Published online Jul 27, 2025. doi: 10.4240/wjgs.v17.i7.105956
Revised: March 29, 2025
Accepted: May 29, 2025
Published online: July 27, 2025
Processing time: 161 Days and 8.4 Hours
Irreversible transmural intestinal necrosis (ITIN) is associated with high mortality rates in patients with acute occlusive mesenteric ischemia (AOMI). Currently, there are not many studies on the use of dual energy computed tomography (DECT) for evaluating ITIN.
To evaluate the diagnostic value of DECT for ITIN in AOMI.
The cases and computed tomography (CT) images of 102 patients with clinically diagnosed AOMI (including 48 ITIN) from January 2012 to January 2022 were retrospectively collected. The CT scans included both multidetector CT and DECT. The raw data from DECT portal-venous phase were reconstructed into 120 kVp mixed energy image, 50 keV virtual monoenergetic imaging, and iodine map. Two radiologists independently completed the subjective visual assessment of CT signs related to AOMI. Objective parameters, including the attenuation of the normal and lesion intestinal wall segment (CT50 keV lesion, CT50 keV normal/lesion) and iodine concentrations (IClesion and ICnormal/lesion), were quantified. Furthermore, multivariate logistic regression, receiver operating characteristic curves, and area under the curve (AUC) values were used to evaluate the subjective and objective indicators in predicting ITIN.
Regarding subjective signs, logistic regression analysis revealed reduced or absent bowel wall enhancement [odds ratio (OR) = 5.576, 95% confidence interval (CI): 1.547-20.093], bowel dilation (OR = 11.613, 95%CI: 3.790-35.586), and parenchymatous organ infarction (OR = 4.727, 95%CI: 1.536-14.551) were independent risk factors for the ITIN. CT subjective signs had a high diagnostic efficacy for ITIN (AUC = 0.853). The two DECT objective parameters also exhibited excellent diagnostic value for ITIN, with an AUC of 0.79, a cut-off value of CT50 keV normal/lesion = 2.81, and an AUC of 0.777 with a cut-off value of ICnormal/lesion = 2.39. The Delong test showed that there was no significant difference in the efficacy of subjective CT signs and objective DECT parameters (P > 0.05). Importantly, we observed that ICnormal/lesion combined with subjective signs (bowel dilation and parenchymatous organ infarction) had the highest predictive performance (AUC = 0.894), sensitivity (100%), and specificity (70.83%), which was statistically different from the AUC of CT subjective signs (P = 0.017).
ICnormal/lesion (DECT-based features) combined with CT subjective signs (bowel dilatation and parenchymatous organ infarction) showed favorable predictive performance for ITIN in AOMI, which may help clinicians develop timely treatment strategies.
Core Tip: Acute occlusive mesenteric ischemia is an intestinal ischemic injury caused by sudden interruption of intestinal blood supply. If not treated promptly, acute occlusive mesenteric ischemia usually develops into irreversible transmural intestinal necrosis (ITIN). Accurate diagnosis of ITIN is challenging because of its non-specific clinical manifestations. Currently, there is limited research on predicting ITIN using dual energy computed tomography (DECT). We use DECT objective parameters including iodine concentration and virtual monoenergetic imaging to quantify the degree of intestinal wall ischemia, and then evaluate their predictive performance for ITIN. The results demonstrated that DECT quantitative parameters can provide important information about whether ITIN occurs.
- Citation: Yang JS, Xu ZY, Chen FX, Wang MR, Fan XL, He BS. Diagnostic value of dual-energy computed tomography in irreversible transmural intestinal necrosis in patients with acute occlusive mesenteric ischemia. World J Gastrointest Surg 2025; 17(7): 105956
- URL: https://www.wjgnet.com/1948-9366/full/v17/i7/105956.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i7.105956
Acute mesenteric ischemia (AMI) is caused by a sudden interruption in blood supply to the intestine, which can result in cellular damage and intestinal ischemic injury. If not treated promptly, it usually develops into necrosis of the intestinal wall, leading to death[1]. The main causes of AMI were further defined as occlusive ischemia [acute occlusive mesenteric ischemia (AOMI), arterial embolism, arterial thrombosis, and venous thrombosis; accounts for 2/3 of cases] and non-occlusive ischemia[2]. In general, the overall incidence of AMI is low, accounting for 0.09%-0.2% of all acute admissions to the emergency department, and is a rare cause of abdominal pain. However, it has a high mortality rate of approximately 50%-80%[3]. AOMI can be categorized into two stages (early and late) and is associated with different prognoses. In the early ischemic stage, lesions are reversible and conservative management or endovascular techniques are essential. Advanced disease is characterized by irreversible transmural intestinal necrosis (ITIN), which requires surgical resection of the necrotic intestinal wall to control disease progression[4]. Clinical research has found that if left untreated or treated too late, the probability of ITIN development in patients with AOMI increases rapidly from 0% to 82% within 24 hours, followed by a sharp increase in mortality[5]. Therefore, rapid diagnosis and intervention are essential to limit patient mortality. However, accurate diagnosis of ITIN is challenging because of its non-specific clinical manifestations.
Radiologists play a crucial role in the rapid diagnosis of AMI. According to the current guidelines, multidetector computed tomography (MDCT) is recommended as the first-line method for assessing AMI[6]. Previous studies have shown that the MDCT signs in patients with AMI are associated with ITIN. For example, Calame et al[7] reported that AOMI was characterized by reduced or absent bowel wall enhancement and less mesenteric fat stranding; Tang et al[8] found that superior mesenteric artery (SMA) thrombus density on MDCT was considered an independent risk factor for ITIN, and SMA > 36.2 Hounsfield units (HU) can predict the occurrence of thromboembolic ITIN. Although MDCT has high sensitivity and specificity for the diagnosis of ischemia caused by arterial and venous occlusion (approximately 90%), it has low sensitivity for the prediction of obstruction and ITIN (approximately 49%)[9]. Dual-energy computed tomography (DECT) has emerged as a new tool for evaluating AMI. DECT utilizes X-ray attenuation at two different photon energy levels (low and high energy) to characterize tissue composition[10]. In addition, DECT post-processing techniques such as virtual monoenergetic image (VMI) reconstruction and iodine localization can increase the visibility of iodized contrast agents to improve the visualization of reduced intestinal wall enhancement[11]. Therefore, it may become increasingly valuable for the diagnosis and monitoring of bowel-related diseases. However, only a few studies have investigated the relationship between DECT and ITIN in patients with AOMI. Moreover, a comparison of the efficacy of conventional MDCT and DECT in predicting ITIN is lacking.
Consecutive patients with a high suspicion of AOMI were recruited at our hospital between January 2021 and January 2022. The diagnostic criteria for AOMI have been described previously[12]. Patients were required to meet the following three requirements simultaneously: (1) Acute abdominal symptoms; (2) Stenosis and occlusion of the main trunk and/or branches of the SMA and/or superior mesenteric vein (SMV); and (3) Computed tomography (CT) showing abnormal changes in the wall of the small bowel. The surgical patients underwent surgical resection within 12 hours of their CT scan, and the corresponding clinical excision specimens were subsequently subjected to retrospective pathological evaluation. Structural disruption of the entire small bowel wall (mucosa, submucosa, muscular, and plasma layers), as well as intestinal perforation, is considered ITIN. In addition, clinical data (including telephone interviews) 3 months after admission were reviewed. After anticoagulation or endovascular intervention, patients with AOMI who did not require surgical bowel resection and whose clinical symptoms/signs disappeared were classified as non-ITIN. Patients with AOMI who underwent conventional abdominal MDCT or plain and two-phase enhanced DECT scan were included in this study. Notably, cases were excluded for the following reasons: Patients with chronic mesenteric ischemia (duration of disease > 4 weeks); CT examination of advanced AOMI patients with signs of free abdominal gas, intestinal wall gas, and mesenteric venous gas at first admission; quantitative measurement affected by the appearance of thinning of the intestinal wall on CT, stenosis, or occlusion of mesenteric vessels caused by tumor invasion or cancer-associated thrombus; intestinal wall abnormalities caused by non-mesenteric vascular obstruction; CT image artifacts affecting the quantitative and qualitative evaluation of lesions; and incomplete clinical data. This retrospective study was approved by the ethics committee of our hospital and the requirement for informed patient consent was waived.
Participants were examined using 64-section and 128-section CT machines, and DECT data were acquired using the SOMATOM Force (third-generation) dual-source CT system (Siemens Healthcare, Forchheim, Germany). All patients underwent standardized imaging protocols, including plain abdominal, arterial, and portal vein-phase imaging. For DECT scanning, the plain scan adopted Care Dose 4D auto-adjustment technology with a voltage setting of 120 kVp. The specific parameters were as follows: Detector collimation was 64 mm × 0.6 mm; spiral pitch was 0.8; rotational speed was 0.5 s/r; kernel coefficient was B25f smooth. For DECT enhancement scanning, two X-ray tubes with different voltages were used for detection based on Care Dose 4D technology: Tube A voltage of 90 kVp and tube B voltage of Sn 150 kPv. The scanning parameters were as follows: Collimator 64 mm × 0.6 mm; field of view 512 mm × 512 mm; pitch 0.8; and tube rotation speed 0.5 s/r. The arterial phase was initiated using the TEST Bolus, with the threshold for the start CT value set to 100 Hounsfield units and a delay of 4 seconds to start the scan. Meanwhile, the portal-venous phase began to be collected approximately 75 seconds after the injection of the contrast agent. The contrast agent was injected into the right anterior elbow of all patients using a double-barrel syringe; tube A contained Ultravist (370 mgI/mL, 60-80 mL), and tube B contained normal saline (50 mL, injection rate 3.5 mL/seconds). In addition, the Care Dose 4D technology was employed to conduct conventional MDCT three-phase scanning with a tube voltage of 120 kVp.
Images of the portal-venous phase: The intestinal wall abnormalities were evaluated using images of the portal-venous phase. For conventional CT, the collected raw data were reconstructed using a convolution kernel (soft-tissue, B25f, Siemens) and iterative reconstruction technique (SAFIRE-3), and the images (layer thickness: 1.0 mm; spacing: 0.7 mm) were transferred to a post-processing workstation (Syngo MMWP VE40A). Multiplanar reconstruction was performed according to the requirements with a thickness and spacing of 2 mm. For DECT, the double-energy raw data (90 kVp and Sn 150 kVp from the portal-venous phase) were reconstructed using a convolution kernel (soft-tissue, B25f) and an iterative reconstruction technique (ADMIRE, strength level 3), and the images were then transferred to a Siemens post-processing workstation (Syngo.Via, version 30A). The low- and high-kV data were then fused via linear fusion (ratio: 6:4; fusion coefficient: M_0.6) to simulate a conventional 120 kVp mixed energy image. In addition, it has been found that the best image quality can be obtained from 50 keV[13]. Thus, we utilized a dual-energy Monoenergetic Plus (Mono+) to reconstruct 50-KeV VMI, and iodine density maps were acquired using liver nailfold video capillaroscopy software.
Images of the arterial phase: The lesion area and extent of SMA were evaluated using images of the arterial phase. Raw data of arterial phase from the DECT scan were simulated by linear fusion to simulate conventional 120-kVp hybrid energy images[14].
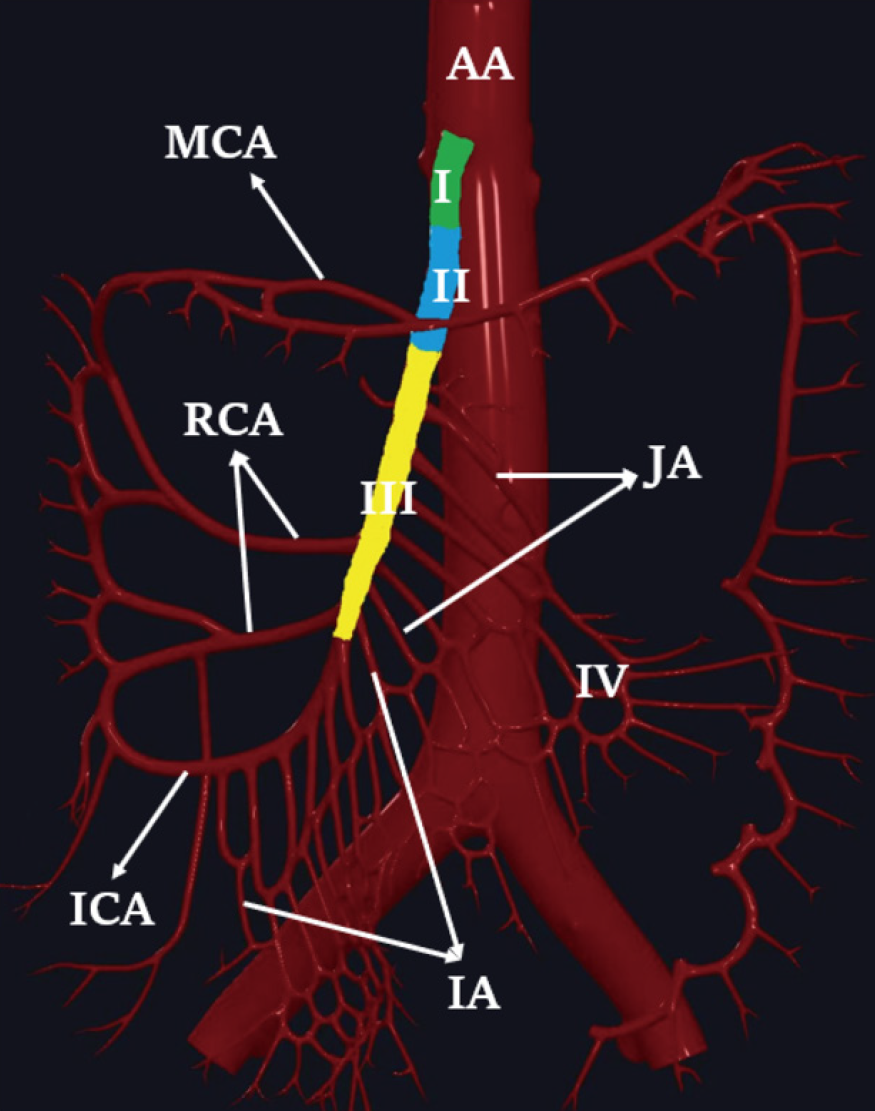
Subjective image analysis: Two radiologists, A and B (with 7 and 10 years of experience in abdominal CT diagnosis, respectively), independently and blindly assessed the images. They were all unaware of the patients’ final diagnoses and evaluated subjective CT signs on 120kVp images. The final subjective CT signs were determined by two senior radiologists C and D (with 20 years of experience in abdominal CT diagnosis) based on the patient’s pathology and other final diagnostic results in a post-view and finally reached an agreement after discussions. SMA partitioning was applied to determine the location of SMA stenosis or occlusion. The SMA was divided into the following four regions: Region I, the starting part of the SMA; region II, the main SMA trunk containing the initiation of the middle colic artery; region III, the SMA trunk below the initiation of the middle colic artery; and region IV, the branch arteries around the main SMA trunk, including the middle colic artery, jejunal artery, ileal artery, and ileocolic artery (Figure 1). The extent of SMA lesion involvement was categorized as localized (≤ 20 mm), segmental (20 to 50 mm), and diffuse (≥ 50 mm or ≥ 2 localized and/or segmental lesions).
The subjective signs of AOMI major intestinal wall and extra-intestinal abnormalities on CT were as follows: (1) Intestinal wall thickening, thickness ≥ 3 mm; (2) High density of the intestinal wall, intestinal wall of the lesion segment was visually hyperdense compared with adjacent normal segment intestinal wall on non-enhanced CT; (3) Intestinal wall enhancement, intestinal wall was highly strengthened compared with adjacent normal segment intestinal wall; (4) Reduced or absent bowel wall enhancement, the strengthening of intestinal wall in the lesioned segment was diminished compared with adjacent normal segment intestinal wall; (5) Dilatation of the intestinal cavity, with pipe diameter ≥ 25 mm; (6) Mesenteric exudation; (7) Abdominal free fluid; and (8) Parenchymatous organ infarcts, mainly including liver, spleen, and kidney, were seen in cuneiform hypoperfusion area.
Objective analysis of images: Objective image analysis of DECT-acquired data was conducted by radiologists C and D according to the patient’s final diagnosis. If there was a dispute between two individuals regarding the identification of normal and diseased intestinal walls, a consensus was reached through negotiation. Objective measures included CT50 keV lesions, CT50 keV normal/lesions, iodine concentrations (IClesions), and ICnormal/lesions. CT50 keV lesions referred to the attenuation of the intestinal wall of lesion segment on 50 keV images, CT50 keV normal/lesion referred to the ratio of attenuation between the intestinal wall of the normal and lesion segment on 50 keV images, IClesions referred to iodine value of the intestinal wall of lesion segment measured on the iodine map, while ICnormal/lesion referred to the ratio of iodine values between the intestinal wall of the normal and lesion segment on the iodine map. During the measurement, the image was enlarged three times, and the region of interest (ROI) was outlined by selecting the most obvious area of the lesion, avoiding areas with obvious small blood vessels, gas, and artifacts. Individual heterogeneity exists in the shape and size of the patient’s ROI owing to differences in intestinal wall thickness and the degree of bowel dilation, the area range of ROI is 0.3-1 cm3. The average of two measurements was used to minimize measurement error. Objective parameters, including the attenuation of the normal and lesion intestinal wall segment (CT50 keV lesion, CT50 keV normal/lesion), IClesion, and ICnormal/lesion, were quantified.
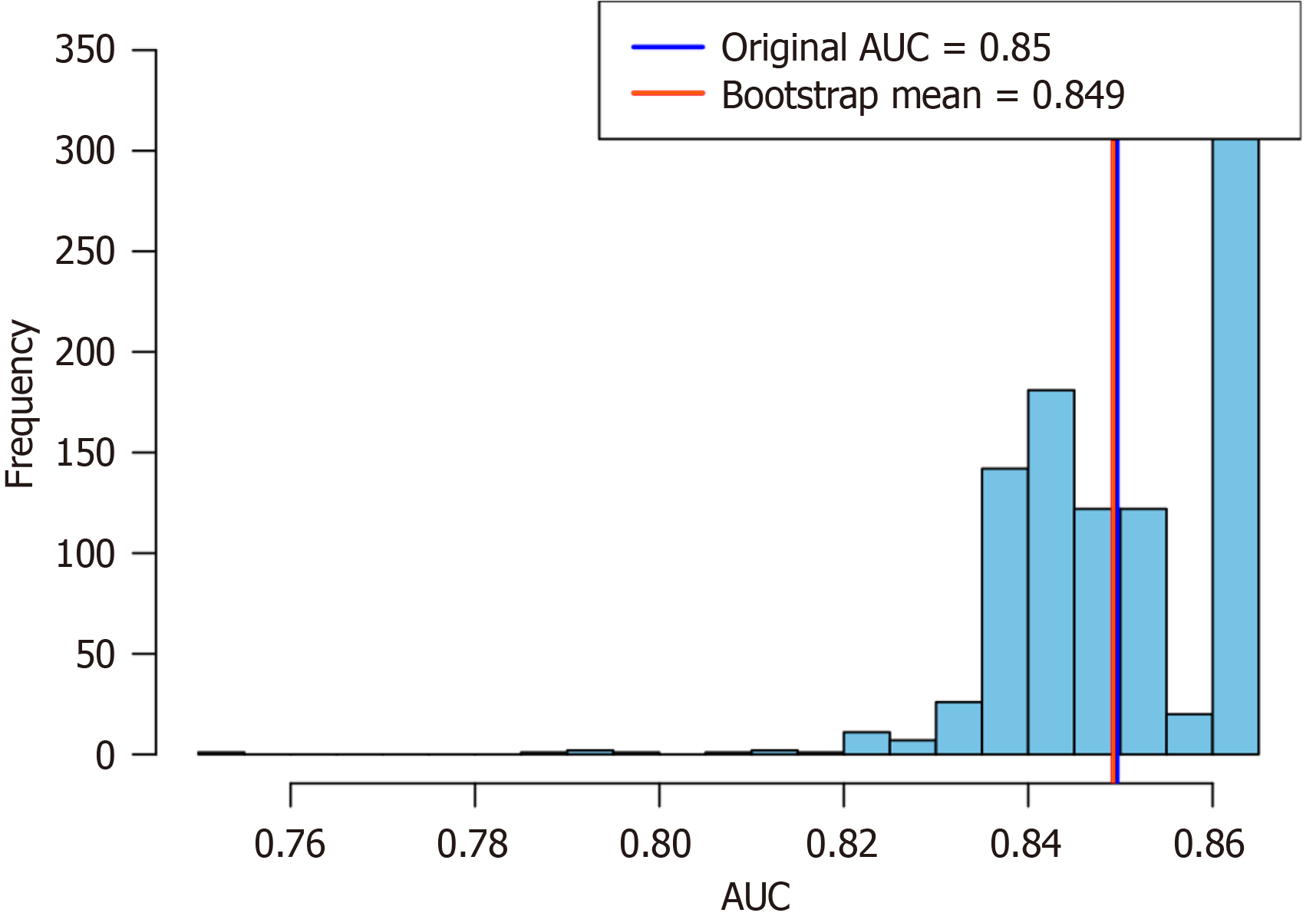
The Kolmogorov-Smirnov test was used to test the normality of the data distribution. For the DECT objective indicators, normally distributed continuous variables were compared between the groups using the independent t-test, whereas non-normally distributed variables were compared using the Mann-Whitney U test. For subjective CT signs, Pearson’s χ2-test or Fisher’s exact probability method was used for comparison between groups. The consistency between the radiologists A and B was evaluated based on a Cohen’s kappa coefficient. A kappa value of < 0.20 was considered to show poor confidence; 0.21-0.40, slight confidence; 0.41-0.60, moderate confidence; 0.61-0.80, high confidence; and 0.81-1.00, excellent confidence. With the occurrence of ITIN as the dependent variable, multivariate logistic regression was used to analyze the correlation between subjective CT signs and ITIN, and the odds ratio (OR) and 95% confidence interval (CI) of each factor were calculated. The area under the curve (AUC) values for different indicators were compared using the Delong test. Internal validation was performed using the bootstrap method with 1000 resampling iterations to calculate the AUC values and their mean. The distribution of these values was visualized using histograms. All analyses were conducted with SPSS software (version 26.0, IBM Corp), MedCalc version 20.0 and R version 4.1.0. Statistical significance was set at P < 0.05.
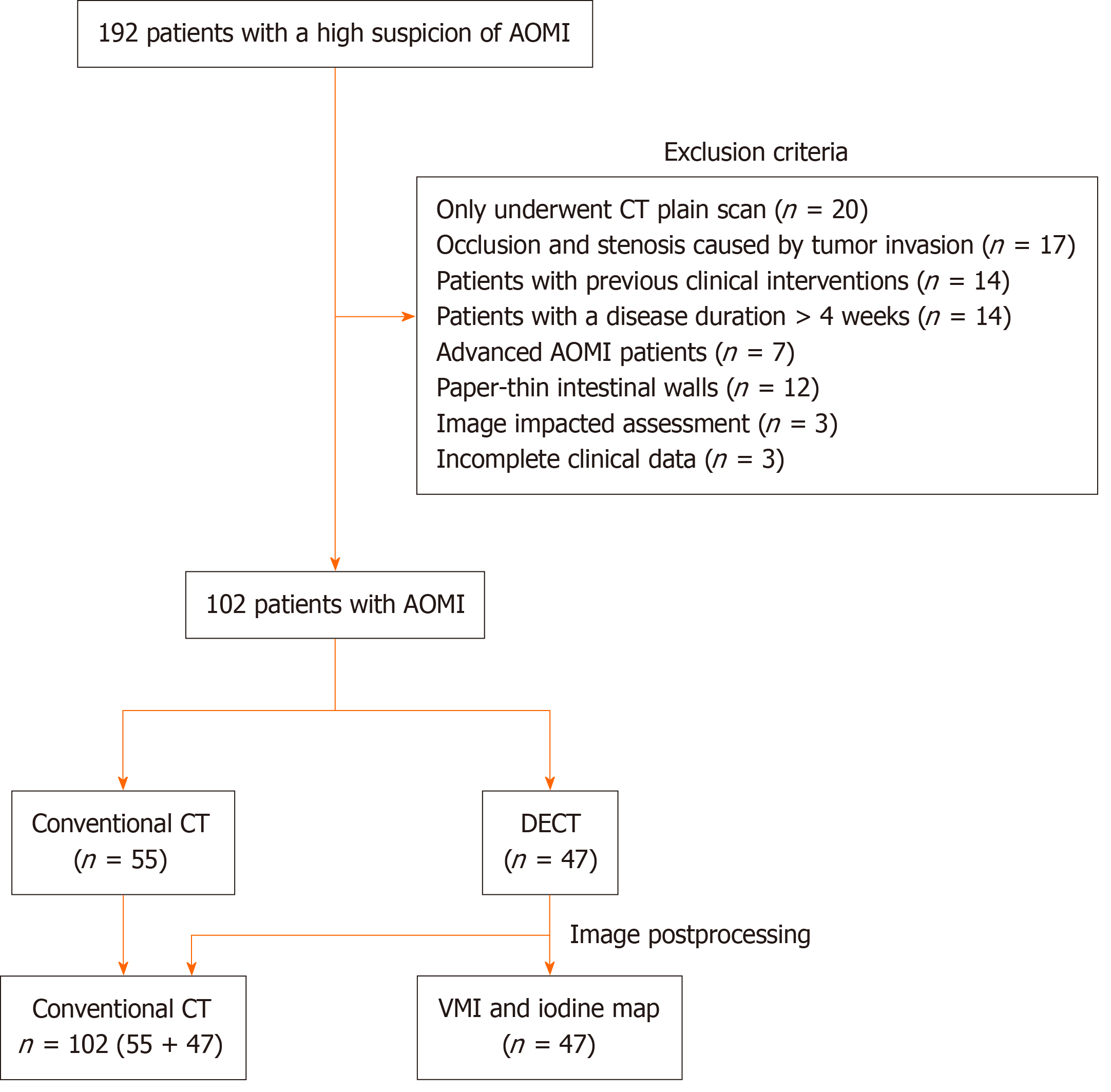
A total of 192 patients with AOMI were consecutively collected, and the following cases that did not meet the criteria were excluded: Only underwent CT plain scan (20 cases); occlusion and stenosis caused by tumor invasion of mesenteric arteries and veins (17 cases); patients with previous clinical interventions (14 cases); patients with a disease duration > 4 weeks (14 cases); CT showed signs of abdominal free gas, intestinal wall gas, and mesenteric venous gas in advanced patients (7 cases); intestinal wall pattern of patients was thin and could not be accurately measured (12 cases); image impacted assessment (3 cases); and clinical data were incomplete (3 cases). Ultimately, 102 patients were included in this study (Figure 2). Of these patients, 62 had SMA thrombosis or SMA embolism, 29 had superior mesenteric venous thrombosis, 11 had both, and 48 had ITIN. The clinical characteristics of the patients are summarized in Table 1.
| All cohort (n = 102) | Non-ITIN (n = 54) | ITIN (n = 48) | t/χ2 | P value | |
| Age, years | 70.00 ± 13.33 | 66.50 ± 13.72 | 73.94 ± 11.82 | -2.914 | 0.004 |
| Sex, male | 54 (52.9) | 32 (59.3) | 22 (45.8) | 1.839 | 0.175 |
| Complication | |||||
| Hypertension | 50 (49.0) | 27 (50.0) | 23 (47.9) | 0.044 | 0.834 |
| Diabetes | 16 (15.7) | 9 (16.7) | 7 (14.6) | 0.083 | 0.773 |
| Heart disease | 51 (50.0) | 25 (46.3) | 26 (54.2) | 0.630 | 0.427 |
| History of cerebrovascular disease | 16 (15.7) | 8 (14.8) | 8 (16.7) | 0.066 | 0.797 |
| Scanning mode | 0.123 | 0.725 | |||
| DECT | 47 (46.1) | 24 (44.4) | 23 (47.9) | ||
| Conventional MDCT | 55 (53.9) | 30 (55.6) | 25 (52.1) | ||
| Vascular involvement | 3.970 | 0.137 | |||
| SMA | 62 (60.8) | 33 (61.1) | 29 (60.4) | ||
| SMV | 29 (28.4) | 18 (33.3) | 11 (22.9) | ||
| SMA + SMV | 11 (10.8) | 3 (5.6) | 8 (16.7) |
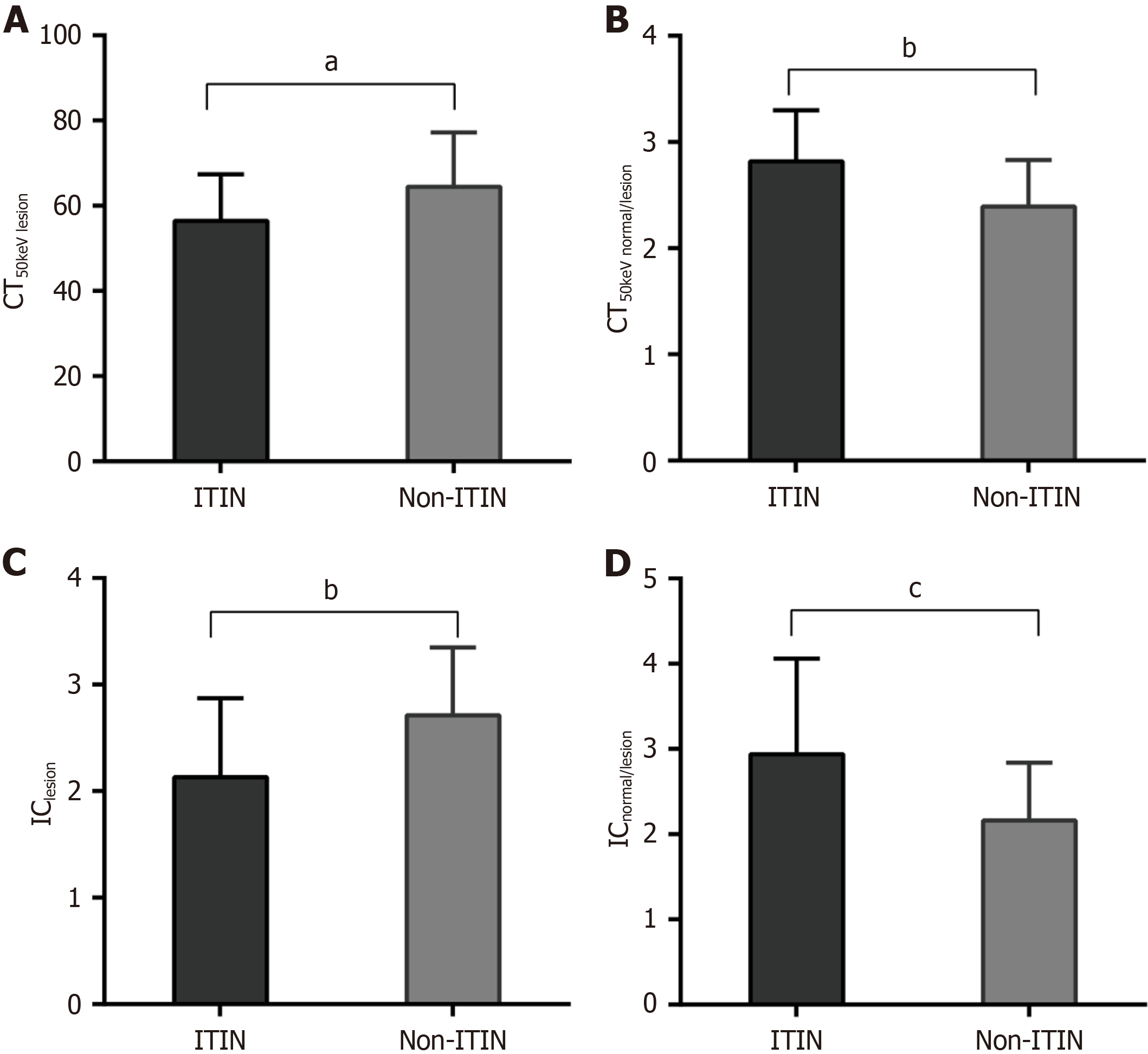
Forty-seven patients with AOMI, including 23 patients with ITIN, were scanned using DECT. The CT50 keV lesion value in the ITIN group was significantly lower than that in the non-ITIN group (P = 0.027, Figure 3A), whereas the CT50 keV normal/lesion in the ITIN group was significantly higher than that in the non-ITIN group (P = 0.003, Figure 3B). Similar results were observed for IC-related indicators; that is, patients in the ITIN group had markedly lower IClesion values than those in the non-ITIN group (P = 0.006, Figure 3C), whereas patients in the ITIN group had higher ICnormal/lesion values than those in the non-ITIN group (P = 0.001, Figure 3D).
In the evaluation of CT subjective signs, the consistency between radiologists A and B of CT signs of parenchymatous organ infarction, swelling and exudation in the mesentery and seroperitoneum was excellent; the consistency of intestinal wall thickening, reduced or absent bowel wall enhancement and bowel dilation was high; the consistency of intestinal wall enhancement was slight (Table 2). Among 102 patients, the incidence of reduced or absent bowel wall enhancement
| CT signs | Kappa (95%CI) | P value |
| Intestinal wall thickening | 0.781 (0.646, 0.898) | < 0.001 |
| Reduced or absent bowel wall enhancement | 0.739 (0. 601, 0.866) | < 0.001 |
| Intestinal wall enhancement | 0.370 (0.023, 0.764) | < 0.001 |
| High density of the intestinal wall | 0.593 (0.297, 0.889) | < 0.001 |
| Bowel dilation | 0.800 (0.672, 0.902) | < 0.001 |
| Swelling and exudation in the mesentery | 0.859 (0.759, 0.959) | < 0.001 |
| Seroperitoneum | 0.902 (0.818, 0.986) | < 0.001 |
| Parenchymatous organ infarction | 0.960 (0.896, 1.000) | < 0.001 |
| CT signs | Total | ITIN | Non-ITIN | χ2 | P value |
| Vascular CT sign | 62 | 29 | 33 | ||
| SMA stenosis/occlusion involved range | 3.267 | 0.195 | |||
| ≤ 20 mm | 8 (12.9) | 2 (6.9) | 6 (18.2) | ||
| 20-50 mm | 16 (25.8) | 6 (20.7) | 10 (30.3) | ||
| ≥ 50 mm | 38 (61.3) | 21 (72.4) | 17 (51.5) | ||
| SMA narrow/blocked area | 9.939 | 0.148 | |||
| I | 5 (8.1) | 2 (6.9) | 3 (9.1) | ||
| II | 0 (0) | 0 (0) | 0 (0) | ||
| III | 2 (3.2) | 0 (0) | 2 (6.1) | ||
| IV | 8 (12.9) | 1 (3.4) | 7 (21.2) | ||
| I + II | 1 (1.6) | 0 (0) | 1 (3.0) | ||
| III + IV | 24 (38.7) | 14 (48.3) | 10 (30.3) | ||
| I + II + III | 4 (6.5) | 1 (3.4) | 3 (9.1) | ||
| II + III + IV | 11 (17.7) | 6 (20.7) | 5 (15.2) | ||
| I + II + III + IV | 7 (11.3) | 5 (17.2) | 2 (6.1) | ||
| Intestinal wall CT sign | 102 | 48 | 54 | ||
| Intestinal wall thickening | 62 (60.8) | 32 (66.7) | 30 (55.6) | 1.949 | 0.163 |
| Reduced or absent bowel wall enhancement | 74 (72.5) | 43 (89.6) | 31 (57.4) | 4.543 | 0.033 |
| Intestinal wall enhancement | 4 (3.9) | 0 (0) | 4 (7.4) | 0.122 | |
| High density of the intestinal wall | 8 (7.8) | 4 (8.3) | 4 (7.4) | < 0.001 | 1 |
| Bowel dilation | 47 (46.1) | 34 (70.8) | 13 (24.1) | 24.095 | < 0.001 |
| Parenteral CT sign | |||||
| Swelling and exudation in the mesentery | 61 (59.8) | 30 (62.5) | 31 (57.4) | 0.588 | 0.443 |
| Seroperitoneum | 47 (46.1) | 24 (50.0) | 23 (42.6) | 0.872 | 0.350 |
| Parenchymatous organ infarction | 45 (44.1) | 27 (56.3) | 18 (33.3) | 5.413 | 0.020 |
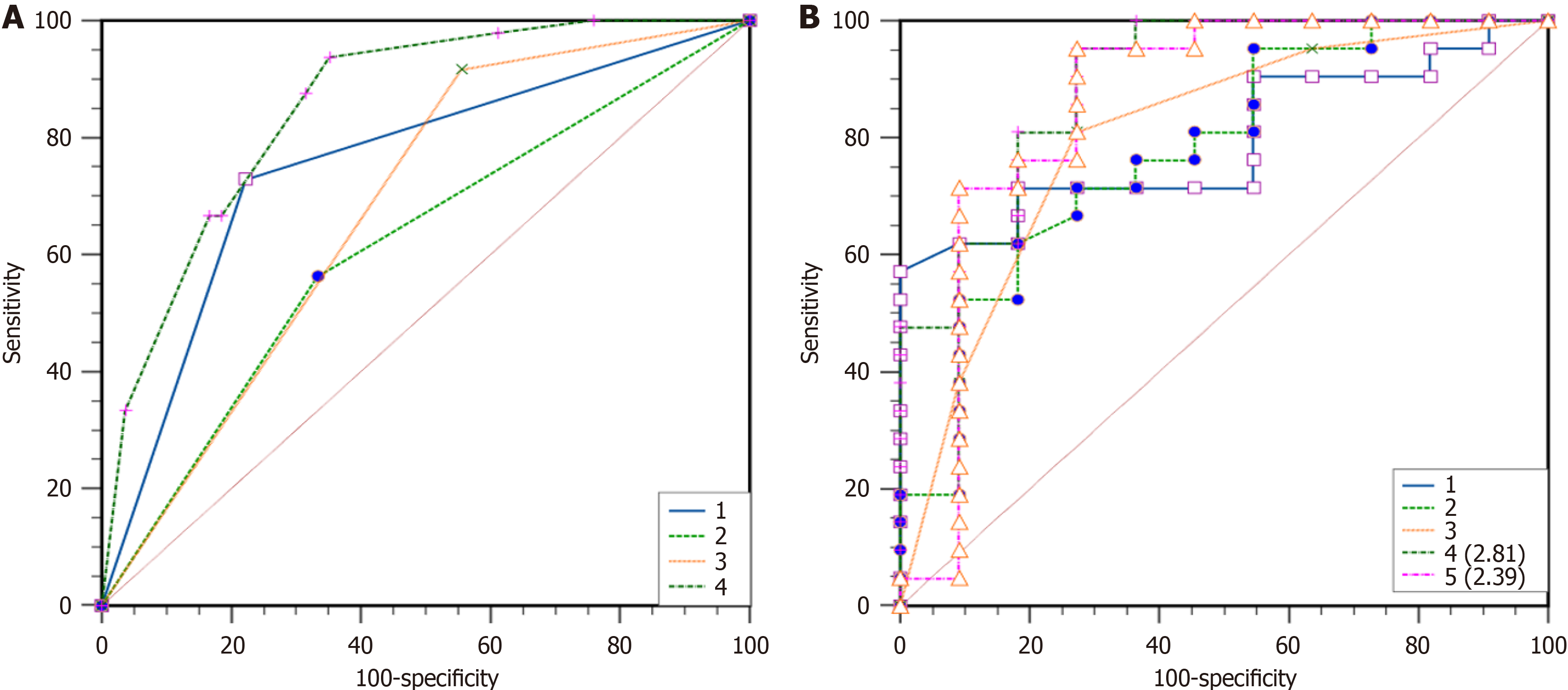
As shown in Table 4, the binary logistic regression analysis revealed reduced or absent bowel wall enhancement (OR = 5.576, P = 0.009), bowel dilatation (OR = 11.613. P < 0.001), and parenchymatous organ infarction (OR = 4.727, P = 0.007) were independent risk factors for ITIN. The AUC of reduced or absent bowel wall enhancement, bowel lumen dilatation, and substantial organ infarction for predicting ITIN were 0.681, 0.753, and 0.615, respectively. Additionally, the AUC of the three subjective CT signs combined to predict ITIN was 0.8530. The Delong test showed that the AUC for the combined prediction of ITIN based on subjective signs was higher than that for reduced or absent intestinal wall enhancement (P < 0.0001), intestinal dilatation (P = 0.0006), and organ infarction (P < 0.0001). There was no significant difference in the AUC between reduced or absent intestinal wall enhancement, bowel dilation, and organ infarction (all P > 0.05, Table 5 and Figure 4A).
| CT signs | B value | Wald value | O1 | 95%CI | P value |
| Reduced or absent bowel wall enhancement | 1.718 | 6.903 | 5.576 | 1.547-20.093 | 0.009 |
| Bowel dilation | 2.452 | 18.420 | 11.613 | 3.790-35.586 | < 0.001 |
| Parenchymatous organ infarction | 1.553 | 7.332 | 4.727 | 1.536-14.551 | 0.007 |
| CT signs | AUC (95%CI) | P value | Sensitivity (95%CI) | Specificity (95%CI) |
| Reduced or absent bowel wall enhancement | 0.681 (0.581-0.769) | 0.002 | 91.67 (80.0-97.7) | 44.44 (30.9-58.6) |
| Bowel dilation | 0.753 (0.658-0.833) | < 0.001 | 72.92 (58.2-84.7) | 77.78 (64.4-88.0) |
| Parenchymatous organ infarction | 0.615 (0.513-0.709) | 0.046 | 56.26 (41.2-70.5) | 66.67 (52.5-78.9) |
| Combination signs | 0.853 (0.769-0.915) | < 0.001 | 93.75 (82.8-98.7) | 64.81 (50.6-77.3) |
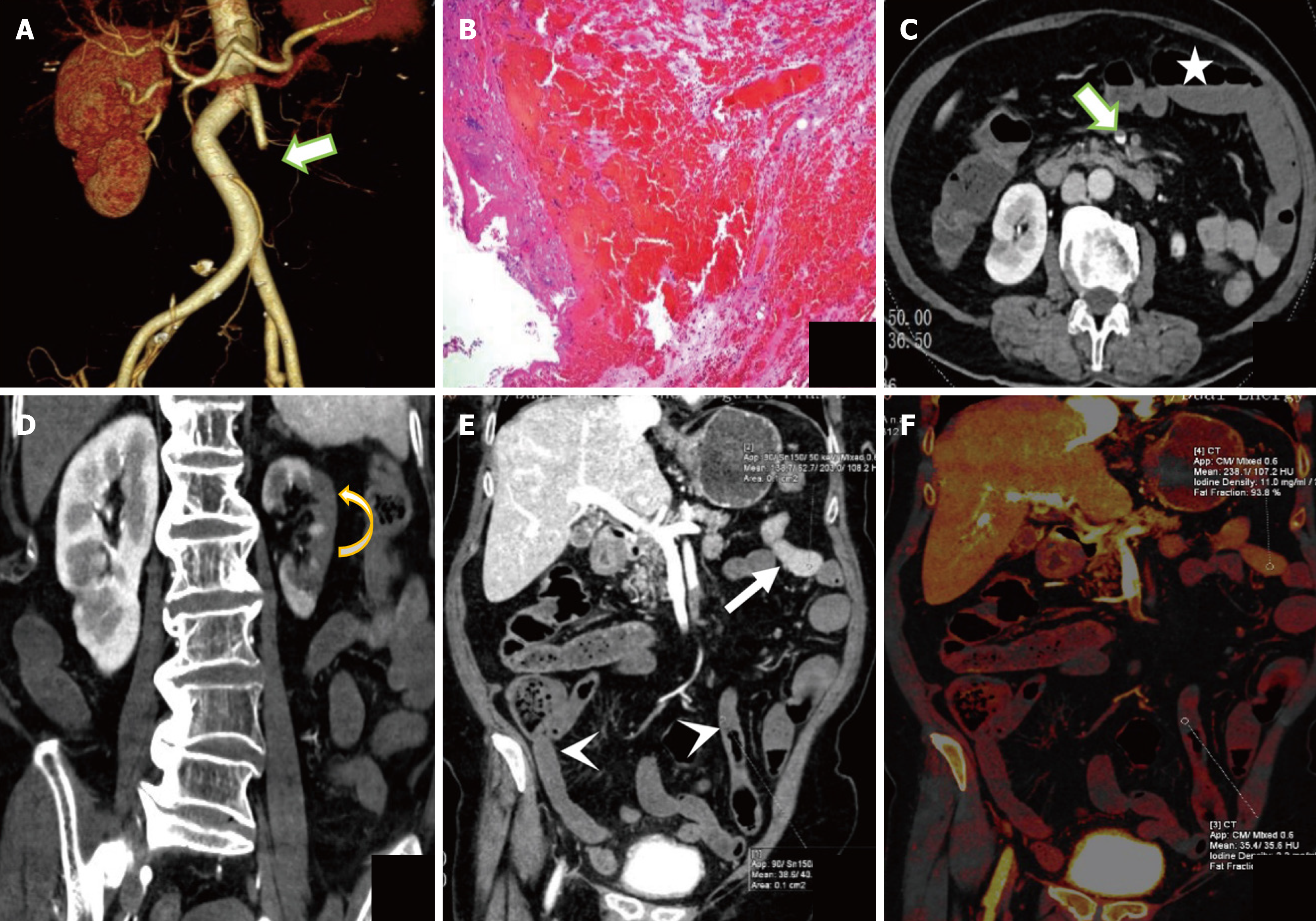
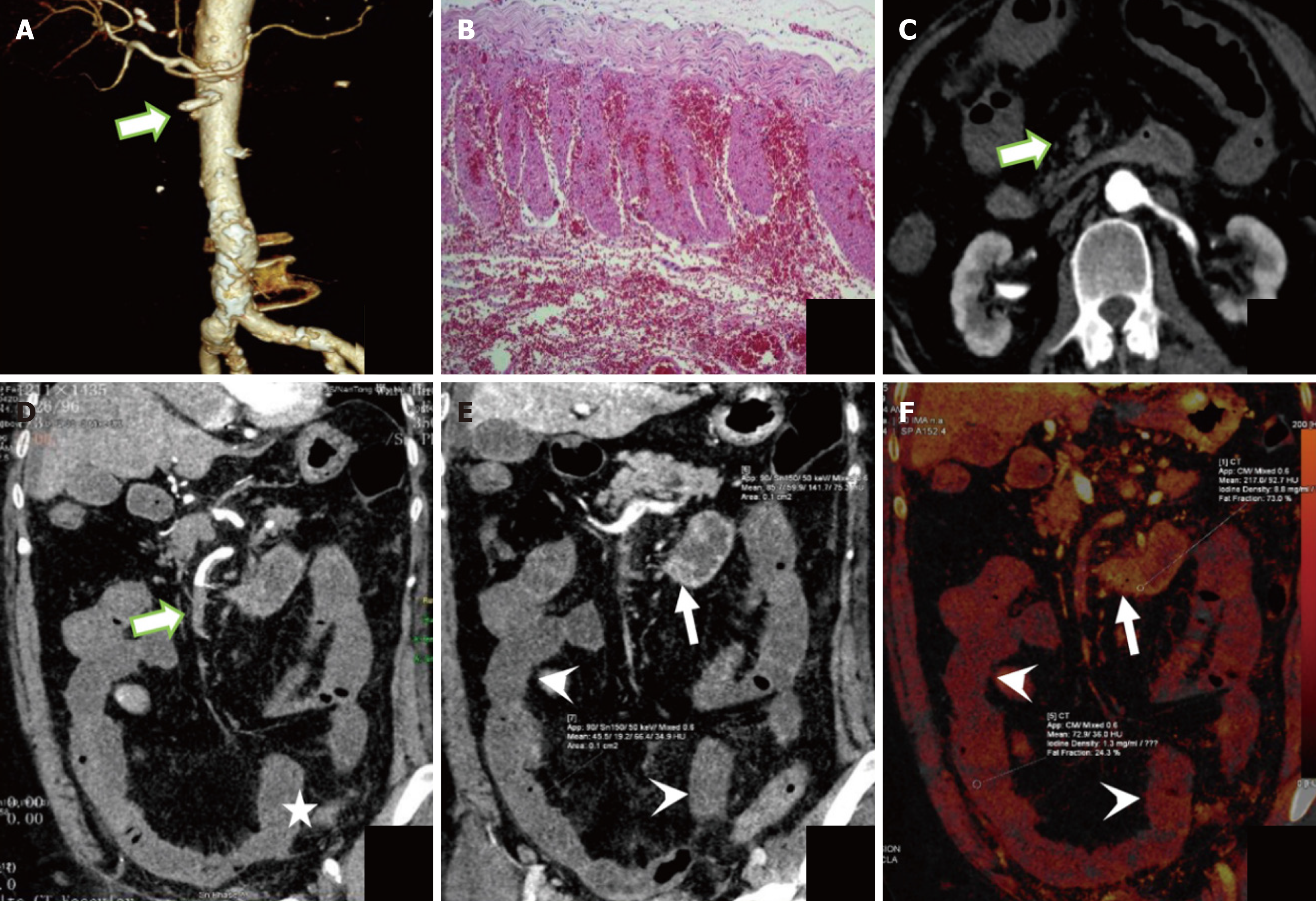
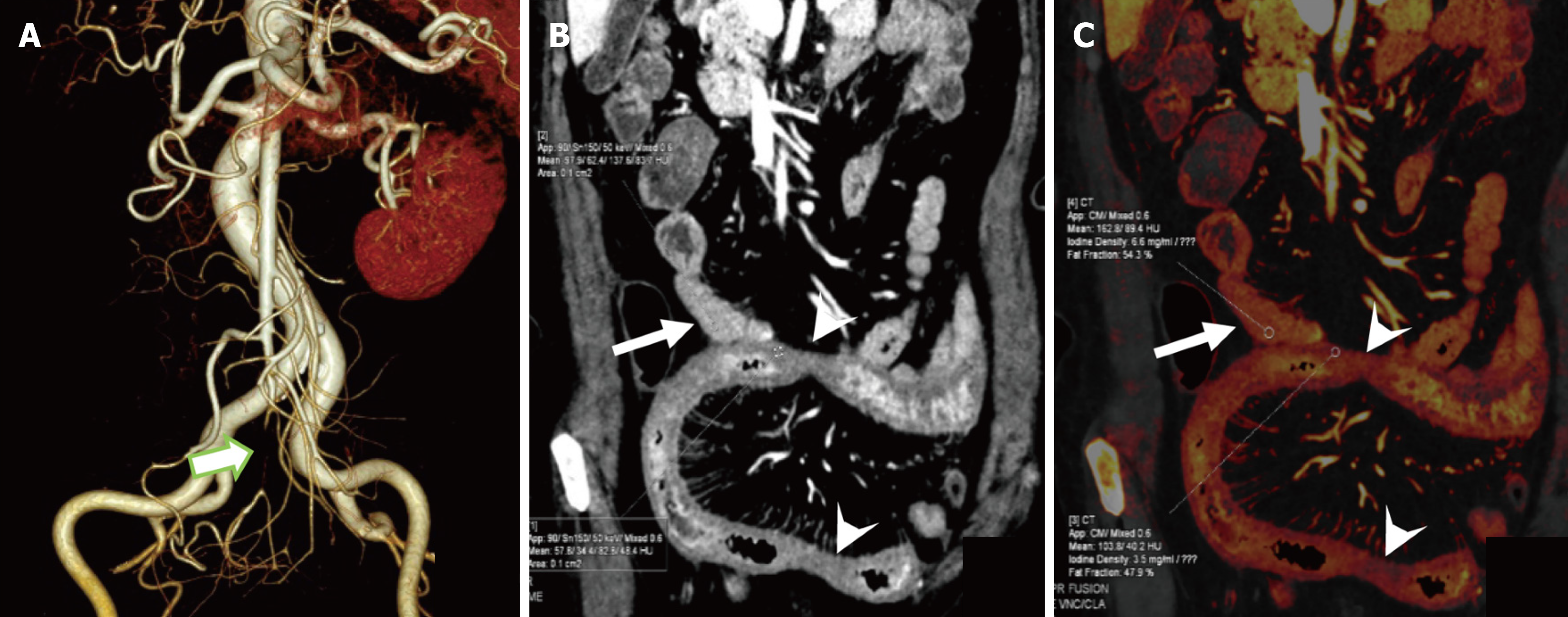
In the 47 patients detected by DECT, the AUC for predicting ITIN was 0.794 with a cutoff value of CT50 keV normal/lesion = 2.81, while its AUC was 0.777 with a cutoff value of ICnormal/lesion = 2.39. In these patients, the AUC for CT combined subjective signs was 0.801. The Delong test showed no significant difference in the AUC for predicting ITIN between the DECT objective indicators (CT50 keV normal/lesion and ICnormal/lesion) and CT combined subjective signs (P > 0.05). Thus, we replaced some subjective signs with objective indicators. When CT50 keV normal/lesion and ICnormal/lesion were used instead of reduced and absent intestinal wall reinforcement in the subjective sign combination, the predicted ITIN AUC were 0.857 and 0.894, respectively. Among them, there was a difference in the AUC between ICnormal/lesions combined with intestinal lumen dilatation and parenchymal organ infarction and CT subjective signs (Table 6 and Figure 4B). Bootstrap analysis, based on 1000 resampling iterations, yielded an average AUC of 0.888, demonstrating the robust stability and reliable predictive performance of the model (Figure 5). Furthermore, there were no significant differences among the other groups. Figures 6, 7, and 8 exhibit representative DECT images of three cases: Two with ITIN and one without.
| CT parameters | AUC (95%CI) | P value | Sensitivity (95%CI) | Specificity (95%CI) |
| CT50 KeV normal/lesion | 0.794 (0.615-0.916) | 0.005 | 56.52 (34.5-76.8) | 87.50 (67.6-97.3) |
| ICnormal/lesion | 0.777 (0.596-0.904) | 0.001 | 73.91 (51.6-89.8) | 79.17 (57.8-92.9) |
| Combined subjective signs | 0.801 (0.622-0.920) | 0.006 | 80.95 (58.1-94.6) | 72.73 (39.0-94.0) |
| CT50 KeV normal/lesion combined with intestinal dilatation and parenchymatous organ infarction | 0.857 (0.688-0.955) | 0.001 | 95.65 (78.1-99.9) | 79.17 (57.8-92.9) |
| ICnormal/lesion combined with intestinal dilatation and parenchymatous organ infarction | 0.894 (0.737-0.976) | < 0.001 | 100 (85.2-100) | 70.83 (49.8-87.4) |
AMI is a rare and often misdiagnosed disease that is usually diagnosed late and has a high mortality rate[15]. Therefore, the diagnosis and treatment of patients with suspected AMI, particularly those at risk for ITIN, should be highly valued. Unfortunately, the lack of specific clinical symptoms and experimental parameters often lead to significant delays in diagnosis and targeted therapy. In this study, we aimed to evaluate the potential of DECT in predicting the occurrence of ITIN in AOMI. We also observed the significance of a combination of CT subjective signs and objective DECT indicators in the diagnosis of ITIN.
In the assessment of subjective CT imaging parameters, we found that reduced or absent bowel wall enhancement, bowel dilation, and parenchymatous organ infarction were independent risk factors for ITIN. Notably, the combination of the three CT parameters had favorable performance in predicting ITIN, with an AUC of 0.8530 (sensitivity: 93.75%), which was higher than that of a single indicator. Hypoenhancement of the bowel wall is essential for the early detection of AMI[12]. Abdominal CT showing reduced or absent bowel wall enhancement is a specific but insensitive finding that indicates reduced or absent arterial blood flow. Previous studies have revealed that this parameter is correlated with transmural necrosis of the small intestinal wall, particularly in cases of intravenous AMI. In the early stages of AOMI, the regional arterial blood flow is below a critical threshold, suggesting that the bowel wall is still alive and the damage is reversible[16]. Reduced local arterial inflow occurs only in the late stages of occlusive AMI, and enhanced CT exhibits decreased or no enhancement, indicating possible irreversible damage to the intestinal wall[17]. The evidence suggests that bowel dilation is more common in patients with arterial AOMI[18]. This shows that disruption of intestinal peristalsis is a reflection of ischemic injury and causes irreversible whole-wall ischemic damage to the intestinal wall. Hence, the rate of surgical resection increases when dilatation is present[19]. Consistent with these studies, we found that patients in the ITIN group were more likely to have intestinal dilatation (71%, 34/48). Parenchymatous organ infarction is common in patients with embolic AMI, and its presence as an auxiliary finding is highly specific[20]. In this study, the incidence of parenchymatous organ infarction was 56 % (27/48) in the ITIN group. After organ infarction, it causes abnormal biochemical parameters such as blood creatinine and bilirubin, which may lead to organ failure[21]. Organ failure is an independent risk factor for ITIN and may be associated with high mortality rates. Taken together, it is necessary to have a higher degree of suspicion and familiarity with the CT spectral manifestations of AOMI, which can assist in the accurate diagnosis of ITIN and determination of the therapeutic strategy.
Although CT signs have a good predictive value, they mainly rely on the subjective evaluation of observers, especially reduced or absent bowel wall enhancement. Furthermore, the specificity of three combined CT signs for predicting ITIN was unsatisfactory (64.81). Therefore, we analyzed the DECT results. In this study, we quantified the degree of intestinal wall ischemia in patients using IC measurement and attenuation measurement (VMI, CT50 keV) and then evaluated the predictive performance of DECT objective parameters on TINI. There was no significant difference in the predictive abilities of DECT and CT signs for ITIN. Next, to reduce the subjective heterogeneity, we used quantitative measures in DECT to replace some subjective CT signs. The results demonstrated that replacing reduced or absent bowel wall enhancement in the combination model with IC normal/lesions enhanced the predictive efficacy of ITIN (from 0.853 to 0.894), and sensitivity (100%) and specificity (70.83%). The observed low specificity may be attributed to several factors. First, the limited sample size could have introduced statistical bias. Second, the optimal diagnostic model incorporated not only quantitative metrics, such as the IC normal/lesions, but also subjective features, including intestinal dilation and parenchymal organ infarction. Given the inherently subjective nature of these latter indicators, interobserver variability may have influenced the model’s specificity. This limitation highlights a broader challenge associated with traditional diagnostic approaches that rely heavily on subjective criteria in the evaluation of ITIN. In recent years, DECT has emerged as a diagnostic technology for the clinical detection of various diseases[22]. IC is the most commonly used quantitative parameter in DECT and is considered equivalent to the actual enhancement value. In the bowel, Lourenco et al[23] found that iodine mapping and 40-keV reconstruction techniques reduced the subjectivity and difficulty of wall enhancement and provided the greatest visual difference between non-ischemic and ischemic bowel segments, allowing early and accurate detection of intestinal ischemia. Mazzei et al[24] also found that DECT with iodograms and low-energy images helped assess intestinal wall vascularization more accurately. Consistent with these studies, we also found that iodograms and low-energy images (50 keV) provided superior image quality for the effective assessment of intestinal wall lesions and the accuracy of ITIN diagnosis. In routine diagnosis, physicians can directly calculate CT50 keV normal/lesion and ICnormal/lesion values to initially assess whether ITIN occurs in patients with AMI. These quantitative parameters are more accurate and reliable than the subjective CT signs alone.
Numerous prior studies have demonstrated that CT findings such as paper-thin intestinal walls, pneumatosis intestinalis, free intra-abdominal air, or gas within the portal venous system are indicative of advanced-stage ischemia in AOMI patients, suggesting a high likelihood of established or imminent ITIN. In such cases, intestinal resection is often unavoidable. To focus on early- and mid-stage AOMI, this study deliberately excluded patients exhibiting these late-stage imaging features. By doing so, our aim was to enhance the predictive accuracy for ITIN at earlier stages, potentially enabling timely intervention to prevent or minimize the need for bowel resection. This methodological distinction represents a key innovation of our study compared with previous research. This is the first study to confirm the application of DECT in the evaluation of ITIN. This study had two major limitations. First, the number of patients who underwent DECT was small. This is mainly due to the lack of typical clinical manifestations in patients with early-stage AOMI. Second, there are differences in MDCT signs between arterial and venous AMI. Due to the limited sample size, the etiology of AOMI has not yet been classified in detail.
In summary, portal-venous phase CT50 keV normal/lesion and ICnormal/lesion were effective objective parameters for predicting ITIN, of which ICnormal/lesion combined with subjective CT signs (bowel dilatation and parenchymatous organ infarction) had the best predictive efficacy. Accordingly, DECT serves as a valuable tool for the early detection of ITIN in patients with AOMI, providing critical information to guide prompt clinical decision-making and potentially improve patient outcomes.
| 1. | Kärkkäinen JM. Acute Mesenteric Ischemia: A Challenge for the Acute Care Surgeon. Scand J Surg. 2021;110:150-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Gnanapandithan K, Feuerstadt P. Review Article: Mesenteric Ischemia. Curr Gastroenterol Rep. 2020;22:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Bala M, Catena F, Kashuk J, De Simone B, Gomes CA, Weber D, Sartelli M, Coccolini F, Kluger Y, Abu-Zidan FM, Picetti E, Ansaloni L, Augustin G, Biffl WL, Ceresoli M, Chiara O, Chiarugi M, Coimbra R, Cui Y, Damaskos D, Di Saverio S, Galante JM, Khokha V, Kirkpatrick AW, Inaba K, Leppäniemi A, Litvin A, Peitzman AB, Shelat VG, Sugrue M, Tolonen M, Rizoli S, Sall I, Beka SG, Di Carlo I, Ten Broek R, Mircea C, Tebala G, Pisano M, van Goor H, Maier RV, Jeekel H, Civil I, Hecker A, Tan E, Soreide K, Lee MJ, Wani I, Bonavina L, Malangoni MA, Koike K, Velmahos GC, Fraga GP, Fette A, de'Angelis N, Balogh ZJ, Scalea TM, Sganga G, Kelly MD, Khan J, Stahel PF, Moore EE. Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2022;17:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 4. | Chabert S, Porcheron J, Balique JG. [Management of acute intestinal arterial ischemia]. J Chir (Paris). 1999;136:130-135. [PubMed] |
| 5. | Tendler DA. Acute intestinal ischemia and infarction. Semin Gastrointest Dis. 2003;14:66-76. [PubMed] |
| 6. | Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology. 2010;256:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Calame P, Malakhia A, Turco C, Grillet F, Piton G, Delabrousse E. Transmural Bowel Necrosis From Acute Mesenteric Ischemia and Strangulated Small-Bowel Obstruction: Distinctive CT Features. AJR Am J Roentgenol. 2020;214:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Tang W, Zhang J, Kuang LQ, Yi KM, Li CX, Wang Y. Relationship of superior mesenteric artery thrombus density with transmural intestinal necrosis on multidetector computed tomography in acute mesenteric ischemia. Quant Imaging Med Surg. 2021;11:3120-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Cox VL, Tahvildari AM, Johnson B, Wei W, Jeffrey RB. Bowel obstruction complicated by ischemia: analysis of CT findings. Abdom Radiol (NY). 2018;43:3227-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Chellini D, Kinman K. Dual-Energy CT Principles and Applications. Radiol Technol. 2020;91:561CT-576CT. [PubMed] |
| 11. | Nehnahi M, Simon G, Moinet R, Piton G, Camelin C, Ronot M, Delabrousse É, Calame P. Quantifying iodine concentration in the normal bowel wall using dual-energy CT: influence of patient and contrast characteristics. Sci Rep. 2023;13:22714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, Joly F, Castier Y, Vilgrain V, Paugam C, Panis Y, Bouhnik Y, Cazals-Hatem D, Corcos O. Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia: Prospective Study from an Intestinal Stroke Center. Am J Gastroenterol. 2017;112:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Martin SS, Pfeifer S, Wichmann JL, Albrecht MH, Leithner D, Lenga L, Scholtz JE, Vogl TJ, Bodelle B. Noise-optimized virtual monoenergetic dual-energy computed tomography: optimization of kiloelectron volt settings in patients with gastrointestinal stromal tumors. Abdom Radiol (NY). 2017;42:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Albrecht MH, Scholtz JE, Hüsers K, Beeres M, Bucher AM, Kaup M, Martin SS, Fischer S, Bodelle B, Bauer RW, Lehnert T, Vogl TJ, Wichmann JL. Advanced image-based virtual monoenergetic dual-energy CT angiography of the abdomen: optimization of kiloelectron volt settings to improve image contrast. Eur Radiol. 2016;26:1863-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (1)] |
| 16. | Paterno F, Longo WE. The etiology and pathogenesis of vascular disorders of the intestine. Radiol Clin North Am. 2008;46:877-885, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 17. | Zeng Y, Yang F, Hu X, Zhu F, Chen W, Lin W. Radiological predictive factors of transmural intestinal necrosis in acute mesenteric ischemia: systematic review and meta-analysis. Eur Radiol. 2023;33:2792-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Copin P, Zins M, Nuzzo A, Purcell Y, Beranger-Gibert S, Maggiori L, Corcos O, Vilgrain V, Ronot M. Acute mesenteric ischemia: A critical role for the radiologist. Diagn Interv Imaging. 2018;99:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Scaglione M, Galluzzo M, Santucci D, Trinci M, Messina L, Laccetti E, Faiella E, Beomonte Zobel B. Small bowel obstruction and intestinal ischemia: emphasizing the role of MDCT in the management decision process. Abdom Radiol (NY). 2022;47:1541-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Yu H, Kirkpatrick IDC. An Update on Acute Mesenteric Ischemia. Can Assoc Radiol J. 2023;74:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Guillaume A, Pili-Floury S, Chocron S, Delabrousse E, De Parseval B, Koch S, Samain E, Capellier G, Piton G. Acute Mesenteric Ischemia Among Postcardiac Surgery Patients Presenting with Multiple Organ Failure. Shock. 2017;47:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Hamid S, Nasir MU, So A, Andrews G, Nicolaou S, Qamar SR. Clinical Applications of Dual-Energy CT. Korean J Radiol. 2021;22:970-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Lourenco PDM, Rawski R, Mohammed MF, Khosa F, Nicolaou S, McLaughlin P. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia. AJR Am J Roentgenol. 2018;211:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Mazzei MA, Gentili F, Volterrani L. Dual-Energy CT Iodine Mapping and 40-keV Monoenergetic Applications in the Diagnosis of Acute Bowel Ischemia: A Necessary Clarification. AJR Am J Roentgenol. 2019;212:W93-W94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/