Published online Apr 27, 2025. doi: 10.4240/wjgs.v17.i4.103483
Revised: February 6, 2025
Accepted: February 26, 2025
Published online: April 27, 2025
Processing time: 113 Days and 21.8 Hours
Esophageal cancer constitutes one of the most aggressive malignant neoplasms associated with poor clinical outcomes. While surgical resection remains the cornerstone of curative intervention, optimization of perioperative care protocols has emerged as an essential strategy to reduce postoperative complications and potentially improve long-term survival rates in patients undergoing esophagec
To identify perioperative factors affecting prognosis after radical esophagectomy, aiming to improve patient outcomes through targeted interventions.
A retrospective study analyzed 378 patients with esophageal cancer who underwent radical esophagectomy (McKeown, Sweet, or Ivor-Lewis procedures) from January 2022 through December 2023. All operations were performed by experienced surgeons following standardized perioperative protocols. The inves
Multivariate Cox proportional hazards analysis identified three independent predictors of survival: Tumor-node-metastasis staging [Hazard ratio (HR) = 2.31, 95% confidence interval (CI): 1.72-3.10, P < 0.001], tumor differentiation (moderate: HR = 1.46, 95%CI: 1.02-2.09, P = 0.038; poor: HR = 2.15, 95%CI: 1.47-3.14, P < 0.001), and extended postoperative analgesic use (> 5 days) (HR = 1.43, 95%CI: 1.08-1.89, P = 0.012). Kaplan-Meier analysis demonstrated significantly lower overall survival rates in patients requiring analgesics for > 5 days compared to ≤ 5 days (P = 0.003), with consistent patterns observed for both opioid (P = 0.019) and nonsteroidal anti-inflammatory drug use (P = 0.028). The extended analgesic group exhibited a higher proportion of elderly patients (48.47% vs 35.57%, P = 0.015), while other baseline characteristics and tumor features remained comparable between groups.
Tumor-node-metastasis staging, tumor differentiation, and duration of postoperative analgesic use independently predict survival following radical esophagectomy, underscoring the significance of optimal pain management protocols.
Core Tip: In this retrospective analysis of 378 patients who underwent radical esophagectomy for esophageal cancer, we identified tumor-node-metastasis staging, tumor differentiation, and extended postoperative analgesic use (> 5 days) as independent predictors of overall survival. Of particular significance was the adverse impact of prolonged administration of both opioids and nonsteroidal anti-inflammatory drugs on survival outcomes. These findings emphasize the critical importance of precise perioperative management, specifically regarding optimal pain control protocols, in enhancing patient outcomes. Further validation through multicenter, prospective studies and investigation of underlying mechanisms is warranted to optimize perioperative care strategies and improve long-term survival rates in this patient population.
- Citation: Liu SG, Xu XJ, He M, Zhao JD, Pei L. Perioperative risk factors for prognosis in patients undergoing radical esophagectomy: A retrospective study. World J Gastrointest Surg 2025; 17(4): 103483
- URL: https://www.wjgnet.com/1948-9366/full/v17/i4/103483.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i4.103483
Esophageal cancer is a malignant neoplasm originating from esophageal epithelial cells that can develop in any segment of the esophagus, with squamous cell carcinoma being the predominant histologic type. While early-stage disease may be asymptomatic, advanced cases typically manifest with dysphagia and food obstruction[1]. Global cancer statistics from 2020 indicate that esophageal cancer ranks seventh among all malignancies worldwide, with China accounting for over half of incident cases[2]. In China, as of 2016, esophageal cancer ranked sixth in incidence and fifth in mortality among all malignancies, constituting a substantial public health burden[3]. The disease is characterized by high malignant potential and poor outcomes, with advanced-stage patients in China exhibiting only a 26% five-year survival rate[4].
Currently, surgical intervention remains the most definitive curative approach for esophageal cancer[5]. Improved surgical mortality rates can be attributed to advances in operative techniques, standardized perioperative protocols, and widespread implementation of multidisciplinary treatment strategies. However, radical esophagectomy remains a highly invasive procedure characterized by prolonged operative duration, challenging surgical exposure, and complex anato
Perioperative management is fundamental to esophageal cancer treatment, with surgical outcomes and patient prognosis directly linked to the quality of perioperative care[7]. Despite technological and therapeutic advances, esophageal cancer surgery continues to carry substantial morbidity and mortality risks. Key perioperative challenges include inadequate pain control, respiratory complications, delayed gastrointestinal recovery, and poor nutritional status[8,9]. Pain management is particularly crucial, as insufficient analgesia can precipitate respiratory compromise, impair early mobilization, and amplify stress response, potentially compromising both immediate recovery and long-term outcomes.
While numerous studies have investigated perioperative risk factors[10-12], including age, nutritional status, comor
Our study aims to comprehensively evaluate perioperative factors influencing outcomes following radical esophagec
This retrospective study analyzed clinical data from 378 patients who underwent radical esophagectomy for esophageal cancer at the Department of Thoracic Surgery, Fourth Hospital of Hebei Medical University between January 2022 and December 2023. Patients were eligible for inclusion if they met the following criteria: (1) Histologically confirmed esophageal cancer by preoperative endoscopic biopsy; (2) Absence of distant metastasis on preoperative imaging studies, including contrast-enhanced chest and abdominal computed tomography, cervical ultrasonography, and whole-body positron emission tomography-computed tomography; (3) Medical fitness for surgery with no contraindications; (4) Completion of standard radical esophagectomy; and (5) Availability of complete clinical records. Exclusion criteria comprised: (1) Prior neoadjuvant therapy; (2) History of other malignancies; (3) Presence of severe cardiac, pulmonary, hepatic, or renal dysfunction; (4) Intraoperative discovery of distant metastases precluding complete resection; (5) Perioperative mortality; and (6) Loss to follow-up or incomplete follow-up data.
All procedures were performed by experienced surgeons (associate chief physician or above) with a minimum of 15 years of expertise in esophageal cancer surgery. The surgical approach (McKeown three-incision, Sweet left thoracic, or Ivor-Lewis two-incision) was selected based on tumor location, patient characteristics, and surgical expertise. All operations adhered to standard oncological principles of radical esophagectomy, ensuring complete resection of the diseased esophageal segment and regional lymph nodes with negative margins.
The surgical procedure was performed under general anesthesia with double-lumen endotracheal intubation. Patient positioning varied by approach: Supine with contralateral head rotation for cervical access, lateral decubitus with 30° elevation for thoracic access, and supine for abdominal access. The thoracic phase involved esophageal mobilization and systematic lymphadenectomy of the periesophageal, subcarinal, paratracheal, and supradiaphragmatic nodes. The abdominal phase included gastric mobilization along greater and lesser curvatures while preserving the right gastroepiploic artery, accompanied by lymph node dissection around the cardia, left gastric artery, and celiac axis.
Gastrointestinal continuity was restored using a 3-4 cm wide gastric tube, created with a linear stapler along the greater curvature. The gastric conduit was routed through either the posterior mediastinum or retrosternal space for esophagogastric anastomosis, performed either mechanically or manually, based on anatomical considerations and surgeon preference. Standard placement of thoracic drainage and nasogastric tubes was performed. Strict adherence to oncological principles included “no-touch” technique to minimize tumor manipulation. All specimens underwent pathological examination for margin status and lymph node involvement.
Preoperative preparation: All patients underwent comprehensive preoperative evaluation upon admission. Standard assessment included complete blood count, liver and kidney function tests, coagulation profile, and cardiopulmonary function evaluation. The American Society of Anesthesiologists (ASA) classification was applied to patients aged 65 years or older or those with comorbidities. Nutritional status was evaluated using the Nutritional Risk Screening 2002 scoring system; patients scoring ≥ 3 or with serum albumin < 35 g/L received nutritional supplementation. Anemia (hemoglobin < 120 g/L in men, < 110 g/L in women) was corrected preoperatively. Pulmonary function was assessed through detailed history, physical examination, spirometry, and arterial blood gas analysis. Patients with impaired pulmonary function received respiratory physiotherapy and nebulization treatment. Medical conditions including hypertension and diabetes were optimized to achieve target parameters. Bowel preparation was conducted one day preoperatively using oral polyethylene glycol electrolyte solution or mannitol, with 8-hour fasting and 4-hour clear liquid restrictions prior to surgery. Intravenous access was established 24 hours preoperatively, and prophylactic second-generation cephalosporins were administered 30 minutes before incision. All patients received psychological support and detailed operative instructions.
Anesthesia method: Combined general and thoracic epidural anesthesia was utilized. Standard monitoring included electrocardiogram, blood pressure, and pulse oximetry, complemented by invasive arterial pressure monitoring. Anesthesia induction was performed with midazolam (0.05 mg/kg), sufentanil (0.5 μg/kg), propofol (1.5-2.0 mg/kg), and cisatracurium (0.2 mg/kg). A double-lumen endobronchial tube was positioned using fiberoptic bronchoscopic guidance. Anesthesia maintenance was achieved through continuous propofol (4-12 mg/kg/h) and remifentanil (0.1-0.3 μg/kg/minute) infusion, with intermittent cisatracurium administration. Volume-controlled ventilation was sustained with 6-8 mL/kg tidal volume, 12-14 respirations/min, and end-tidal CO2 maintained at 35-45 mmHg. Single-lung ventilation parameters were modified accordingly, with positive end-expiratory pressure applied as needed. Mean arterial pressure was maintained at or above 65 mmHg using vasoactive medications when required. Temperature, urine output, and blood gas values were monitored to preserve physiological homeostasis.
Postoperative management: Patients were transferred to the intensive care unit for immediate postoperative monitoring. Analgesia was administered through a patient-controlled analgesia pump (sufentanil + tropisetron) in conjunction with thoracic epidural analgesia. Continuous monitoring included vital parameters, drainage characteristics, and urinary output. Early mobilization, pulmonary exercises, and nebulization therapy were implemented to prevent respiratory complications. Endotracheal suctioning was performed at 2-4 hours intervals. Nutritional support followed a systematic approach: Enteral nutrition via nasogastric tube was initiated 24 hours postoperatively, supplemented with parenteral nutrition. Oral intake commenced between postoperative days 3-5 following verification of anastomotic integrity, progressing from clear liquids to soft diet. Prophylactic antibiotic therapy was typically discontinued within 48-72 hours. Deep venous thrombosis prophylaxis included early ambulation, intermittent pneumatic compression, and subcutaneous low-molecular-weight heparin administration. Thoracic drainage tubes were removed when output was < 200 mL/day and serous. Patients were transferred to the general ward upon clinical stabilization, with immediate intervention for any complications.
Baseline characteristics collected included demographic variables (age, sex, body mass index), medical history (hypertension, diabetes mellitus, chronic gastritis), lifestyle factors (smoking status, alcohol consumption), and clinical parameters [ASA classification, New York Heart Association (NYHA) functional classification, preoperative glomerular filtration rate]. The ASA physical status classification system was defined as follows: (1) Class I: Normal healthy patient; (2) Class II: Patient with mild systemic disease; (3) Class III: Patient with severe systemic disease limiting activity; (4) Class IV: Patient with severe systemic disease that poses constant threat to life; (5) Class V: Moribund patient not expected to survive without surgery; and (6) The NYHA functional classification was categorized as: Class I: No limitation of physical activity; class II: Mild limitation with symptoms during ordinary activity; class III: Marked limitation with symptoms during less than ordinary activity; and class IV: Symptoms at rest with any physical activity causing discomfort.
Operative data included anesthetic technique, surgical approach, anastomotic location, operative duration, length of postoperative analgesia, adjuvant therapy (chemotherapy or radiotherapy), and postoperative complications (pneumonia, pleural effusion). Tumor characteristics were evaluated according to the 8th edition of the American Joint Committee on Cancer staging system, including tumor-node-metastasis (TNM) stage, tumor location and maximum diameter, histologic grade, depth of invasion, perineural invasion, lymphovascular invasion, and lymph node status.
TNM Staging was classified as: Stage 0: Carcinoma in situ; stage I: Local tumor invasion without nodal involvement; stage II: (1) Muscular invasion without nodal involvement; and (2) With regional lymph node metastasis; stage III: Advanced local invasion with regional lymph node involvement; and stage IV: Distant metastasis. The primary endpoint was all-cause mortality, defined as death from any cause during the follow-up period. Mortality was determined through active follow-up with participants or their next of kin. Date and cause of death were documented. Participants were followed from the index date until death, loss to follow-up, or study completion, whichever occurred first.
Statistical analyses were conducted using SPSS software (version 26.0, IBM Corporation, Armonk, NY, United States). The normality of continuous variables’ distribution was assessed using the Shapiro-Wilk test. Normally distributed continuous variables were expressed as mean ± SD and compared using independent-samples t-tests, while non-normally distributed variables were presented as median (interquartile range) and analyzed using the Mann-Whitney U test. Variance homogeneity was evaluated using Levene's test. Categorical variables were expressed as frequencies (percentages) and analyzed using Pearson’s χ2 test or Fisher's exact test when expected cell counts were less than 5.
Overall survival was calculated using the Kaplan-Meier method, with intergroup differences assessed using the log-rank test. The proportional hazards assumption for Cox regression analysis was verified through examination of Schoenfeld residuals. Prognostic factors were identified through univariate and multivariate Cox proportional hazards models. To control for potential confounding factors, the following variables were selected a priori based on clinical significance and published literature: Age, sex, body mass index, ASA physical status classification, comorbidities, tumor stage, surgical approach, and operative time. Variables showing P ≤ 0.1 in univariate analysis were included in the multivariate Cox regression model. The magnitude and precision of associations were quantified using hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). All statistical tests were two-sided, with statistical significance defined as P < 0.05.
Baseline characteristics: Advanced age (≥ 65 years; HR = 1.52, 95%CI: 1.18-1.96, P = 0.001), ASA physical status class IV (HR = 1.45, 95%CI: 1.08-1.95, P = 0.01), and NYHA functional class III (HR = 1.68, 95%CI: 1.12-2.52, P = 0.01) demonstrated significant associations with decreased overall survival. Hypertension and diabetes exhibited borderline significance (P < 0.10) (Table 1).
| Baseline characteristic | n = 378 | Hazard ratio | 95%CI | P value |
| Age | ||||
| <65 years | 215 (56.88) | 1.00 | Reference | - |
| ≥ 65 years | 163 (43.12) | 1.52 | 1.18-1.96 | 0.001 |
| Gender | ||||
| Male | 292 (77.25) | 1.00 | Reference | - |
| Female | 86 (22.75) | 0.89 | 0.67-1.19 | 0.44 |
| BMI (kg/m²), mean ± SD | 23.10 ± 3.20 | 0.97 | 0.93-1.02 | 0.24 |
| Hypertension | ||||
| No | 246 (65.08) | 1.00 | Reference | - |
| Yes | 132 (34.92) | 1.28 | 0.99-1.65 | 0.06 |
| Diabetes | ||||
| No | 298 (78.84) | 1.00 | Reference | - |
| Yes | 80 (21.16) | 1.31 | 0.98-1.75 | 0.07 |
| Smoking history | ||||
| No | 156 (41.27) | 1.00 | Reference | - |
| Yes | 222 (58.73) | 1.22 | 0.94-1.58 | 0.13 |
| History of drinking | ||||
| No | 169 (44.70) | 1.00 | Reference | - |
| Yes | 209 (55.29) | 1.15 | 0.89-1.48 | 0.28 |
| Chronic gastritis | ||||
| No | 271 (71.69) | 1.00 | Reference | - |
| Yes | 107 (28.31) | 1.12 | 0.85-1.47 | 0.43 |
| ASA classification | ||||
| III | 312 (82.54) | 1.00 | Reference | - |
| IV | 66 (17.46) | 1.45 | 1.08-1.95 | 0.01 |
| NYHA classification | ||||
| I | 256 (67.72) | 1.00 | Reference | - |
| II | 89 (23.54) | 1.33 | 0.99-1.78 | 0.06 |
| III | 33 (8.74) | 1.68 | 1.12-2.52 | 0.01 |
| Glomerular filtration rate | ||||
| < 90 mL/minute/1.73 m² | 142 (37.57) | 1.00 | Reference | - |
| ≥ 90 mL/minute/1.73 m² | 236 (62.43) | 0.81 | 0.63-1.05 | 0.11 |
Surgery-related factors: Extended postoperative analgesic administration (> 5 days; HR = 1.62, 95%CI: 1.24-2.12, P < 0.001), postoperative pneumonia (HR = 1.58, 95%CI: 1.21-2.06, P = 0.001), pleural effusion (HR = 1.42, 95%CI: 1.07-1.88, P = 0.02), and adjuvant chemoradiotherapy (HR = 1.45, 95%CI: 1.12-1.88, P = 0.01) were associated with inferior survival outcomes. Prolonged operative time showed marginal correlation with adverse outcomes (HR = 1.00, P = 0.05) (Table 2).
| Surgery-related factors | n = 378 | Hazard ratio | 95%CI | P value |
| Type of anesthesia | ||||
| General anesthesia | 203 (53.70) | 1.00 | Reference | - |
| Epidural anesthesia | 175 (46.30) | 0.92 | 0.71-1.19 | 0.52 |
| Surgical methods | ||||
| Open surgery | 245 (64.81) | 1.00 | Reference | - |
| Minimally invasive surgery | 133 (35.19) | 0.78 | 0.60-1.02 | 0.07 |
| Location of anastomotic site | ||||
| Cervical | 228 (60.32) | 1.00 | Reference | - |
| Thoracic | 150 (39.68) | 1.25 | 0.97-1.62 | 0.09 |
| Operative time, minutes, mean ± SD | 266.01 ± 83.13 | 1.00 | 0.99-1.01 | 0.05 |
| Duration of analgesic use | ||||
| ≤ 5 days | 149 (39.42) | 1.00 | Reference | - |
| > 5 days | 229 (60.58) | 1.62 | 1.24-2.12 | < 0.001 |
| Postoperative radiotherapy or chemotherapy | ||||
| No | 198 (52.38) | 1.00 | Reference | - |
| Yes | 180 (47.62) | 1.45 | 1.12-1.88 | 0.01 |
| Postoperative pneumonia | ||||
| No | 265 (70.11) | 1.00 | Reference | - |
| Yes | 113 (29.89) | 1.58 | 1.21-2.06 | 0.001 |
| Postoperative pleural effusion | ||||
| No | 284 (75.13) | 1.00 | Reference | - |
| Yes | 94 (24.87) | 1.42 | 1.07-1.88 | 0.02 |
Tumor pathological characteristics: Advanced TNM stage (III/IV; HR = 2.85, 95%CI: 2.15-3.78, P < 0.001), increased tumor size (≥ 3 cm; HR = 1.68, 95%CI: 1.29-2.19, P < 0.001), submucosal invasion (HR = 1.92, 95%CI: 1.46-2.52, P < 0.001), poor differentiation (HR = 2.42, 95%CI: 1.67-3.51, P < 0.001), perineural invasion (HR = 1.75, 95%CI: 1.33-2.30, P < 0.001), vascular invasion (HR = 1.89, 95%CI: 1.42-2.52, P < 0.001), and lymph node metastasis (HR = 2.32, 95%CI: 1.77-3.04, P < 0.001) demonstrated significant correlation with decreased survival (Table 3).
| Pathological characteristic | n = 378 | Hazard ratio | 95%CI | P value |
| TNM stage | ||||
| I or II stage | 167 (44.18) | 1.00 | Reference | - |
| III or IV stage | 211 (55.82) | 2.85 | 2.15-3.78 | < 0.001 |
| Tumor location | ||||
| Upper segment | 72 (19.05) | 1.00 | Reference | - |
| Middle segment | 198 (52.38) | 0.92 | 0.66-1.28 | 0.62 |
| Lower segment | 108 (28.57) | 0.88 | 0.61-1.27 | 0.50 |
| Tumor diameter | ||||
| < 3 cm | 156 (41.27) | 1.00 | Reference | - |
| ≥ 3 cm | 222 (58.73) | 1.68 | 1.29-2.19 | < 0.001 |
| Depth of tumor infiltration | ||||
| On the muscle layer | 143 (37.83) | 1.00 | Reference | - |
| Subcutaneous muscle layer | 235 (62.17) | 1.92 | 1.46-2.52 | < 0.001 |
| Histological differentiation | ||||
| Well differentiated | 89 (23.54) | 1.00 | Reference | - |
| Moderately differentiated | 196 (51.85) | 1.58 | 1.12-2.23 | 0.01 |
| Poorly differentiated | 93 (24.61) | 2.42 | 1.67-3.51 | < 0.001 |
| Perineural invasion | ||||
| No | 286 (75.66) | 1.00 | Reference | - |
| Yes | 92 (24.34) | 1.75 | 1.33-2.30 | < 0.001 |
| Tumor thrombus | ||||
| No | 301 (79.63) | 1.00 | Reference | - |
| Yes | 77 (20.37) | 1.89 | 1.42-2.52 | < 0.001 |
| Lymph node metastasis | ||||
| No | 184 (48.68) | 1.00 | Reference | - |
| Yes | 194 (51.32) | 2.32 | 1.77-3.04 | < 0.001 |
The extended analgesic group demonstrated a higher proportion of elderly patients (≥ 65 years; 48.47% vs 35.57%, P = 0.015) (Table 4). Other baseline characteristics, surgery-related parameters (Table 5), and tumor pathological features (Table 6) revealed no significant differences between groups (all P > 0.05).
| Baseline characteristic | Short-term analgesic use (n = 149) | Long-term analgesic use (n = 229) | t value or χ2 | P value |
| Age | 5.87 | 0.02 | ||
| < 65 years | 96 (64.43) | 118 (51.53) | ||
| ≥ 65 years | 53 (35.57) | 111 (48.47) | ||
| Gender | 0.22 | 0.64 | ||
| Male | 117 (78.52) | 175 (76.42) | ||
| Female | 32 (21.48) | 54 (23.58) | ||
| BMI (kg/m²), mean ± SD | 23.25 ± 3.15 | 23.01 ± 3.24 | 0.72 | 0.47 |
| Hypertension | 0.64 | 0.42 | ||
| No | 107 (71.81) | 139 (60.70) | ||
| Yes | 42 (28.19) | 90 (39.30) | ||
| Diabetes | 0.55 | 0.46 | ||
| No | 126 (84.56) | 172 (75.11) | ||
| Yes | 23 (15.44) | 57 (24.89) | ||
| Smoking history | 0.74 | 0.39 | ||
| No | 66 (44.30) | 90 (39.30) | ||
| Yes | 83 (55.70) | 139 (60.70) | ||
| History of drinking | 0.89 | 0.35 | ||
| No | 71 (47.65) | 98 (42.79) | ||
| Yes | 78 (52.35) | 131 (57.21) | ||
| Chronic gastritis | 0.52 | 0.47 | ||
| No | 104 (69.80) | 167 (72.93) | ||
| Yes | 45 (30.20) | 62 (27.07) | ||
| ASA classification | 0.65 | 0.42 | ||
| III | 133 (89.26) | 179 (78.17) | ||
| IV | 16 (10.74) | 50 (21.83) | ||
| NYHA classification | 1.24 | 0.54 | ||
| I | 108 (72.48) | 148 (64.63) | ||
| II | 34 (22.82) | 55 (24.02) | ||
| III | 7 (4.70) | 26 (11.35) | ||
| Glomerular filtration rate | 2.24 | 0.14 | ||
| < 90 mL/minute/1.73 m² | 50 (33.56) | 92 (40.17) | ||
| ≥ 90 mL/minute/1.73 m² | 99 (66.44) | 137 (59.83) | ||
| Surgery-related factors | Short-term analgesic use (n = 149) | Long-term analgesic use (n = 229) | t value or χ2 | P value |
| Type of anesthesia | 0.33 | 0.57 | ||
| General anesthesia | 77 (51.68) | 126 (55.02) | ||
| Epidural anesthesia | 72 (48.32) | 103 (44.98) | ||
| Surgical methods | 1.96 | 0.17 | ||
| Open surgery | 83 (55.70) | 162 (70.74) | ||
| Minimally invasive surgery | 66 (44.30) | 67 (29.26) | ||
| Location of anastomotic site | 1.34 | 0.25 | ||
| Cervical | 78 (52.35) | 150 (65.50) | ||
| Thoracic | 71 (47.65) | 79 (34.50) | ||
| Operative time, minutes, mean ± SD | 265.83 ± 76.28 | 266.35 ± 85.42 | 1.73 | 0.09 |
| Duration of analgesic use | 2.74 | 0.10 | ||
| ≤ 5 days | 85 (57.05) | 113 (49.34) | ||
| > 5 days | 64 (42.95) | 116 (50.66) | ||
| Postoperative radiotherapy or chemotherapy | 1.84 | 0.18 | ||
| No | 118 (79.19) | 147 (64.19) | ||
| Yes | 31 (20.81) | 82 (35.81) | ||
| Postoperative pneumonia | 1.95 | 0.16 | ||
| No | 122 (81.88) | 162 (70.74) | ||
| Yes | 27 (18.12) | 67 (29.26) | ||
| Pathological characteristic | Short-term analgesic use (n = 149) | Long-term analgesic use (n = 229) | t value or χ² | P value |
| TNM stage | 1.90 | 0.17 | ||
| I or II stage | 78 (52.35) | 88 (38.43) | ||
| III or IV stage | 71 (47.65) | 141 (61.57) | ||
| Tumor location | 0.16 | 0.92 | ||
| Upper segment | 29 (19.46) | 43 (18.78) | ||
| Middle segment | 77 (51.68) | 121 (52.84) | ||
| Lower segment | 43 (28.86) | 65 (28.38) | ||
| Tumor diameter | 1.40 | 0.24 | ||
| < 3 cm | 74 (49.66) | 81 (35.37) | ||
| ≥ 3 cm | 75 (50.34) | 148 (64.63) | ||
| Depth of tumor infiltration | 1.13 | 0.29 | ||
| On the muscle layer | 65 (43.62) | 78 (34.06) | ||
| Subcutaneous muscle layer | 84 (56.38) | 151 (65.94) | ||
| Histological differentiation | 2.74 | 0.26 | ||
| Well differentiated | 42 (28.19) | 47 (20.52) | ||
| Moderately differentiated | 81 (54.36) | 115 (50.22) | ||
| Poorly differentiated | 26 (17.45) | 67 (29.26) | ||
| Perineural invasion | 1.49 | 0.22 | ||
| No | 124 (83.22) | 162 (70.74) | ||
| Yes | 25 (16.78) | 67 (29.26) | ||
| Tumor thrombus | 1.22 | 0.27 | ||
| No | 125 (83.89) | 176 (76.86) | ||
| Yes | 24 (16.11) | 53 (23.14) | ||
| Lymph node metastasis | 1.90 | 0.17 | ||
| No | 86 (57.72) | 98 (42.79) | ||
| Yes | 63 (42.28) | 131 (57.21) | ||
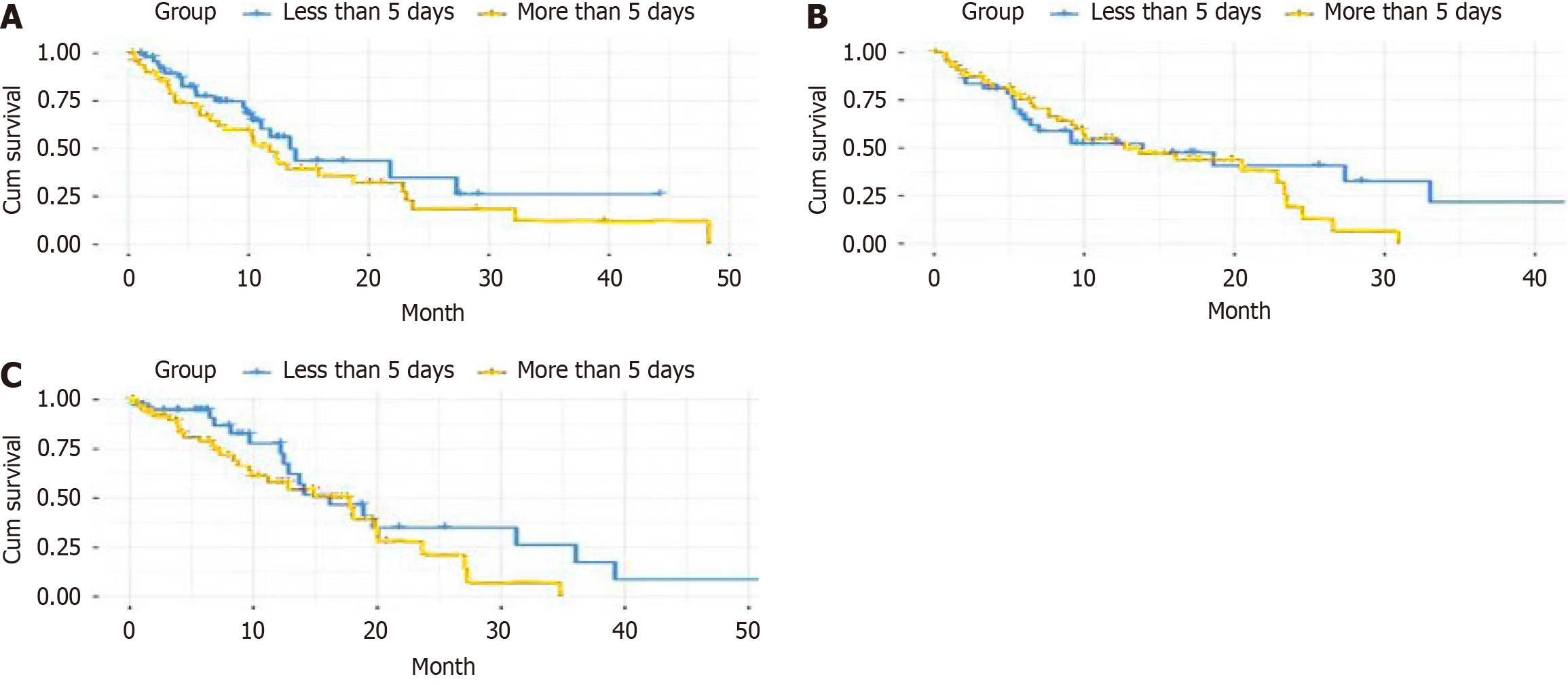
Kaplan-Meier analyses revealed significantly improved survival in patients with ≤ 5 days of analgesic administration compared to > 5 days (P = 0.003) (Figure 1A). Consistent patterns were observed for both opioid (P = 0.019) (Figure 1B) and nonsteroidal anti-inflammatory drug (NSAID) administration (P = 0.028) (Figure 1C).
After adjusting for potential confounding variables (P ≤ 0.1 in univariate analysis), TNM stage (HR = 2.31, 95%CI: 1.72-3.10, P < 0.001), tumor differentiation (moderate: HR = 1.46, 95%CI: 1.02-2.09, P = 0.038; poor: HR = 2.15, 95%CI: 1.47-3.14, P < 0.001), and extended analgesic administration (> 5 days) (HR = 1.43, 95%CI: 1.08-1.89, P = 0.012) emerged as independent predictors of decreased survival (Table 7).
| Variables | Hazard ratio | 95%CI | P value |
| TNM staging | - | - | - |
| Phase I and II | 1.00 | Reference | - |
| Phase III and IV | 2.31 | 1.72-3.10 | < 0.001 |
| Degree of tumor differentiation | - | - | - |
| Well differentiated | 1.00 | Reference | - |
| Moderately differentiated | 1.46 | 1.02-2.09 | 0.038 |
| Poorly differentiated | 2.15 | 1.47-3.14 | < 0.001 |
| Postoperative analgesics used for more than 5 days | - | - | - |
| ≤ 5 days | 1.00 | Reference | - |
| > 5 days | 1.43 | 1.08-1.89 | 0.012 |
Our multivariate Cox proportional hazards analysis identified TNM stage, tumor differentiation, and extended post
Tumor differentiation emerged as another crucial prognostic indicator, with both moderate (HR = 1.46, 95%CI: 1.02-2.09, P = 0.038) and poor differentiation (HR = 2.15, 95%CI: 1.47-3.14, P < 0.001) associated with inferior outcomes. This gradient relationship likely reflects the enhanced invasiveness, complex molecular alterations, and treatment resistance of poorly differentiated tumors[19,20]. The independent prognostic value of TNM stage and differentiation suggests they capture distinct aspects of tumor biology-anatomical progression and biological behavior, respectively. The integration of both TNM staging and tumor differentiation may provide more comprehensive prognostic stratification and guide therapeutic decision-making. Specifically, patients with early-stage disease but poor differentiation may benefit from intensified multimodal therapy, whereas selected patients with advanced-stage but well-differentiated tumors might remain candidates for surgical intervention, provided they meet appropriate physiological criteria for operability.
Notably, extended postoperative analgesic administration (> 5 days) independently predicted decreased survival (HR = 1.43, 95%CI: 1.08-1.89, P = 0.012), validating previous findings that reported a comparable hazard ratio (HR = 1.38) for extended analgesic use in gastrointestinal cancer patients[21]. Kaplan-Meier analyses demonstrated significantly decreased survival with prolonged administration of both opioids (P = 0.019) and NSAIDs (P = 0.028). Several mecha
Firstly, extended analgesic requirements may indicate complicated recovery, particularly in elderly patients who constituted a larger proportion of the > 5 days group (48.47% vs 35.57%, P = 0.015). Secondly, regarding opioid administration, our findings support the expanding evidence suggesting that opioids may influence tumor progression[22]. Recent molecular studies have demonstrated that opioids enhance tumor angiogenesis and suppress apoptosis through μ-opioid receptor activation[23]. The immunosuppressive effects of opioids, specifically on natural killer cell activity and T lymphocyte function[24], may explain the increased early recurrence risk observed in our study. Thirdly, our results indicate that extended postoperative administration may be detrimental. This aligns with Zhao-Fleming et al’s research[25], which demonstrated that prolonged NSAID use can result in complications affecting overall recovery. Furthermore, our observations support the findings of Arron et al[26], who documented increased surgical site complications with extended NSAID use, potentially compromising long-term outcomes.
These findings collectively suggest that optimizing perioperative pain management requires precise balance between adequate pain control and minimizing the potential adverse impacts of extended analgesic administration. This per
Several limitations warrant consideration in this investigation. Primarily, the single-center retrospective design introduces potential selection bias, despite stringent inclusion and exclusion criteria. The institutional source limitation may affect the external validity and generalizability of our findings. Additionally, the retrospective methodology inherently presents challenges in data collection, particularly regarding critical perioperative parameters such as intraoperative blood loss and precise analgesic dosing, potentially affecting our analytical accuracy. Furthermore, while we identified an association between extended postoperative analgesic administration and adverse outcomes, the causal relationship remains uncertain due to potential unmeasured confounding variables, including underlying complications or compromised physical status that might independently influence prognosis. Lastly, the relatively brief follow-up period may inadequately capture long-term survival outcomes.
Future research directions should address these limitations through: (1) Implementation of multicenter, prospective trials with expanded cohorts and comprehensive perioperative data collection, particularly regarding pharmacological interventions; (2) Mechanistic studies investigating analgesic-mediated effects on prognosis, emphasizing molecular biological pathways; (3) Development of individualized perioperative analgesic protocols that optimize pain manage
In conclusion, this comprehensive analysis demonstrates that TNM staging, tumor differentiation, and duration of postoperative analgesic administration serve as independent prognostic indicators in patients undergoing radical esophagectomy for esophageal carcinoma. These findings emphasize the critical importance of judicious perioperative analgesic management and the optimization of comprehensive perioperative care protocols. The implementation of evidence-based strategies in perioperative management may significantly influence clinical outcomes in this patient population. Further investigation into these prognostic determinants may facilitate the development of more individualized therapeutic approaches and ultimately enhance patient survival outcomes.
| 1. | Sugase T, Kanemura T, Matsuura N, Ushimaru Y, Masuike Y, Yanagimoto Y, Mori R, Kitakaze M, Amisaki M, Kubo M, Mukai Y, Komatsu H, Sueda T, Kagawa Y, Nishimura J, Wada H, Yasui M, Omori T, Miyata H. Prognostic impact of dysphagia scores in patients with advanced resectable esophageal cancer who underwent radical esophagectomy after preoperative treatment. J Gastrointest Surg. 2024;28:1853-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68631] [Article Influence: 13726.2] [Reference Citation Analysis (201)] |
| 3. | Chen YS, Wang J, Ding LL, Zhang YH, Chen JG, Zhu J. [Epidemic characteristics of esophageal cancer mortality in Qidong, 1972-2016]. Zhonghua Zhong Liu Za Zhi. 2022;44:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | He Y, Liang D, Du L, Guo T, Liu Y, Sun X, Wang N, Zhang M, Wei K, Shan B, Chen W. Clinical characteristics and survival of 5283 esophageal cancer patients: A multicenter study from eighteen hospitals across six regions in China. Cancer Commun (Lond). 2020;40:531-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Deboever N, Jones CM, Yamashita K, Ajani JA, Hofstetter WL. Advances in diagnosis and management of cancer of the esophagus. BMJ. 2024;385:e074962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 6. | Mizobe R, Tarasawa K, Fushimi K, Fujimori K. Relationship between Hospital Volume and Short-term Postoperative Outcomes in Thoracoscopic Esophageal Cancer Surgery: A Study of Mortality and Postoperative Complications Using a Nationwide Database in Japan. Tohoku J Exp Med. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Bandidwattanawong C. Multi-disciplinary management of esophageal carcinoma: Current practices and future directions. Crit Rev Oncol Hematol. 2024;197:104315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G, Davies A, D'Journo XB, Gisbertz SS, Griffin SM, Hardwick R, Hoelscher A, Hofstetter W, Jobe B, Kitagawa Y, Law S, Mariette C, Maynard N, Morse CR, Nafteux P, Pera M, Pramesh CS, Puig S, Reynolds JV, Schroeder W, Smithers M, Wijnhoven BPL. Benchmarking Complications Associated with Esophagectomy. Ann Surg. 2019;269:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 600] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 9. | Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol. 2016;14:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, Chen YP, Zhang H. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol. 2013;8:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 11. | Serralheiro P, Rankin A, Clark A, Holyoake D, Cheong E. Pre- and postoperative prognostic factors for resectable esophageal adenocarcinoma. Surg Oncol. 2020;35:132-138. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Ahmadinejad M, Soltanian A, Maghsoudi LH. Risk factors and therapeutic measures for postoperative complications associated with esophagectomy. Ann Med Surg (Lond). 2020;55:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Oncology and Anesthesiology Group of Chinese Society of Anesthesiology; Society of Oncological Anesthesia and Analgesia, Chinese Anti-Cancer Association. [Expert consensus on anesthesia in Enhanced Recovery after Surgery for esophageal cancer surgery]. Zhonghua Yi Xue Za Zhi. 2024;104:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Jiang D, Wang H, Song Q, Wang H, Wang Q, Tan L, Hou Y. Comparison of the prognostic difference between ypTNM and equivalent pTNM stages in esophageal squamous cell carcinoma based on the 8th edition of AJCC classification. J Cancer. 2020;11:1808-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Qiu MJ, Yang SL, Wang MM, Li YN, Jiang X, Huang ZZ, Xiong ZF. Prognostic evaluation of esophageal cancer patients with stages I-III. Aging (Albany NY). 2020;12:14736-14753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Guo W, Zhou B, Dou L, Guo L, Li Y, Qin J, Wang Z, Huai Q, Xue X, Li Y, Ying J, Xue Q, Gao S, He J. Single-cell RNA sequencing and spatial transcriptomics of esophageal squamous cell carcinoma with lymph node metastases. Exp Mol Med. 2025;57:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Reticker-Flynn NE, Zhang W, Belk JA, Basto PA, Escalante NK, Pilarowski GOW, Bejnood A, Martins MM, Kenkel JA, Linde IL, Bagchi S, Yuan R, Chang S, Spitzer MH, Carmi Y, Cheng J, Tolentino LL, Choi O, Wu N, Kong CS, Gentles AJ, Sunwoo JB, Satpathy AT, Plevritis SK, Engleman EG. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell. 2022;185:1924-1942.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 249] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 18. | Cho JW, Choi SC, Jang JY, Shin SK, Choi KD, Lee JH, Kim SG, Sung JK, Jeon SW, Choi IJ, Kim GH, Jee SR, Lee WS, Jung HY; Korean ESD Study Group. Lymph Node Metastases in Esophageal Carcinoma: An Endoscopist's View. Clin Endosc. 2014;47:523-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | França GS, Baron M, King BR, Bossowski JP, Bjornberg A, Pour M, Rao A, Patel AS, Misirlioglu S, Barkley D, Tang KH, Dolgalev I, Liberman DA, Avital G, Kuperwaser F, Chiodin M, Levine DA, Papagiannakopoulos T, Marusyk A, Lionnet T, Yanai I. Cellular adaptation to cancer therapy along a resistance continuum. Nature. 2024;631:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 20. | Shi ZD, Pang K, Wu ZX, Dong Y, Hao L, Qin JX, Wang W, Chen ZS, Han CH. Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies. Signal Transduct Target Ther. 2023;8:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 151] [Reference Citation Analysis (0)] |
| 21. | Ma Y, Wu H, Wei X, Yang Y, Xu Z, Chen Y. Comparison of different pain management strategies during the perioperative period of esophageal squamous cell carcinoma: a retrospective cohort study. Perioper Med (Lond). 2025;14:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Fu G, Xu L, Chen H, Lin J. State-of-the-art anesthesia practices: a comprehensive review on optimizing patient safety and recovery. BMC Surg. 2025;25:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Zhou D, Gu J, Qu M, Guo K, Chen W, Miao C. Targeting the mu-Opioid Receptor for Cancer Treatment. Curr Oncol Rep. 2021;23:111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Sun Q, Li Z, Wang Z, Wang Q, Qin F, Pan H, Lin W, Mu X, Wang Y, Jiang Y, Ji J, Lu Z. Immunosuppression by opioids: Mechanisms of action on innate and adaptive immunity. Biochem Pharmacol. 2023;209:115417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Zhao-Fleming H, Hand A, Zhang K, Polak R, Northcut A, Jacob D, Dissanaike S, Rumbaugh KP. Effect of non-steroidal anti-inflammatory drugs on post-surgical complications against the backdrop of the opioid crisis. Burns Trauma. 2018;6:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Arron MNN, Lier EJ, de Wilt JHW, Stommel MWJ, van Goor H, Ten Broek RPG. Postoperative administration of non-steroidal anti-inflammatory drugs in colorectal cancer surgery does not increase anastomotic leak rate; A systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:2167-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS, Gan TJ, Kennedy RH, Ljungqvist O, Lobo DN, Miller T, Radtke FF, Ruiz Garces T, Schricker T, Scott MJ, Thacker JK, Ytrebø LM, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/