Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.106901
Revised: April 14, 2025
Accepted: May 28, 2025
Published online: July 27, 2025
Processing time: 133 Days and 3.6 Hours
Elderly patients often display age-related physiological decline, which increases their susceptibility to complications during medical procedures. Therefore, it is clinically imperative to refine anesthetic protocols for painless gastroscopy in this vulnerable population.
To explore the effects of the etomidate-propofol combination on anesthesia quality, compliance, and adverse reactions in elderly patients undergoing painless gastrointestinal endoscopy.
A total of 103 elderly patients scheduled for painless gastrointestinal endoscopy at the Hospital of Wuhan Economic and Technological Development Zone (Hannan District) between October 2022 and October 2024 were enrolled. The participants were divided into a control group (n = 50) receiving propofol anesthesia and an observation group (n = 53) that received a combination of etomidate and propofol anesthesia. The anesthesia quality (including induction time, recovery time, and orientation recovery time), compliance, hemodynamic parameters (heart rate, oxygen saturation, systolic/diastolic blood pressure), adverse reactions (muscle tremors, injection pain, respiratory depression, hypotension, and nausea/vomi
The observation group had significantly shorter anesthesia induction, recovery, and orientation recovery times than the control group. Moreover, the observation group showed higher compliance; greater hemodynamic stability at preanesthesia (T0), during anesthesia (T1), and postrecovery (T2) time points; and a significantly lower incidence of adverse reactions. The VAS and Ramsay scores at 5, 30, and 60 minutes after anesthesia recovery were also significantly lower in the observation group than in the control group.
The etomidate-propofol combination for painless gastrointestinal endoscopy in elderly patients may provide superior anesthesia quality and improved compliance and safety, making it a promising approach for clinical application.
Core Tip: The choice of anesthesia plays a pivotal role in ensuring both safety and efficacy during painless gastroscopy. This study enrolled 103 elderly patients scheduled for painless gastrointestinal endoscopy and systematically compared propofol monotherapy with a combined etomidate-propofol regimen. Comprehensive evaluations of anesthesia quality, patient compliance, hemodynamic stability, adverse events, and sedation-analgesia efficacy demonstrated that the etomidate-propofol combination provides distinct clinical advantages for such patients. These results support its adoption as a preferred anesthetic strategy for elderly patients undergoing painless gastrointestinal endoscopy.
- Citation: Zhang YY. Etomidate-propofol combination in painless gastrointestinal endoscopy for elderly patients: A comparative study. World J Gastrointest Surg 2025; 17(7): 106901
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/106901.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.106901
Painless gastrointestinal endoscopy, a widely adopted diagnostic and therapeutic modality, is extensively used for the screening and diagnosis of various gastrointestinal disorders, such as reflux esophagitis, esophageal carcinoma, gastric and duodenal ulcers, and gastric malignancies, facilitating the timely and accurate identification of gastrointestinal pathologies[1]. Worldwide, millions of such procedures are performed annually[2]. Conventional gastrointestinal endoscopy without anesthesia is associated with significant physiological stress, potentially causing adverse events such as asphyxia, nausea, vomiting, increased catecholamine secretion, tachycardia, cerebral hemorrhage, and myocardial ischemia, thereby causing considerable discomfort and distress to patients[3]. Conversely, painless gastrointestinal endoscopy, which involves the administration of anesthesia to induce a state of unconsciousness, effectively mitigates these adverse physiological responses and reduces the risk of complications, such as bleeding and perforation, resulting from poor patient compliance[4-6]. This approach aligns with the increasing emphasis on patient comfort and satisfaction in modern medical practice[7]. Consequently, the selection of safe and effective anesthetic agents is of paramount importance for optimizing the outcomes of painless gastroscopy.
Elderly patients, who often face a higher risk of complications due to age-related physiological decline and comor
Despite these insights, research on the etomidate-propofol combination in elderly patients undergoing painless gastrointestinal endoscopy is limited, particularly regarding its effect on anesthesia quality, patient compliance, and the incidence of adverse reactions. Therefore, this study was conducted to address this gap by investigating the efficacy and safety of this combined anesthetic approach, with the aim of providing a more effective and safe option for anesthesia management in such patients.
A cohort of 103 elderly patients scheduled for painless gastrointestinal endoscopy at the Hospital of Wuhan Economic and Technological Development Zone (Hannan District) was enrolled in this study. The recruitment period was extended from October 2022 to October 2024. Participants were divided into the control group (n = 50), which received propofol anesthesia, and the observation group (n = 53), which received a combined regimen of etomidate and propofol anesthesia.
Inclusion criteria: Patients indicated for painless gastrointestinal endoscopy with American Society of Anesthesiologists (ASA) physical status classification of I-II[15], clear clinical indications for both gastrointestinal endoscopy and anesthesia[16], normal auditory and verbal communication abilities, no history of surgical anesthesia within the previous month, nonemergency cases, excluding conditions such as acute gastrointestinal hemorrhage, and availability of complete clinical documentation were included.
Exclusion criteria: Patients with hypersensitivity to propofol, etomidate, or related anesthetic agents; active asthma exacerbation or pulmonary heart disease; history of cerebrovascular disorders, traumatic brain injury, or intracranial surgery; immune system dysfunction; concurrent diagnosis of other malignant neoplasms; impaired cardiac function (e.g., heart failure); documented history of anesthesia-related allergic reactions; severe metabolic or endocrine dysfunction; primary cognitive impairment or dementia; and history of chronic alcoholism or psychiatric disorders were excluded.
One day before gastrointestinal endoscopy, patients were advised to adopt a semiliquid diet and complete all necessary preprocedural preparations. A fasting period of 8-12 hours was enforced, during which patients abstained from both food and fluids. Upon arrival, patients were positioned in the left lateral decubitus position, and intravenous access was established. Oxygen supplementation was provided using a face mask, and the vital signs was continuously monitoring throughout the procedure.
Patients in the control group received propofol as the sole anesthetic agent, which was administered intravenously at a dosage of 1-1.5 mg/kg administered over a period of 60 seconds.
Patients in the observation group received a combined anesthetic regimen consisting of etomidate and propofol. The anesthesia protocol was implemented as follows: First, an intravenous infusion of a mixture containing fentanyl (50 µg) and lidocaine (30 mg) was administered, and then propofol was dripped at a dosage of 1-1.5 mg/kg for 60 seconds. Next, intravenous administration of etomidate was provided at a dosage of 0.15-0.3 mg/kg. All medications were administered via slow intravenous injection. The depth of anesthesia was evaluated by the loss of the eyelash reflex and the absence of response to verbal commands, which served as indicators of successful anesthesia induction. After achieving these criteria, gastroscopy was started. In the event of intraoperative complications, such as coughing, body movement, and other signs of inadequate anesthesia, supplemental doses of propofol were administered to maintain the desired anesthetic depth.
Anesthesia quality: Anesthesia quality was evaluated by measuring and recording the anesthesia induction, recovery, and orientation recovery times for both groups.
Compliance: Patient compliance was evaluated using a standardized, institution-developed questionnaire. Healthcare providers objectively evaluated patient compliance during the procedure, categorizing it as follows: Full compliance (80-100 points), wherein patients demonstrated complete cooperation; partial compliance (60-79 points), wherein patients showed moderate cooperation; and noncompliance (≤ 59 points), wherein patients demonstrated poor cooperation. The overall compliance rate was calculated as the percentage of patients achieving full or partial compliance compared with the total cohort.
Hemodynamic parameters: Hemodynamic stability was monitored using a patient monitoring system at the following three predefined time points: Preanesthesia (T0), during anesthesia (T1), and postrecovery (T2). The parameters measured were heart rate (HR), oxygen saturation (SpO2), systolic blood pressure (SBP), and diastolic blood pressure (DBP).
Adverse reactions: The incidence of adverse reactions, including muscle tremors, injection pain, respiratory depression, hypotension, and nausea/vomiting, was documented for both groups, after which the total adverse reaction rate was calculated.
Analgesic and sedative effects: Analgesic and sedative effects were evaluated at 5, 30, and 60 minutes postanesthesia recovery. Analgesia was evaluated using the visual analog scale (VAS), with scores ranging from 0 (no pain) to 10 (worst pain), with higher scores indicating inferior analgesic efficacy. Sedation levels were measured using the Ramsay Sedation Scale, which was scored as follows: 1 point: Agitated or restless; 2 points: Cooperative and calm; 3 points: Drowsy but responsive to commands; 4 points: Asleep but easily aroused; 5 points: Sluggish response to stimuli; and 6 points: Deeply asleep, unarousable. A score of 2-4 was classified as satisfactory sedation, whereas a score of > 4 indicated oversedation[17].
Continuous variables are expressed as mean ± SEM. Intergroup comparisons of continuous data were performed using independent samples t-tests, and intragroup comparisons (e.g., pretreatment and posttreatment) were performed using paired t-tests. Categorical variables were expressed as proportions (percentages), and intergroup comparisons were conducted using χ2 tests. All statistical analyses were performed using SPSS version 20.0. P < 0.05 indicated a statistically significant difference.
Baseline characteristics, including sex, age, weight, body mass index, and ASA classification, were comparable between the observation and control groups (P > 0.05). The detailed data are summarized in Table 1.
| Baseline characteristics | Control group (n = 50) | Observation group (n = 53) | χ2/t | P value |
| Gender (male/female) | 26/24 | 31/22 | 0.439 | 0.508 |
| Age (years) | 68.56 ± 4.90 | 69.55 ± 6.14 | 0.901 | 0.370 |
| Weight (kg) | 69.74 ± 10.55 | 69.30 ± 10.47 | 0.212 | 0.832 |
| BMI (kg/m2) | 23.58 ± 2.23 | 23.19 ± 2.44 | 0.845 | 0.400 |
| ASA classification (I/II) | 28/22 | 29/24 | 0.017 | 0.896 |
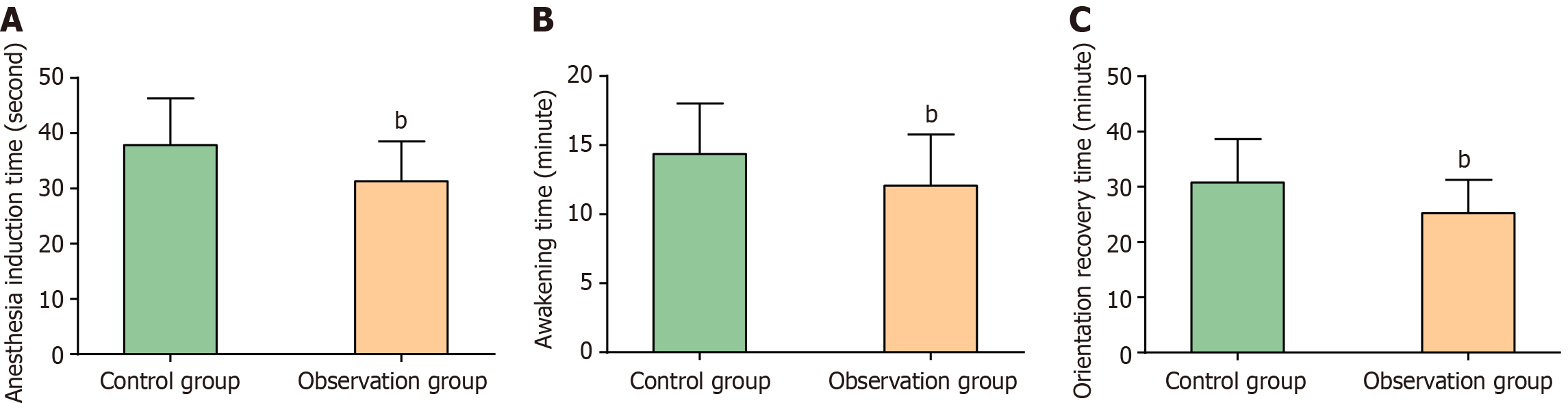
Significant differences in anesthesia quality were observed between the two groups. The observation group exhibited markedly shorter anesthesia induction, recovery, and orientation recovery times than the control group (P < 0.01). These data are illustrated in Figure 1.
Compliance rates were significantly different between the two groups. In the control group, 18 patients showed full compliance, 20 demonstrated partial compliance, and 12 were noncompliant. In contrast, 28 patients showed full compliance, 22 exhibited partial compliance, and only 3 were noncompliant in the observation group. The overall compliance rate was significantly higher in the observation group (P < 0.05). The detailed results are presented in Table 2.
| Compliance | Control group (n = 50) | Observation group (n = 53) | χ2 | P value |
| Full compliance | 18 (36.00) | 28 (52.83) | ||
| Partial compliance | 20 (40.00) | 22 (41.51) | ||
| Non-compliance | 12 (24.00) | 3 (5.66) | ||
| Patient compliance | 38 (76.00) | 50 (94.34) | 6.955 | 0.008 |
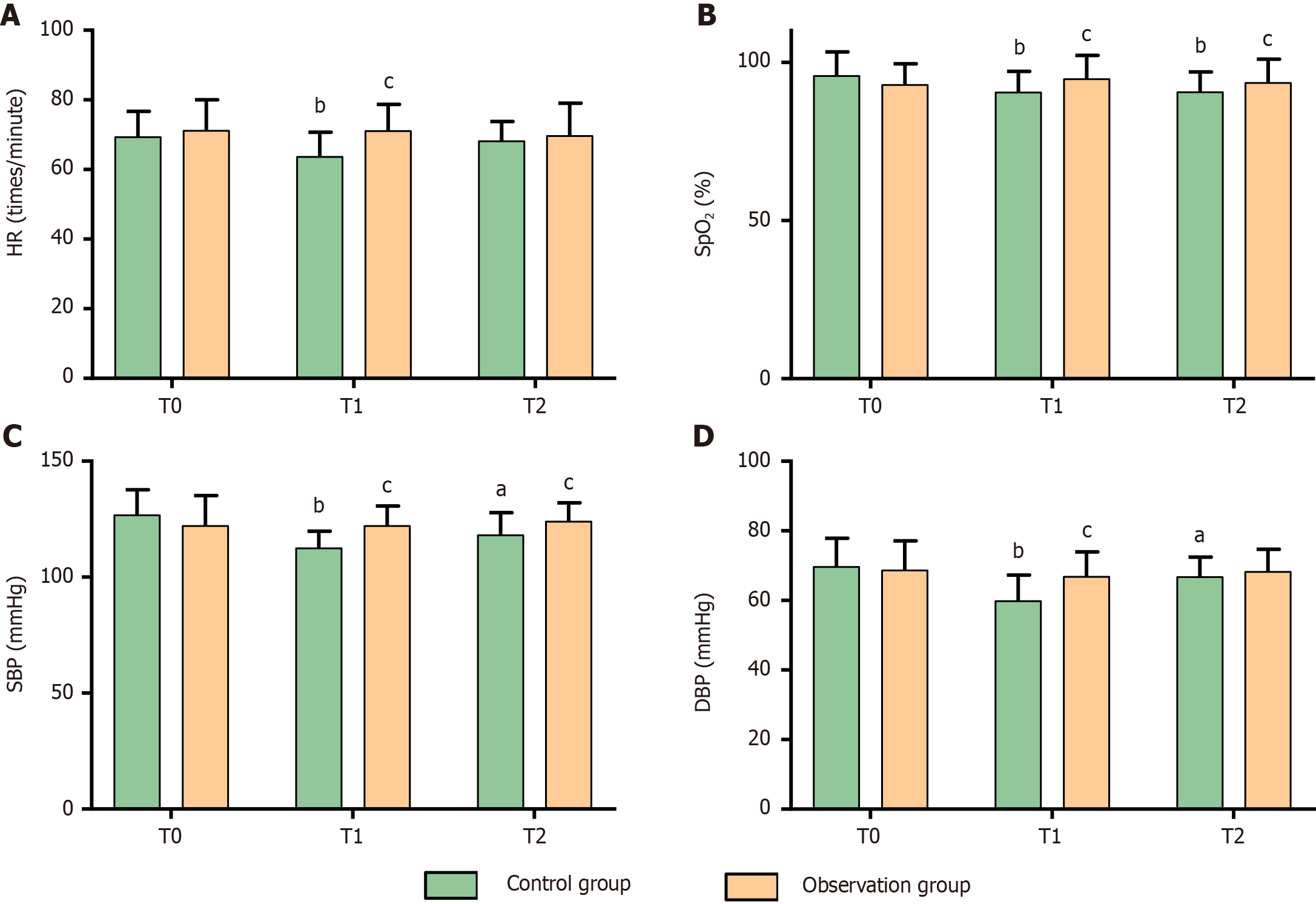
Regarding hemodynamic stability, there were no significant intergroup differences in HR, SpO2, SBP, or DBP at T0 (P > 0.05). In the control group, all hemodynamic parameters significantly declined at T1 (P < 0.05) and partially recovered at T2 (except for SpO2, P < 0.05). Conversely, the observation group maintained stable hemodynamic parameters at T1 and T2 compared with those at T0 (P > 0.05). Remarkably, the observation group demonstrated significantly higher hemodynamic values at T1 and higher SpO2 and SBP at T0 than the control group (P < 0.05). These data are depicted in Figure 2.
Adverse events in the control group included muscle tremors (4 cases), injection pain (5 cases), respiratory depression (2 cases), hypotension (3 cases), and nausea/vomiting (4 cases). These events were less frequent in the observation group, with 1 case of muscle tremors, 3 cases of injection pain, 0 cases of respiratory depression, 1 case of hypotension, and 2 cases of nausea/vomiting. The total adverse reaction rate was significantly lower in the observation group than in the control group (P < 0.05). Table 3 presents the detailed results.
| Adverse reactions | Control group (n = 50) | Observation group (n = 53) | χ2 | P value |
| Muscle tremors | 4 (8.00) | 1 (1.89) | ||
| Injection pain | 5 (10.00) | 3 (5.66) | ||
| Respiratory depression | 2 (4.00) | 0 (0.00) | ||
| Hypotension | 3 (6.00) | 1 (1.89) | ||
| Nausea/vomiting | 4 (8.00) | 2 (3.77) | ||
| Total | 18 (36.00) | 7 (13.21) | 7.272 | 0.007 |
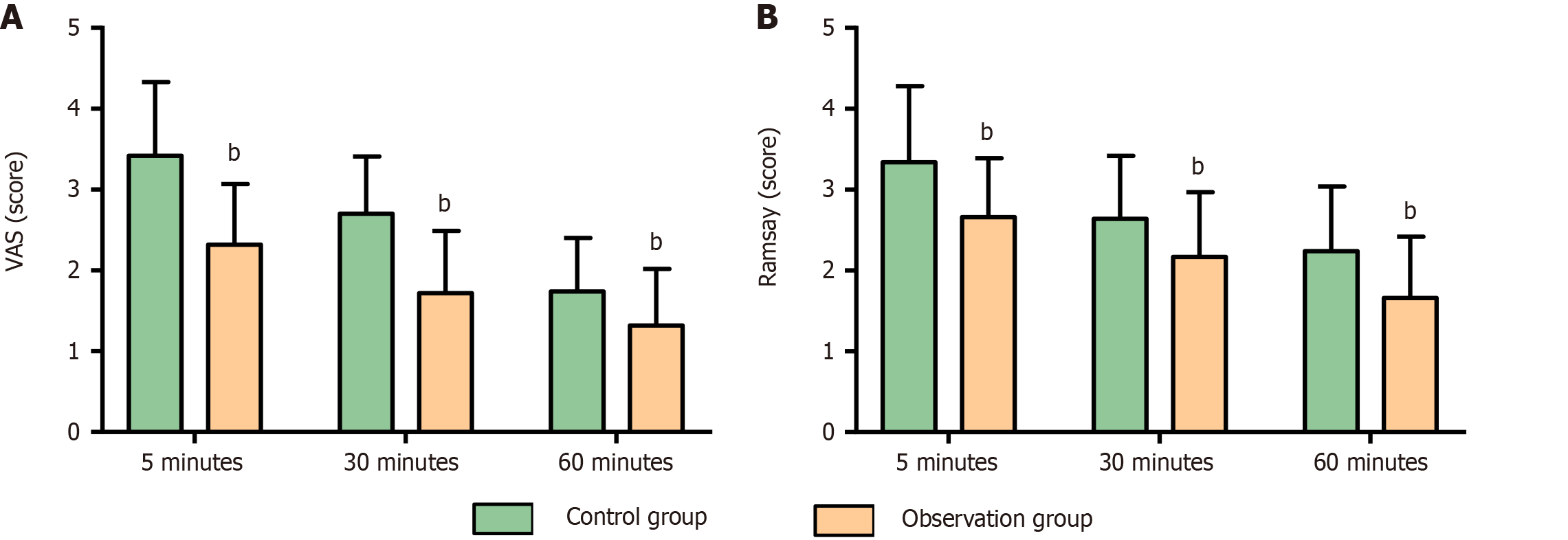
The analgesic and sedative effects were evaluated at 5, 30, and 60 minutes postanesthesia. The observation group consistently achieved significantly lower VAS and Ramsay scores than the control group (P < 0.01). These findings are depicted in Figure 3.
Elderly patients, who are characterized by compromised respiratory function and a high prevalence of comorbidities, are particularly vulnerable to significant discomfort (e.g., reflexive spasms, nausea, and vomiting) during painless gastrointestinal endoscopy. These adverse reactions often engender fear and reluctance toward the procedure, thereby substantially diminishing patient compliance[18]. Consequently, the selection of an optimal anesthetic regimen is imperative to ensure both efficacy and safety in this demographic population.
The present study demonstrated that the etomidate-propofol combination confers superior anesthetic quality for elderly patients undergoing painless gastrointestinal endoscopy. Specifically, this regimen is associated with significantly reduced anesthesia induction time, recovery time, and orientation recovery time compared with propofol monotherapy. Furthermore, patients receiving the combined regimen exhibited improved procedural compliance. Hemodynamically, the etomidate-propofol combination demonstrated greater stability than propofol alone, maintaining more consistent hemodynamic parameters throughout the procedure. From a safety perspective, the incidence of adverse reactions-such as myoclonus, injection pain, respiratory depression, hypotension, nausea, and vomiting-was markedly lower in the etomidate-propofol group. Moreover, the combined regimen provided superior analgesic and sedative effects at 5, 30, and 60 minutes postanesthesia. The clinical efficacy of etomidate can be partially attributed to its active metabolite, hydroxyimidazole carboxylic acid, which mitigates respiratory depression and promotes hemodynamic stability, which partially explains the clinical advantages of etomidate anesthesia for elderly patients undergoing painless gastrointestinal endoscopy[19-21]. In addition, the etomidate-propofol combination allows for a reduction in the required dosage of each agent. The 20% medium- and long-chain triglycerides in etomidate effectively dilute the propofol concentration and inhibit bradykinin release, thereby minimizing vascular irritation. This combination also attenuates the cardiovascular and respiratory suppression associated with high-dose propofol administration, contributing to improved hemodynamic stability and overall safety during anesthesia[22,23]. Supporting our findings, Luo et al[24] reported that the etomidate-propofol combination in patients undergoing bidirectional endoscopy reduced propofol dosage, decreased the incidence of cardiovascular adverse events and respiratory depression, and improved satisfaction among patients, endoscopists, and anesthesiologists. Similarly, Zhang et al[25] observed that the etomidate-propofol combination provided superior hemodynamic stability compared with remimazolam besylate monotherapy in ASA I-II elderly patients undergoing gastrointestinal endoscopy, consistent with our results. Liu et al[26] further highlighted the favorable hemodynamic profile of the etomidate-propofol mixture in patients undergoing radical gastrectomy under general anesthesia, further corroborating our findings. Furthermore, evidence has demonstrated that a 1:1 mixture of 10 mL etomidate and 10 mL propofol, when combined with dezocine, provides stable hemodynamics and high clinical safety in painless gastroscopy, emphasizing the potential advantages of this anesthetic combination[27]. Another study reported a significantly lower incidence of injection pain with the etomidate-propofol combination, suggesting its intrinsic analgesic properties, which is consistent with our observations[28]. Zhou et al[29] also reported that the sedative efficacy of the etomidate-propofol combination during gastroscopy surpassed that of propofol alone, further validating our findings.
This study provides three significant advances in geriatric anesthesia practice: First, the findings establish a clinically superior anesthetic approach for elderly patients undergoing painless gastrointestinal endoscopy, providing practitioners with an evidence-based alternative to conventional protocols. Second, the demonstrated reduction in postoperative pain and agitation with the combined regimen translates into measurable improvements in patient compliance, comfort, and procedural tolerance, improving patient experience and increasing the acceptance of gastrointestinal endoscopy in this population. Most importantly, this study addresses a critical knowledge gap in literature on geriatric anesthesia by providing robust clinical data to guide decision-making for this vulnerable patient group. However, several limitations should be acknowledged. First, the moderate sample size (n = 103), although adequate for initial evaluation, warrants expansion in subsequent multicenter trials to confirm generalizability across diverse clinical settings and evaluate the long-term effects of the anesthesia regimen on delayed adverse reactions or cognitive function. Second, our study design did not incorporate dose-response analyses; future investigations should systematically investigate optimal etomidate-propofol ratios and develop precision dosing algorithms tailored to individual patient characteristics. Perhaps most remarkably, the mechanistic aspects of our observed clinical benefits-particularly regarding stress response modulation and inflammatory pathway regulation-remain to be fully characterized. These knowledge gaps represent important directions for our ongoing research programs.
The etomidate-propofol combination is a superior anesthetic strategy for elderly patients undergoing painless gastrointestinal endoscopy than propofol monotherapy. This approach not only shortens anesthesia induction, recovery, and orientation recovery times but also ensures hemodynamic stability and improves procedural compliance and clinical safety. Moreover, this combination facilitates rapid cognitive recovery, making it a highly effective and safe option for this vulnerable patient population. Considering these compelling findings, we strongly advocate for the inclusion of etomidate-propofol combination therapy as a recommended option in clinical practice guidelines for anesthesia management during painless gastrointestinal endoscopy in geriatric populations. Should subsequent cost-benefit evaluations confirm the economic advantages of this combined regimen, proactive measures should be implemented to incorporate it into national health insurance coverage policies, thereby improving patient access and alleviating financial constraints associated with endoscopic procedures.
| 1. | Mi SC, Wu LY, Xu ZJ, Zheng LY, Luo JW. Effect of modified ShengYangYiwei decoction on painless gastroscopy and gastrointestinal and immune function in gastric cancer patients. World J Gastrointest Endosc. 2023;15:376-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Sebastian S, Dhar A, Baddeley R, Donnelly L, Haddock R, Arasaradnam R, Coulter A, Disney BR, Griffiths H, Healey C, Hillson R, Steinbach I, Marshall S, Rajendran A, Rochford A, Thomas-Gibson S, Siddhi S, Stableforth W, Wesley E, Brett B, Morris AJ, Douds A, Coleman MG, Veitch AM, Hayee B. Green endoscopy: British Society of Gastroenterology (BSG), Joint Accreditation Group (JAG) and Centre for Sustainable Health (CSH) joint consensus on practical measures for environmental sustainability in endoscopy. Gut. 2023;72:12-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Wu X, Chen Y, Luo T, Liu Y, Zeng L. The Study of Remazolam Combined With Propofol on Painless Gastroscopy: A Randomized Controlled Trial. J Perianesth Nurs. 2025;40:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Zheng LY, Mi SC, Wu LY, Xu ZJ, Lu H. Study of wrist-ankle acupuncture therapy for optimizing anaesthesia scheme of painless gastroscopy and improving painless gastroscopy related complications. World J Gastrointest Endosc. 2023;15:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Sinonquel P, Jans A, Bisschops R. Painless colonoscopy: fact or fiction? Clin Endosc. 2024;57:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Sun H, Zhang L, Liu Z, Zhang Y, Li J, Pang K. Study on different sequences of painless gastroenteroscopy in patients with difficult airway. Biotechnol Genet Eng Rev. 2024;40:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wu X, Zeng L, Zhang T, Wu W, Tian Y, Dong S. The study of different dosages of remazolam combined with sufentanil and propofol on painless gastroscopy: A randomized controlled trial. Medicine (Baltimore). 2023;102:e34731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Wang G, Zhen B, Li JJ, Jin CN, Jia J, Liu BH, Bai YH. Insights into anesthesia administration for elderly individuals undergoing painless gastroenteroscopy: A bibliometric study. World J Gastrointest Endosc. 2025;17:101382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Hu S, Wang M, Li S, Zhou W, Zhang Y, Shi H, Ye P, Sun J, Liu F, Zhang W, Zheng L, Hou Q, Wang Y, Sun W, Chen Y, Lu Z, Ji Z, Liao L, Lv X, Wang Y, Wang X, Yang H. Intravenous Lidocaine Significantly Reduces the Propofol Dose in Elderly Patients Undergoing Gastroscopy: A Randomized Controlled Trial. Drug Des Devel Ther. 2022;16:2695-2705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Liu G, Xiong Y. Analysis of Stress Response and Analgesic Effect of Remazolam Combined with Etomidate in Painless Gastroenteroscopy. Contrast Media Mol Imaging. 2022;2022:4863682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Chen X, Guo P, Yang L, Liu Z, Yu D. Comparison and Clinical Value of Ciprofol and Propofol in Intraoperative Adverse Reactions, Operation, Resuscitation, and Satisfaction of Patients under Painless Gastroenteroscopy Anesthesia. Contrast Media Mol Imaging. 2022;2022:9541060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Zheng L, Wang Y, Ma Q, Liang W, Zhang X, Ren Z, Qin W, Meng F, Li Y, Fan G, Yin N. Efficacy and Safety of a Subanesthetic Dose of Esketamine Combined with Propofol in Patients with Obesity Undergoing Painless Gastroscopy: A Prospective, Double-Blind, Randomized Controlled Trial. Drug Des Devel Ther. 2023;17:1347-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Falk J, Zed PJ. Etomidate for procedural sedation in the emergency department. Ann Pharmacother. 2004;38:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Hong JT, Park SW. Etomidate versus propofol for sedation in gastrointestinal endoscopy: A systematic review and meta-analysis of outcomes. Medicine (Baltimore). 2023;102:e32876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Porcaro AB, Rizzetto R, Bianchi A, Gallina S, Serafin E, Panunzio A, Tafuri A, Cerrato C, Migliorini F, Zecchini Antoniolli S, Novella G, De Marco V, Brunelli M, Siracusano S, Cerruto MA, Polati E, Antonelli A. American Society of Anesthesiologists (ASA) physical status system predicts the risk of postoperative Clavien-Dindo complications greater than one at 90 days after robot-assisted radical prostatectomy: final results of a tertiary referral center. J Robot Surg. 2023;17:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Sidhu R, Turnbull D, Haboubi H, Leeds JS, Healey C, Hebbar S, Collins P, Jones W, Peerally MF, Brogden S, Neilson LJ, Nayar M, Gath J, Foulkes G, Trudgill NJ, Penman I. British Society of Gastroenterology guidelines on sedation in gastrointestinal endoscopy. Gut. 2024;73:219-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 17. | Xia Y, Sun Y, Liu J. Effects of dezocine on PAED scale and Ramsay sedation scores in patients undergoing NUSS procedure. Am J Transl Res. 2021;13:5468-5475. [PubMed] |
| 18. | Zhou Y, Li YP. Safety and efficacy of etomidate in combination with oxycodone in painless gastroscopic procedures in the elderly: A prospective randomized controlled trial study. Medicine (Baltimore). 2023;102:e32612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Yang X, Luethy A, Zhang H, Luo Y, Xue Q, Yu B, Lu H. Mechanism and Development of Modern General Anesthetics. Curr Top Med Chem. 2019;19:2842-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Hulsman N, Hollmann MW, Preckel B. Newer propofol, ketamine, and etomidate derivatives and delivery systems relevant to anesthesia practice. Best Pract Res Clin Anaesthesiol. 2018;32:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Goel KK, Thapliyal S, Kharb R, Joshi G, Negi A, Kumar B. Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure-Activity Relationship Studies. Pharmaceutics. 2023;15:2208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Mets B. Management of hypotension associated with angiotensin-axis blockade and general anesthesia administration. J Cardiothorac Vasc Anesth. 2013;27:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Chen L, Liang X, Tan X, Wen H, Jiang J, Li Y. Safety and efficacy of combined use of propofol and etomidate for sedation during gastroscopy: Systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Luo HR, Chen AD, Lin JF, Ye P, Chen YJ, Lin MX, Chen PZ, Chen XH, Zheng XC. Effect of etomidate added to propofol target-controlled infusion in bidirectional endoscopy: A randomized clinical trial. World J Gastrointest Endosc. 2025;17:100722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Zhang Q, Zhao R, Wu Y, Zhang L, Feng Y. Etomidate Combined with Propofol versus Remimazolam for Sedation in Elderly Patients During Gastrointestinal Endoscopy: A Randomized Prospective Clinical Trial. Drug Des Devel Ther. 2024;18:2681-2692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Liu J, Yang J, Yang X, Yin G, Li T, Li R, Wang A. Application of dexmedetomidine combined with propofol-etomidate mixture in radical gastrectomy under general anesthesia. Medicine (Baltimore). 2024;103:e39669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Tang S, Zheng Y, Li X, Zhang Y, Zhang Z. Optimizing sedation in gastroscopy: a study on the etomidate/propofol mixture ratio. Front Med (Lausanne). 2024;11:1392141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Hao L, Hu X, Zhu B, Li W, Huang X, Kang F. Clinical observation of the combined use of propofol and etomidate in painless gastroscopy. Medicine (Baltimore). 2020;99:e23061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Zhou X, Li BX, Chen LM, Tao J, Zhang S, Ji M, Wu MC, Chen M, Zhang YH, Gan GS, Song XY. Etomidate plus propofol versus propofol alone for sedation during gastroscopy: a randomized prospective clinical trial. Surg Endosc. 2016;30:5108-5116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/