Published online Mar 27, 2025. doi: 10.4240/wjgs.v17.i3.101940
Revised: November 25, 2024
Accepted: January 6, 2025
Published online: March 27, 2025
Processing time: 145 Days and 4.3 Hours
The cellular prion protein (PrPC), traditionally associated with neurodegenerative disorders, plays an important role in cancer progression and metastasis by in
To investigate the influence of PrPC expression in cholangiocarcinoma (CCA) on patient outcomes following surgical resection.
Patients who underwent curative surgical resection for either intrahepatic or hilar CCA were enrolled in this retrospective study. Based on the immunohistochemical staining results of the surgical specimens, patients were categorized into two groups: The low PrPC group (negative or 1+) and the high PrPC group (2+ or 3+). Survival analyses, including overall survival and recurrence-free survival, were conducted using the Kaplan-Meier method and compared using the log-rank test.
In total, seventy-six patients diagnosed with CCA (39 with intrahepatic and 37 with hilar CCA) underwent curative hepatectomy from January 2011 to November 2021. Among these patients, 38 (50%) demonstrated high PrPC expression, whereas the remaining 38 (50%) showed low expression of PrPC. During a median follow-up period of 31.2 months (range: 1 to 137 months), the high PrPC group had a significantly shorter median overall survival than the low PrPC group (40.4 months vs 137.9 months, respectively; P = 0.041). Moreover, the high PrPC group had a significantly shorter median recurrence-free survival than the low PrPC group (13.3 months vs 23.8 months, respectively; P = 0.026).
PrPC expression is significantly associated with early recurrence and decreased survival period in CCA patients following surgical resection. Thus, PrPC may be used as a prognostic factor in treatment planning.
Core Tip: High cellular prion protein (PrPC) expression is significantly associated with early recurrence and decreased survival period in cholangiocarcinoma patients following surgical resection. PrPC expression serves as an independent prognostic factor for overall survival and recurrence-free survival. These findings suggest that PrPC could be a valuable prognostic biomarker following curative surgery.
- Citation: Shin DW, Cho YA, Moon SH, Kim TH, Park JW, Lee JW, Choe JY, Kim MJ, Kim SE. High cellular prion protein expression in cholangiocarcinoma: A marker for early postoperative recurrence and unfavorable prognosis. World J Gastrointest Surg 2025; 17(3): 101940
- URL: https://www.wjgnet.com/1948-9366/full/v17/i3/101940.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i3.101940
Cholangiocarcinoma (CCA) arises from the epithelial cells lining the bile duct and ranks as the second most common primary liver cancer, following hepatocellular carcinoma[1]. CCA is anatomically classified into three subtypes: Intrahepatic, hilar, and distal CCA[2,3]. The global incidence of CCA is increasing, especially intrahepatic CCA, which is growing more rapidly than extrahepatic CCA[4-6]. Surgical resection remains the only curative treatment for CCA; however, only 35% of patients are considered suitable for curative resection at the time of diagnosis[7,8]. CCA has a dismal prognosis, with a five-year survival rate of less than 20%[6,9]. Even with standard chemotherapy (gemcitabine plus cisplatin), the median survival period for patients with advanced, unresectable CCA does not exceed 12 months[10]. Molecular genetic profiling of tumors and targeted therapies are emerging as important areas of research[11,12]. Despite these ongoing research efforts, the development of personalized chemotherapeutic agents is urgently needed, and the identification of novel prognostic biomarkers plays a critical role in next-generation CCA treatment.
The prion protein is a cell surface glycoprotein predominantly expressed in the central and peripheral nervous systems[13]. Prion proteins have two isoforms: The cellular prion protein (PrPC) and the scrapie prion protein (PrPSC)[14]. Misfolding of PrPC into PrPSC can lead to fatal neurodegenerative disorders[15]. PrPC plays an important role in the nervous and immune systems, influencing cell proliferation, differentiation, survival, and programmed cell death[16,17]. PrPC is emerging as a potential target for chemotherapy, given its implicated involvement in tumor growth, metastasis, resistance to chemotherapy-induced cell death, and overall cancer progression across different cancer types[18,19]. However, the role of PrPC expression in CCA remains unknown. In this study, we aim to investigate two questions: (1) The expression of PrPC in CCA; and (2) The effects of PrPC on long-term prognosis such as recurrence and survival following surgical resection.
This retrospective study enrolled patients who underwent curative surgical resection for intrahepatic and hilar CCA at Hallym University Sacred Heart Hospital (Anyang, Republic of Korea) between January 2011 and November 2021. The inclusion criteria were as follows: (1) Adults aged 18 years or older; (2) Those who underwent radical resection for CCA; and (3) Histologically confirmed adenocarcinoma in surgical specimens. Exclusion criteria were as follows: (1) A history of being diagnosed with cancer other than primary CCA; (2) The presence of malignant ascites, peritoneal metastasis, or distant metastasis at diagnosis; (3) Prior neoadjuvant chemotherapy or chemoradiation therapy; (4) Uncontrolled infections, diabetes mellitus, hypertension, ischemic heart disease, or myocardial infarction within the last 6 months; and (5) Neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s disease.
After surgery, patients were monitored every three months for the first year and then every six months thereafter. Postoperative tumor recurrence evaluation included imaging tests, such as computed tomography or magnetic resonance imaging, and blood tests, including serum carbohydrate antigen 19-9 (CA19-9). If tumor recurrence was detected, appropriate treatment was administered. Data on demographics, radiologic and pathologic characteristics (tumor size, location, number of metastatic lymph nodes, resection margin, differentiation), blood tests (CA19-9 and carcinoembryonic antigen), along with survival and recurrence information, were retrospectively collected from electronic medical records. Tumor stages were assessed according to the American Joint Committee on Cancer staging system, eighth edition[20].
Tissue microarrays (TMAs) utilized a 2 mm tissue core from the designated region, which was subsequently re-embedded. Each sample was meticulously arranged in duplicate to minimize tissue loss. These TMA blocks were subsequently sliced into 4 μm thick sections, which were used for immunohistochemical staining. Prior to staining, TMA sections underwent deparaffinization with xylene and hydration through a graded ethanol series. Following established protocols, they were treated with a peroxidase-blocking solution (Dako, Copenhagen, Denmark) for 10 minutes to inhibit endogenous peroxidase activity. After blocking with a protein solution (Dako) for 60 minutes at room temperature, the sections were incubated with a diluted rabbit polyclonal antibody against PrPC (8H4; Sigma-Aldrich, St. Louis, MO, United States). Detection was carried out following the primary antibody incubation, using the Dako EnVision Detection System kit (Dako), in accordance with the manufacturer’s guidelines.
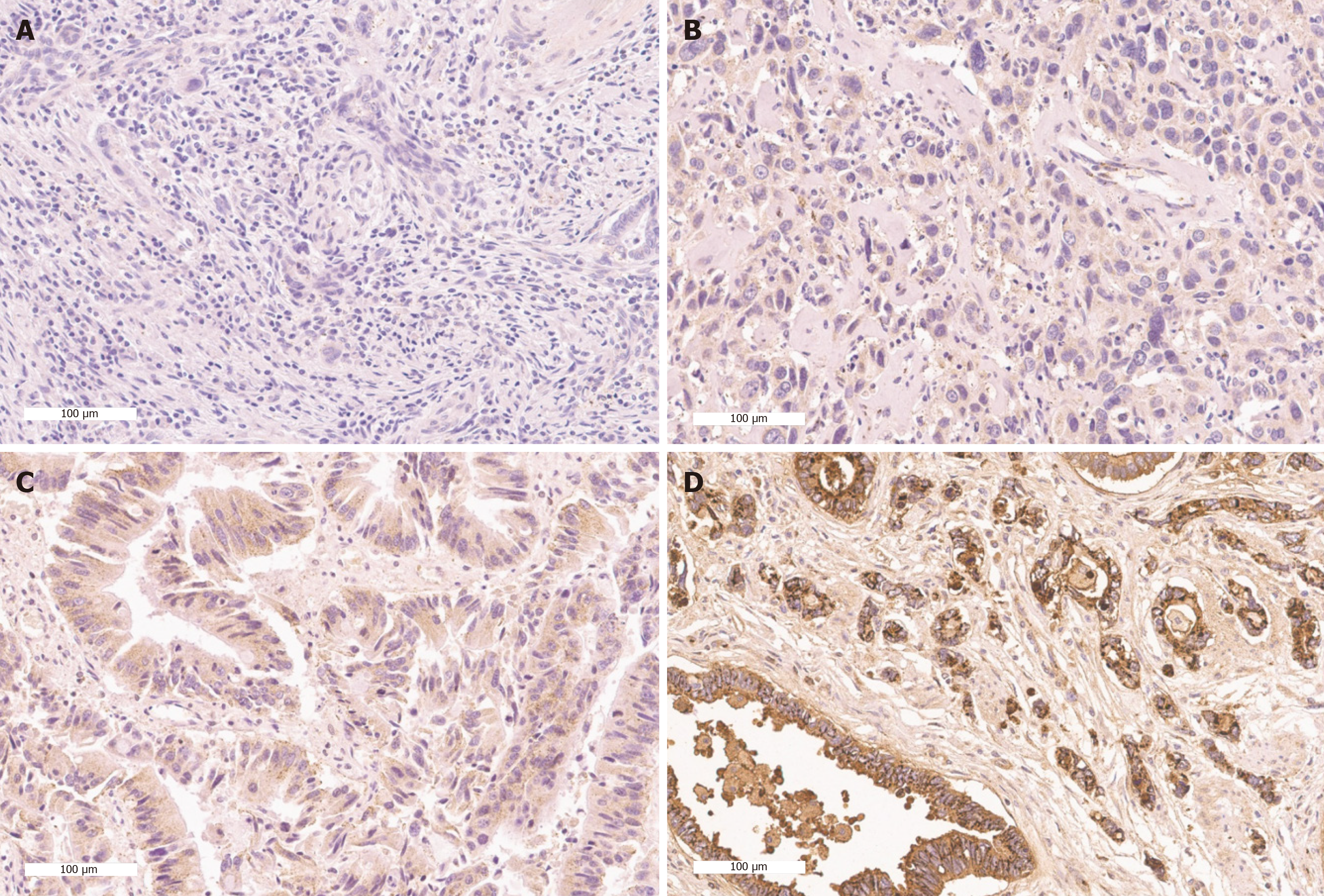
The TMA slides were analyzed to determine the proportion of tumor cells with positive PrPC staining in their nuclei. An expert pancreaticobiliary pathologist (Cho YA) without any knowledge of the patients’ personal or clinical data evaluated the PrPC stainings. Immunoreactivity was assessed based on the histochemical scoring (H-score) system. The H-score was calculated by a semi-quantitative assessment of both the intensity of staining (graded as: 0, no evidence of staining; 1+, weak staining; 2+, moderate staining; or 3+, strong staining, using adjacent normal mucosa as the median) and the percentage of positive cells. Expression levels of each component were classified into low PrPC or high PrPC groups based on the median value of the H-score (Figure 1).
Overall survival (OS) and recurrence-free survival (RFS) were compared between the two groups. OS was calculated from the date of surgery to either the date of death or the last follow-up. RFS was defined as the time from the date of surgery to recurrence or death from any cause.
Continuous variables are presented as means ± SD, whereas categorical variables are presented as frequencies and proportions. Categorical data were analyzed using the χ2 test or Fisher’s exact test, as appropriate. Continuous data were analyzed using Student’s t-test. Survival analyses for both RFS and OS were conducted using Kaplan-Meier curves, and differences in survival were tested using the log-rank test. Multivariate analyses for recurrence and survival were conducted using the Cox proportional hazards regression model. Variables with a P value < 0.1 in univariate analysis were included in the multivariate Cox regression model. To ensure the stability of the model, the ratio of events to independent variables was maintained at a minimum of 10 events per variable. Statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Chicago, IL, United States) and R version 3.5.3 (The R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value of less than 0.05 was considered statistically significant.
Table 1 summarizes the demographic and clinical characteristics of the patients. The study included seventy-six patients who underwent surgical resection for CCA. Among these patients, 38 (50%) demonstrated high PrPC expression, whereas the other 38 (50%) showed low expression of PrPC. The median age was 66.7 years, with most patients being men (64.5%). Nearly half of the study population had intrahepatic CCA (48.7%), whereas all other patients had hilar CCA (51.3%). All patients underwent curative-intent hepatectomy. The median tumor size was 4.7 ± 2.8 cm, and 44.7% of patients had lymph node involvement. According to the American Joint Committee on Cancer 8th edition staging system, 11 (14.5%), 17 (22.4%), and 48 (63.2%) patients were classified as stages I, II, and III, respectively. Margin-negative (R0) resection was achieved in 63.2% of patients, whereas 35.5% had R1 resection. Most tumors were either well-differentiated (26.3%) or moderately differentiated (65.8%). The mean concentrations of CA19-9 and carcinoembryonic antigen were 957.8 ± 2237.2 U/mL and 8.1 ± 21.0 ng/dL, respectively. Baseline characteristics showed no significant differences between the low and high PrPC groups.
| Characteristics | Category | Subgroup (based on immunostaining) | Total (n = 76) | P value | |
| Low PrPC (n = 38) | High PrPC (n = 38) | ||||
| Age (years), mean ± SD | 66.7 ± 9.5 | 66.8 ± 9.8 | 66.7 ± 9.6 | 0.953 | |
| Male | 22 (57.9) | 27 (71.1) | 49 (64.5) | 0.338 | |
| BMI (kg/m2), mean ± SD | 24.4 ± 2.9 | 23.4 ± 2.7 | 23.9 ± 2.8 | 0.139 | |
| Location | Hilar CCA | 22 (57.9) | 15 (39.5) | 37 (48.7) | 0.169 |
| Intrahepatic CCA | 16 (42.1) | 23 (60.5) | 39 (51.3) | ||
| Tumor size (cm), mean ± SD | 4.1 ± 1.9 | 5.3 ± 3.5 | 4.7 ± 2.8 | 0.056 | |
| Positive LN | 17 (44.7) | 17 (44.7) | 34 (44.7) | 1.000 | |
| TNM stage | I | 6 (15.8) | 5 (13.2) | 11 (14.5) | 0.890 |
| II | 9 (23.7) | 8 (21.1) | 17 (22.4) | ||
| III | 23 (60.5) | 25 (65.8) | 48 (63.2) | ||
| Resection margin | R0 | 27 (71.1) | 21 (55.3) | 48 (63.2) | 0.262 |
| R1 | 11 (28.9) | 16 (42.1) | 27 (35.5) | ||
| R2 | 0 (0.0) | 1 (2.6) | 1 (1.3) | ||
| Differentiation | WD | 8 (21.1) | 12 (31.6) | 20 (26.3) | 0.177 |
| MD | 25 (65.8) | 25 (65.8) | 50 (65.8) | ||
| PD | 5 (13.2) | 1 (2.6) | 6 (7.9) | ||
| Laboratory tests | CEA (ng/dL) | 5.6 ± 4.4 | 11.1 ± 31.0 | 8.1 ± 21.0 | 0.406 |
| CA19-9 (U/mL) | 813.7 ± 2038.1 | 1115.9 ± 2461.6 | 957.8 ± 2237.2 | 0.591 | |
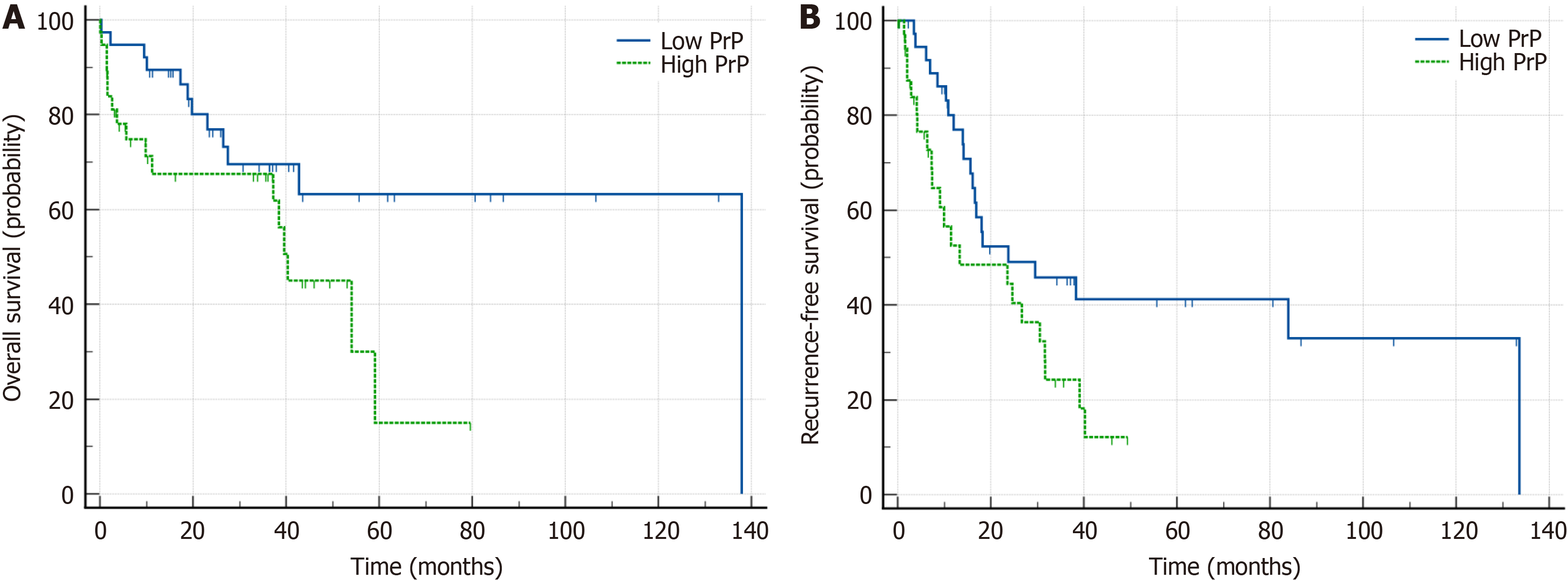
During the median follow-up period of 31.2 months (ranging from 1 to 137 months), 20 patients (26.3%) were alive without recurrence. The high PrPC group exhibited a shorter median OS of 40.4 months [95% confidence interval (CI): 11.2-59.1] compared to the low PrPC group with a median OS of 137.9 months (95%CI: 72.8-147.1; log-rank test, P = 0.041; Table 2, Figure 2A). Furthermore, the high PrPC group had a shorter median RFS of 13.3 months compared to the low PrPC group with a median RFS of 23.8 months (P = 0.026; Table 2, Figure 2B).
| Characteristics | Low PrPC (n = 38) | High PrPC (n = 38) | P value |
| Median OS (95%CI), months | 137.9 (72.8-147.1) | 40.4 (11.2-59.1) | 0.041 |
| 3-year survival rate (%) | 28 (73.7) | 27 (71.1) | - |
| 5-year survival rate (%) | 27 (71.1) | 21 (55.3) | - |
| Median RFS (95%CI), months | 23.8 (15.6-133.6) | 13.3 (7.3-31.6) | 0.026 |
| 3-year recurrence-free rate (%) | 20 (52.6) | 18 (47.4) | - |
| 5-year recurrence-free rate (%) | 19 (50.0) | 16 (42.1) | - |
Table 3 presents the results of the univariate and multivariate analyses on the prognostic factors influencing OS. The univariate analysis identified tumor size ≥ 5 cm [hazard ratio (HR) = 2.609; 95%CI: 1.218-5.591; P = 0.014], lymph node metastasis (HR = 2.222; 95%CI: 1.039-4.753; P = 0.040), and high PrPC expression (HR = 2.187; 95%CI: 1.015-4.709; P = 0.046) as independent prognostic factors. In the multivariate analysis, tumor size ≥ 5 cm [adjusted HR (aHR) = 2.448; 95%CI: 1.130-5.306; P = 0.023], lymph node metastasis (aHR = 2.633; 95%CI: 1.168-5.936; P = 0.020), and high PrPC expression (aHR = 2.877; 95%CI: 1.260-6.572; P = 0.012) remained independent prognostic factors for OS. Table 4 details the results of univariate and multivariate analyses affecting RFS. The univariate analysis revealed that high PrPC expression (HR = 1.985; 95%CI: 1.071-3.679; P = 0.029) was the only significant prognostic factor.
| Subgroup | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | aHR (95%CI) | P value | |
| Age ≥ 65 years | 0.764 (0.357-1.635) | 0.488 | - | - |
| Male | 1.496 (0.658-3.400) | 0.336 | - | - |
| Tumor size ≥ 5 cm | 2.609 (1.218-5.591) | 0.014 | 2.448 (1.130-5.306) | 0.023 |
| LN metastasis | 2.222 (1.039-4.753) | 0.040 | 2.633 (1.168-5.936) | 0.020 |
| Resection margin | 2.036 (0.954-4.349) | 0.066 | - | - |
| MD tumor (WD as a reference) | 1.321 (0.552-3.158) | 0.532 | - | - |
| PD tumor (WD as a reference) | 1.073 (0.222-5.180) | 0.930 | - | - |
| CA19-9 ≥ 37 U/mL | 2.248 (0.954-5.296) | 0.064 | - | - |
| High PrPC | 2.187 (1.015-4.709) | 0.046 | 2.877 (1.260-6.572) | 0.012 |
| Subgroup | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | aHR (95%CI) | P value | |
| Age ≥ 65 years | 0.968 (0.510-1.840) | 0.921 | - | - |
| Male | 1.163 (0.617-2.193) | 0.641 | - | - |
| Tumor size ≥ 5 cm | 1.605 (0.816-3.157) | 0.170 | - | - |
| LN metastasis | 1.484 (0.803-2.746) | 0.208 | - | - |
| Resection margin | 1.070 (0.563-2.034) | 0.837 | - | - |
| MD tumor (WD as a reference) | 1.385 (0.686-2.796) | 0.363 | - | - |
| PD tumor (WD as a reference) | 0.290 (0.037-2.247) | 0.236 | - | - |
| CA19-9 ≥ 37 U/mL | 1.355 (0.724-2.534) | 0.342 | - | - |
| High PrPC | 1.985 (1.071-3.679) | 0.029 | 1.985 (1.071-3.679) | 0.029 |
To the best of our knowledge, this is the first study investigating the expression of PrPC in CCA. We found that patients with high intratumoral PrPC expression had significantly shorter OS and RFS compared to those with low intratumoral PrPC expression. Multivariate analysis identified PrPC expression as an independent prognostic factor, similar to established factors such as tumor size and lymph node metastasis. These findings suggest that PrPC expression may serve as a postoperative prognostic biomarker of CCA, akin to pancreatic cancer or hepatocellular carcinoma[21,22].
PrPC is a glycosylphosphatidylinositol-anchored cell surface protein that consists of 208 amino acids[19]. The structure of PrPC includes a flexible coil in the N-terminal domain and a globular C-terminal domain containing three α-helices and two β-sheets[23]. PrPC is found not only in the nucleus but also in the mitochondria and Golgi complex[24-26]. Expression of PrPC begins during embryogenesis and reaches its highest level in adulthood[27,28]. Prion disease is an untreatable, fatal, transmissible neurodegenerative disorder caused by the accumulation of misfolded PrPSC in the brain[29]. Transmissible spongiform encephalopathies caused by PrPSC include sporadic Creutzfeldt-Jakob disease, variant Creutzfeldt-Jakob disease, fatal familial insomnia, Gerstmann-Straussler-Scheinker Syndrome, and Kuru[30-32]. PrPSC also plays a substantial role in other neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease by interacting with amyloid-β and α-synuclein[33,34].
PrPC is not only expressed in the central nervous system but also in non-neuronal tissues such as the heart, lungs, lymphocytes, and gastrointestinal tract[28,35,36]. Interestingly, PrPC is involved in several physiological processes, including stress protection, cellular differentiation, mitochondrial homeostasis, circadian rhythm, myelin homeostasis, and immune modulation[27,37]. In addition, PrPC inhibits apoptosis and supports tumor progression by enhancing cancer cell proliferation and metastasis[37-44]. Therefore, PrPC is involved in the development of human cancers including hepatocellular carcinoma[21], pancreatic cancer[45,46], gastric cancer[47], colorectal cancer[48], melanoma[49], and glioblastoma[50] beyond prion disease. PrPC has also been identified in exosomes secreted by cancer cells[51], and such exosomes can promote tumor metastasis by increasing the permeability of endothelial cells and the secretion of angiogenic factors[52]. Recent studies suggest that PrPC contributes to cancer development through various pathways that regulate tumor growth, differentiation, migration, and invasion[53-57]. Cancer tissue exhibits greater genetic instability and increased expression of the PRNP gene[44]. Using The Cancer Genome Atlas database, genome-wide association studies analyzing data from 10967 patients with cancer revealed 48 mutations, with eight somatic mutations (G131V, D167N, V180I, D202N, V203I, R208C, R208H, and E211Q) identified as pathogenic[44,58]. Somatic mutations in the PRNP gene have been found in various cancers, including lung adenocarcinoma, colorectal adenocarcinoma, endometrial cancer, head and neck squamous cell carcinoma, and melanoma[44]. Overexpression of PrPC is associated with malignant phenotypes of cancer stem cells in various solid tumors[46,59,60]. In contrast, the suppression of PrPC expression impairs proliferation and migration, thereby reducing invasiveness[61-63].
Hypoxia, a common condition in tumor microenvironments due to rapid cell growth outpacing the development of new blood vessels, has a close relationship with PrPC. Under hypoxic conditions, cells often increase survival pathways to adapt to stress, and PrPC has been observed to interact with hypoxia-inducible factor 1-alpha (HIF-1α), a key transcription factor that manages the cellular response to low oxygen levels[64,65]. Since HIF-1α regulates the expression of genes involved in angiogenesis, metabolism, and cell survival, PrPC may indirectly help cancer cells adapt to hypoxic conditions, thus promoting tumor proliferation[66-68]. Growing evidence links increased PrPC expression to the in
High levels of PrPC expression are associated with increased resistance to various types of drugs in glioblastoma, gastric cancer, breast cancer, and colorectal cancer[71-74]. Conversely, the inhibition or knockdown of PrPC induces sensitivity to chemotherapy[35]. In colorectal cancer cells, PrPC is involved in 5-fluorouracil resistance by activating the phosphatidylinositol 3-kinase/protein kinase B signaling pathway and increasing cell survival and proliferation through the expression of cell cycle-related proteins[75]. PrPC can promote multi-drug resistance by upregulating the multidrug resistance p-glycoprotein and inhibiting apoptosis in gastric cancer and breast cancer cells[63,76]. PrPC levels are sig
This study has some potential limitations that should be considered when interpreting the results. First, this is a small-scale study conducted at a single institution, focusing only on intrahepatic and hilar CCA. Therefore, a large-scale study is needed to understand the expression of PrPC in bile duct cancer, including extrahepatic CCA. Second, as this is a retrospective study using existing data, it cannot control for variables that may have been inconsistently or inaccurately recorded, which could lead to selection bias. Third, research on the pathophysiological mechanisms of PrPC in CCA is lacking. Further research is needed to confirm the functional role of PrPC in CCA. Despite these limitations, this study has several strengths. This is the first study, to our knowledge, that explores PrPC expression in patients with surgically removed CCA, offering new insights into the biology of its aggressiveness. Increasing evidence suggests that PrPC is a promising target for cancer treatment, indicating the need for continued research in this field.
This study shows that CCA patients with high PrPC expression have shorter postoperative OS and RFS than those with low PrPC expression. The study results suggest that the level of PrPC expression in CCA may serve as a significant prognostic marker following curative surgery.
| 1. | Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 2. | Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, Carpino G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 254] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 3. | Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 4. | McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 546] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 6. | Ali H, Tedder B, Waqar SH, Mohamed R, Cate EL, Ali E. Changing incidence and survival of intrahepatic cholangiocarcinoma based on Surveillance, Epidemiology, and End Results Database (2000-2017). Ann Hepatobiliary Pancreat Surg. 2022;26:235-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 7. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-17; discussion 517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 983] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 8. | Jung P, Cho EH, Kim SB, Kim RG. Comparison of the clinical results of surgical resection for extrahepatic cholangiocarcinomas: Hilar cholangiocarcinoma and mid-to-distal cholangiocarcinoma. Ann Hepatobiliary Pancreat Surg. 2019;23:319-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Tran TB, Ethun CG, Pawlik TM, Schmidt C, Beal EW, Fields RC, Krasnick B, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Idrees K, Isom CA, Hatzaras I, Shenoy R, Maithel SK, Poultsides GA. Actual 5-Year Survivors After Surgical Resection of Hilar Cholangiocarcinoma. Ann Surg Oncol. 2019;26:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3345] [Article Influence: 209.1] [Reference Citation Analysis (15)] |
| 11. | Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 600] [Article Influence: 42.9] [Reference Citation Analysis (2)] |
| 12. | Ilyas SI, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1207] [Article Influence: 150.9] [Reference Citation Analysis (0)] |
| 13. | Wulf MA, Senatore A, Aguzzi A. The biological function of the cellular prion protein: an update. BMC Biol. 2017;15:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 14. | Atkinson CJ, Zhang K, Munn AL, Wiegmans A, Wei MQ. Prion protein scrapie and the normal cellular prion protein. Prion. 2016;10:63-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Aguzzi A, Heppner FL. Pathogenesis of prion diseases: a progress report. Cell Death Differ. 2000;7:889-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Biasini E, Turnbaugh JA, Unterberger U, Harris DA. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012;35:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, Brentani RR. Physiology of the prion protein. Physiol Rev. 2008;88:673-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 447] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 18. | Santos TG, Lopes MH, Martins VR. Targeting prion protein interactions in cancer. Prion. 2015;9:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Go G, Lee SH. The Cellular Prion Protein: A Promising Therapeutic Target for Cancer. Int J Mol Sci. 2020;21:9208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4708] [Article Influence: 523.1] [Reference Citation Analysis (4)] |
| 21. | Kim MJ, Cho YA, Kim E, Choe JY, Park JW, Lee J, Lee JW, Moon SH, Kim YS, Kim SE, Choi EK. Cellular Prion Protein Is Closely Associated with Early Recurrence and Poor Survival in Patients with Hepatocellular Carcinoma. Diagnostics (Basel). 2022;12:1635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Bianchini M, Giambelluca MA, Scavuzzo MC, Di Franco G, Guadagni S, Palmeri M, Furbetta N, Gianardi D, Funel N, Pollina LE, Di Candio G, Fornai F, Morelli L. The occurrence of prion protein in surgically resected pancreatic adenocarcinoma. Pancreatology. 2020;20:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Acevedo-Morantes CY, Wille H. The structure of human prions: from biology to structural models-considerations and pitfalls. Viruses. 2014;6:3875-3892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Gu Y, Hinnerwisch J, Fredricks R, Kalepu S, Mishra RS, Singh N. Identification of cryptic nuclear localization signals in the prion protein. Neurobiol Dis. 2003;12:133-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Hachiya NS, Yamada M, Watanabe K, Jozuka A, Ohkubo T, Sano K, Takeuchi Y, Kozuka Y, Sakasegawa Y, Kaneko K. Mitochondrial localization of cellular prion protein (PrPC) invokes neuronal apoptosis in aged transgenic mice overexpressing PrPC. Neurosci Lett. 2005;374:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Magalhães AC, Silva JA, Lee KS, Martins VR, Prado VF, Ferguson SS, Gomez MV, Brentani RR, Prado MA. Endocytic intermediates involved with the intracellular trafficking of a fluorescent cellular prion protein. J Biol Chem. 2002;277:33311-33318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Grimaldi I, Leser FS, Janeiro JM, da Rosa BG, Campanelli AC, Romão L, Lima FRS. The multiple functions of PrP(C) in physiological, cancer, and neurodegenerative contexts. J Mol Med (Berl). 2022;100:1405-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 28. | Makrinou E, Collinge J, Antoniou M. Genomic characterization of the human prion protein (PrP) gene locus. Mamm Genome. 2002;13:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Soto C, Satani N. The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol Med. 2011;17:14-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. Transmissions to mice indicate that 'new variant' CJD is caused by the BSE agent. Nature. 1997;389:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1328] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 31. | Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of 'new variant' CJD. Nature. 1996;383:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1163] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 32. | Caughey B, Chesebro B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol. 1997;7:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Cox TO, Gunther EC, Brody AH, Chiasseu MT, Stoner A, Smith LM, Haas LT, Hammersley J, Rees G, Dosanjh B, Groves M, Gardener M, Dobson C, Vaughan T, Chessell I, Billinton A, Strittmatter SM. Anti-PrP(C) antibody rescues cognition and synapses in transgenic alzheimer mice. Ann Clin Transl Neurol. 2019;6:554-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Linden R. The Biological Function of the Prion Protein: A Cell Surface Scaffold of Signaling Modules. Front Mol Neurosci. 2017;10:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Mehrpour M, Codogno P. Prion protein: From physiology to cancer biology. Cancer Lett. 2010;290:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Pammer J, Cross HS, Frobert Y, Tschachler E, Oberhuber G. The pattern of prion-related protein expression in the gastrointestinal tract. Virchows Arch. 2000;436:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Castle AR, Gill AC. Physiological Functions of the Cellular Prion Protein. Front Mol Biosci. 2017;4:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 38. | Dupiereux I, Falisse-Poirrier N, Zorzi W, Watt NT, Thellin O, Zorzi D, Pierard O, Hooper NM, Heinen E, Elmoualij B. Protective effect of prion protein via the N-terminal region in mediating a protective effect on paraquat-induced oxidative injury in neuronal cells. J Neurosci Res. 2008;86:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Graner E, Mercadante AF, Zanata SM, Martins VR, Jay DG, Brentani RR. Laminin-induced PC-12 cell differentiation is inhibited following laser inactivation of cellular prion protein. FEBS Lett. 2000;482:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Ramljak S, Asif AR, Armstrong VW, Wrede A, Groschup MH, Buschmann A, Schulz-Schaeffer W, Bodemer W, Zerr I. Physiological role of the cellular prion protein (PrPc): protein profiling study in two cell culture systems. J Proteome Res. 2008;7:2681-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicke T, Moser M, Oesch B, McBride PA, Manson JC. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature. 1996;380:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 420] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 42. | Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P, Steele AD, Toyka KV, Nave KA, Weis J, Aguzzi A. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci. 2010;13:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 43. | Haddon DJ, Hughes MR, Antignano F, Westaway D, Cashman NR, McNagny KM. Prion protein expression and release by mast cells after activation. J Infect Dis. 2009;200:827-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Kim YC, Won SY, Jeong BH. Identification of Prion Disease-Related Somatic Mutations in the Prion Protein Gene (PRNP) in Cancer Patients. Cells. 2020;9:1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Bianchini M, Giambelluca MA, Scavuzzo MC, Di Franco G, Guadagni S, Palmeri M, Furbetta N, Gianardi D, Funel N, Ricci C, Gaeta R, Pollina LE, Falcone A, Vivaldi C, Di Candio G, Biagioni F, Busceti CL, Morelli L, Fornai F. Detailing the ultrastructure's increase of prion protein in pancreatic adenocarcinoma. World J Gastroenterol. 2021;27:7324-7339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 46. | Wang Y, Yu S, Huang D, Cui M, Hu H, Zhang L, Wang W, Parameswaran N, Jackson M, Osborne B, Bedogni B, Li C, Sy MS, Xin W, Zhou L. Cellular Prion Protein Mediates Pancreatic Cancer Cell Survival and Invasion through Association with and Enhanced Signaling of Notch1. Am J Pathol. 2016;186:2945-2956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu N, Zhang G, Jin H, Gao J, Xie H, Wang J, Liu Z, Fan D. Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 2006;20:1886-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | de Lacerda TC, Costa-Silva B, Giudice FS, Dias MV, de Oliveira GP, Teixeira BL, Dos Santos TG, Martins VR. Prion protein binding to HOP modulates the migration and invasion of colorectal cancer cells. Clin Exp Metastasis. 2016;33:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Li C, Yu S, Nakamura F, Pentikäinen OT, Singh N, Yin S, Xin W, Sy MS. Pro-prion binds filamin A, facilitating its interaction with integrin beta1, and contributes to melanomagenesis. J Biol Chem. 2010;285:30328-30339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Ryskalin L, Busceti CL, Biagioni F, Limanaqi F, Familiari P, Frati A, Fornai F. Prion Protein in Glioblastoma Multiforme. Int J Mol Sci. 2019;20:5107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Wiegmans AP, Saunus JM, Ham S, Lobb R, Kutasovic JR, Dalley AJ, Miranda M, Atkinson C, Foliaki ST, Ferguson K, Niland C, Johnstone CN, Lewis V, Collins SJ, Lakhani SR, Al-Ejeh F, Möller A. Secreted cellular prion protein binds doxorubicin and correlates with anthracycline resistance in breast cancer. JCI Insight. 2019;5:e124092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Yun CW, Lee JH, Go G, Jeon J, Yoon S, Lee SH. Prion Protein of Extracellular Vesicle Regulates the Progression of Colorectal Cancer. Cancers (Basel). 2021;13:2144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Provenzano L, Ryan Y, Hilton DA, Lyons-Rimmer J, Dave F, Maze EA, Adams CL, Rigby-Jones R, Ammoun S, Hanemann CO. Cellular prion protein (PrP(C)) in the development of Merlin-deficient tumours. Oncogene. 2017;36:6132-6142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Iglesia RP, Prado MB, Cruz L, Martins VR, Santos TG, Lopes MH. Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells. Stem Cell Res Ther. 2017;8:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Mouillet-Richard S, Ghazi A, Laurent-Puig P. The Cellular Prion Protein and the Hallmarks of Cancer. Cancers (Basel). 2021;13:5032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Gao Z, Peng M, Chen L, Yang X, Li H, Shi R, Wu G, Cai L, Song Q, Li C. Prion Protein Protects Cancer Cells against Endoplasmic Reticulum Stress Induced Apoptosis. Virol Sin. 2019;34:222-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Ryskalin L, Biagioni F, Busceti CL, Giambelluca MA, Morelli L, Frati A, Fornai F. The Role of Cellular Prion Protein in Promoting Stemness and Differentiation in Cancer. Cancers (Basel). 2021;13:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Mead S, Uphill J, Beck J, Poulter M, Campbell T, Lowe J, Adamson G, Hummerich H, Klopp N, Rückert IM, Wichmann HE, Azazi D, Plagnol V, Pako WH, Whitfield J, Alpers MP, Whittaker J, Balding DJ, Zerr I, Kretzschmar H, Collinge J. Genome-wide association study in multiple human prion diseases suggests genetic risk factors additional to PRNP. Hum Mol Genet. 2012;21:1897-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Du L, Rao G, Wang H, Li B, Tian W, Cui J, He L, Laffin B, Tian X, Hao C, Liu H, Sun X, Zhu Y, Tang DG, Mehrpour M, Lu Y, Chen Q. CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res. 2013;73:2682-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Chieng CK, Say YH. Cellular prion protein contributes to LS 174T colon cancer cell carcinogenesis by increasing invasiveness and resistance against doxorubicin-induced apoptosis. Tumour Biol. 2015;36:8107-8120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Wang JH, DU JP, Li SJ, Zhai LP, Yang XY, Wang ZH, Wu ZT, Han Y. Octarepeat peptides of prion are essential for multidrug resistance in gastric cancer cells. J Dig Dis. 2012;13:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Meslin F, Hamaï A, Gao P, Jalil A, Cahuzac N, Chouaib S, Mehrpour M. Silencing of prion protein sensitizes breast adriamycin-resistant carcinoma cells to TRAIL-mediated cell death. Cancer Res. 2007;67:10910-10919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Li QQ, Cao XX, Xu JD, Chen Q, Wang WJ, Tang F, Chen ZQ, Liu XP, Xu ZD. The role of P-glycoprotein/cellular prion protein interaction in multidrug-resistant breast cancer cells treated with paclitaxel. Cell Mol Life Sci. 2009;66:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Park JY, Jeong JK, Lee JH, Moon JH, Kim SW, Lee YJ, Park SY. Induction of cellular prion protein (PrPc) under hypoxia inhibits apoptosis caused by TRAIL treatment. Oncotarget. 2015;6:5342-5353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Yun SP, Han YS, Lee JH, Yoon YM, Yun CW, Rhee P, Lee SH. Role of hypoxiamediated cellular prion protein functional change in stem cells and potential application in angiogenesis (Review). Mol Med Rep. 2017;16:5747-5751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Rashid M, Zadeh LR, Baradaran B, Molavi O, Ghesmati Z, Sabzichi M, Ramezani F. Up-down regulation of HIF-1α in cancer progression. Gene. 2021;798:145796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 67. | Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 68. | Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 460] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 69. | Ramljak S, Herlyn H, Zerr I. Cellular Prion Protein (PrP(c)) and Hypoxia: True to Each Other in Good Times and in Bad, in Sickness, and in Health. Front Cell Neurosci. 2016;10:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Lee JH, Han YS, Yoon YM, Yun CW, Yun SP, Kim SM, Kwon HY, Jeong D, Baek MJ, Lee HJ, Lee SJ, Han HJ, Lee SH. Role of HSPA1L as a cellular prion protein stabilizer in tumor progression via HIF-1α/GP78 axis. Oncogene. 2017;36:6555-6567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Zhuang D, Liu Y, Mao Y, Gao L, Zhang H, Luan S, Huang F, Li Q. TMZ-induced PrPc/par-4 interaction promotes the survival of human glioma cells. Int J Cancer. 2012;130:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Zhao Y, You H, Liu F, An H, Shi Y, Yu Q, Fan D. Differentially expressed gene profiles between multidrug resistant gastric adenocarcinoma cells and their parental cells. Cancer Lett. 2002;185:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Cheng Y, Tao L, Xu J, Li Q, Yu J, Jin Y, Chen Q, Xu Z, Zou Q, Liu X. CD44/cellular prion protein interact in multidrug resistant breast cancer cells and correlate with responses to neoadjuvant chemotherapy in breast cancer patients. Mol Carcinog. 2014;53:686-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 74. | Atkinson CJ, Kawamata F, Liu C, Ham S, Győrffy B, Munn AL, Wei MQ, Möller A, Whitehall V, Wiegmans AP. EGFR and Prion protein promote signaling via FOXO3a-KLF5 resulting in clinical resistance to platinum agents in colorectal cancer. Mol Oncol. 2019;13:725-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Lee JH, Yun CW, Lee SH. Cellular Prion Protein Enhances Drug Resistance of Colorectal Cancer Cells via Regulation of a Survival Signal Pathway. Biomol Ther (Seoul). 2018;26:313-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Go G, Yun CW, Yoon YM, Lim JH, Lee JH, Lee SH. Role of PrP(C) in Cancer Stem Cell Characteristics and Drug Resistance in Colon Cancer Cells. Anticancer Res. 2020;40:5611-5620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Bernardino-Sgherri J, Siberchicot C, Auvré F, Busso D, Brocas C, El Masri G, Lioutsko A, Ferri F, Radicella JP, Romeo PH, Bravard A. Tumor resistance to radiotherapy is triggered by an ATM/TAK1-dependent-increased expression of the cellular prion protein. Oncogene. 2021;40:3460-3469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Lee JH, Yoon YM, Han YS, Yun CW, Lee SH. Melatonin Promotes Apoptosis of Oxaliplatin-resistant Colorectal Cancer Cells Through Inhibition of Cellular Prion Protein. Anticancer Res. 2018;38:1993-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/