INTRODUCTION
Diverticulosis is a disease primarily affecting elderly people in Western civilization. On the one hand, diverticulosis is age-related, as only 1% to 2% of patients are younger than 30 years, but more than 63% of patients are over 60 years of age. On the other hand, nutrition has a strong impact on the development of the disease[1,2]. About 10% to 25% of patients with diverticulosis suffer from diverticulitis[3]. Diverticula are mainly restricted to the colon, in particular to the left part of the colon[4]. According to scientific knowledge, three aspects are responsible for diverticulosis development: Structural anomalies in the large intestinal wall, disordered motility, and lack of dietary fiber[3,5,6]. In comparison to diverticulosis of the colon, diverticulosis of the small intestine is considered a rare disease. Colonic diverticulosis has a prevalence between 5% and 45%[7], whereas small bowel diverticulosis has an incidence ranging from 0.3% to 1.3% in the general population, with a rate of 2.3% observed in autopsy studies[8]. This is due to the differences between the large and small intestine in terms of anatomy, function[9], and intraluminal pressure[10], as well as factors such as diet, lifestyle[11,12], and neuromuscular disorders[13]. Duodenal diverticula occur five times more often than jejunoileal diverticula[14]. Jejunal diverticulosis has a reported clinical incidence of 0.5%[15]. In total, 80% of diverticula in the small intestine occur in the jejunum, 15% in the ileum, and 5% in both[16]. The etiology of jejunal diverticulosis is broad and ranges from progressive systemic sclerosis[17-19] to visceral neuropathies and myopathies[20], which affect the smooth muscle or myenteric plexus of the small intestine[17]. These conditions are believed to disrupt normal peristalsis and increase intraluminal pressure, leading to the formation of multiple pouch-like protrusions (diverticula) in the weakened areas of the jejunal intestinal wall[17]. The elevated pressure causes the mucosa (inner lining) and submucosa (layer beneath the mucosa) to herniate through the muscularis propria (muscle layer), resulting in diverticula[8]. Comparable to diverticulosis in the colon[5], a combination of structural anomalies in the intestinal wall and disordered motility seems to be responsible for the development of diverticulosis in the small intestine. Nevertheless, the exact cause of jejunal diverticulosis remains unknown. In many cases, multiple jejunal diverticula are asymptomatic[21]. However, possible symptoms include abdominal pain or discomfort, nausea, vomiting, diarrhea or constipation, malabsorption, and in severe cases, gastrointestinal bleeding. Rare complications, such as diverticulitis, can occur, leading to pain, fever, and other symptoms[22]. In uncomplicated cases, no treatment is required[23,24]. However, if symptoms are present, management may involve dietary modifications and symptom-relieving medications. In severe cases, surgical intervention may be necessary to remove the affected part of the jejunum[22,25].
In this report, we present a case of jejunal diverticulosis from both an anatomical and histological perspective. Several aspects of our findings support the theory that pathologic changes in the macroscopic and microscopic architecture contributed to the extensive case of jejunal diverticulosis in this case.
CASE PRESENTATION
Chief complaints
During body donor dissection for student education in our institute, a case of multiple jejunal diverticulosis was observed in a 72-year-old male. A release from medical confidentiality obligations was given by the bereaved. This allowed us to retrieve the clinical records from the 3 years preceding death.
History of present illness
In the described case, we observed an extensive jejunal diverticulosis in a 72-year-old male body donor. The patient’s medical history revealed that this condition had remained undiagnosed during his lifetime. Death certificate states the cause of death as multiple organ failure. Approximately 7 weeks before his death, the patient was admitted to the hospital with chest pain and hypotension. He was diagnosed with perimyocarditis and started on colchicine (0.5 mg twice daily). However, a chest radiograph revealed free intraperitoneal gas. A laparoscopy was subsequently performed, but no pathological intestinal perforation was found. It is known that jejunoileal diverticulosis is usually asymptomatic but can lead to diagnostic challenges[21]. Four weeks later, the patient developed vomiting and worsening of chronic diarrhea accompanied by severe metabolic acidosis and exsiccosis, necessitating intensive care unit treatment. Due to these symptoms and the presence of severe microcytic anemia, requiring blood transfusion, a colonoscopy and gastroscopy were performed 10 days before death. These examinations revealed a dilated post-bulbar duodenum with an atrophic mucosa, which appeared translucent from the underlying vessels. Microbiological analyses of stool samples were negative for pathogenic microorganisms. Subsequently, the patient developed a urinary tract infection and pneumonia and experienced progression of his chronic compensated renal failure to acute renal failure. He ultimately succumbed to these complications.
History of past illness
As we were only able to retrieve the patient’s medical records from the last 3 years before death, we could not determine the exact dates of initial diagnoses. However, records from 3 years prior to death indicate that the patient suffered from gastroesophageal reflux disease, accompanied by chronic esophagitis, chronic gastritis with foveolar hyperplasia, and sigmoid diverticulosis. Additionally, the patient experienced exocrine pancreatic insufficiency due to chronic pancreatitis that required pancreatic enzyme replacement therapy. The medical history revealed opioid abuse, likely in response to chest pain of unknown origin, as well as an alcohol use disorder. Furthermore, 2 years before death, the patient was diagnosed with coronary heart disease, insufficiency of the aortic valve, and compensated chronic renal failure.
Personal and family history
The patient's family history is unknown.
Physical examination
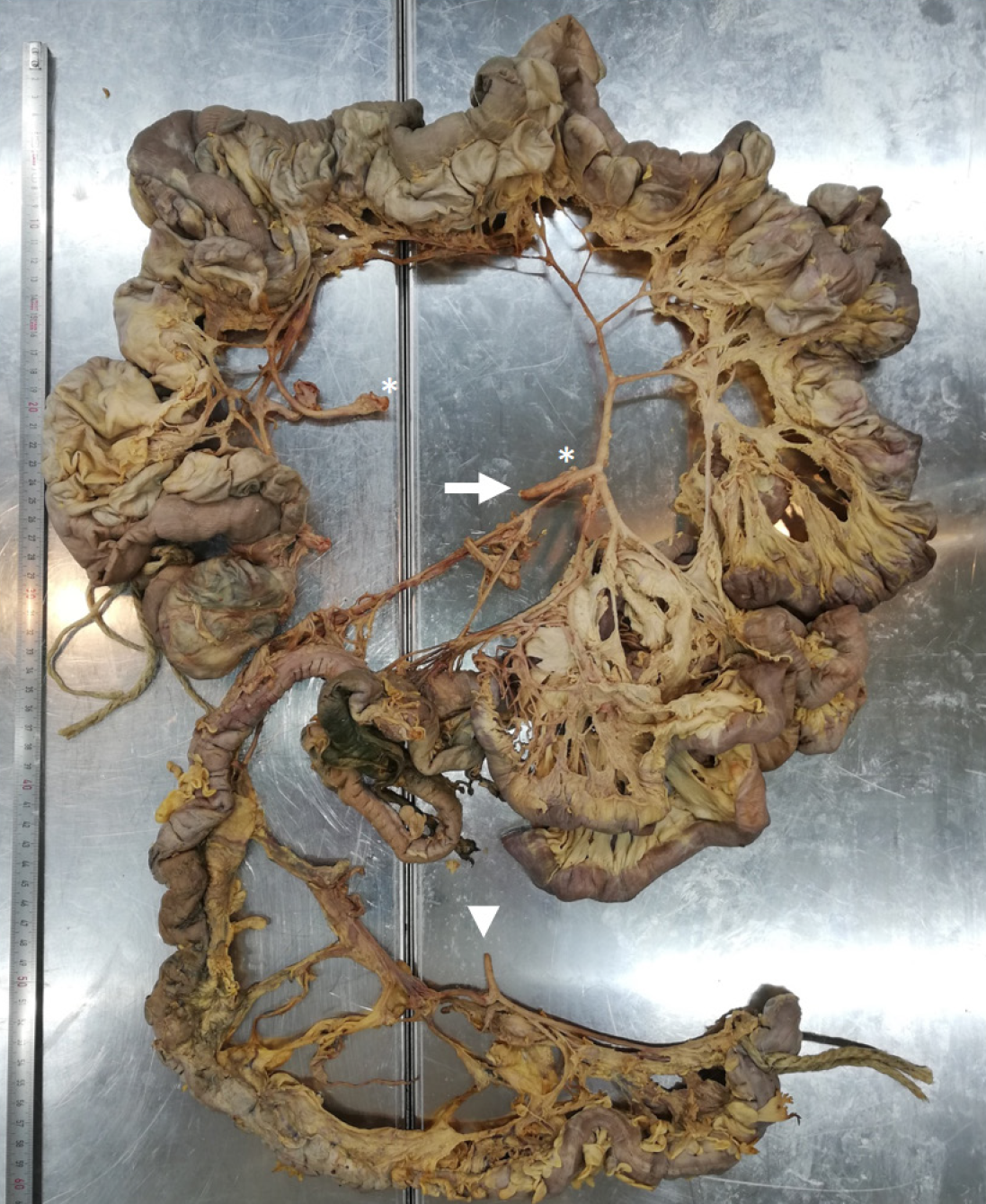
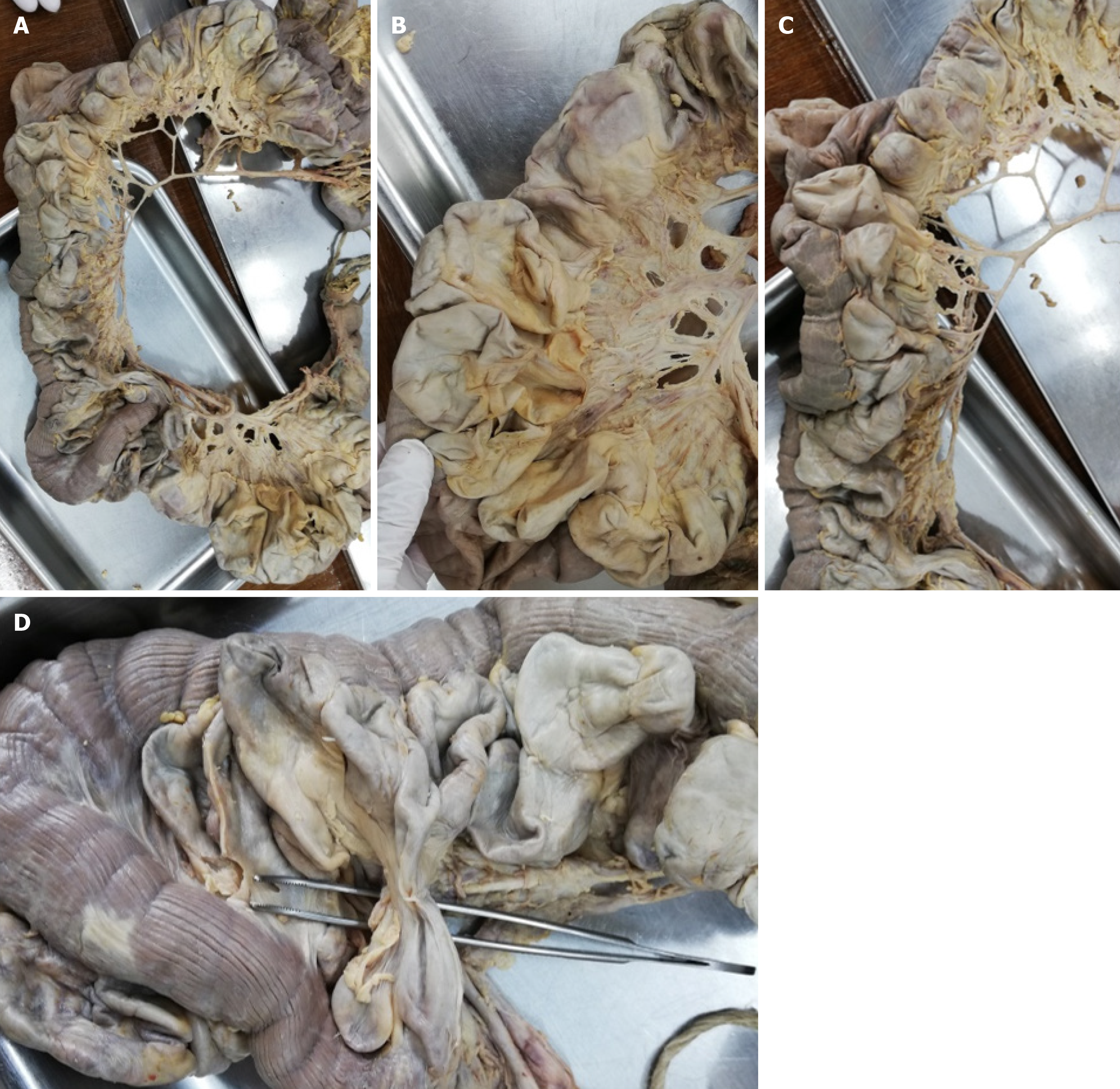
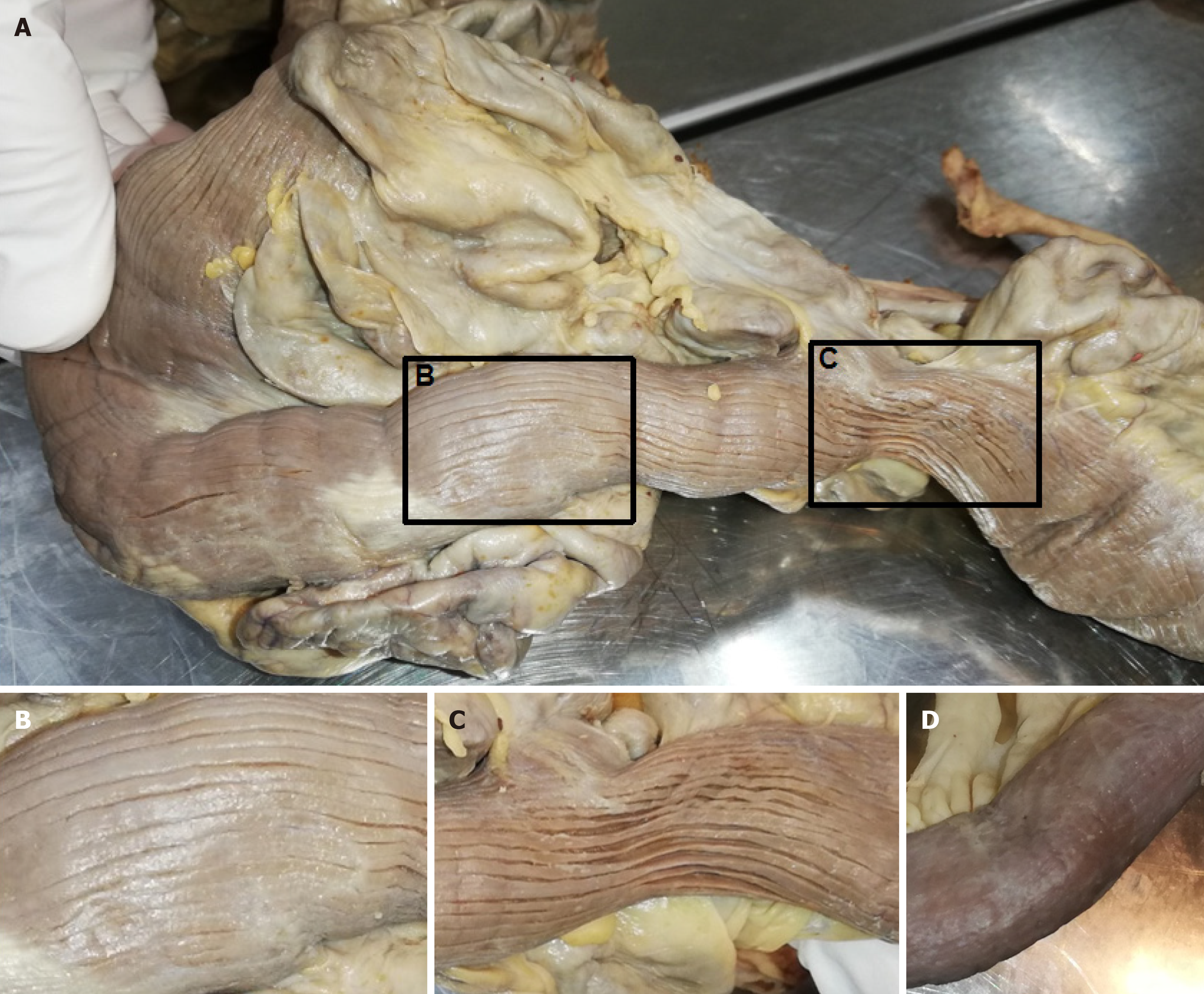
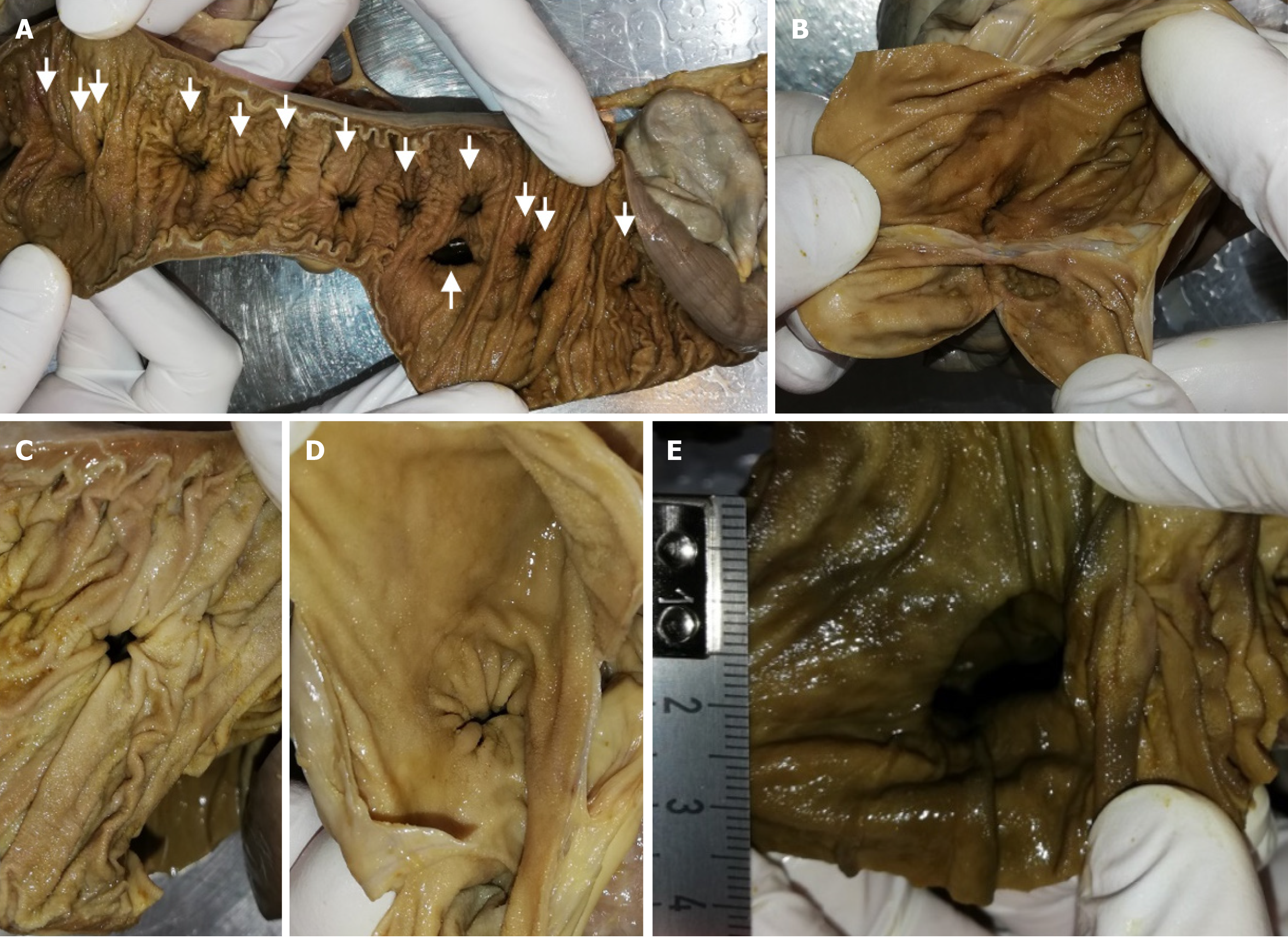
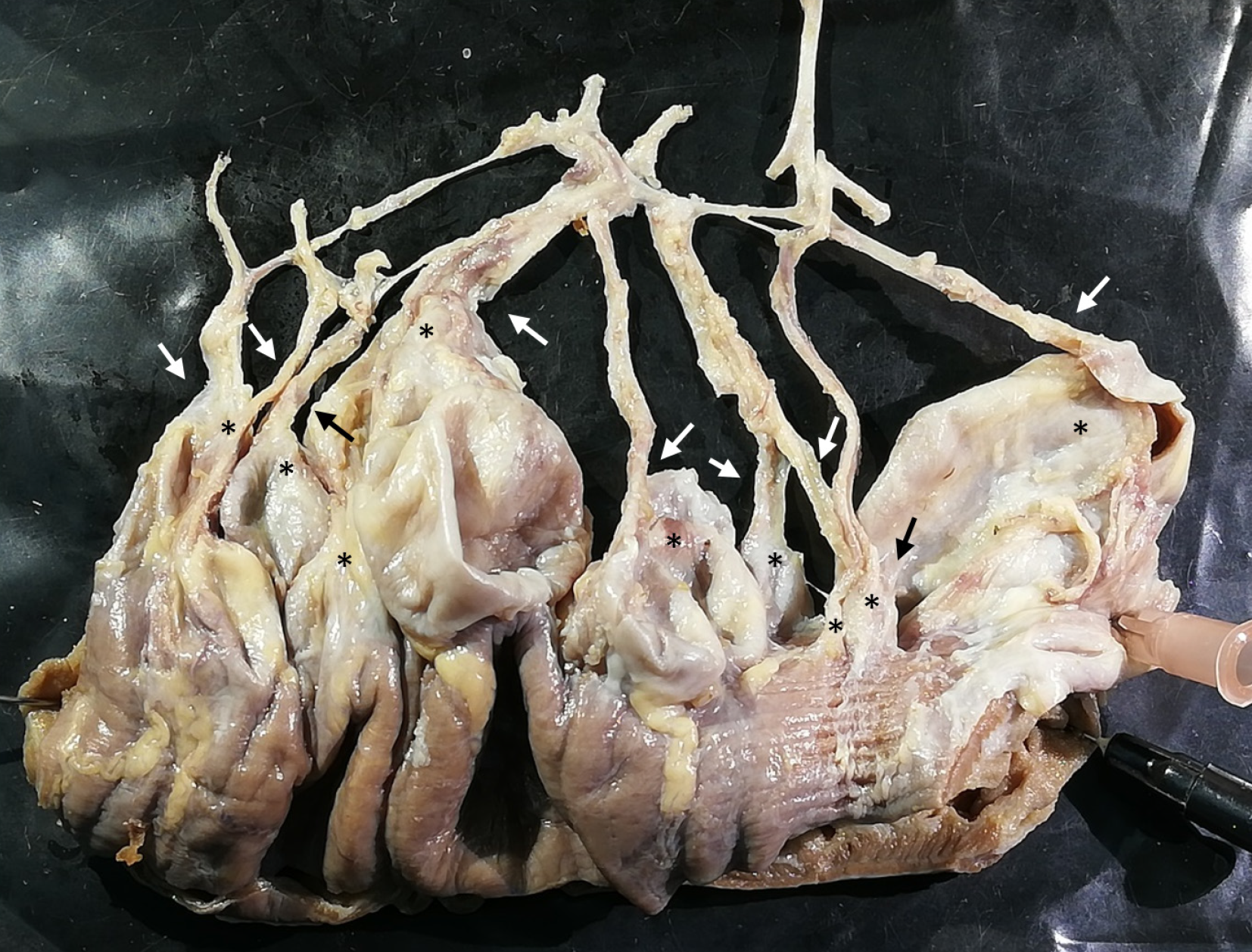
The intraperitoneal intestine, together with the mesentery, was removed from the abdominal cavity. Surrounding fat tissue, lymphatic tissue, and the majority of veins were removed to expose the intestinal tract with the superior and inferior mesenteric artery and all branches supplying the intestinal tract, as well as the diverticula. The extracted intestinal segment was photographed. Length of the jejunum hosting diverticula was measured manually utilizing a flexible string and a tape measure. The intestinal segment was cut longitudinally, and selected diverticula were opened. The remaining chyme was removed to expose and photographically record the intestinal lumen and diverticula entries. Diverticula entries were counted manually utilizing a bulb-headed probe to ensure that entries open into a diverticulum. A representative part of the jejunum (8 cm) with diverticula was excised and dissected separately, utilizing a dissecting microscope to examine the relationship between vessels and diverticula. All remaining fat tissue and tunica serosa covering the intestine were removed. Vessels were stretched out, and entry sites were exposed. We found multiple jejunal diverticula (232 in count) along a total length of 208 cm of intestine (Figure 1). Diverticula covered the whole jejunum and were predominantly larger and more voluminous than the jejunum itself (Figure 2A-C). We also observed two connected diverticula building a shortcut between two intestinal parts (Figure 2D). Interestingly, we observed an extreme longitudinal striation on intestinal parts hosting diverticula (Figure 3A-C). This longitudinal striation could not be found in the intestinal parts without diverticula (Figure 3D).
Figure 1 Overview of intestine harboring diverticula.
Intestinal tract including jejunum, ileum, and colon. Jejunum hosting diverticula over a length of 208 cm. Arrows mark superior mesenteric artery. Asterisks mark cut connection of artery branch due to preparation and visualization. Arrowheads mark inferior mesenteric artery.
Figure 2 Diverticula.
A-C: Representative diverticula; D: Shortcut between two intestinal parts provided by diverticula.
Figure 3 Longitudinal striation of jejunum with diverticula.
A: Intestinal part hosting diverticula showed remarkable longitudinal striation. Black box marks magnified part shown in B and C; B and C: Magnification of longitudinal striation; D: Intestinal part without diverticula.
Laboratory examinations
Tissue with diverticula (n = 9) or control tissue (n = 3) of different parts of the jejunum used for histochemistry or immunohistochemistry were washed in 0.1 M phosphate buffer and embedded in paraffin (Paraplast Plus®; Leica, Nussloch, Germany). Hematoxylin and eosin (H&E) staining was applied to tissue sections (5 µm) of all collected specimens. For immunostaining, primary antibody was applied to 4-18 tissue sections (5 µm) of jejunum with diverticula (n = 2) and control tissue (n = 1) and repeated in an independent experiment. Unspecific protein binding sites were saturated by incubation with 4% horse serum (PAA Laboratories Inc., Pasching, Austria) and 1% bovine serum albumin (Sigma Aldrich/Merck, Darmstadt, Germany) in 0.005 M phosphate buffer for 2 hours. Samples were incubated in primary and secondary antibodies overnight, respectively, rinsed, post-fixed for 10 minutes in 4% paraformaldehyde, and mounted in Mowiol (Sigma Aldrich/Merck). Primary antibodies were mouse-anti-smooth muscle actin (SMA) (1:2000 dilution; monoclonal mouse-anti-actin, α-smooth muscle-FITC, F3777; Sigma Aldrich/Merck) to label SMA of the intestinal tract muscle layers; goat-anti-choline acetyltransferase (ChAT) (AB144P, 1:800 dilution; Merck Millipore/Merck, Darmstadt, Germany) to label ChAT of cholinergic nerve fibers, which promote intestinal motility; guinea pig-anti-protein gene product 9.5 (PGP9.5) (ab5898, 1:500 dilution; Merck Millipore/Merck) as neuronal marker to label the PGP9.5 of the myenteric and submucosal plexus; rat-anti-substance P (SP) (sc-21715, 1:400 dilution; Santa Cruz Biotechnology, Dallas TX, United States) to label the neuropeptide SP, which promotes intestinal motility; rabbit-anti-neuronal nitric oxide synthase (nNOS) (No. 61-7000, 1:800 dilution; ZYMED, San Francisco, CA, United States) to label nNOS, which inhibits intestinal motility; guinea pig-anti-vasoactive intestinal peptide (VIP) (No. 16071, 1:4000 dilution; PROGEN, Heidelberg, Germany) to label neuropeptide VIP, which inhibits intestinal motility; and rabbit-anti-villin-1 (N-term, AP6774a, 1:400 dilution; ABGENT, San Diego, CA, United States) to label intestinal the brush border. Secondary antibodies were donkey-anti-rabbit IgG (H + L) biotin-SP-conjugated (Biotin, No. 711-065-152, 1:200 dilution; Dianova, Hamburg, Germany), streptavidin cyanine 5-conjugated (Cy5, 45-000-734, 1:1600 dilution; Thermo Fisher Scientific Inc., Waltham, MA, United States), donkey-anti-rat IgG (H + L) fluorescein isothiocyanate-conjugated (FITC) (No. 712-095-153, 1:1000 dilution; Dianova), donkey-anti-guinea pig IgG (H + L) cyanine 3-conjugated (Cy3) (No. 706-165-148, 1:800 dilution; Dianova), and donkey-anti-goat IgG Cy3 (AP180C, 1:1600 dilution; Merck Millipore/Merck). Specificity of secondary reagents was validated by omission of primary antibodies. All sections were rinsed and cover slipped with carbonate-buffered glycerol (pH 8.6; Merck Millipore/Merck). Sections were evaluated by microscopy (Axioplan 2, Zeiss, Wetzlar, Germany; BX51 equipped with an automatic stage, Olympus, Shinjuku, Tokyo, Japan; DM750 with ICC50 HD, Leica, Nussloch, Germany). Coloring of black and white images was performed using ImageJ software (https://imagej.nih.gov/ij).
Imaging examinations
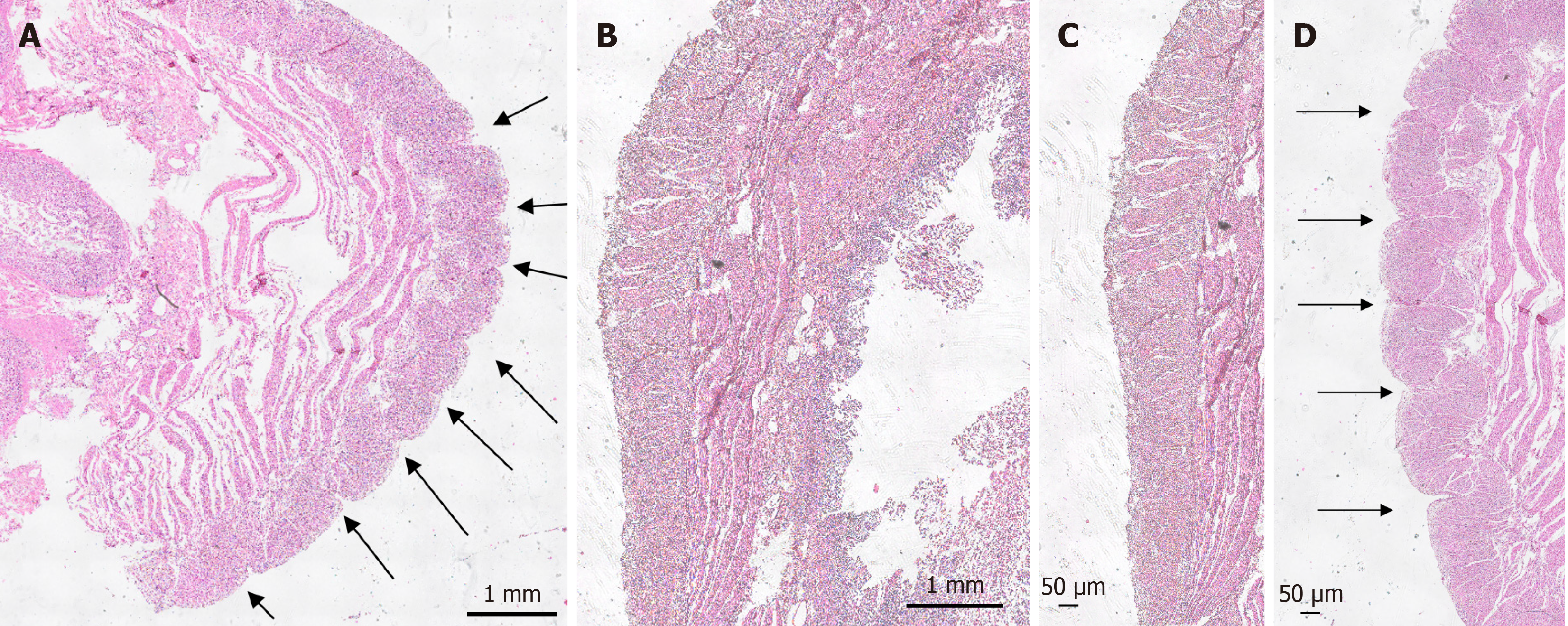
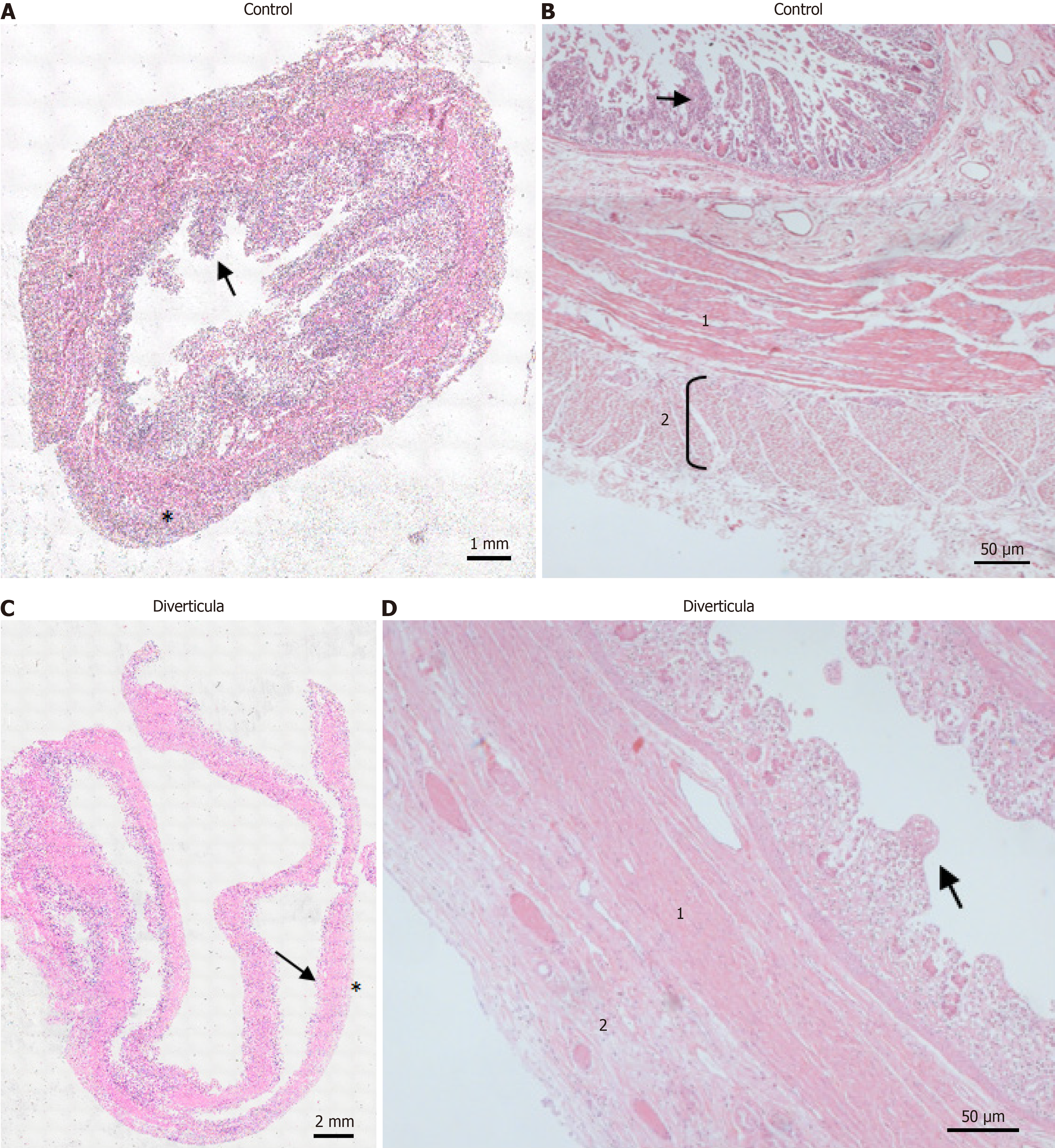
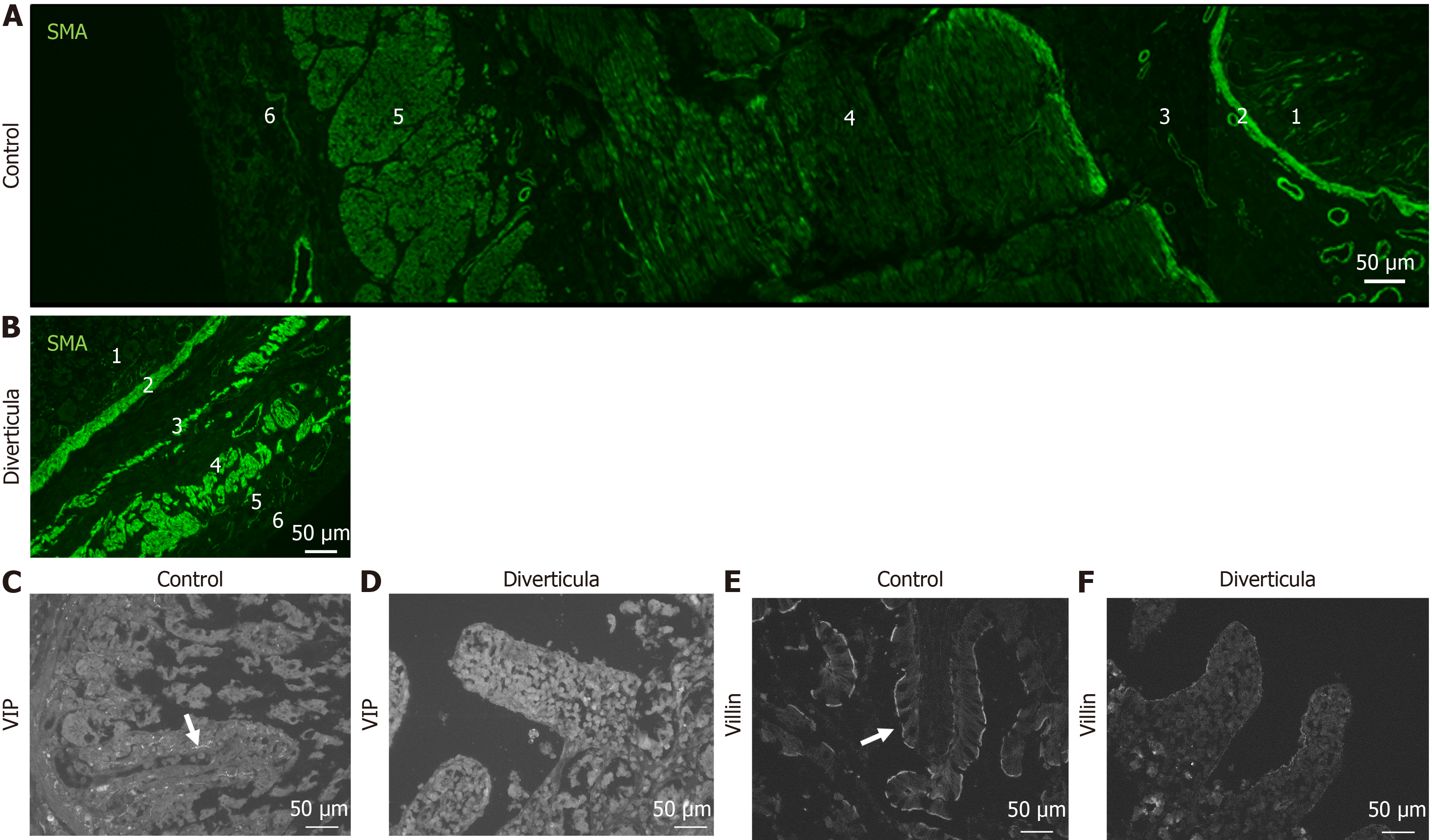
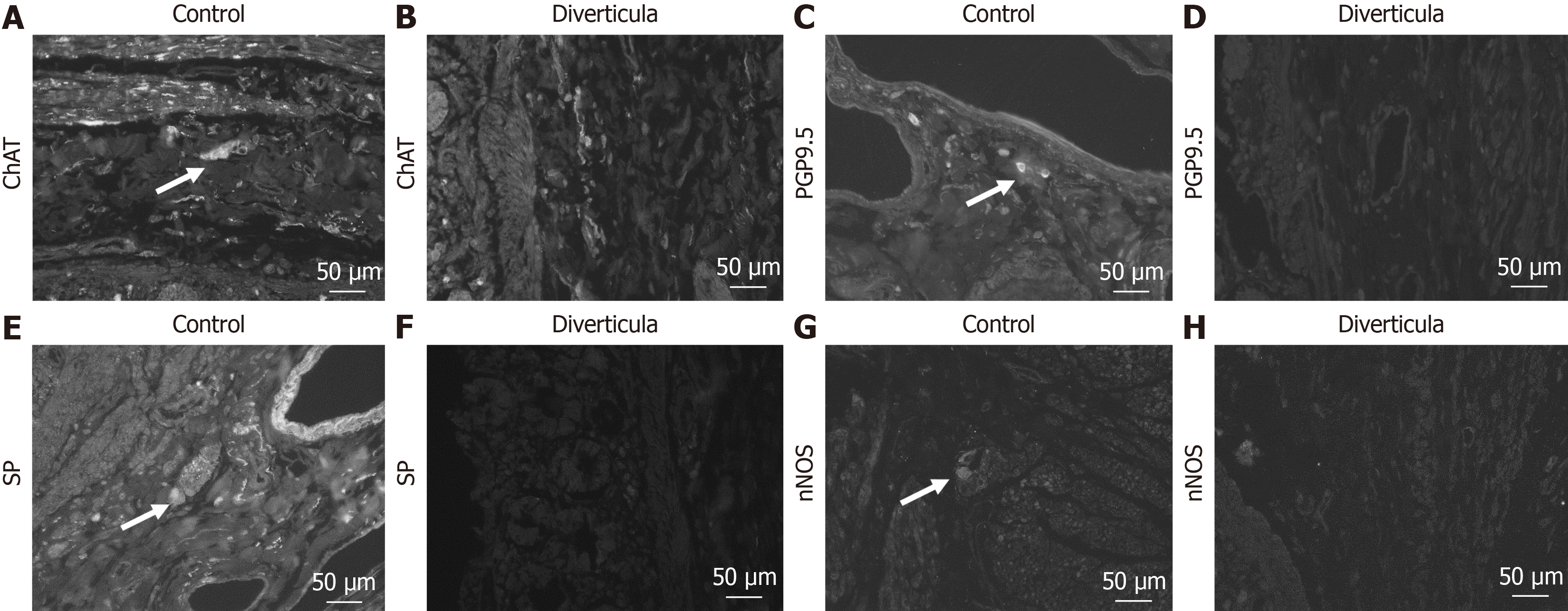
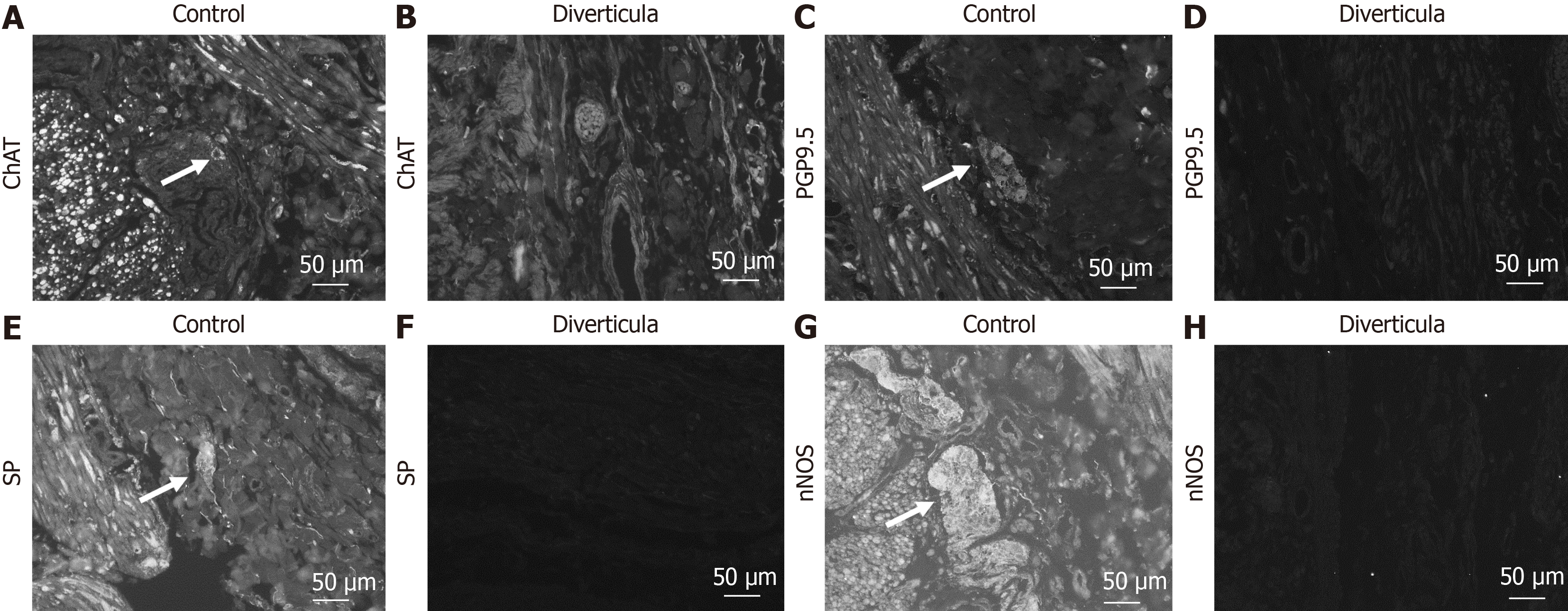
Histological investigation of H&E-stained tissue sections showed that this longitudinal striation on intestinal parts hosting diverticula could also be observed on the microscopic level (Figure 4A). Comparable to macroscopic investigation, longitudinal striation was missing from the intestinal parts without diverticula (Figure 4B). A higher magnification shows that the indentations responsible for the striation are of natural origin and not due to the treatment during the fixation process (Figure 4C and D). After opening the intestinal tract, we counted 232 jejunal diverticulum entry points with a diameter of up to 2 cm (Figure 5). We dissected an 8 cm long representative part of the jejunum with diverticula, utilizing the dissecting microscope to display the relationship between vessels and diverticula. This particularly thorough vessel preparation showed that all investigated arteriae rectae end in diverticula and all investigated diverticula were supplied by a vessel (Figure 6). Histological investigation of H&E-stained samples revealed that the intestinal villi of diverticula were smaller and less prominent than control tissue (Figure 7A and B), and the stratum longitudinale was much thinner in comparison to control tissue (Figure 7C and D). Labeling of SMA with antibodies confirmed the observation of a thinner stratum longitudinale and a thinner stratum circular in the diverticula. The anti-SMA antibody clearly marked the lamina muscularis mucosae in both the diverticula and the control tissue. Lamina muscularis mucosae appeared comparable and inconspicuous in both tissues (Figure 8A and B). VIP-positive fibers could only be detected in control tissue, and the brush border labeled with villin antibody was not present in the diverticula (Figure 8C-F). Clear identification of submucosal and mesenteric plexus was possible in control tissue, utilizing antibodies against ChAT, SP, PGP9.5, and nNOS. Neither submucosal plexus (Figure 9) nor mesenteric plexus (Figure 10) could be detected in the investigated tissue of the diverticula. The specimen showed neither macroscopic nor microscopic signs of inflammation, i.e. diverticulitis.
Figure 4 Microscopic investigation of longitudinal striation of jejunum with diverticula.
A and B: Hematoxylin and eosin (H&E)-stained diverticula and control tissue; C and D: Magnification of H&E-stained diverticula and control tissue. Arrows mark indentations responsible for longitudinal striation.
Figure 5 Lumen of intestine and diverticula.
A: Intestinal tract was cut longitudinally. Arrows mark diverticula entry points; B: Representative diverticula were opened; C and D: Diverticula entry points pictured from intestinal site (C) and from diverticulum site (D); E: Large diverticulum entry points with a diameter of 2 cm.
Figure 6 Relationship between vessels and diverticula.
Representative part of the jejunum (8 cm) with diverticula was cut out and dissected utilizing a dissecting microscope. All remaining fat tissue and intestine covering tunica serosa was removed, vessels were stretched out, and entry sites were exposed. Arrows mark arteria recta entering diverticula (marked with an asterisk). All investigated vessels end in diverticula, and all investigated diverticula were supplied by a vessel.
Figure 7 Histological investigation of diverticula.
A and C: Hematoxylin and eosin (H&E)-stained control tissue (A) and diverticula (C). Arrows mark intestinal villi. Asterisks mark stratum longitudinale; B and D: H&E-stained control tissue (B) and diverticula (D) in a higher magnification. 1: Stratum circular; 2: Stratum longitudinale. Arrows mark intestinal villi.
Figure 8 Immunohistochemical investigation of diverticula.
A and B: Immunohistochemical staining with smooth muscle actin antibody of control tissue (A) and diverticula (B); C-F: Investigation of intestinal villi in control tissue (C and E) and diverticula (D and F) utilizing vasoactive intestinal peptide (VIP; C and D) and villin (E and F) to mark VIP-positive fibers and brush border, respectively. 1: Intestinal villi; 2: Lamina muscularis mucosa; 3: Lamina submucosae; 4: Stratum circular; 5: Stratum longitudinale; 6: Adventitia/serosa.
Figure 9 Immunohistochemical investigation of submucosal plexus in diverticula.
A-H: Detection of submucosal plexus in control tissue (A, C, E, and G) and diverticula (B, D, F, and H) utilizing choline acetyltransferase (ChAT) (A and B), protein gene product 9.5 (PGP9.5) (C and D), substance P (SP) (E and F) and neuronal nitric oxide synthase (nNOS) (G and H) antibody. Arrows mark positive cells in the plexus.
Figure 10 Immunohistochemical investigation of myenteric plexus in diverticula.
A-H: Detection of myenteric plexus in control tissue (A, C, E, and G) and diverticula (B, D, F, and H) utilizing choline acetyltransferase (ChAT) (A and B), protein gene product 9.5 (PGP9.5) (C and D), substance P (SP) (E and F) and neuronal nitric oxide synthase (nNOS) (G and H) antibody. Arrows mark positive cells in the plexus.
FINAL DIAGNOSIS
We observed a jejunal diverticulosis of tremendous extent.
DISCUSSION
The etiology of jejunal diverticula is associated with abnormalities in the smooth muscle or myenteric plexus, visible at the microscopic level[17], that leads to distorted contractions of the smooth muscles in the affected small intestine. The degeneration and fibrosis of smooth muscle cells are indicative of a visceral myopathy, as well as neuronal and axonal degeneration suggestive of visceral neuropathy. Increased intraluminal pressure results from these abnormalities, leading to herniation of the mucosa and submucosa through the muscle layer and forming diverticula[8]. In the presented case, we detected 232 diverticula on a 208 cm length of intestine. Four key observations should be highlighted: (1) Macroscopic and microscopic abnormalities in the smooth muscle architecture; (2) Absence of the submucosal and mesenteric plexus, resulting in a lack of innervation within the diverticula; (3) Termination of all investigated arteriae rectae within the diverticula; and (4) The diverticula did not appear to result from a herniation of the mucosa and submucosa through the muscularis propria, which we like to emphasize in the reported case. Instead, all tissue layers exhibited thinning. Review of the patient’s history revealed that this finding was previously unknown. Even though the patient suffered from gastrointestinal diseases, the performed laparoscopy excluded a pathological perforation. Nevertheless, it is known that jejunoileal diverticulosis usually does not show any symptoms but can lead to diagnostic challenges[21]. The patient’s medical history included opioid abuse due to chest pain of unknown origin and an alcohol use disorder. Whether these factors contribute to an increased risk of diverticulosis remains unclear. While some studies have demonstrated a clear association between high alcohol consumption and an increased risk for diverticulosis[22,26,27], a large meta-analysis found no significant influence of alcohol on its development[28]. Endoscopy of the upper and lower gastrointestinal tract reveals a dilated postbulbar duodenum, which shows an atrophic mucosa that is translucent to underlying vessels. Interestingly, we could already detect changes in the jejunal intestinal wall on a macroscopic level. The longitudinal striation was clearly visible on the intestinal segments hosting diverticula. Microscopic investigation supports these findings. Initially, we hypothesized that this effect might be due to the fixation process, but higher magnification shows that the indentations responsible for the striation are of natural origin. Investigation of the vessel supply reveals a clear interaction between diverticula and blood vessels. All investigated vessels ended in a diverticulum. This supports the theory that diverticula are built by herniation through the weakest mesenteric site of the intestinal wall. This structural vulnerability is inadvertently induced by paired blood vessels from the mesentery, which arise from the supplying, paired blood vessels from the mesentery[8]. Intriguingly, approximately 11-12 vessels supply 10 cm of intestine[29]. We detected 232 diverticula on 208 cm of intestine, and our particularly thorough vessel preparation shows that all investigated arteriae rectae terminate in diverticula. This implies the conclusion that, in our presented case, the majority of blood vessel entry sites from the mesentery are transformed into diverticula. Pathophysiological models propose that increased intraluminal pressure and structural weaknesses in the intestinal wall are the primary mechanisms underlying diverticulosis[13]. The frequent occurrence of diverticula at sites where blood vessels (vasa recta) penetrate the muscularis propria provides further support for this model, as these vascular entry points represent natural weak spots prone to herniation under elevated luminal pressure[3]. This aligns with the hypothesis that structural vulnerabilities in the intestinal wall, when combined with increased intraluminal pressure, contribute significantly to the formation and progression of diverticula. An alternative explanation, the ischemic hypothesis, proposes that compression of the vasa recta at the neck of diverticula leads to localized ischemia and inflammatory responses, potentially resulting in perforation. This viewpoint underscores the role of vascular factors, such as impaired blood supply, in the onset and progression of diverticular disease[13]. Our findings support both theories, although interestingly, the ischemia hypothesis is associated with cholinergic denervation[13], which is supported by our immunohistochemical observations, lacking cholinergic nerve fibers in the diverticula. Whether vascular dysfunction occurs independently of motility disorders remains unknown. If vascular dysfunction does occur independently, this would suggest a distinct primary mechanism rather than just a secondary contributing factor. This perspective could shift research focus toward investigating vascular biomarkers or developing therapeutic strategies targeting microvascular integrity. In general, histological processing of the tissue supports our observations from a macroscopic level. Diverticula showed an abnormality of the muscle layers, reduced brush border, fewer intestinal villi, and no submucosal or myenteric plexus. A study involving 10 patients with jejunal diverticulosis found that disorders of the smooth muscle or myenteric plexus may contribute to diverticula formation[17], and in some cases, visceral myopathy has been identified as a potential cause of jejunal diverticulosis[20]. Our findings strongly support these theories. In our case, we were unable to detect any submucosal or myenteric plexus within the diverticula. It is reasonable to assume that there was also a lack of innervation of the corresponding area, which likely led to a disorder of the smooth muscles. However, it remains unclear whether this was the cause or the consequence of the diverticulosis. The association between histologic abnormalities of the jejunal diverticula and the onset of fatal chronic diarrhea that finally led to multiorgan failure remains unclear and cannot be excluded. However, it seems likely that at least some symptoms described in the patient’s history may be related to the extensive jejunal diverticulosis. In addition, colchicine treatment, which was continued until the patient’s death, may have aggravated his symptoms. Individual reports show that jejunal diverticulosis may be linked to chronic symptoms, including abdominal pain, nausea, chronic pancreatitis, and malabsorption[30]. In addition, it may cause acute complications, such as inflammation, bleeding, obstruction, and/or perforation[25]. Therefore, it is essential to consider this disease in patients presenting with unspecified chronic abdominal symptoms. An adequate diagnosis is the prerequisite for adequate treatment that may include pancreatic enzyme supplementation and optimized nutrition. However, surgical treatment may also be discussed[25]. Our study provides evidence for a previously undescribed type of jejunal diverticula characterized by a lack of herniation of the mucosa and submucosa through the muscularis propria, absence of cholinergic innervation, and alterations in blood vessel morphology. Even though the symptoms associated with this observation may be similar to “normal hernia,” we were not able to provide any conclusions regarding the development of this hernia subtype. Further studies on innervation and absence of the submucosal and mesenteric plexus in diverticula and the associated smooth muscle architecture and vascular involvement should also be the focus of further investigations. The present study displays a case report, describing one specimen; therefore, no statistical analyses of the observations are possible, and different phenotypes cannot be compared. Furthermore, it should be mentioned that we examined a donated cadaver; for that reason, the tissue was fixed for a substantial time and exhibits the corresponding fixation artifacts and postmortem changes due to the delayed fixation after death.
CONCLUSION
In sum, we report a case of extensive jejunal diverticulosis, which arises from the mesenteric vessel border, with 232 diverticula entries on 208 cm of intestine. The visible changes in the smooth muscle architecture strongly support the theory of abnormalities in smooth muscle affecting the intestine, which are usually only visible by microscopic investigation. In the diverticula, we observed an absence of the submucosal and mesenteric plexus and associated absence of innervation to thinned tissue layers. These macroscopic and histological findings correlate with the previously observed symptoms i.e. including vomiting and chronic gastroesophageal reflux, esophagitis, chronic gastritis, and diarrhea, and may have been associated with the exacerbated diarrhea, which finally contributed to the fatal multiple organ failure.
ACKNOWLEDGEMENTS
The authors wish to sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially improve patient care and increase mankind's overall knowledge. Therefore, these donors and their families deserve our highest gratitude. We also thank M. Bodenbenner skillful technical assistance. We gratefully thank Dante Grace Minichetti from Brigham and Women's Hospital in Boston for language editing and proofreading.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Anatomische Gesellschaft; Deutsche Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie e.V.
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade B, Grade B
Novelty: Grade A, Grade B, Grade B, Grade C
Creativity or Innovation: Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade A, Grade B, Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Nagamine T; Osman H S-Editor: Lin C L-Editor: Filipodia P-Editor: Xu ZH