Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.106341
Revised: April 26, 2025
Accepted: June 6, 2025
Published online: July 27, 2025
Processing time: 113 Days and 2.7 Hours
Colorectal cancer (CRC) is a globally prevalent gastrointestinal malignant cancer, especially in elderly patients. Currently, surgery resection remains the primary treatment due to its favorable therapeutic outcomes. However, postoperative deterioration in nutritional status and quality of life (QoL) remains a concern. The geriatric nutritional risk index (GNRI), which is calculated based on serum albumin levels and the ratio of normal body weight to ideal body weight, is easily accessible and accurate, making it increasingly popular in clinical practice.
To investigate the impact of GNRI-guided tiered nutritional interventions on postoperative nutritional recovery and QoL in elderly CRC patients.
A retrospective analysis was conducted on 135 elderly CRC patients undergoing radical resection at our hospital from September 2022 to December 2024. Participants were divided into two cohorts: The research group (n = 61) received GNRI-based graded nutritional support, while the control group (n = 65) received conventional nutritional intervention. Clinical indicators, such as postoperative passage of gas by anus, incidence/duration of postoperative fever, hospitalization length and costs, were compared between the two groups. Nutritional bio
The research group showed significantly faster postoperative passage of gas by anus, fewer instances of fever, reduced fever duration, shorter hospitalization duration, and lower costs compared with the control group (P < 0.05). Following intervention, the research group exhibited higher levels of hemoglobin, prealbumin, and transferrin, and lower Patient-Generated Subjective Global Assessment scores vs the control group (P < 0.05). Scores for physical function, social function, material life, and psychological function showed substantial improvement (P < 0.05). Levels of IgG, IgA, and IgM were significantly elevated in the research group (P < 0.05), while nuclear factor kappa B, IL-1, tumor necrosis factor-α, and IL-8 levels were noticeably lowered vs the control group (P < 0.05). The incidence of overall complications within the research group reached 24.59%, notably lower than that (43.08%) observed in the control group (P < 0.05).
GNRI-based graded nutritional intervention in elderly CRC patients can significantly improve postoperative recovery, enhance their nutritional status and QoL, promote immune function recovery, attenuate inflammation, and lower the incidence of postoperative complications. This protocol represents a clinically viable strategy for optimizing postoperative care.
Core Tip: A total of 135 elderly colorectal cancer patients subjected to radical resection at our hospital between September 2022 and December 2024 were involved in the retrospective study. In this research, comparisons were made regarding clinical indicators, nutritional status, quality of life, immune function, inflammatory factor levels, and the incidence of complications between the two groups. Results showed geriatric nutritional risk index - based graded nutritional intervention improved clinical indicators, nutrition, and quality of life, promoted immune recovery, alleviated inflammation, reduced complications, confirming its reliability as a postoperative strategy.
- Citation: Ni SS, Du Y. Effect of graded nutritional intervention on elderly colorectal cancer patients’ postop status. World J Gastrointest Surg 2025; 17(7): 106341
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/106341.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.106341
The global rise in colorectal cancer (CRC) incidence, driven by aging populations and shifting dietary patterns, has established CRC as a critical public health challenge. Currently, it is the third leading cause of cancer-related deaths, with individuals aged 65-74 years diagnosed[1]. In China, this burden is particularly pronounced, with 517000 new cases and 245000 deaths reported in 2018 alone - representing 28% of global CRC-related mortality during that period[2]. While surgical resection remains the most widely used and effective treatment for elderly CRC patients[3,4], postoperative recovery is often complicated by surgical trauma. Tumor progression and metastasis exacerbate systemic resource depletion, leading to reduced dietary intake, chronic pain, and subsequent declines in nutritional and immune resilience[5,6], negatively affecting prognosis and diminishing quality of life (QoL). Therefore, it is crucial to implement effective interventions for improving patient outcomes.
The geriatric nutritional risk index (GNRI), a validated tool combining serum albumin and body weight metrics, is designed to identify and predict nutritional risks in the elderly. Previous studies have confirmed its prognostic utility for the prognosis of elderly patients with various solid tumors, including pancreatic, esophageal, gastrointestinal cancers, and non-small cell lung cancer[7-9]. Notably, Zhang et al[10] found that elderly gastric cancer surgery patients with lower GNRI scores were more likely to develop postoperative complications compared with those with higher GNRI scores (odds ratio = 1.768; 95% confidence interval: 1.445-2.163, P < 0.001), highlighting GNRI as a valuable predictor of long-term prognosis and complications in gastric cancer surgery patients. However, clinical implementation of GNRI-guided care often overlooks the heterogeneity of malnutrition risk among individuals.
This study investigates the clinical utility of GNRI-driven graded nutritional interventions in elderly CRC patients undergoing radical resection at our institution. By conducting the preoperative nutritional risk assessments, we aim to develop and apply the tailored nutritional intervention strategies and evaluate its effects on postoperative recovery, immune restoration, inflammatory modulation, and long-term QoL - addressing a critical gap in personalized perioperative care.
This retrospective study included 135 elderly CRC patients subjected to radical resection at our hospital between September 2022 and December 2024. The inclusion criteria were as follows: (1) Aged ≥ 65 years; (2) Confirmed primary CRC diagnosis via clinical and histopathological evaluation; (3) Completion of radical CRC surgery; and (4) Availability of complete clinical and follow-up data. The exclusion criteria were as follows: (1) Patients undergoing preoperative chemotherapy, radiotherapy, or neoadjuvant radiotherapy; (2) Patients with other primary or secondary malignancies; (3) Patients with concomitant hematological diseases, autoimmune diseases, or infectious diseases; (4) Patients who had undergone emergency surgery recently; (5) Patients requiring a second surgery due to local recurrence; (6) Patients with severe underlying diseases or malnutrition; and (7) Patients who had received nutritional preparations prior to admission. This study received ethical approval from the Ethics Committee of our hospital and was conducted in keeping with the ethical guidelines set forth in the Declaration of Helsinki by the World Medical Association.
Baseline characteristics of patients, such as age, sex, tumor location, and clinical staging, were retrospectively collected from the electronic medical record system. Based on the case selection criteria mentioned above, 126 patients were analyzed. Of these, 71 were male and 55 were female, with their ages ranging from 65 years to 89 years and a mean age of (73.48 ± 5.12) years. The patients were allocated into two groups according to the type of nutritional intervention: The research group received GNRI-based graded nutritional intervention (n = 61), and the control group was subjected to conventional nutritional support (n = 65).
The control group received conventional nutritional intervention. Nurses provided explanations about the disease and surgery, conducted nutritional screening, and organized meal schedules for the patients. They also informed the patients about potential postoperative complications and advised them to consume high-nutrient and digestible foods. Nutritional education materials, such as pamphlets on dietary and nutrition knowledge, were distributed, and postoperative dietary guidance posters for CRC patients were displayed in the hospital’s bulletin boards.
In the research group, 61 patients received GNRI-based graded nutritional intervention. The specific approach was as follows: (1) Nutritional risk assessment: Nursing staff performed nutritional risk assessments using the GNRI tool, where GNRI was calculated as: GNRI = 1.489 × albumin (g/L) + 41.7 × (actual body weight/ideal body weight). Ideal body weight for males = height (cm) – 100 - [(height - 150)/4]; for females = height (cm) - 100 - [(height - 150)/2.5]. Patients with a GNRI below 82 were assigned to the high-risk group (30 patients), those with a GNRI of 82-91 to the medium-risk group (22 patients), and those with a GNRI between 92 and 98 to the low-risk group (9 patients). Dietary intervention measures were then tailored for each group based on risk levels; (2) Dietary intervention for low nutritional risks: For patients in the low-risk group, data such as age, body weight, daily activity level, and occurrence of complications were collected. Their daily protein and calorie requirements were calculated, along with their water and sodium intake based on dialysis frequency, urine volume, and biochemical markers. Energy supply was provided at 30-35 kcal/kg/day, protein intake was controlled at 0.8-1 g/kg/day, with at least 30% of protein from high-quality sources, and salt intake was limited to 3-4 g/day; (3) Dietary intervention for medium nutritional risks: For patients in the medium-risk group, energy supply was adjusted to 35-40 kcal/kg/day based on physical activity, with protein intake set at 0.9-1.1 g/kg/day and at least 40% of protein from high-quality sources. Salt intake was the same as in the low-risk group. Dietary health education was enhanced in the hospital, using methods such as visual dialogue and scenario simulations to educate patients on the importance of dietary adjustments in managing their primary diseases. Patients were advised to avoid high-sodium foods like pickled vegetables and cured meats, and high-potassium foods such as oranges, apples, and bananas. A telephone follow-up system was also established to provide regular guidance on home dietary practices; and (4) Dietary intervention for high nutritional risks: For patients in the high-risk group, energy supply was set at 40-45 kcal/kg/day based on physical activity, and protein intake was adjusted to 1.0-1.2 g/kg/day, with more than 50% of protein from high-quality sources. Additionally, compound α-Ketoacid (0.08-0.12 g/kg/day) was administered. Dietary health education was reinforced both inside and outside the hospital. Building on the strategies for the medium-risk group, a food diary system was implemented, where patients or their families were asked to record their dietary intake over three days, including fruits, vegetables, staple foods, meats, water, and seasoning usage. A professional nutritionist assessed the dietary structure, made necessary adjustments, and provided remote guidance on dietary changes through nursing staff. Feedback and adjustments were made every three days.
Postoperative clinical indicators: Postoperative clinical indicators, including time to passage of gas by anus, number of fever episodes, duration of fever, length of hospital stay, and hospitalization costs, were compared between the two groups.
Nutritional status: Fasting blood samples were drawn from the elbow vein of patients in both groups before intervention (day 1 after surgery) and after intervention (day 1 after intervention completion), respectively. Hemoglobin (Hb), prealbumin (PAB), and transferrin (TRF) levels were measured using a fully automatic biochemical analyzer. Additionally, the nutritional status of both groups was assessed with the use of the Patient-Generated Subjective Global Assessment (PG-SGA). This scale covers functional status, symptoms and signs, dietary conditions, and body weight, with a total score ranging from 0 to 35. A lower score denotes better nutritional status.
QoL: Preceding and following intervention, the Generic QoL Inventory-74[11] was harnessed to evaluate the QoL of patients in both groups. This scale comprises four dimensions: Physical function, social function, material life, and psychological function. Scores for each dimension range from 0 to 100, with higher scores indicating better QoL.
Immune response: Fasting venous blood samples were harvested from both groups preceding and following intervention. After centrifugation, the supernatant was collected and mixed well with antibodies. Subsequent to a water bath for 20 minutes, an immunoturbidimetric assay was performed to determine the levels of immunoglobulin (Ig) G, IgA, and IgM to confirm the immune function of the patients[12].
Inflammatory responses: Prior to and following the intervention, enzyme-linked immunosorbent assay (ELISA)[13] was performed to measure the sera levels of nuclear factor kappa B (NF-κB), interleukin (IL)-1, tumor necrosis factor-α
Postoperative complications: The incidence of complications such as nausea and vomiting, abdominal distension and diarrhea, abnormal blood glucose levels, wound infections, anastomotic fistula, and pulmonary infections were observed and recorded for both groups of patients.
All data in this study were analyzed using SPSS22.0 software. Measurement data were represented as mean ± SD, and intergroup comparisons were performed using t-tests. Enumeration data were presented as percentages, with the χ2 test applied. A P-value < 0.05 was regarded as statistical significance.
The research group encompassed 61 patients, including 35 males and 26 females, with an average age of 73.66 ± 4.82 years. Among them, 31 had colon cancer and 30 had rectal cancer. Tumor-node-metastasis (TNM) staging was as follows: 26 cases in stage I and 35 cases in stage II. Educational attainment included junior high school or lower (n = 16), senior high school (n = 28), and university-level education (n = 17). In the control group (n = 65, 36 males and 29 females, mean age 73.31 ± 5.76 years) consisted of 39 colon cancer and 26 rectal cancer cases, with TNM staging of 32 stage I and 33 stage II tumors. Educational levels were junior high school or lower (n = 14), senior high school (n = 30), and university or higher education (n = 30). No statistically significant differences were observed between groups in sex, age, tumor type, TNM stage, or educational background (P > 0.05), confirming baseline comparability (Table 1).
| Group | n | Sex (cases) | Age (years), mean ± SD | Tumor type (cases) | Clinical staging (cases) | Education level (cases) | |||||
| Male | Female | Colon cancer | Rectal cancer | Stage I | Stage II | Junior high school or lower | Senior high school | University or higher | |||
| Research group | 61 | 35 | 26 | 73.66 ± 4.82 | 31 | 30 | 26 | 35 | 16 | 28 | 17 |
| Control group | 65 | 36 | 29 | 73.31 ± 5.76 | 39 | 26 | 32 | 33 | 14 | 30 | 21 |
| χ2/t | - | 0.051 | 0.369 | 1.074 | 0.553 | 0.497 | |||||
| P value | - | 0.822 | 0.713 | 0.300 | 0.457 | 0.780 | |||||
The postoperative time to passage of gas by anus, number of fever cases, duration of fever, length of hospital stays, and costs for the research group were 52.63 ± 8.22 hours, 6 cases, 49.68 ± 6.01 days, 6.51 ± 0.73 days, and 42500 ± 6600 RMB, respectively, all of which were significantly lower than those in the control group (P < 0.05). See Table 2.
| Group | n | Postoperative time to passage of gas by anus (hour) | Fever (cases) | Duration of fever (hour) | Length of hospital stay (day) | Hospitalization costs (ten thousand yuan) |
| Research group | 61 | 52.63 ± 8.22 | 6 | 49.68 ± 6.01 | 6.51 ± 0.73 | 4.25 ± 0.66 |
| Control group | 65 | 67.45 ± 7.68 | 15 | 64.33 ± 5.37 | 8.47 ± 0.95 | 5.33 ± 0.79 |
| χ2/t | - | 10.463 | 3.972 | 14.447 | 12.925 | 6.224 |
| P value | - | < 0.001 | 0.046 | < 0.001 | < 0.001 | < 0.001 |
No significant were observed in baseline nutritional markers, including the levels of Hb, PAB, TRF, and PG-SGA between the two groups pre-intervention (P > 0.05). Following intervention, the research group demonstrated significantly elevated levels of Hb (121.67 ± 13.13 g/L), PAB (245.54 ± 23.01 mg/L), and TRF (2.31 ± 0.20 g/L) compared with the control group (P < 0.05). The PG-SGA score for the research group attained 4.55 ± 0.48, significantly lower than that observed in the control group (P < 0.05). Refer to Table 3. Our findings indicated that GNRI-based graded nutritional intervention significantly enhanced postoperative nutritional status relative to conventional care.
| Group | n | Hb (g/L) | PAB (mg/L) | TRF (g/L) | PG-SGA (points) | ||||
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | ||
| Research group | 61 | 102.33 ± 10.52 | 121.67 ± 13.13 | 152.32 ± 18.71 | 245.54 ± 23.01 | 1.41 ± 0.15 | 2.31 ± 0.20 | 10.29 ± 1.33 | 4.55 ± 0.48 |
| Control group | 65 | 104.28 ± 9.47 | 113.46 ± 14.85 | 151.78 ± 16.52 | 210.37 ± 22.41 | 1.42 ± 0.13 | 1.98 ± 0.12 | 10.31 ± 1.48 | 5.81 ± 0.56 |
| t | - | 1.095 | 3.279 | 0.172 | 8.690 | 0.401 | 11.311 | 0.080 | 13.519 |
| P value | - | 0.276 | 0.001 | 0.864 | < 0.001 | 0.689 | < 0.001 | 0.967 | < 0.001 |
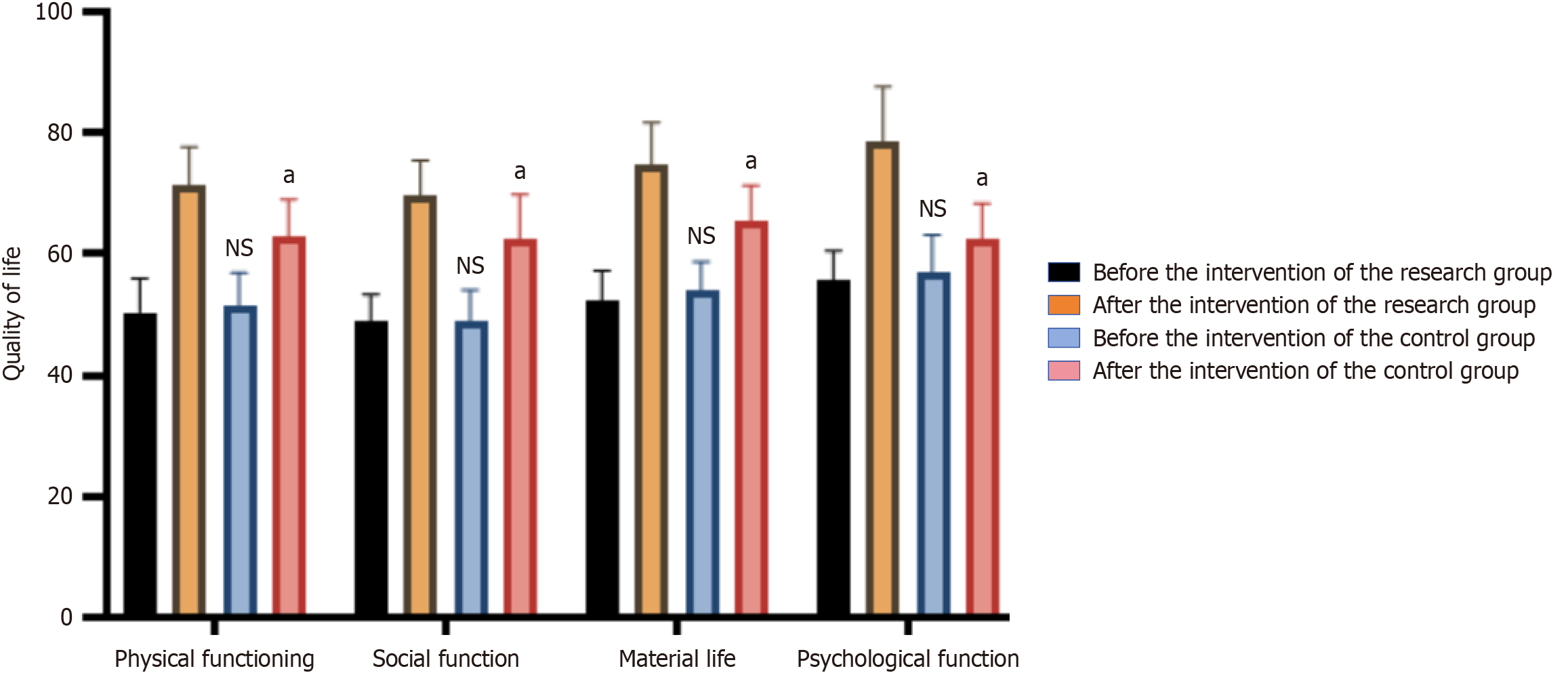
Prior to intervention, there were no significant difference between the two groups regarding physical function, social function, material life, and psychological function (P > 0.05). Post-intervention, the research group scored 71.35 ± 6.31 in physical function, 69.71 ± 5.69 in social function, 74.85 ± 6.84 in material life, and 78.45 ± 9.12 in psychological function, all of which were significantly higher than the control group (P < 0.05), as presented in Figure 1, suggesting that GNRI-based graded nutritional intervention improved the postoperative QoL in elderly CRC patients.
Preceding intervention, no significant difference were found between the two groups in terms of IgG, IgA, and IgM levels (P > 0.05). Following intervention, the research group demonstrated IgG levels of 8.40 ± 0.87 g/L, IgA levels of 1.64 ± 0.18 g/L, and IgM levels of 1.51 ± 0.19 g/L, all of which were significantly higher than those observed in the control group (P < 0.05), as exhibited in Table 4, implying that GNRI-based graded nutritional intervention improved the restore of immune function in patients after surgery.
| Group | n | IgG (g/L) | IgA (g/L) | IgM (g/L) | |||
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | ||
| Research group | 61 | 6.25 ± 0.59 | 8.40 ± 0.87 | 1.32 ± 0.15 | 1.64 ± 0.18 | 1.23 ± 0.11 | 1.51 ± 0.19 |
| Control group | 65 | 6.21 ± 0.68 | 7.31 ± 0.91 | 1.36 ± 0.13 | 1.41 ± 0.13 | 1.20 ± 0.09 | 1.32 ± 0.15 |
| t | - | 0.352 | 6.864 | 1.602 | 8.260 | 1.680 | 6.250 |
| P value | - | 0.726 | < 0.001 | 0.112 | < 0.001 | 0.096 | < 0.001 |
Pre-intervention analysis revealed no significant differences in inflammatory markers between groups in terms of NF-κB, IL-1, TNF-α, and IL-8 Levels (P > 0.05). Following intervention, the research group exhibited NF-κB levels of 13.21 ± 1.02 U/L, IL-1 Levels of 32.18 ± 3.88 g/L, TNF-α levels of 22.12 ± 2.08 μg/L, and IL-8 levels of 48.71 ± 6.94 μg/L, all of which were significantly reduced than those within the control group (P < 0.05), as detailed in Table 5. These findings demonstrate that GNRI-stratified nutritional intervention significantly attenuated postoperative inflammatory responses compared to conventional nutritional support, suggesting enhanced recovery potential through modulation of systemic inflammation.
| Group | n | NF-κB (U/L) | IL-1 (g/L) | IL-8 (μg/L) | TNF-α (μg/L) | ||||
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | ||
| Research group | 61 | 17.55 ± 1.83 | 13.21 ± 1.02 | 50.48 ± 5.92 | 32.18 ± 3.88 | 29.28 ± 2.50 | 22.12 ± 2.08 | 61.35 ± 6.14 | 48.71 ± 6.94 |
| Control group | 65 | 17.63 ± 1.25 | 15.08 ± 1.23 | 51.12 ± 6.48 | 40.75 ± 4.11 | 29.85 ± 2.63 | 25.81 ± 2.16 | 60.89 ± 5.87 | 52.64 ± 7.18 |
| t | - | 0.288 | 9.257 | 0.578 | 12.018 | 1.245 | 9.756 | 0.430 | 3.120 |
| P value | - | 0.774 | < 0.001 | 0.565 | < 0.001 | 0.215 | < 0.001 | 0.668 | < 0.001 |
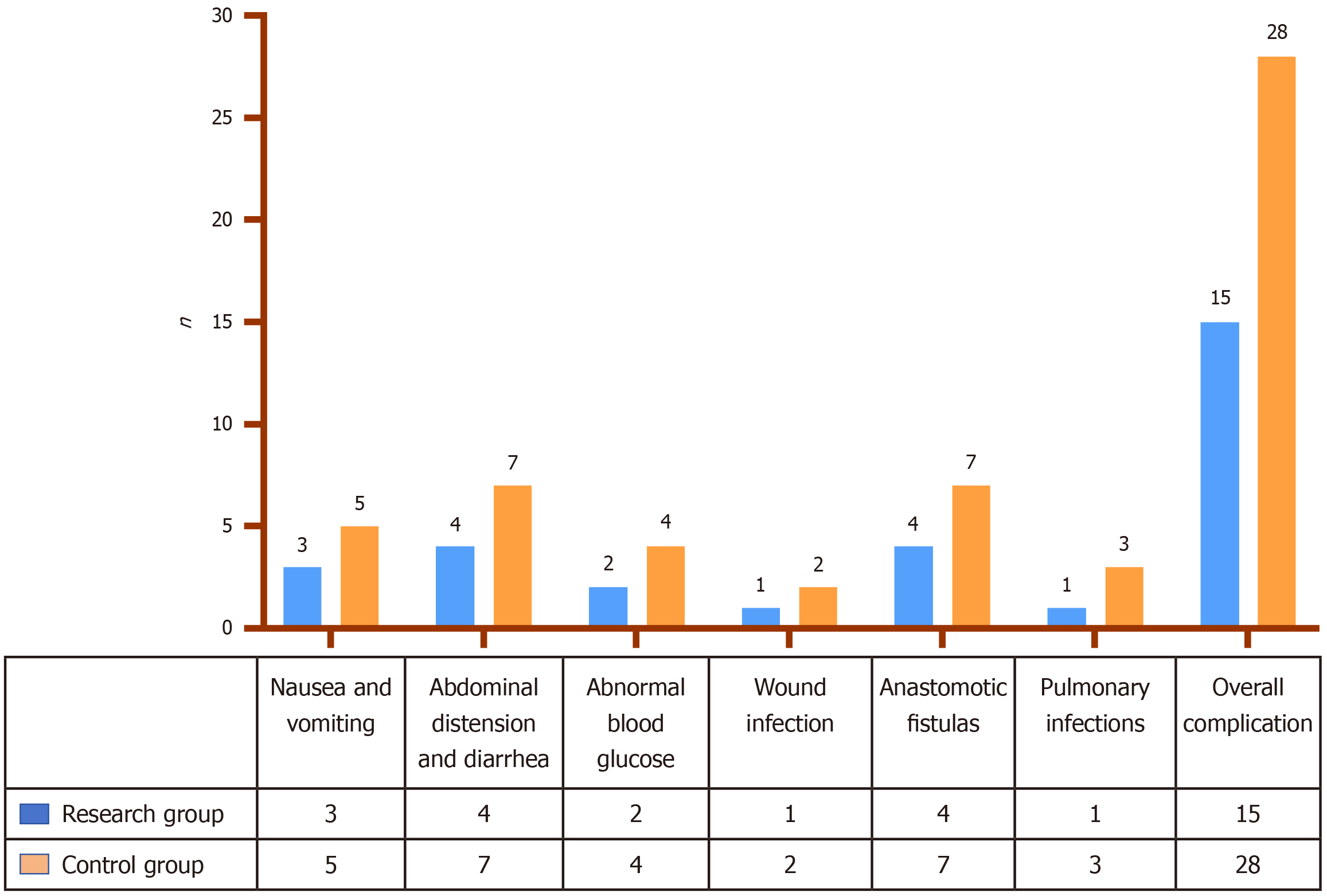
Nausea and vomiting, abdominal distension and diarrhea, abnormal blood glucose levels, incision infections, anastomotic fistulas, and pulmonary infections are the most prevalent postoperative complications in elderly patients with CRC. The overall complication rate in the research group attained 24.59%, significantly lower than the 43.08% observed in the control group (P < 0.05; Figure 2).
Individuals aged ≥ 60 years constitute a high-risk population for CRC due to age-related physiological changes. Pro
To address these clinical challenges, the GNRI has emerged as a valuable assessment tool. Originally developed by American clinicians in 2005, GNRI provides a simple and accurate method for quantifying the nutritional status of elderly patients and predicting morbidity and mortality risks during hospitalization. This index has demonstrated significant prognostic value in various benign and malignant diseases[20-22]. For instance, Xu et al[23] analyzed data from 3239 CRC patients through 8 retrospective studies and found that a low GNRI score could be served as an important predictor for postoperative complications and long-term low survival rates in Asian CRC patients. With GNRI-based graded nutritional intervention for CRC patients, it enables personalized risk-adapted intervention strategies in clinics. This approach addresses a critical gap in geriatric surgical oncology, where standardized nutritional protocols often fail to account for individual risk variability[24,25]. By implementing GNRI-based stratification, we aim to investigate the effects of GNRI-based graded nutritional intervention on the postoperative nutritional status and QoL in elderly CRC individuals.
Our findings demonstrated that GNRI-stratified nutritional intervention yielded superior outcomes compared to conventional care in elderly CRC patients across multiple postoperative parameters. The intervention group showed accelerated surgical recovery (shorter time to first flatus, reduced febrile episodes, and decreased fever duration), significant reductions in hospital stay length and treatment costs, improved nutritional biomarkers (elevated Hb, PAB, and TRF levels with better PG-SGA scores), enhanced QoL across all measured domains (physical, social, material, and psychological functioning), strengthened immune response (increased IgG, IgA, and IgM levels), reduced systemic inflammation (lower NF-κB, IL-1, TNF-α, and IL-8 concentrations), decreased overall complication rates. These findings indicated that GNRI-based graded nutritional intervention effectively improves postoperative recovery while mitigating inflammatory responses and complication risks in elderly CRC patients. Our findings align with previous research by Liao et al[26], which discovered GNRI as an independent predictor of surgical complications and survival outcomes, with lower GNRI scores correlating with lower overall survival and disease-free survival rates. Similarly, Mao et al[27] emphasized the prognostic indicator of GNRI for CRC patients undergoing surgery. Patients with low GNRI scores displayed poor disease-free survival and higher complication rates. Therefore, patients may benefit from the personalized treatment strategies based on GNRI.
The superiority of GNRI-stratified nutritional intervention may be attributed to precision nutritional assessment and risk-adapted interventions. This systematic approach enables clinicians to objectively assess patients’ malnutrition risk levels, implement tiered nutritional support protocols based on individualized risk stratification, adjust nutritional intake appropriately[28]. The intervention's effectiveness is further enhanced through innovative patient engagement strategies, such as scenario simulations and visual dialogues. This comprehensive framework fosters patient empowerment through nutritional literacy, active participation in treatment adherence and sustainable dietary behavior modification. Ultimately, this promotes the establishment of a healthy, effective, and sustainable optimized nutritional diet structure[29], contributing to enhanced nutritional status, strengthened immune function, better recovery capacity, reduced inflammation, improved QoL, and a lower incidence of complications.
This study introduces several important innovations to perioperative nutritional management in elderly CRC patients. For intervention model, we developed and implemented a graded nutritional intervention model based on the GNRI, which demonstrated superior clinical outcomes compared to conventional nutritional support approaches. However, there are several limitations. The retrospective study design may introduce potential selection biases, and our single-center experience with a limited sample size restricts the generalizability of findings. Additionally, due to the sample size limitations, we did not control for or stratify patients by educational level, which could represent an important confounding variable in nutritional intervention studies. Future studies should be conducted with prospective experiments with large sample sizes and multiple centers to validate our findings. Additional studies should also evaluate the cost-effectiveness of this intervention and assess its real-world implementation feasibility across diverse healthcare settings.
In summary, GNRI-based graded nutritional intervention can effectively improve clinical indicators, enhance nutritional status and QoL in elderly CRC patients, promote immune function recovery, alleviate inflammation, and reduce the incidence of postoperative complications, highlighting the importance of potential postoperative intervention approach.
| 1. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 1368] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56688] [Article Influence: 7086.0] [Reference Citation Analysis (135)] |
| 3. | Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y, Lu H. Neoadjuvant immunotherapy for colorectal cancer: Right regimens, right patients, right directions? Front Immunol. 2023;14:1120684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Zeng S, Wu W, Zhang X, Qiu T, Gong P. The significance of anatomical variation of the inferior mesenteric artery and its branches for laparoscopic radical resection of colorectal cancer: a review. World J Surg Oncol. 2022;20:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Lin Y, Sun Y, Lin H, Huang Y, Jiang W, Xu Z, Huang S, Ye D, Chi P. Prediction of prolonged resolution of chylous ascites after radical D3 resection for colorectal cancer: A population-based experience from a high-volume center. Eur J Surg Oncol. 2022;48:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Chen W, Yu D, Ren Q, Shen Z, Huang G, Chen X, Dong Q, Yu Z. Predictive value of Global Leadership Initiative on Malnutrition criteria combined with handgrip strength for postoperative outcomes in overweight colorectal cancer patients. J Gastroenterol Hepatol. 2024;39:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Lu W, Shen J, Zou D, Li P, Liu X, Jian Y. Predictive role of preoperative geriatric nutritional risk index for clinical outcomes in surgical gastric cancer patients: A meta-analysis. Front Surg. 2022;9:1020482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Zhao H, Xu L, Tang P, Guo R. Geriatric Nutritional Risk Index and Survival of Patients With Colorectal Cancer: A Meta-Analysis. Front Oncol. 2022;12:906711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Shen F, Ma Y, Guo W, Li F. Prognostic Value of Geriatric Nutritional Risk Index for Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lung. 2022;200:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 10. | Zhang Q, Zhang L, Jin Q, He Y, Wu M, Peng H, Li Y. The Prognostic Value of the GNRI in Patients with Stomach Cancer Undergoing Surgery. J Pers Med. 2023;13:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 11. | Xie YE, Huang WC, Li YP, Deng JH, Huang JT. Dynamic interaction nursing intervention on functional rehabilitation and self-care ability of patients after aneurysm surgery. World J Clin Cases. 2022;10:4827-4835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 12. | Schneider F, Failing K, Wehrend A. [Measurement of IgG concentration in bovine colostrum by immunoturbidimetric assay in comparison to ELISA-based assessment]. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2020;48:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Lei P, Li Z, Hua Q, Song P, Gao L, Zhou L, Cai Q. Ursolic Acid Alleviates Neuroinflammation after Intracerebral Hemorrhage by Mediating Microglial Pyroptosis via the NF-κB/NLRP3/GSDMD Pathway. Int J Mol Sci. 2023;24:14771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 14. | Guo CG, Ma W, Drew DA, Cao Y, Nguyen LH, Joshi AD, Ng K, Ogino S, Meyerhardt JA, Song M, Leung WK, Giovannucci EL, Chan AT. Aspirin Use and Risk of Colorectal Cancer Among Older Adults. JAMA Oncol. 2021;7:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 15. | Ozluk AA, Outlaw D, Akce M, Fowler ME, Hess DL, Giri S, Williams GR. Management of Older Adults With Colorectal Cancer: The Role of Geriatric Assessment. Clin Colorectal Cancer. 2023;22:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gausman V, Dornblaser D, Anand S, Hayes RB, O'Connell K, Du M, Liang PS. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol. 2020;18:2752-2759.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 17. | Li Z, Zou Z, Lang Z, Sun Y, Zhang X, Dai M, Mao S, Han Z. Laparoscopic versus open radical resection for transverse colon cancer: evidence from multi-center databases. Surg Endosc. 2021;35:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Shen N, Wen J, Chen C, Chen X, Zhang W, Garijo PD, Wei MY, Chen W, Xue X, Sun X. The relationship between GLIM-malnutrition, post-operative complications and long-term prognosis in elderly patients undergoing colorectal cancer surgery. J Gastrointest Oncol. 2023;14:2134-2145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Arora S, Fowler ME, Harmon C, Al-Obaidi M, Outlaw D, Hollis R, Gbolahan O, Khushman M, Giri S, Williams GR. Differences in Pretreatment Frailty Across Gastrointestinal Cancers in Older Adults: Results From the Cancer and Aging Resilience Evaluation Registry. JCO Oncol Pract. 2022;18:e1796-e1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1728] [Article Influence: 82.3] [Reference Citation Analysis (7)] |
| 21. | Abd Aziz NAS, Teng NIMF, Abdul Hamid MR, Ismail NH. Assessing the nutritional status of hospitalized elderly. Clin Interv Aging. 2017;12:1615-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 22. | Sagasaki M, Maruyama Y, Nakashima A, Fukui A, Yokoo T. Association between the serum zinc level and nutritional status represented by the geriatric nutritional Rrisk index. Clin Exp Nephrol. 2024;28:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Xu J, Sun Y, Gong D, Fan Y. Predictive Value of Geriatric Nutritional Risk Index in Patients with Colorectal Cancer: A Meta-Analysis. Nutr Cancer. 2023;75:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 24. | Schult D, Maurer HC, Frolova M, Ringelhan M, Mayr U, Ulrich J, Heilmaier M, Rasch S, Lahmer T, Reitmeier S, Hennig C, Gassner C, Thur N, Will T, Janssen KP, Steiger K, Jesinghaus M, Neuhaus K, Quante M, Haller D, Abdelhafez M, Schmid RM, Middelhoff M. Systematic Evaluation of Clinical, Nutritional, and Fecal Microbial Factors for Their Association With Colorectal Polyps. Clin Transl Gastroenterol. 2024;15:e00660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Chu AH, Lin K, Croker H, Kefyalew S, Markozannes G, Tsilidis KK, Park Y, Krebs J, Weijenberg MP, Baskin ML, Copson E, Lewis SJ, Seidell JC, Chowdhury R, Hill L, Chan DS, Lee DH, Giovannucci EL. Dietary-Lifestyle Patterns and Colorectal Cancer Risk: Global Cancer Update Programme (CUP Global) Systematic Literature Review. Am J Clin Nutr. 2025;121:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Liao CK, Chern YJ, Hsu YJ, Lin YC, Yu YL, Chiang JM, Yeh CY, You JF. The Clinical Utility of the Geriatric Nutritional Risk Index in Predicting Postoperative Complications and Long-Term Survival in Elderly Patients with Colorectal Cancer after Curative Surgery. Cancers (Basel). 2021;13:5852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Mao Y, Lan J. Prognostic value of the geriatric nutritional index in colorectal cancer patients undergoing surgical intervention: A systematic review and meta-analysis. Front Oncol. 2022;12:1066417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Dai C, Yan D, Xu M, Huang Q, Ren W. Geriatric Nutritional Risk Index is related to the risk of stroke-associated pneumonia. Brain Behav. 2022;12:e2718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Sato R, Oikawa M, Kakita T, Okada T, Abe T, Tsuchiya H, Akazawa N, Ohira T, Harada Y, Okano H, Ito K, Tsuchiya T. Low Geriatric Nutritional Risk Index (GNRI) Predicts Poorer Survival in Patients with Obstructive Colorectal Cancer Who Had a Self-Expandable Metallic Stent (SEMS) Inserted as a Bridge to Curative Surgery. J Anus Rectum Colon. 2023;7:63-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/