Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.102174
Revised: December 17, 2024
Accepted: January 9, 2025
Published online: March 27, 2025
Processing time: 136 Days and 18.5 Hours
Peutz-Jeghers (PJ) syndrome (PJS) is a rare autosomal dominant genetic disease characterized by the association of intestinal polyposis, mucosal skin pigmen
A 62-year-old male presented with intermittent left lower abdominal pain after drinking or consuming cold beverages that was accompanied by occasional hematochezia. Abdominal contrast-enhanced computed tomography indicated an isolated sigmoid colon grape-like lesion. Subsequently, the patient underwent la
The age of onset and lesion location of this case are different from those of typical or isolated PJS patients.
Core Tip: Peutz-Jeghers syndrome (PJS) is a rare autosomal dominant hereditary illness defined by the connection of intestinal polyposis, mucosal skin pigmentation, and cancer risk. Due to the older age, the clinical features of mucosal hyperpigmentation were atypia in this case study. Due to the location of the polyp in the sigmoid colon, the common in
- Citation: Tian ZS, Ma XP, Ruan HX, Yang Y, Zhao YL. Rare large sigmoid hamartomatous polyp in an elderly patient with atypical Peutz-Jeghers syndrome: A case report. World J Gastrointest Surg 2025; 17(3): 102174
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/102174.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.102174
Peutz-Jeghers (PJ) syndrome (PJS) is a rare autosomal dominant genetic disorder that is primarily manifested by three characteristics, including pigmentation in the mucous membranes of the lips, fingers, toes and perineum, multiple polyps in the gastrointestinal tract, and a familial hereditary tendency. The incidence rate is 1/50000 to 1/200000[1]. The diagnostic criteria for PJS are as follows: (1) Three or more PJ polyps confirmed by histology; (2) Individuals with a family history of PJS developing any number of PJ polyps; (3) Individuals with a family history of PJS presenting with mucocutaneous pigmentation; and (4) Any number of PJ polyps accompanied by mucocutaneous pigmentation (diagnosis of this disease can be made if any of the above criteria are met). The hamartomatous polyps of PJS most commonly occur in the small intestine (in order of prevalence: Jejunum, ileum, and duodenum) followed by the stomach and are relatively rare in the large intestine[2]. The size of PJS-related gastrointestinal polyps varies, ranging from a few millimeters to several centimeters. The polyps can be sessile or pedunculated and often occur in adolescents and during early adulthood, but they are relatively rare in elderly patients[3,4]. Xu et al[5] analyzed 566 patients with PJS, where male patients accounted for 55.3%, female patients accounted for 44.7%, the median age of skin and mucosal pigmentation was 2 years, and the median time interval between the age of skin pigmentation and the onset of gastrointestinal symptoms was 10 years. Matsubara et al[6] investigated 701 Japanese patients with PJS and juvenile polyposis syndrome (JPS) and determined that males accounted for 53.5% and 59.6% of the PJS and JPS groups, respectively. The mean age at diagnosis of PJS is 23 years in men and 26 years in women, the incidence of PJS is comparable in men and women, and there is no ethnic tendency for PJS[7].
The typical clinical manifestations of PJS are gastrointestinal symptoms such as abdominal pain, diarrhea, bloody stool, constipation, and others. Gastric and intestinal polyps can also cause anemia, intestinal obstruction, and intestinal volvulus, among other complications[5]. Approximately half of the patients experience bleeding, obstruction, and abdominal pain around the age of 20[8]. Generally, the gastrointestinal symptoms of PJS are primarily related to small intestinal polyps, while the impact of colon and rectal polyps on the gastrointestinal tract is minimal. This results in patients with PJS often experiencing lesions in the colon and rectum that are difficult to detect, leading to a later age of diagnosis.
Previous case reports of PJS predominantly focused on children and adolescents, and cases of PJS in older patients were rarely reported. Additionally, with the increase in age, melanin pigmentation on the face and palms of older patients with PJS gradually fades, particularly when PJS polyps are located in atypical sites or are solitary polyps, and the pigmentation becomes even less obvious[9]. As the faces of the elderly are often accompanied by seborrheic keratosis, clinical skin physical examinations are prone to being overlooked. At such times, it is even more necessary for clinical practitioners to pay close attention to the pigmentation in concealed areas such as the oral mucosa and perineum of patients.
This report is of a 62-year-old male who presented with left lower abdominal pain and hematochezia following alcohol or cold beverage consumption that was attributed to a giant hamartomatous polyp in the sigmoid colon. The patient underwent laparoscopic resection of a segment of the sigmoid colon that was pathologically diagnosed as PJ-like polyp. Physical examination revealed melanin deposition in the lower lip, oral buccal mucosa, fingers, and perineal area, leading to a diagnosis of PJS. The patient experienced satisfactory recovery and improvement postoperatively. Notably, this case exhibited an older age at onset, unique disease localization, and rare occurrence of an isolated giant hamartoma polyp.
A 62-year-old man presented at the Anus and Intestine Surgery Department of our hospital with a one-week history of intermittent dull pain in the left lower abdomen accompanied by bloody stools.
The patient began experiencing intermittent dull pain in the left lower abdomen with bloody stools one week earlier after consuming alcohol and cold drinks. There were no symptoms such as fever, defecation rhythm, tenesmus, anal heaviness, emaciation, fatigue, nausea, and vomiting. Although the patient had previously noticed pain in the left lower abdomen after consuming alcohol or cold drinks, this time it was noticeably worse. The pain could resolve spontaneously in previous episodes, but this time it lasted for a week and was associated with bloody stools.
The patient exhibited a history of hypertension for 20 years. The patient took irbesartan and hydrochlorothiazide regularly, and his blood pressure was well controlled. There was no history of diabetes mellitus or coronary heart disease.
The patient exhibited a 40-year history of smoking and drinking alcohol. There was no history of allergy or family history. No one in the family experienced similar symptoms and diseases.
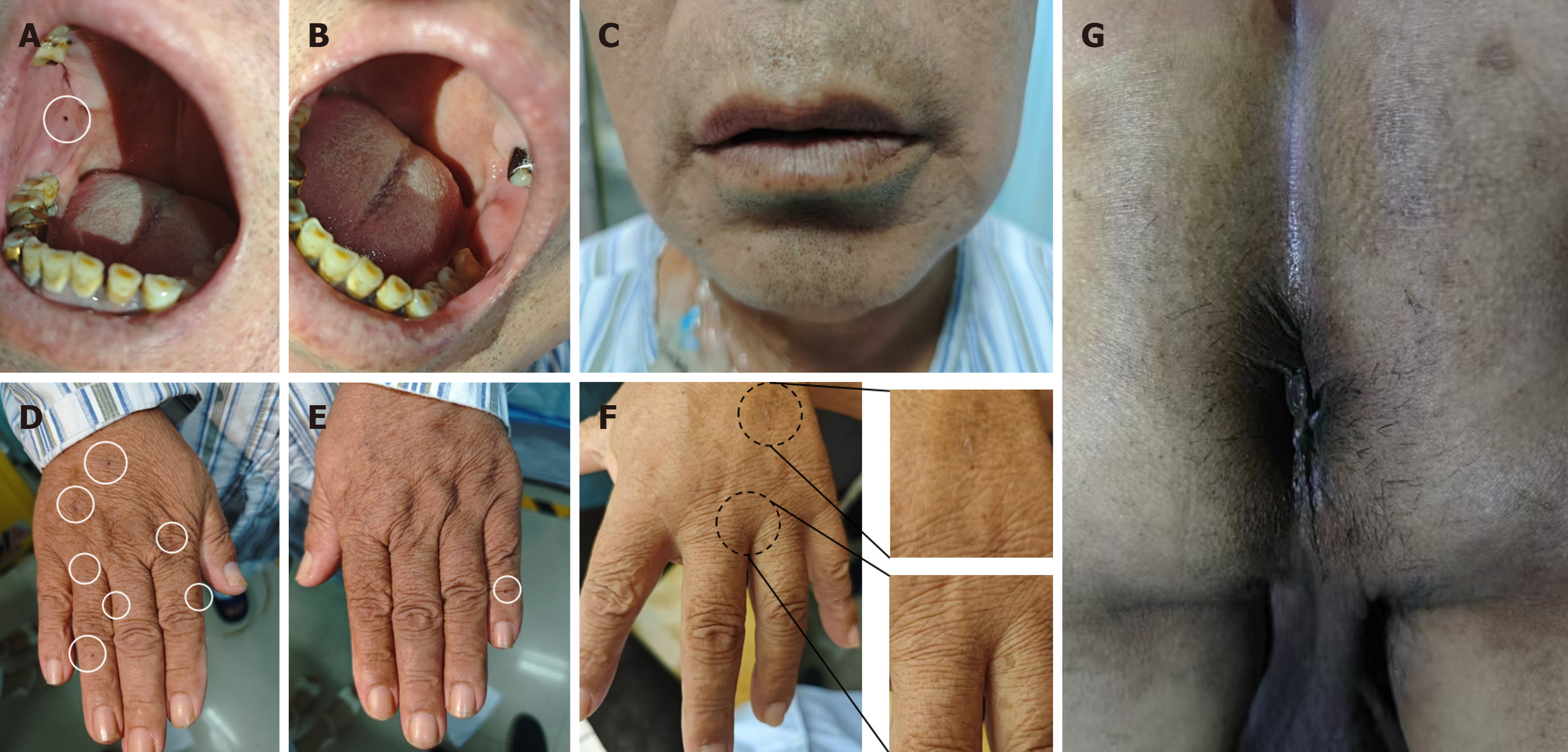
The patient's temperature was 36.5 °C, pulse was 58 beats per minute, respiration was 17 breaths per minute, and blood pressure was 153/104 mmHg. Upon examination, the patient's vital signs remained stable. Dark brown spots were visible on the lower lip and back of the hand; however, these pigmentation spots appeared lighter compared to those typically observed in previous patients. An oral mucosa examination revealed a melanotic spot on the right buccal mucosa. Prominent pigmentation of the perineal skin was noted (Figure 1). Abdominal examination did not reveal any significant abnormal signs. Digital rectal examination was performed without detecting any masses or bleeding.
All of the patient’s laboratory examinations yielded normal results.
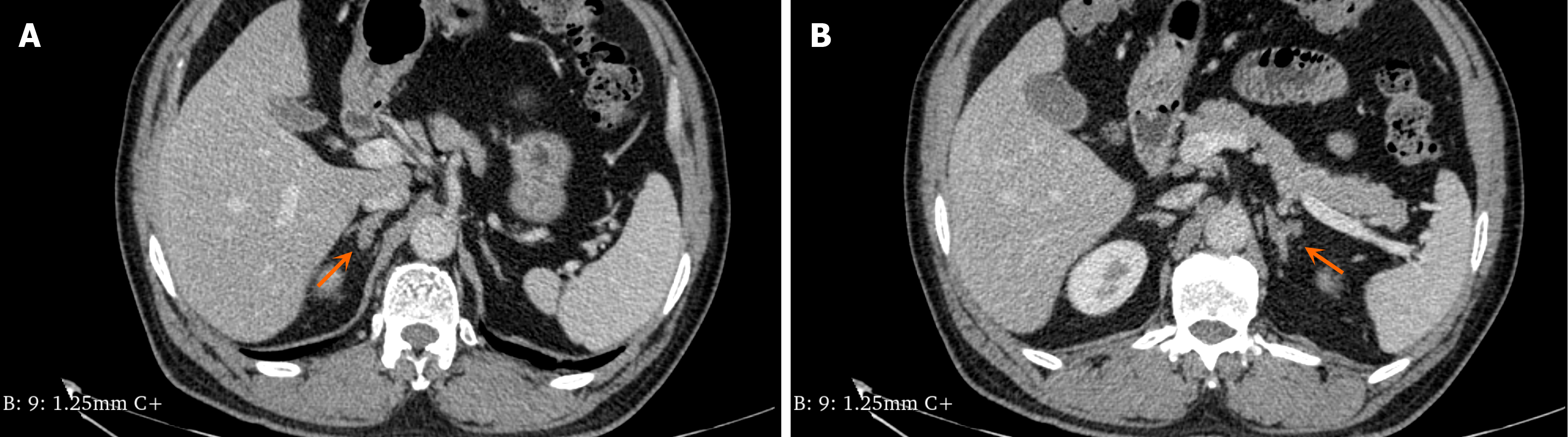
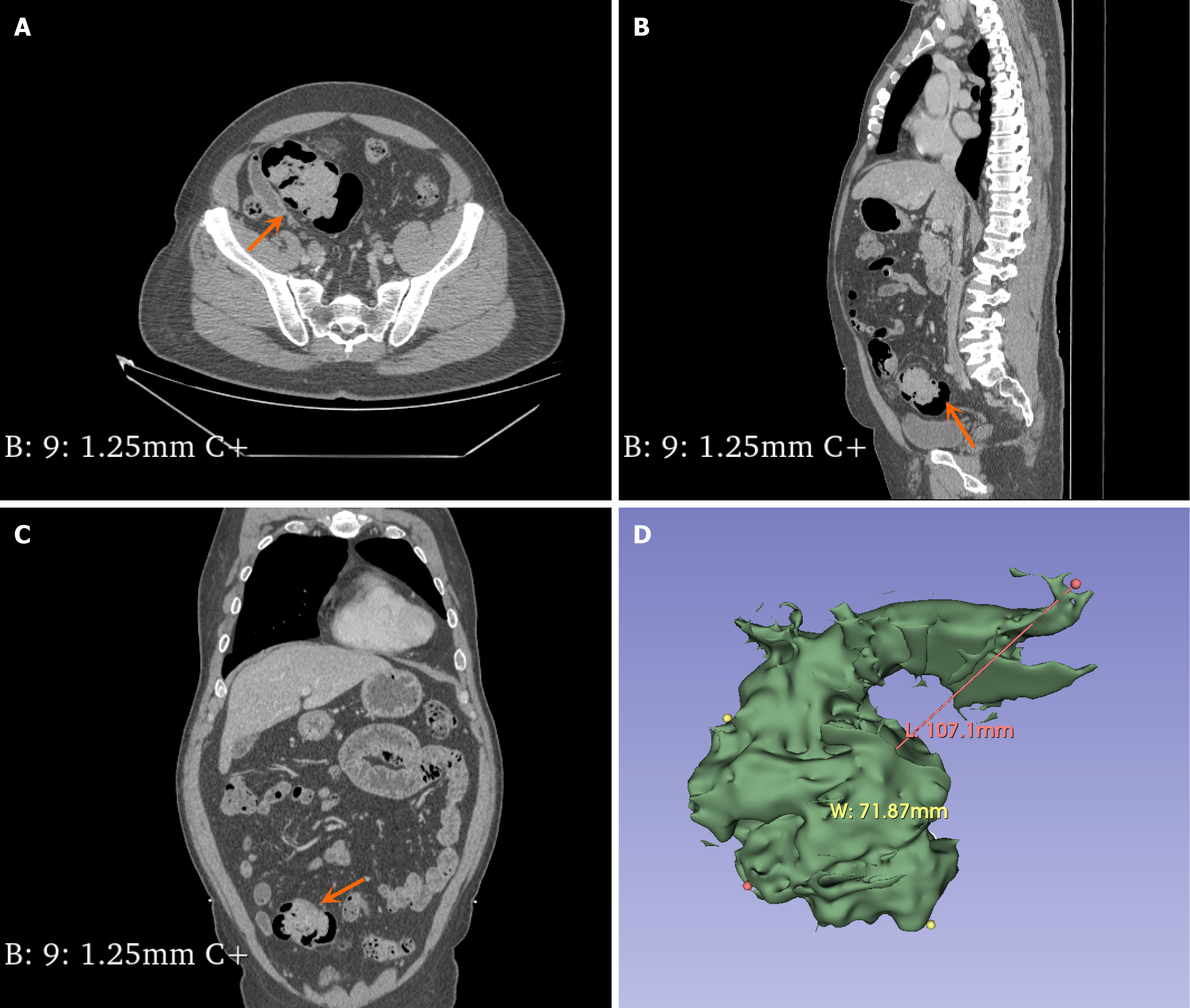
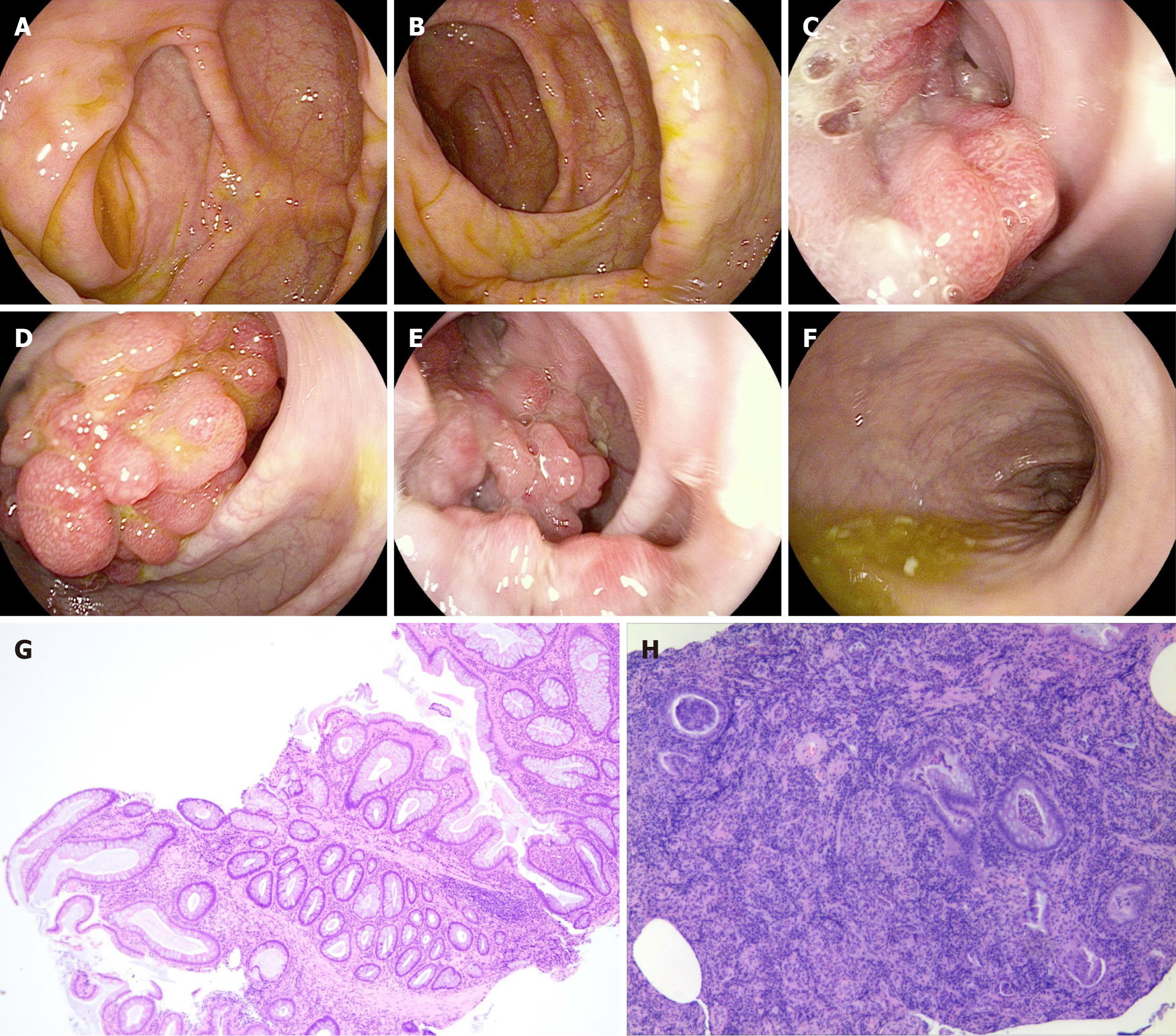
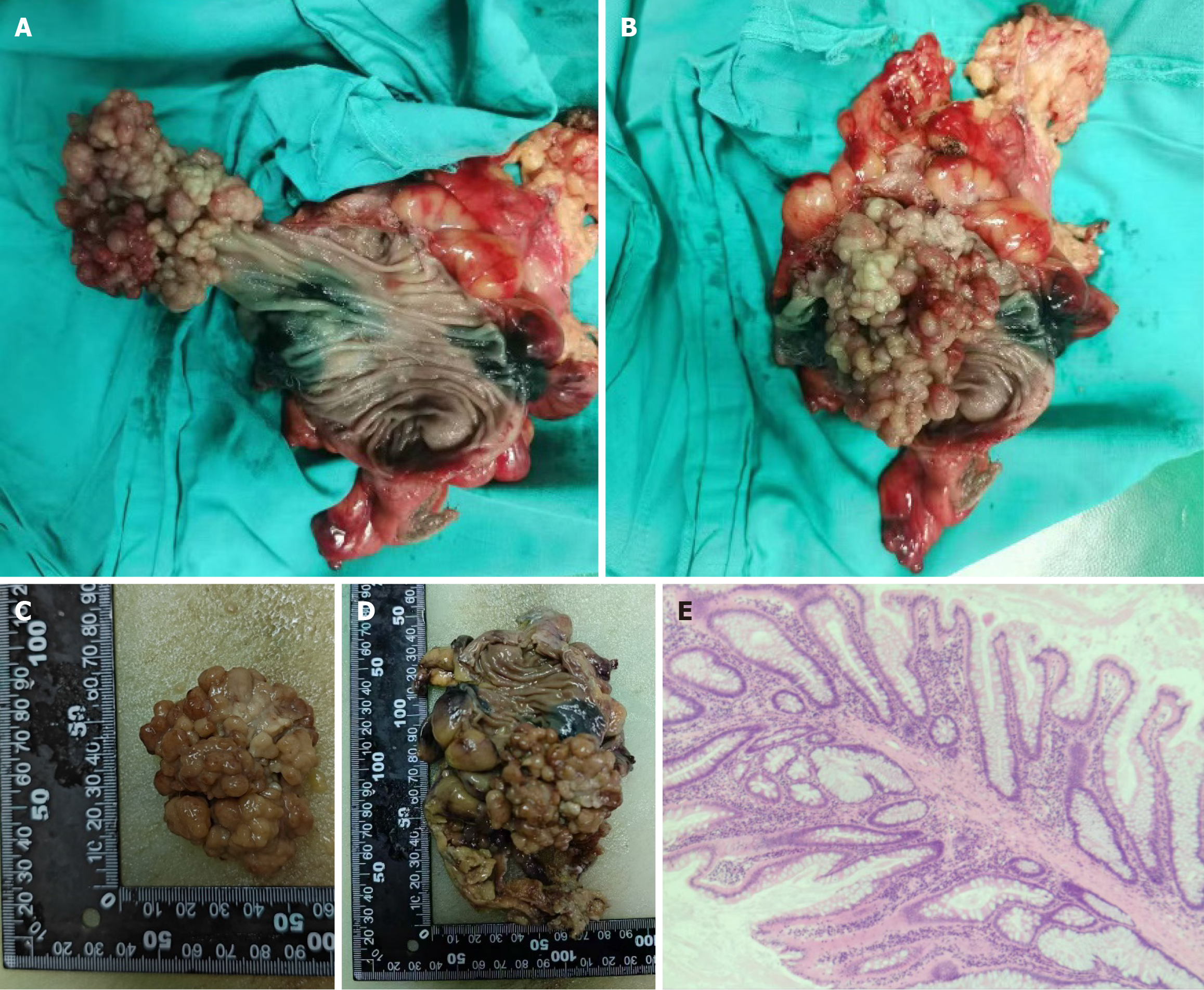
The abdominal contrast-enhanced computed tomography examination of the patient revealed bilateral adrenal gland thickening with an uneven pattern, accompanied by a nodular shadow. The left nodule was relatively large, measuring approximately 0.7 cm in size, and it exhibited significant enhancement on the contrast-enhanced scan (Figure 2). Additionally, there was an irregular soft tissue mass in the sigmoid colon measuring approximately 10.71 cm × 7.187 cm with a computed tomography (CT) value of approximately 46 HU (Figure 3). Colonoscopy demonstrated multiple grape-like masses located in the colon at a distance of 30-35 cm from the anal verge. The mucosa of the ascending colon, transverse colon, descending colon, sigmoid colon, and rectum appeared smooth with clear vascular texture, and no ulcers or vegetations were observed. Enteroscopy was not performed in this case. An endoscopic biopsy was conducted on the lesion tissue present in the sigmoid colon that indicated hamartomatous polyps and mucosal prodromal polyps (Figure 4). Fecal occult blood testing yielded positive results, while preoperative blood routine analysis indicated normal values. Plasma free cortisol levels and rhythm tests did not exhibit any abnormal changes.
Based on postoperative pathological examination, colonoscopic biopsy, and skin pigmentation, the patient was finally diagnosed with PJS.
Laparoscopic partial resection of the sigmoid colon was performed. The resected bowel measured approximately 15 cm in length. The lesion was 3 cm from one stump and 6 cm from the other. The polyp size was approximately 6 cm × 5 cm × 5 cm (Figure 5).
Hematoxylin-eosin staining of the lesion samples revealed dendritic extension of smooth muscle from the mucosal muscle layer to the epithelial cells, with normal mucosal epithelium covering its surface. Local growth of the lesion exhibited active progression without abnormal hyperplasia, and there were no notable changes observed in both ends of adjacent bowel or surrounding lymph nodes (Figure 5). The pathological diagnosis confirmed PJ polyp, and this com
The definition of PJS is an autosomal dominant disorder, also known as familial hamartomatous polyposis syndrome[10]. It is characterized by the presence of hamartomatous polyps in the gastrointestinal tract, particularly in the small intestine, along with pigmented mucocutaneous lesions[11,12]. Hamartomatous polyps are characterized by bundles of smooth muscle that extend dendritically into the lamina propria and are overlaid with nearly normal epithelium. The surface epithelium of hamartomatous polyps was identical to the adjacent normal mucosal epithelium. Mucocutaneous pigmentation typically occurs in infancy and resolves later in life, typically in late adolescence[13]. These lesions are due to the presence of pigment-rich macrophages in the dermis, dark brown or blue-brown color, and sizes ranging from 1 mm to 5 mm.
PJS is an autosomal dominant inherited disease caused by the mutation of the tumor suppressor gene STK11/LKB1, and this disease is characterized by multiple polyps in the gastrointestinal tract, skin and mucosal pigmentation, and susceptibility to malignant tumors[14,15]. Li et al[16] reported that compared to normal gastrointestinal mucosa, the promoter methylation level of the LKB1 gene in PJS polyps was typically increased, but the methylation pattern of different PJS polyps was also different. The study observed that hypomethylation in PJS patients was closely related to gastrointestinal malignant tumors. The change in methylation level may be the result of the influence of diet, the environment, and other factors. Due to family economic difficulties, chromosome and family tree gene analyses were not performed. The patient indicated that no other family members experienced symptoms, and we recommended that his children undergo regular gastroenteroscopy.
In general, PSJ often occurs in young children, and the increase and enlargement of gastrointestinal polyps can cause various complications such as intussusception, intestinal obstruction, gastrointestinal bleeding, cancerization, and malnutrition. In addition to the typical skin mucosa and multiple polyps of the gastrointestinal tract, some patients can be characterized as only melanin with no gastrointestinal polyps or as only gastrointestinal polyps with no melanin, and this is called incomplete PJS and is an incomplete explicit genetic performance that may be related to environmental factors[17,18]. In this case, the affected area is the sigmoid colon, and it exists as a solitary giant hamartoma that is very rare in PJS. Due to anatomical reasons, intussusception in adults is less common than it is in children, and intussusception in the rectum and colon is less common than that in the proximal intestine[19]. The patient's age and the unusual position of the PJ polyps led to late onset of symptoms, with the predominant symptoms being hematochezia and intestinal spastic abdominal pain caused by alcohol or cold drinks. Although the pigmentation of the lower lip, palm, and fingers had faded with age, the pigmentation of the oral mucosa and perineal region remained visible. Hypopigmentation in elderly patients poses a considerable challenge for our diagnosis of PJS. Hence, we need to collect and follow up on a large-scale PJS patients in the following steps and systematically categorize their characteristics based on different age groups. Additionally, seborrheic keratosis is often present on the faces of elderly individuals. To discriminate this from the depigmented macules of PJS, clinicians need to carefully observe the skin conditions of the limbs, oral mucosa, and perineum to avoid misdiagnosis of PJS.
Additionally, it is interesting that the abdominal CT of this patient revealed nodules in the adrenal gland. Combined with the pigmentation of the patient, we considered whether the patient’s pigmentation was caused by adrenal nodules and adrenal insufficiency[20]. Subsequently, we performed adrenocortical hormone measurements that indicated normal adrenocortical hormone secretion, and this also ultimately supported the diagnosis of PSJ. This case prompts our thinking and clinical differential diagnosis of skin hyperpigmentation related diseases. Skin pigmentation is a prominent feature of primary adrenal insufficiency such as Addison's disease[21]. This pigmentation caused by Addison's disease is observed primarily on sun-exposed areas, scars, armpits, nipples, palm folds, pressure points, and mucous membranes (including the cheeks, vagina, vulva, and anus)[22]. The cause of this hyperpigmentation is generally believed to be due to increased stimulation of the melanocyte-stimulating hormone receptor (MC1R) by adrenocorticotropic hormone (ACTH) itself[23]. Pigmentation in PJS occurs primarily in the lower lip, mouth, limbs, and perineum, it appears at birth and in early childhood, and it increases during adolescence. They may become inconspicuous in adulthood but are typically still present on the buccal mucosa without abnormal hormonal changes.
Patients with PJS exhibit a higher risk of malignancies at different sites, including gastrointestinal and extra-gastroin
In conclusion, the age, location, predisposing factors, and differential diagnosis of this case can provide important reference value for clinical practice. The report of this case will enrich our understanding of the view that PJS patients of different ages exhibit different signs. Concurrently, for elderly patients with abdominal pain and hematochesia, we should pay more attention to some local signs that may provide us with unexpected ideas for the diagnosis of related diseases.
| 1. | Wang R, Qi X, Liu X, Guo X. Peutz-Jeghers syndrome: Four cases in one family. Intractable Rare Dis Res. 2016;5:42-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Jelsig AM, Qvist N, Brusgaard K, Nielsen CB, Hansen TP, Ousager LB. Hamartomatous polyposis syndromes: a review. Orphanet J Rare Dis. 2014;9:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Aslan PG, Çağlayan AO, Bora E, Koç A, Yücel H, Ülgenalp A, Öztürk Y, Şeker G, Akarsu M. Clinical and Molecular Analysis in Patients with Peutz-Jeghers Syndrome. Turk J Gastroenterol. 2024;35:374-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Kim SH, Kim ER, Park JJ, Kim ES, Goong HJ, Kim KO, Nam JH, Park Y, Lee SP, Jang HJ; KSGE; Research Group of Capsule Endoscopy and Artificial Intelligence in Imaging. Cancer risk in patients with Peutz-Jeghers syndrome in Korea: a retrospective multi-center study. Korean J Intern Med. 2023;38:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | Xu ZX, Jiang LX, Chen YR, Zhang YH, Zhang Z, Yu PF, Dong ZW, Yang HR, Gu GL. Clinical features, diagnosis, and treatment of Peutz-Jeghers syndrome: Experience with 566 Chinese cases. World J Gastroenterol. 2023;29:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (3)] |
| 6. | Matsubara Y, Nakamura Y, Nakayama Y, Yano T, Ishikawa H, Kumagai H, Umeno J, Uchida K, Jimbo K, Yamamoto T, Ishida H, Suzuki O, Okamoto K, Kakuta F, Koike Y, Kawasaki Y, Sakamoto H. Prevalence and Incidence of Peutz-Jeghers Syndrome and Juvenile Polyposis Syndrome in Japan: A Nationwide Epidemiological Survey in 2022. J Gastroenterol Hepatol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol. 2006;4:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Latchford AR, Neale K, Phillips RK, Clark SK. Peutz-Jeghers syndrome: intriguing suggestion of gastrointestinal cancer prevention from surveillance. Dis Colon Rectum. 2011;54:1547-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Oncel M, Remzi FH, Church JM, Goldblum JR, Zutshi M, Fazio VW. Course and follow-up of solitary Peutz-Jeghers polyps: a case series. Int J Colorectal Dis. 2003;18:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Duarte M, Milikowski C. Gastrointestinal polyposis with associated cutaneous manifestations. Pathology. 2022;54:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Yamamoto H, Sakamoto H, Kumagai H, Abe T, Ishiguro S, Uchida K, Kawasaki Y, Saida Y, Sano Y, Takeuchi Y, Tajika M, Nakajima T, Banno K, Funasaka Y, Hori S, Yamaguchi T, Yoshida T, Ishikawa H, Iwama T, Okazaki Y, Saito Y, Matsuura N, Mutoh M, Tomita N, Akiyama T, Yamamoto T, Ishida H, Nakayama Y. Clinical Guidelines for Diagnosis and Management of Peutz-Jeghers Syndrome in Children and Adults. Digestion. 2023;104:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Korsse SE, Dewint P, Kuipers EJ, van Leerdam ME. Small bowel endoscopy and Peutz-Jeghers syndrome. Best Pract Res Clin Gastroenterol. 2012;26:263-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Xu Z, Gu G. Cancer Risk of Peutz-Jeghers Syndrome and Treatment Experience: A Chinese Medical Center. Clin Colon Rectal Surg. 2023;36:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Tacheci I, Kopacova M, Bures J. Peutz-Jeghers syndrome. Curr Opin Gastroenterol. 2021;37:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Duan SX, Wang GH, Zhong J, Ou WH, Fu MX, Wang FS, Ma SH, Li JH. Peutz-Jeghers syndrome with intermittent upper intestinal obstruction: A case report and review of the literature. Medicine (Baltimore). 2017;96:e6538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Li T, Lin W, Zhao Y, Zhu J, Sun T, Ren L. Distinct promoter methylation patterns of LKB1 in the hamartomatous polyps of Peutz-Jeghers syndrome and its potential in gastrointestinal malignancy prediction. Orphanet J Rare Dis. 2020;15:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Khsiba A, Bradai S, Nakhli A, Chelbi E, Mahmoudi M, Mohamed AB, Medhioub M, Hamzaoui L, Azouz MM. Solitary gastric Peutz-Jeghers polyp: a case report. Pan Afr Med J. 2022;41:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Zou BC, Wang FF, Zhao G, Lu XL, Zhang L, Zhao P, Shi HT, Qin B, Guo XD, Zhang J. A giant and extensive solitary Peutz-Jeghers-type polyp in the antrum of stomach: Case report. Medicine (Baltimore). 2017;96:e8466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Ganesan S, Xavier J. A rare case of sigmoid intussusception. A case report. Int J Surg Case Rep. 2023;110:108705. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Stratakis CA. Carney complex: A familial lentiginosis predisposing to a variety of tumors. Rev Endocr Metab Disord. 2016;17:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Sandru F, Petca A, Dumitrascu MC, Petca RC, Carsote M. Peutz-Jeghers syndrome: Skin manifestations and endocrine anomalies (Review). Exp Ther Med. 2021;22:1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Thawabteh AM, Jibreen A, Karaman D, Thawabteh A, Karaman R. Skin Pigmentation Types, Causes and Treatment-A Review. Molecules. 2023;28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 23. | Fernandez-Flores A, Cassarino DS. Histopathologic Findings of Cutaneous Hyperpigmentation in Addison Disease and Immunostain of the Melanocytic Population. Am J Dermatopathol. 2017;39:924-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Wang Z, Wang Z, Wang Y, Wu J, Yu Z, Chen C, Chen J, Wu B, Chen Y. High risk and early onset of cancer in Chinese patients with Peutz-Jeghers syndrome. Front Oncol. 2022;12:900516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/