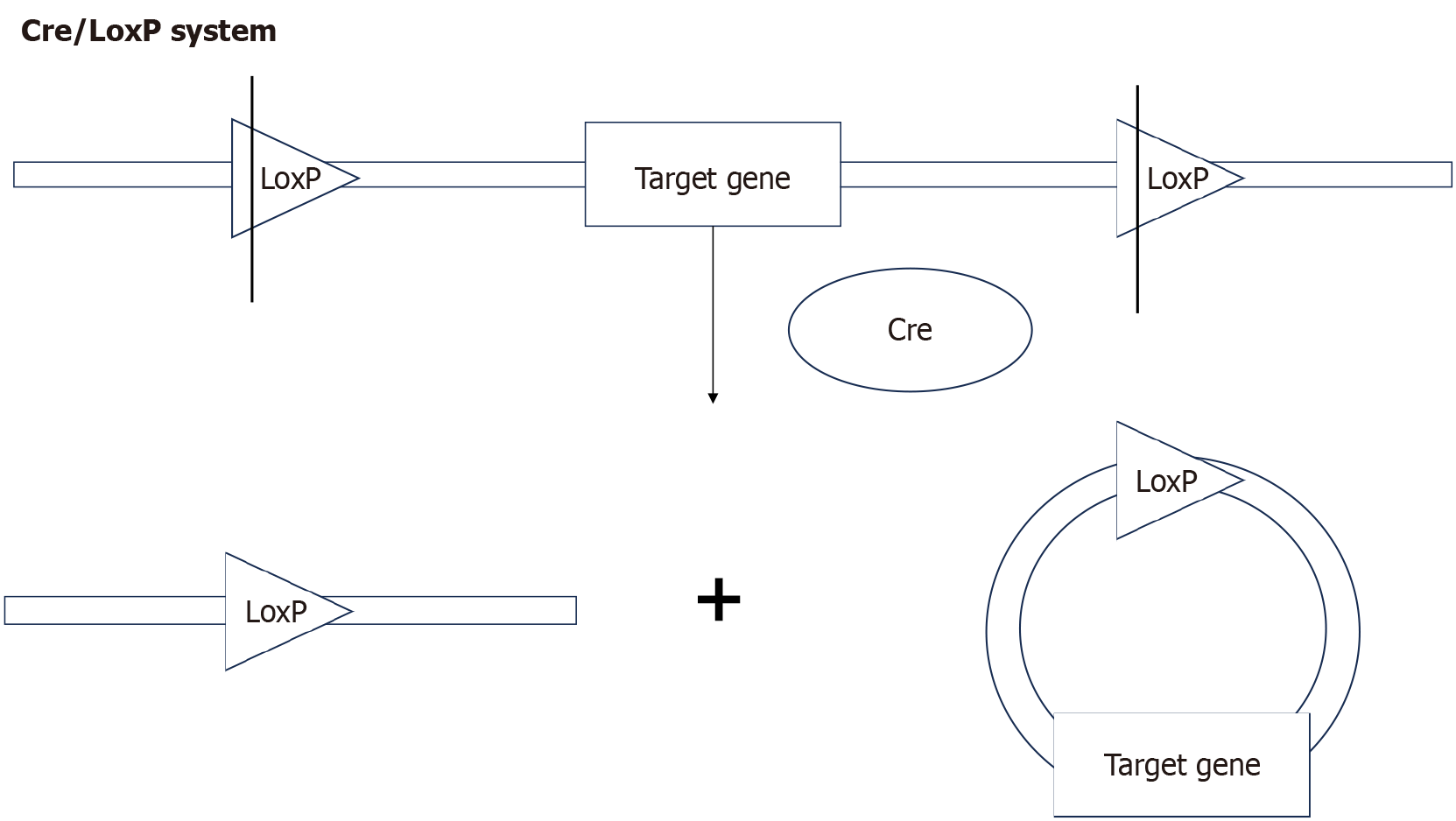
Genomic sequencing study showed that the protein-coding genes of mice and humans share a high degree of similarity[4]. Through precise genetic manipulation, genetically engineered mouse models (GEMMs) can model the development of human PLGC, replicating key molecular events. Commonly use DNA pronuclear microinjection, site-specific nuclease technology, and the cyclization recombinase (Cre)/locus of X-over P1 (LoxP) system. DNA pronuclear microinjection involves the injection of exogenous DNA into the pronucleus of fertilized eggs, effectively transferring the target gene into mice[5]. Site-specific nuclease technology is based on engineered nucleases composed of specific DNA-binding domains fused with nonspecific DNA-cutting modules. These chimeric nucleases induce target DNA double-strand breaks, which stimulate cellular DNA repair mechanisms, including non-homologous end joining and homology-directed repair, thereby achieving gene knockout or insertion[6]. This category includes zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated proteins (Cas), with CRISPR/Cas becoming the mainstream technology due to its high efficiency (Figure 1). In the Cre-LoxP system, a single Cre enzyme recognizes repeated LoxP sites, excising the flanked target DNA (Figure 2). Conditional knockout can be achieved by fusing the Cre enzyme with a drug-binding domain or placing it under the control of a specific tissue promoter[7].
This review systematically summarizes the application of GEMMs in the study of the pathological mechanisms of PLGC, with a focus on their role in gastrin/acid regulation, inflammatory factors, oncogenes/tumor suppressor genes, and apoptosis. It particularly emphasizes their unique value in simulating pathological processes and elucidating molecular mechanisms, as well as predicting disease progression and therapeutic responses.
Gastrin is a gastrointestinal hormone secreted by G cells in the gastric antrum, promoting gastric acid secretion and participating in various normal and abnormal biological processes, including the maintenance of gastric mucosa, the proliferation of enterochromaffin-like cells, and tumorigenesis[8]. Infection with Helicobacter pylori (H. pylori) can result in hypergastrinemia, which is viewed as a contributing factor to the development of GC[9]. INS-GAS mice, under the control of the insulin gene promoter, overexpress the gastrin gene, resulting in high circulating gastrin levels and consistently developing atrophic gastritis with hypochlorhydria[10], can spontaneously develop atrophic gastritis, IM, dysplasia, and adenocarcinoma by approximately 20 months[11,12]. The INS-GAS mouse model is often used in combination with Helicobacter felis (H. felis) infection, after infection, INS-GAS mice exhibit accelerated carcinogenesis, severe atrophic gastritis, and gastric body dysplasia, without dysplasia observed in the antrum, indicating a differential carcinogenic effect of hypergastrinemia on the gastric body and antrum[13]. Additionally, INS-GAS mice exhibit strain and gender differences, with FVB/N mice being more sensitive than C57BL/6 mice. Male gastric tissue tends to exhibit a more pronounced response to H. pylori infection, a high-salt diet, and their combination, resulting in more severe pathological changes, which is consistent with higher GC incidence in males compared to females[14]. Furthermore, ovariectomized (OVX) INS-GAS mice develop more severe gastric mucosal diseases than intact mice, suggesting that estradiol may have a protective effect on the gastric mucosa of female INS-GAS mice[15]. Further findings indicate that exogenous estradiol exerts a protective effect against PLGC by stimulating interleukin (IL)-10 activity, enhancing Th2-mediated immune responses, and inhibiting epithelial cell proliferation[16].
Due to its maturity, the INS-GAS mouse model has also been frequently used in recent years to study the effects of different drugs and strains on the gastrointestinal microbiota. This model accurately reproduces the gastric inflammation observed in human H. pylori infection and the gradual increase in FOXM1 expression (a GC-related protein). Using this model, thiopeptide-producing C. acnes strains can reduce H. pylori-induced gastric inflammation and FOXM1 expression[17]. Another study indicated that long-term vonoprazan treatment can promote gastric damage and gastrointestinal microbiota imbalance in male INS-GAS mice[18]. The research on the microbiota also confirmed the impact of sex differences on the development of PLGC: Male mice and OVX female mice exhibited more severe gastric lesions, while intact female mice had a higher abundance of beneficial bacteria. In contrast, pathogenic bacteria were more abundant in male mice and OVX female mice. Moreover, exchanging gut microbiota (through co-housing) significantly reduced the differences in gastric lesions between OVX and intact female mice[19].
Gastrin-/- mice spontaneously develop body atrophy and antrum hyperplasia, suggesting different regions of the stomach respond differently to the absence of gastrin[20]. One study revealed that trefoil factor 1 (TFF1) is reversibly decreased in Gastrin-/- mice. Gastrin activates TFF1 transcription through a mitogen-activated protein kinase kinase 1-, rapidly accelerated fibrosarcoma 1-, and extracellular signal-regulated kinase (ERK)-dependent but Ras-independent pathway and can also indirectly activate TFF1 through cell-cell interactions or soluble factors[21]. However, another study showed that TFF1 expression did not change significantly in young mice, but was significantly upregulated under long-term achlorhydric conditions. Additionally, achlorhydria in Gastrin-/- mice leads to the upregulation of immune defense genes and genes primarily expressed in the intestine. Exogenous gastrin can restore gastric acidity and reverse gene expression changes, but elderly Gastrin-/- mice develop irreversible IM, possibly related to caudal type homeobox (CDX) 2 expression and CDX1 activation. Achlorhydria also promotes bacterial overgrowth, with these bacteria capable of forming carcinogenic N-nitrosamines[22]. The Gastrin-/- model combined with N-methyl-N-nitrosourea and H. felis model replicates several key features of human GC, including a high mutational burden and increased programmed death-ligand 1 expression, making it a valuable model for studying the mechanisms of resistance to programmed death 1 inhibitors and the role of its ligand programmed death-ligand 1[23].
H+/K+-ATPase, an enzyme predominantly located in the parietal cells of the stomach, utilizes the energy from adenosine triphosphate hydrolysis to exchange intracellular H+ for extracellular K+. The H+ then combines with Cl- to form HCl, aiding in food digestion. The α subunit, encoded by the ATP4A gene, contains the catalytic site and mediates ion transport, while the β subunit, encoded by the ATP4B gene, stabilizes the α subunit[24]. In autoimmune gastritis, the gastric proton pump H+/K+-ATPase is the principal autoantigen recognized by autoreactive T cells[25]. Mice with knockout of either ATP4A or ATP4B genes exhibit achlorhydria and elevated gastrin levels.
Atp4a-/- mice exhibit hyperplasia, and as age increases, the hyperplasia and metaplasia of the gastric mucosa progressively deteriorate, though GC does not develop. Additionally, it was observed that female mice exhibit more pronounced hyperplasia than male mice, consistent with the higher incidence of autoimmune atrophic gastritis (AAG) in females[26,27]. Significant alterations in mucin 2 expression in Atp4a-/- mice were observed, suggesting that gastric acid levels may influence mucus expression. The absence of the α subunit leads to activation of the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway, which subsequently promotes the Warburg effect. It was also found the lifespan of Atp4a-/- mice does not differ significantly from wild-type mice, making them an excellent model for studying the progression from atrophic gastritis to IM[28]. Additionally, knockout of the α subunit does not impair the viability of parietal cells or the differentiation of chief cells, though parietal cells exhibit abnormal secretory membranes and tubulovesicular membranes[29,30].
When using this model to explore the treatment of IM with metformin, it was found that metformin significantly reduced the progression of IM lesions in the Atp4a mouse model, possibly by downregulating the nuclear factor kappa B and phosphatidylinositol 3-kinase/ protein kinase B/ mammalian target of rapamycin/hypoxia-inducible factor 1 alpha signaling pathways[31]. To better understand the relationship between impaired transmembrane proton export capability of parietal cells and tumorigenesis, researchers constructed an ATP4A mutant knock-in mouse model (Atp4ap.R703C mice). This model recapitulates atrophic gastritis and IM by affecting mitochondrial function and biosynthesis, thereby activating reactive oxygen species signaling, triggering parietal cell death, and it suggests that autoimmune gastritis may not be caused by the conventional autoimmune response leading to parietal cell atrophy but rather by genetically mediated ATP4A dysfunction[32].
Atp4b-/- mice exhibit elevated gastrin levels, marked gastric hypertrophy, submucosal cysts, and widespread expression of spasmolytic polypeptide-expressing metaplasia (SPEM) and neutrophil cell markers in the corpus, while these phenomena are not observed in the antrum. This finding is perfectly in line with the characteristics of human AAG[33]. Furthermore, in Atp4b-/- mice, parietal cell development was delayed during embryogenesis, suggesting that H+/K+-ATPase may play a critical role in the developmental pathways of gastric mucosal cells. Moreover, parietal cells exhibit morphological abnormalities, including abnormally dilated tubules and a lack of typical tubulovesicular structures, which are associated with impaired acid secretion function[34]. Currently, there is limited research on the mechanisms of Atp4b-/- mice, warranting further investigation.
Slc26a9 belongs to the Slc26a family of anion transport proteins. In the stomach, it is primarily found on parietal cells and functions as a chloride channel/bicarbonate exchanger[35]. Slc26a9-/- mice exhibited achlorhydria and a loss of tubulovesicular structures in parietal cells, indicating that Slc26a9 is essential for gastric acid secretion by affecting the activity of tubulovesicular membranes and regulating chloride secretion in parietal cells[36]. Moreover, complete and parietal cell-specific knockout of Slc26a9 in mice could both accelerate the carcinogenesis. Additionally, selective deletion of Slc26a9 in parietal cells led to dysregulated differentiation of stem and progenitor cells, induced hyperproliferation, and inhibited apoptosis in an inflammatory environment[37].
Interferon-γ (IFN-γ) is an inflammatory cytokine produced by activated immune cells such as T cells and natural killer (NK) cells. Some studies suggest that IFN-γ has antitumor properties, while others indicate it may promote tumor growth and progression[38]. One research showed that H+/K+-ATPase-IFN-γ mice overexpress IFN-γ under the control of the H+/K+-ATPase α subunit promoter, resulting in spontaneous inflammation, SPEM, atrophy of parietal and chief cells, gland atrophy, and dysplasia, with the development of adenomas and adenocarcinomas. IFN-γ can directly induce gastric epithelial cell death, playing a crucial role in the progression from gastritis to atrophy and metaplasia, and is essential for the development of metaplasia[39]. Another research showed that H+/K+-ATPase-IFN-γ mice do not develop spontaneous atrophic gastritis or metaplasia, with histopathology resembling normal tissue. High levels of IFN-γ have been reported to inhibit gastric progenitor cell proliferation and reduce epithelial cell apoptosis through autophagy, thereby cooperatively inhibiting bacterial infection and carcinogenesis in the gastric mucosa[40]. In non-small cell lung cancer, studies have indicated that low levels of IFN-γ confer cancer stem cell-like properties, while high levels of IFN-γ induce apoptosis[41]. It is hypothesized that different levels of IFN-γ lead to distinctly different experimental outcomes.
IL-11, as both an anti-inflammatory and pro-inflammatory cytokine, has been shown to be involved in various inflammation-associated cancers, primarily due to its ability to activate the Janus kinase-signal transducers and activators of transcription (STAT) 3 pathway[42]. To investigate whether locally elevated expression of the IL-11 ligand can initiate GC pathogenesis independently of gp130-Janus kinase-STAT pathway mutations, researchers developed K19-IL11Tg mice. These mice specifically express IL-11 in both gastric corpus and antrum, with no expression in other tissues. And K19-IL11Tg mice spontaneously develop PLGC, demonstrating that persistent abnormal elevation of IL-11 alone is sufficient to initiate the GC cascade[43]. However, in K19-IL11Tg mice, high-grade dysplasia and malignant lesions are usually not observed, and the progression of carcinogenesis is slow, localized to the gastric corpus. Another study indicated that IL-11 is a cytokine secreted by parietal cells, it inhibits gastric acid secretion by downregulating the expression of ion transport genes in parietal cells, as well as the expression of cholecystokinin B receptors and histamine H2 receptors[44].
Gp130 is not a pro-inflammatory cytokine, however, as the shared signaling subunit for IL-6 family cytokines, it participates in inflammatory responses and contains two distinct functional modules that signal through STAT1/3 and Src homology 2 domain-containing phosphatase 2/ERK pathways[45]. The Gp130 757F/F mouse is a knock-in mutant carrying Y757F and V760A mutations, which disrupt the pY757xxV760 SHP2 binding domain, inhibiting the SHP2-Ras-ERK signaling cascade while elevating IL-6 and IL-11 levels. At 6-8 weeks of age, Gp130 757F/F mice spontaneously develop hyperplastic lesions in the antral mucosa, and at 3 months, they spontaneously develop gastric adenomas. It is hypothesized that the hyperplastic lesions due to enhanced STAT3 activity, in the absence of negative feedback signaling from the SHP2-Ras-ERK pathway[46]. Moreover, knocking out one allele of STAT3 in Gp130 757F/F mice suppresses the growth of gastric adenomas, confirming this hypothesis[47]. Furthermore, to investigate the role of IL-6, Gp130 757F/F mice with IL-6 knockout were studied, revealing that the lack of IL-6 did not affect tumor development, providing further evidence that IL-11 alone is sufficient to initiate the GC cascade. Further crossing Gp130 757F/F mice with RAG1 mice showed that the lack of mature T, B, and NK T cells did not affect tumor incidence, size, or IL-6 and IL-11 synthesis, indicating that T, B, and NK T cells are not the primary sources of IL-6 and IL-11[48].
IL-1β is a potent pro-inflammatory mediator, typically expressed at low levels by macrophages, and its production can be induced by H. pylori infection[49]. H+/K+-ATPase-hIL-1β mice, driven by the H+/K+-ATPase promoter, expressing high IL-1β level, parietal cells that continuously secrete IL-1β, leading to spontaneous inflammation, atrophy, metaplasia, and dysplasia. The histopathological scores of H+/K+-ATPase-IL-1β mice expressing high levels of IL-1β were significantly higher than those of H+/K+-ATPase-IL-1β mice expressing lower levels of IL-1β. It was also found that IL-1β overexpression accelerated the progression of gastritis and cancer development in H. felis-infected mice[50]. Conversely, in IL-1R knockout mice, following H. pylori infection, gastric atrophy was more frequently observed in WT mice with functional IL-1β signaling compared to IL-1R1-/- mice. Additionally, E-cadherin methylation was not detected in IL-1R1-/- mice, suggesting that reducing IL-1β activity may alleviate the burden of inflammatory diseases[51]. Given that H+/K+-ATPase-IL-1β mice continuously secrete IL-1β through parietal cells, leading to the early mobilization and recruitment of myeloid-derived suppressor cells, this model has been employed to explore the involvement of myeloid-derived suppressor cells in GC.
The Kras gene is a common oncogene, with clinical studies indicating mutations in the Kras gene in patients with gastritis and gastric adenoma[52]. K19-kras transgenic mice, utilizing the K19 promoter, overexpress the oncogene K-Ras in gastric epithelial cells. One research showed that K19-kras mice spontaneously develop gastric atrophy, metaplasia, and dysplasia similar to H. felis infection. H. felis infection in K19-kras mice does not accelerate the progression of GC, suggesting that Kras mutations can compensate for the lack of infectious stimuli to induce inflammation and carcinogenesis[53]. In another research, tamoxifen was used to induce the expression of the Kras gene in chief cells of Mist1-Kras mice. This model demonstrated all the PLGC of human, including the differentiation of chief cells into SPEM as the initial step of metaplastic changes, SPEM progressing to IM, and the development of carcinoma. Lineage tracing confirmed that chief cells are the direct origin of metaplasia, and sustained activation of Ras signaling drives the further progression of metaplasia. It was further proposed that using mitogen-activated protein kinase kinase inhibitors could reverse the metaplastic process and allow the normal gastric epithelium to repopulate the mucosa[54].
Carbonic anhydrase IX (CAIX) is mainly expressed on the basolateral membrane of surface cells in the gastric mucosa and is a membrane-bound carbonic anhydrase that plays a critical role in tumor cell metabolism, pH regulation, migration, and invasion[55]. Car9-/- mice exhibit significant hyperplasia, an expanded area of cell proliferation, an increased number of mucus-secreting pit cells, and a decrease in chief and parietal cells. However, their gastric acid secretion, pH, and systemic plasma electrolytes do not differ significantly from those of normal mice. It is hypothesized that CAIX does not directly participate in gastric acid secretion but rather contributes to gastric mucosal morphogenesis and homeostasis by controlling cell proliferation and regulating differentiation programs related to migration[56]. However, another study indicated that the absence of CAIX does not affect the maintenance of cellular pH under neutral conditions, whereas under acidic conditions, the lack of CAIX significantly decreases the pH of gastric mucosal cells, particularly in the tight junction regions of gastric mucosal epithelial cells. It is suggested that CAIX plays a crucial role in regulating gastric mucosal pH and protecting the gastric mucosa from acid injury, especially in the apical membrane and tight junctions of surface cells. Additionally, CAIX enabling the extrusion of protons through its basal pH regulatory mechanisms. Additionally, Car9-/- mice exhibit significant downregulation of claudin-18 (CLDN18), suggesting that the lack of CAIX leads to continuous acid reflux mediated by CLDN18 downregulation, resulting in the loss of parietal cells, hypergastrinemia, and gastric gland hyperplasia[57]. Lastly, strain differences was found in Car9-/- mice, with a higher incidence of atrophy observed in mice with a C57BL/6 background compared to those with a BALB/c background under the same conditions[58].
Under normal conditions, both CDX1 and CDX2 are primarily expressed in intestinal epithelial cells and are not expressed in the gastric mucosa. However, both can be detected in gastric mucosa with IM, with expression levels positively correlated with the degree of IM[59], suggesting that CDX1/2 may be crucial for the development of IM in the human stomach[60,61]. In CDX1/2 transgenic mice, the CDX1/2 genes are typically expressed in parietal cells[62]. The specific manifestations of IM differ between the two, CDX1 transgenic mice exhibit IM replacing the gastric mucosa, involving all four types of intestinal epithelial cells, while CDX2 transgenic mice only exhibit pseudo-pyloric gland metaplasia[63,64]. Since metaplasia refers the transformation from one tissue type to another, researchers hypothesize that this process may be initiated through changes in cellular differentiation states. Further research found that CDX1-mediated induction of spalt-like transcription factor 4 and kruppel-like factor 5, both of which are related to lineage reprogramming and stem cell acquisition, plays a significant role in the differentiation of gastric epithelial cells into an intestinal phenotype[65]. During IM of the gastric mucosa in CDX2 transgenic mice, the CDX2 gene induces endogenous CDX1 binding to the unmethylated region of the CDX1 promoter, indicating that CDX2 may act as a trigger for the development of IM[66,67]. Additionally, CDX2 expression gradually decreases during IM, dysplasia, and carcinogenesis in the human stomach, suggesting that CDX2 have opposing roles at different stages of GC[68]. In the early stage, CDX2 drives the differentiation of the gastric epithelial phenotype to the intestinal epithelial phenotype, functioning as an oncogene. In the later stage, as the IM progresses to GC, CDX2 acts as a tumor suppressor gene, inhibiting the invasion and growth of GC[69].
Adenomatous polyposis coli (APC) is a large multifunctional protein involved in various cellular processes, including cell proliferation, cell migration, cell adhesion, cytoskeletal reorganization, and chromosomal stability. Disruption of specific interactions can result in the loss of one or more functions of APC, thereby promoting tumor formation[70]. APC plays a crucial role in the early stages of GC, and well-differentiated adenocarcinomas of the stomach exhibit mutations in the APC gene[71,72]. And high expression of APC is correlated with differences in genome-wide gene/miRNA/methylation expression and associated cellular functional pathways, suggesting that APC is an adverse prognostic factor in T4 stage GC patients[73]. Apcmin/+ mice are an animal model for studying familial adenomatous polyposis, carrying a dominant mutation in the APC gene induced by N-ethyl-N-nitrosourea mutagenesis, resulting in truncation of the gene product at amino acid 850. At 24 weeks of age, Apcmin/+ mice display epithelial cell proliferation and inflammatory infiltration in the forestomach, glandular atrophy and IM in the corpus, and dysplasia in the antrum[74]. And loss of APC function subsequently activates β-catenin/T cell factor transcription, the accumulation of β-catenin may be an initiating event in GC[75].
TFF1 is a member of the TFF family and is abundantly expressed in the mucin-secreting foveolar cells throughout the stomach. It is packaged with the secreted mucin mucin 5AC in the mucin granules of apical mucin secretory vesicles, ready to be released into the adherent mucus layer[76]. TFF1 participates in the formation of a protective mucus barrier, shielding the stomach from gastric acid and digestive enzymes. And TFF1 is upregulated during early H. pylori infection and can specifically bind to the bacterium, causing it to lose its helical shape and significantly slow its movement in the mucus[77]. TFF1 is frequently lost in GC, and the absence of TFF1 in TFF1-/- mice results in the loss of mucosal protection and the activation of multiple pro-inflammatory and carcinogenic signaling pathways, including STAT3, thereby promoting the development and progression of GC[78]. One research indicated that the gastrin gene is one of the most downregulated genes in the low-grade dysplastic tissues of TFF1-/- mice, suggesting that the knockout of TFF1 may affect normal gastric mucosal function by influencing gastrin gene expression[79]. Conversely, gastrin significantly inhibits antral lesions by positively regulating TFF1 gene expression and through epigenetic silencing[80].
CLDN18 is predominantly located on the basolateral membrane of gastric epithelial cells and is a key component of tight junctions in gastric epithelial cells[81]. CLDN18-/- mice exhibit PLGC by 7 weeks of age, intraepithelial neoplasia with invasive submucosal glands by 20-30 weeks, and develop dysplastic polypoid tumors by 2 years[82]. In the stomach, CLDN18 typically forms an intercellular barrier against H+, and its absence leads to intercellular H+ leakage, upregulation of pro-inflammatory cytokines, chronic recruitment of neutrophils, which is followed by atrophic gastritis and SPEM[83]. CLDN18-/- mice can also independently form gastric tumors without H. pylori infection, with the tumorigenesis process partially resembling that of H. pylori-associated human carcinogenesis[84]. And in adult CLDN18-/- mice, the gradual carcinogenesis process may be attributed to altered functions of transcellular anion (mainly Cl-) transporters[85].
BAK, a pro-apoptotic member of the Bcl-2 protein family, plays an important role in promoting cell apoptosis and is primarily distributed in gastric epithelial cells, particularly in the gastric pits and parietal cells. Compared to normal gastric epithelial cells, the expression levels of BAK are reduced in gastric tumors[86]. In Bak-/- mice induced by gamma radiation, gastric epithelial apoptosis is reduced compared to that in wild-type mice, further demonstrating that the Bak gene promotes gastric epithelial cell apoptosis in mice[87]. Also, Bak-/- mice exhibit reduced numbers of parietal and endocrine cells in the fundic glands and increased fundic gland length, a characteristic specific to BAK gene knockout, indicating that the loss of the BAK gene does not directly lead to significant inflammation. Additionally, long-term infection with H. felis in Bak-/-mice leads to a higher propensity for developing gastric atrophy and dysplasia compared to wild-type mice, suggesting that BAK not only regulates apoptosis but may also play an important role in cell proliferation. However, no significant differences in strain, gender, or H. felis colonization were observed in Bak-/- mice[88].
Extensive epidemiological indicated that H. pylori is closely associated with PLGC of the stomach and is a major cause of these lesions. It induces inflammation in the antrum and parts of the corpus, leading to DNA damage in epithelial cells and initiating the GC cascade[89,90]. Commonly used genetically engineered mice, when infected with H. pylori and H. felis, can develop precancerous PLGC. H. felis, a close relative of H. pylori that is isolated from cat stomachs, has been shown to readily colonize the mouse stomach[91]. C57BL/6 mice exhibit significant resistance to the colonization of various H. pylori strains, leading to the development of a more strongly colonizing strain - H. pylori SS1 - that can colonize the entire glandular area of the mouse stomach. This strain prefers the antral mucosa and the transition zone between the antrum and corpus, but shows lower colonization levels in Balb/c mice[92]. H. pylori accelerates the carcinogenesis process in genetically engineered mice, which provide high control and precision, enabling accurate study of the carcinogenic mechanisms induced by H. pylori infection in mice[93].
Many GEMMs of PLGC have been established (Table 1), recreating key molecular events of PLGC. These models help us gain deeper insights into the pathological mechanisms of PLGC and provide potential targets for early diagnosis and treatment of GC. Most GEMMs can spontaneously develop PLGC, and can be used to model high-risk populations for GC, allowing exploration of the role of genetic inheritance. The INS-GAS mouse model can stably develop PLGC, with predictable progression and high similarity to human lesions, making it a widely used model. Infection with H. pylori or H. felis accelerates the progression of PLGC or GC in GEMMs, which provide a controlled environment for investigating the interaction between genetic and environmental factors[94,95]. Notably, gender-specific disparities observed in GEMMs of PLGC align with human epidemiological data. While males exhibit higher susceptibility to most subtypes of gastric carcinoma and associated PLGC, a distinct reversal is evident in AAG, where female predominance is characteristically observed. Furthermore, genetic engineering techniques allow us to create mouse models with specific pathogenic genes, enabling the study of the isolated effects of these genes in PLGC while effectively excluding the interference of other genes.
| Models | Time/lesions | Subtype | Method | Genetic background | Location | Reference |
| INS-GAS | 12 months | IM; D | Transgenic mouse + H. felis | C57BL/6 | Corpus | [13] |
| INS-GAS | 5 months | D | Transgenic mouse + H. pylori | FVB/N | Corpus/antrum | [14] |
| INS-GAS | 6 months | D | Transgenic mouse + H. pylori | FVB/N | Corpus | [10] |
| INS-GAS | 4 months | IM; D | Transgenic mouse + H. pylori | FVB/N | Corpus | [15] |
| Gastrin-/- | 12 months | D | Knockout mouse + H. felis | C57Bl/6 | Antrum | [13] |
| Gastrin-/- | 9 months | D | Knockout mouse | 129/Sv-C57/BL6 | Antrum | [20] |
| Atp4a (-/-) | 3 months | IM | Knockout mouse | C57Bl/6 | Antrum | [28] |
| Atp4a (-/-) | 3 months | D | Knockout mouse | 129SvJ/ Black Swiss | / | [26] |
| Atp4b (-/-) | 12 months | SPEM | Knockout mouse | BalbC | Corpus | [33] |
| Slc26a9 | 6 months | SPEM; IM | Knockout mouse | S129/svj | / | [37] |
| H+/K+ -IFN-γ | 3 months | D | Transgenic mouse | H+/K+ -IFN-γ944 | Corpus | [39] |
| K19-IL11Tg | 3 months | A | Transgenic mouse | C57BL/ 6J / DBA/2J | Corpus | [43] |
| Gp130 757F/F | 1 months | D | Knock in mouse | 129 Sv-J/C57BL/6 | Antrum | [97] |
| H+/K+-ATPase-IL-1β | 12 months | D | Transgenic mouse | C57BL/6J | Corpus | [50] |
| Mist1-Kras | 3 months | IM | Transgenic mouse | C57BL/6J | Corpus | [54] |
| K19-Kras | 3 months | A; IM; D | Transgenic mouse | C57BL/6 | Corpus | [53] |
| Car9-/- | 5 months | A | Knockout mouse | C57BL/6 | Corpus | [58] |
| CDX1 | 120 days | IM | Transgenic mouse | C57BL/6 | Corpus | [63] |
| Foxa3-CDX2 | 1 months | IM | Transgenic mouse | C57BL/6J | Corpus | [98] |
| CDX2 | 37 days | IM | Transgenic mouse | C57BL/6J | Corpus | [64] |
| Apcmin+ | 6 months | A; IM; D | Point mutation mouse | C57BL/6J | Corpus/antrum | [74] |
| TFF1-/- | 6 months | D | Knockout mouse | C57BL/6J/129/Sv | Antrum | [99] |
| TFF1-/- | 6 months | D | Knockout mouse | C57BL/6J/129/Sv | Antrum | [78] |
| CLDN18-/- | 3 days | A | Knockout mouse | / | Corpus | [83] |
| Bak-/- | 12 months | D | Knockout mouse + H. felis | C57BL/6 | Corpus | [88] |
However, due to the anatomical, physiological, and immunological differences between mice and humans, GEMMs of PLGC may not fully replicate all features of human PLGC. Some GEMMs have shortened lifespans due to genetic defects, while others may develop diseases unrelated to PLGC. Both of which may potentially influence the research process and outcomes. Moreover, the occurrence of PLGC and the colonization ability of H. pylori/H. felis vary among different mouse strains and sexes., potentially affecting the generalizability and predictive value of the models. Thus, these factors should be carefully considered when designing experiments. And certain rare or complex genetic background tumors are difficult to establish using GEMMs. The emergence of patient-derived xenograft (PDX) models and patient-derived organoid (PDO) models has effectively addressed this issue. PDX and PDO can be directly obtained from patient tumor tissues, and they are capable of preserving the tumor's tissue structure and cell-to-cell interactions while partially simulating the tumor microenvironment. Moreover, they hold promise for evaluating treatment responses in individual patients, thereby facilitating the development of personalized treatment regimens. However, the considerable individual differences in PDX and PDO models may lead to inconsistencies between models, which in turn pose challenges for the reproducibility of experiments. In contrast, GEMMs do not have this issue. Additionally, there is a scarcity of GEMMs specifically designed for studying PLGC. The creation of GEMMs demands considerable time and resources and it cannot be guaranteed that all GEMMs will develop PLGC. To overcome this problem, some researchers have proposed combining PDOs with CRISPR screening. PDOs are rapid and efficient in assessing drug sensitivity, while CRISPR screening can identify novel therapeutic targets. The combination of these two approaches is expected to accelerate the development of more effective and personalized treatment strategies[96].
The development of more sophisticated GEMMs incorporating multiple genetic alterations and environmental determinants to mimic human conditions better will be critical for achieving a comprehensive understanding of PLGC. Moreover, there is a need to develop more humanized mouse models, especially in terms of simulating human immune responses, which will provide a valuable platform for immunotherapy and targeted therapy. Advancements in CRISPR/Cas9 technology and other genome-editing platforms will facilitate the generation of models with more sophisticated genetic configurations that better recapitulate the heterogeneity observed in human gastric carcinogenesis. Furthermore, the integration of multi-omics approaches (genomics, transcriptomics, proteomics) with GEMMs will enable deeper insights into the molecular mechanisms underlying PLGC pathogenesis. Moreover, the incorporation of microbiome investigations into GEMM-based studies could further elucidate the functional contributions of gastrointestinal microbiota to PLGC progression, thereby establishing a theoretical foundation for microbiota-targeted therapeutic interventions. Studying mouse models with these gene alterations aids in investigating the functions of these genes. Consequently, these new models will help unravel the complex molecular networks of PLGC and offer new strategies for the prevention and treatment of GC.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12764] [Article Influence: 6382.0] [Reference Citation Analysis (8)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3309] [Article Influence: 551.5] [Reference Citation Analysis (6)] |
| 3. | Zhang M, Zhong J, Song Z, Xu Q, Chen Y, Zhang Z. Regulatory mechanisms and potential therapeutic targets in precancerous lesions of gastric cancer: A comprehensive review. Biomed Pharmacother. 2024;177:117068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Mouse Genome Sequencing Consortium; Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigó R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4964] [Cited by in RCA: 5025] [Article Influence: 209.4] [Reference Citation Analysis (0)] |
| 5. | Ittner LM, Götz J. Pronuclear injection for the production of transgenic mice. Nat Protoc. 2007;2:1206-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2477] [Cited by in RCA: 2561] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 7. | Kim H, Kim M, Im SK, Fang S. Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab Anim Res. 2018;34:147-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 8. | Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Wiedemann T, Hofbaur S, Tegtmeyer N, Huber S, Sewald N, Wessler S, Backert S, Rieder G. Helicobacter pylori CagL dependent induction of gastrin expression via a novel αvβ5-integrin-integrin linked kinase signalling complex. Gut. 2012;61:986-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, Wang TC, Fox JG. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 11. | Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 452] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Pritchard DM, Przemeck SM. Review article: How useful are the rodent animal models of gastric adenocarcinoma? Aliment Pharmacol Ther. 2004;19:841-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, Rickman BH, Rogers AB, Lertkowit N, Varro A, Fox JG, Wang TC. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175:365-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942-950. [PubMed] |
| 15. | Ohtani M, García A, Rogers AB, Ge Z, Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC, Fox JG. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ohtani M, Ge Z, García A, Rogers AB, Muthupalani S, Taylor NS, Xu S, Watanabe K, Feng Y, Marini RP, Whary MT, Wang TC, Fox JG. 17 β-estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis. 2011;32:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lunger C, Shen Z, Holcombe H, Mannion AJ, Dzink-Fox J, Kurnick S, Feng Y, Muthupalani S, Carrasco SE, Wilson KT, Peek RM, Piazuelo MB, Morgan DR, Armijo AL, Mammoliti M, Wang TC, Fox JG. Gastric coinfection with thiopeptide-positive Cutibacterium acnes decreases FOXM1 and pro-inflammatory biomarker expression in a murine model of Helicobacter pylori-induced gastric cancer. Microbiol Spectr. 2024;12:e0345023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Peng C, Xu X, Ouyang Y, Li Y, Lu N, Zhu Y, He C. Spatial Variation of the Gastrointestinal Microbiota in Response to Long-Term Administration of Vonoprazan in Mice With High Risk of Gastric Cancer. Helicobacter. 2024;29:e13117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Peng C, Li X, Li Y, Xu X, Ouyang Y, Li N, Lu N, Zhu Y, He C. Sex-specific effects of gastrointestinal microbiome disruptions on Helicobacter pylori-induced gastric carcinogenesis in INS-GAS mice. Biol Sex Differ. 2025;16:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Kang W, Saqui-Salces M, Zavros Y, Merchant JL. Induction of follistatin precedes gastric transformation in gastrin deficient mice. Biochem Biophys Res Commun. 2008;376:573-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Khan ZE, Wang TC, Cui G, Chi AL, Dimaline R. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology. 2003;125:510-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Friis-Hansen L, Rieneck K, Nilsson HO, Wadström T, Rehfeld JF. Gastric inflammation, metaplasia, and tumor development in gastrin-deficient mice. Gastroenterology. 2006;131:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Kim W, Chu TH, Nienhüser H, Jiang Z, Del Portillo A, Remotti HE, White RA, Hayakawa Y, Tomita H, Fox JG, Drake CG, Wang TC. PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice. Gastroenterology. 2021;160:781-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 24. | Shin JM, Munson K, Sachs G. Gastric H+,K+-ATPase. Compr Physiol. 2011;1:2141-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Lenti MV, Rugge M, Lahner E, Miceli E, Toh BH, Genta RM, De Block C, Hershko C, Di Sabatino A. Autoimmune gastritis. Nat Rev Dis Primers. 2020;6:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 26. | Judd LM, Andringa A, Rubio CA, Spicer Z, Shull GE, Miller ML. Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(-/-)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol. 2005;20:1266-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lahner E, Dilaghi E, Cingolani S, Pivetta G, Dottori L, Esposito G, Marzinotto I, Lampasona V, Buzzetti R, Annibale B. Gender-sex differences in autoimmune atrophic gastritis. Transl Res. 2022;248:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Liu W, Yang LJ, Liu YL, Yuan DS, Zhao ZM, Wang Q, Yan Y, Pan HF. Dynamic characterization of intestinal metaplasia in the gastric corpus mucosa of Atp4a-deficient mice. Biosci Rep. 2020;40:BSR20181881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Spicer Z, Miller ML, Andringa A, Riddle TM, Duffy JJ, Doetschman T, Shull GE. Stomachs of mice lacking the gastric H,K-ATPase alpha -subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem. 2000;275:21555-21565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Franic TV, Judd LM, Robinson D, Barrett SP, Scarff KL, Gleeson PA, Samuelson LC, Van Driel IR. Regulation of gastric epithelial cell development revealed in H(+)/K(+)-ATPase beta-subunit- and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1502-G1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Hu R, Xue X, Sun X, Mi Y, Wen H, Xi H, Li F, Zheng P, Liu S. Revealing the role of metformin in gastric intestinal metaplasia treatment. Front Pharmacol. 2024;15:1340309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Benítez J, Marra R, Reyes J, Calvete O. A genetic origin for acid-base imbalance triggers the mitochondrial damage that explains the autoimmune response and drives to gastric neuroendocrine tumours. Gastric Cancer. 2020;23:52-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Aasarød KM, Waldum HL, Stunes AK, Sandvik AK, Flatberg A, Mjønes P, Syversen U, Bakke I, Fossmark R. Gastric Corpus Mucosal Hyperplasia and Neuroendocrine Cell Hyperplasia, but not Spasmolytic Polypeptide-Expressing Metaplasia, Is Prevented by a Gastrin Receptor Antagonist in H(+)/K(+)ATPase Beta Subunit Knockout Mice. Int J Mol Sci. 2020;21:927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Scarff KL, Judd LM, Toh BH, Gleeson PA, Van Driel IR. Gastric H(+),K(+)-adenosine triphosphatase beta subunit is required for normal function, development, and membrane structure of mouse parietal cells. Gastroenterology. 1999;117:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Liu X, Li T, Tuo B. Physiological and Pathophysiological Relevance of the Anion Transporter Slc26a9 in Multiple Organs. Front Physiol. 2018;9:1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci U S A. 2008;105:17955-17960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Liu X, Li T, Ma Z, Riederer B, Yuan D, Zhu J, Li Y, An J, Wen G, Jin H, Yang X, Seidler U, Tuo B. SLC26A9 deficiency causes gastric intraepithelial neoplasia in mice and aggressive gastric cancer in humans. Cell Oncol (Dordr). 2022;45:381-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 2020;8:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 815] [Cited by in RCA: 909] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 39. | Syu LJ, El-Zaatari M, Eaton KA, Liu Z, Tetarbe M, Keeley TM, Pero J, Ferris J, Wilbert D, Kaatz A, Zheng X, Qiao X, Grachtchouk M, Gumucio DL, Merchant JL, Samuelson LC, Dlugosz AA. Transgenic expression of interferon-γ in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181:2114-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Tu SP, Quante M, Bhagat G, Takaishi S, Cui G, Yang XD, Muthuplani S, Shibata W, Fox JG, Pritchard DM, Wang TC. IFN-γ inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011;71:4247-4259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Song M, Ping Y, Zhang K, Yang L, Li F, Zhang C, Cheng S, Yue D, Maimela NR, Qu J, Liu S, Sun T, Li Z, Xia J, Zhang B, Wang L, Zhang Y. Low-Dose IFNγ Induces Tumor Cell Stemness in Tumor Microenvironment of Non-Small Cell Lung Cancer. Cancer Res. 2019;79:3737-3748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 42. | Xu DH, Zhu Z, Wakefield MR, Xiao H, Bai Q, Fang Y. The role of IL-11 in immunity and cancer. Cancer Lett. 2016;373:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 43. | Buzzelli JN, O'Connor L, Scurr M, Chung Nien Chin S, Catubig A, Ng GZ, Oshima M, Oshima H, Giraud AS, Sutton P, Judd LM, Menheniott TR. Overexpression of IL-11 promotes premalignant gastric epithelial hyperplasia in isolation from germline gp130-JAK-STAT driver mutations. Am J Physiol Gastrointest Liver Physiol. 2019;316:G251-G262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Howlett M, Chalinor HV, Buzzelli JN, Nguyen N, van Driel IR, Bell KM, Fox JG, Dimitriadis E, Menheniott TR, Giraud AS, Judd LM. IL-11 is a parietal cell cytokine that induces atrophic gastritis. Gut. 2012;61:1398-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Osman EEA, Neamati N. Ironing Out the Mechanism of gp130 Signaling. Pharmacol Rev. 2024;76:1399-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 372] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 47. | Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 48. | Howlett M, Judd LM, Jenkins B, La Gruta NL, Grail D, Ernst M, Giraud AS. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology. 2005;129:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Galozzi P, Bindoli S, Doria A, Sfriso P. The revisited role of interleukin-1 alpha and beta in autoimmune and inflammatory disorders and in comorbidities. Autoimmun Rev. 2021;20:102785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 51. | Huang FY, Chan AO, Lo RC, Rashid A, Wong DK, Cho CH, Lai CL, Yuen MF. Characterization of interleukin-1β in Helicobacter pylori-induced gastric inflammation and DNA methylation in interleukin-1 receptor type 1 knockout (IL-1R1(-/-)) mice. Eur J Cancer. 2013;49:2760-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Sabry D, Ahmed R, Abdalla S, Fathy W, Eldemery A, Elamir A. Braf, Kras and Helicobacter pylori epigenetic changes-associated chronic gastritis in Egyptian patients with and without gastric cancer. World J Microbiol Biotechnol. 2016;32:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, Betz KS, Muthupalani S, Rogers AB, Fox JG, Rustgi AK, Wang TC. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res. 2010;70:8435-8445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Choi E, Hendley AM, Bailey JM, Leach SD, Goldenring JR. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology. 2016;150:918-30.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 55. | Becker HM. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer. 2020;122:157-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 56. | Gut MO, Parkkila S, Vernerová Z, Rohde E, Závada J, Höcker M, Pastorek J, Karttunen T, Gibadulinová A, Závadová Z, Knobeloch KP, Wiedenmann B, Svoboda J, Horak I, Pastoreková S. Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology. 2002;123:1889-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 57. | Li T, Liu X, Riederer B, Nikolovska K, Singh AK, Mäkelä KA, Seidler A, Liu Y, Gros G, Bartels H, Herzig KH, Seidler U. Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux. Acta Physiol (Oxf). 2018;222:e12923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Leppilampi M, Karttunen TJ, Kivelä J, Gut MO, Pastoreková S, Pastorek J, Parkkila S. Gastric pit cell hyperplasia and glandular atrophy in carbonic anhydrase IX knockout mice: studies on two strains C57/BL6 and BALB/C. Transgenic Res. 2005;14:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | Silberg DG, Furth EE, Taylor JK, Schuck T, Chiou T, Traber PG. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology. 1997;113:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Koide T, Koyanagi-Aoi M, Uehara K, Kakeji Y, Aoi T. CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells. iScience. 2022;25:104314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Kang JM, Lee BH, Kim N, Lee HS, Lee HE, Park JH, Kim JS, Jung HC, Song IS. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer. J Korean Med Sci. 2011;26:647-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Mutoh H, Sakurai S, Satoh K, Osawa H, Hakamata Y, Takeuchi T, Sugano K. Cdx1 induced intestinal metaplasia in the transgenic mouse stomach: comparative study with Cdx2 transgenic mice. Gut. 2004;53:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka I, Honda S, Osawa H, Kaneko Y, Sugano K. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 213] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 65. | Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, Yamagata Y, Seto Y, Aburatani H, Hatakeyama M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci U S A. 2012;109:20584-20589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 66. | Mutoh H, Hayakawa H, Sakamoto H, Sashikawa M, Sugano K. Transgenic Cdx2 induces endogenous Cdx1 in intestinal metaplasia of Cdx2-transgenic mouse stomach. FEBS J. 2009;276:5821-5831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, Sugano K. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Liu Q, Teh M, Ito K, Shah N, Ito Y, Yeoh KG. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod Pathol. 2007;20:1286-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Chen HY, Hu Y, Lu NH, Zhu Y. Caudal type homeoboxes as a driving force in Helicobacter pylori infection-induced gastric intestinal metaplasia. Gut Microbes. 2020;12:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | van Es JH, Giles RH, Clevers HC. The many faces of the tumor suppressor gene APC. Exp Cell Res. 2001;264:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Tamura G, Maesawa C, Suzuki Y, Tamada H, Satoh M, Ogasawara S, Kashiwaba M, Satodate R. Mutations of the APC gene occur during early stages of gastric adenoma development. Cancer Res. 1994;54:1149-1151. [PubMed] |
| 72. | Nishimura K, Yokozaki H, Haruma K, Kajiyama G, Tahara E. Alterations of the apc gene in carcinoma cell-lines and precancerous lesions of the stomach. Int J Oncol. 1995;7:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Du WB, Lin CH, Chen WB. High expression of APC is an unfavorable prognostic biomarker in T4 gastric cancer patients. World J Gastroenterol. 2019;25:4452-4467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Wang S, Kuang J, Li G, Huang G, Zheng L, Li J, Wang L. Gastric precancerous lesions present in Apc(Min/+) mice. Biomed Pharmacother. 2020;121:109534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Tomita H, Yamada Y, Oyama T, Hata K, Hirose Y, Hara A, Kunisada T, Sugiyama Y, Adachi Y, Linhart H, Mori H. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res. 2007;67:4079-4087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Ruchaud-Sparagano MH, Westley BR, May FE. The trefoil protein TFF1 is bound to MUC5AC in human gastric mucosa. Cell Mol Life Sci. 2004;61:1946-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Eletto D, Vllahu M, Mentucci F, Del Gaudio P, Petrella A, Porta A, Tosco A. TFF1 Induces Aggregation and Reduces Motility of Helicobacter pylori. Int J Mol Sci. 2021;22:1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Soutto M, Chen Z, Bhat AA, Wang L, Zhu S, Gomaa A, Bates A, Bhat NS, Peng D, Belkhiri A, Piazuelo MB, Washington MK, Steven XC, Peek R Jr, El-Rifai W. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat Commun. 2019;10:3039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Znalesniak EB, Salm F, Hoffmann W. Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis. Int J Mol Sci. 2020;21:644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Tomita H, Takaishi S, Menheniott TR, Yang X, Shibata W, Jin G, Betz KS, Kawakami K, Minamoto T, Tomasetto C, Rio MC, Lerkowit N, Varro A, Giraud AS, Wang TC. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140:879-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 81. | Nakayama I, Qi C, Chen Y, Nakamura Y, Shen L, Shitara K. Claudin 18.2 as a novel therapeutic target. Nat Rev Clin Oncol. 2024;21:354-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 82. | Hagen SJ, Ang LH, Zheng Y, Karahan SN, Wu J, Wang YE, Caron TJ, Gad AP, Muthupalani S, Fox JG. Loss of Tight Junction Protein Claudin 18 Promotes Progressive Neoplasia Development in Mouse Stomach. Gastroenterology. 2018;155:1852-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Hayashi D, Tamura A, Tanaka H, Yamazaki Y, Watanabe S, Suzuki K, Suzuki K, Sentani K, Yasui W, Rakugi H, Isaka Y, Tsukita S. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology. 2012;142:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | Suzuki K, Sentani K, Tanaka H, Yano T, Suzuki K, Oshima M, Yasui W, Tamura A, Tsukita S. Deficiency of Stomach-Type Claudin-18 in Mice Induces Gastric Tumor Formation Independent of H pylori Infection. Cell Mol Gastroenterol Hepatol. 2019;8:119-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 85. | Caron TJ, Scott KE, Sinha N, Muthupalani S, Baqai M, Ang LH, Li Y, Turner JR, Fox JG, Hagen SJ. Claudin-18 Loss Alters Transcellular Chloride Flux but not Tight Junction Ion Selectivity in Gastric Epithelial Cells. Cell Mol Gastroenterol Hepatol. 2021;11:783-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Krajewska M, Fenoglio-Preiser CM, Krajewski S, Song K, Macdonald JS, Stemmerman G, Reed JC. Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol. 1996;149:1449-1457. [PubMed] |
| 87. | Przemeck SM, Duckworth CA, Pritchard DM. Radiation-induced gastric epithelial apoptosis occurs in the proliferative zone and is regulated by p53, bak, bax, and bcl-2. Am J Physiol Gastrointest Liver Physiol. 2007;292:G620-G627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Duckworth CA, Abuderman AA, Burkitt MD, Williams JM, O'Reilly LA, Pritchard DM. bak deletion stimulates gastric epithelial proliferation and enhances Helicobacter felis-induced gastric atrophy and dysplasia in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G420-G430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 89. | Shah SC, Wang AY, Wallace MB, Hwang JH. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology. 2025;168:405-416.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 50] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 90. | Fu L, Xie C. A lucid review of Helicobacter pylori-induced DNA damage in gastric cancer. Helicobacter. 2019;24:e12631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 238] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 92. | Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 766] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 93. | He C, Peng C, Xu X, Li N, Ouyang Y, Zhu Y, Lu N. Probiotics mitigate Helicobacter pylori-induced gastric inflammation and premalignant lesions in INS-GAS mice with the modulation of gastrointestinal microbiota. Helicobacter. 2022;27:e12898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 94. | Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Ding L, Sheriff S, Sontz RA, Merchant JL. Schlafen4(+)-MDSC in Helicobacter-induced gastric metaplasia reveals role for GTPases. Front Immunol. 2023;14:1139391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Zhu Z, Shen J, Ho PC, Hu Y, Ma Z, Wang L. Transforming cancer treatment: integrating patient-derived organoids and CRISPR screening for precision medicine. Front Pharmacol. 2025;16:1563198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 97. | Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 98. | Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 358] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 99. | Soutto M, Belkhiri A, Piazuelo MB, Schneider BG, Peng D, Jiang A, Washington MK, Kokoye Y, Crowe SE, Zaika A, Correa P, Peek RM Jr, El-Rifai W. Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J Clin Invest. 2011;121:1753-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/