Published online Feb 27, 2025. doi: 10.4240/wjgs.v17.i2.101365
Revised: October 25, 2024
Accepted: November 25, 2024
Published online: February 27, 2025
Processing time: 132 Days and 5.6 Hours
Duodenal adenocarcinoma (DA), a rare gastrointestinal malignancy, lacks clear natural history and management strategies. This study aimed to investigate the long-term outcomes of patients with DA, focusing on long-term survival and the impact of tumor characteristics, surgery, and adjuvant therapy.
To bridge this knowledge gap, we conducted a hospital-based cohort study in our 15-year experience with DA aimed at investigating the long-term outcomes of the patients with DA, along with analyzing the impact of the tumor characteristics, operations and adjuvant therapy on survival outcomes.
A retrospective analysis of 208 patients diagnosed with non-ampullary DA at a single institution between 2009 and 2023 was performed. This study used SPSS 26.0 software to make a comprehensive statistical analysis of demographic characteristics, clinical presentation, treatment modalities, and survival outcomes. The effectiveness of surgical resection and adjuvant therapy in 5-year oval survival (OS) and disease-free survival was evaluated using Kaplan-Meier survival curves, the Cox proportional hazards model, and statistical comparisons of survival distributions.
The median OS time for the cohort was 39 months, with 3- and 5-year OS rates of 51.2% and 43.6%, respectively. Radical resection was performed in 82.6% of cases, and was significantly associated with an improved 5-year OS, with a rate of 57.8%. Adjuvant therapy showed a survival benefit in the specific patient subsets, particularly in tumor stage II or III tumors, with an improved OS. Adjuvant therapy (hazard ratio= 2.71, 95% confidence interval: 1.30-5.62, P = 0.008), pancreatic invasion and advanced tumor stage were identified as significant predictors of OS in multivariate analyses.
Radical operation for DA is associated with a remarkable improvement in the 5-year OS. Importantly, postoperative adjuvant therapy can significantly prolong the OS time in patients with radical operation, especially in patients with stage III. It highlights the necessity for early diagnosis, tailored surgical approaches, and a nuanced understanding of the role of adjuvant therapy.
Core Tip: Numerous meta-analyses and systematic reviews have delved into the treatment of duodenal adenocarcinoma (DA), yet the majority of the studies retrospective, single-center, and small sample size series, particularly in China. To bridge this knowledge gap, we conducted a hospital-based cohort study in our 15-year experience with DA aimed at investigating the long-term outcomes of the patients with DA, along with analyzing the impact of the tumor characteristics, operations and adjuvant therapy on survival outcomes.
- Citation: Xie QF, Long LS, Luo YY, Lu MT, Ming WK, Zhao LY, Liu H. Long-term survival outcomes of duodenal adenocarcinoma: A cohort study with 15-year single-center experience. World J Gastrointest Surg 2025; 17(2): 101365
- URL: https://www.wjgnet.com/1948-9366/full/v17/i2/101365.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i2.101365
Duodenal adenocarcinoma (DA) remains a relatively rare and enigmatic entity within the realm of gastrointestinal malignancies, accounting for less than 1% of all gastrointestinal cancers yet comprising over 50% of small bowel cancers[1]. This scarcity contributes to the disease's poorly delineated natural history and the persistent ambiguities surrounding optimal management strategies. DA is often compared to colorectal cancer because it shares a location in the gastro
The treatment of DA faces several challenges, including the absence of established guidelines based on scientific evidence worldwide, difficulties in early detection due to the rarity of the disease and lack of specific screening markers, and limited evidence supporting various treatment modalities[6,7]. Surgical resection is the only potentially curative treatment for DA. The complexity of the surgery, often involving pancreaticoduodenectomy (PD), has evolved over the years, with advancements in perioperative care and surgical techniques contributing to reduced morbidity and mortality rates, yet without a uniform improvement in long-term survival outcomes[7-9]. Adjuvant therapy, encompassing che
Numerous meta-analyses and systematic reviews have delved into the treatment of DA, yet the majority of the studies retrospective, single-center, and small sample size series, particularly in China[14,15]. To bridge this knowledge gap, we conducted a hospital-based cohort study in our 15-year experience with DA aimed at investigating the long-term outcomes of the patients with DA, along with analyzing the impact of the tumor characteristics, operations and adjuvant therapy on survival outcomes.
This retrospective analysis was conducted to elucidate the long-term survival outcomes of patients diagnosed with non-ampullary DA. The study spanned over a 15-year period, from 2009 to 2023, capturing a comprehensive dataset that reflects advancements in diagnostic and therapeutic strategies over time. The cohort consisted of 208 patients, meticulously selected to provide a robust dataset for analyzing survival outcomes and identifying prognostic factors influencing overall survival (OS) and disease-free survival (DFS). Stage of DA according to American Joint Committee on Cancer (AJCC) 8th edition of small bowel adenocarcinoma, which included patients with positive lymph nodes.
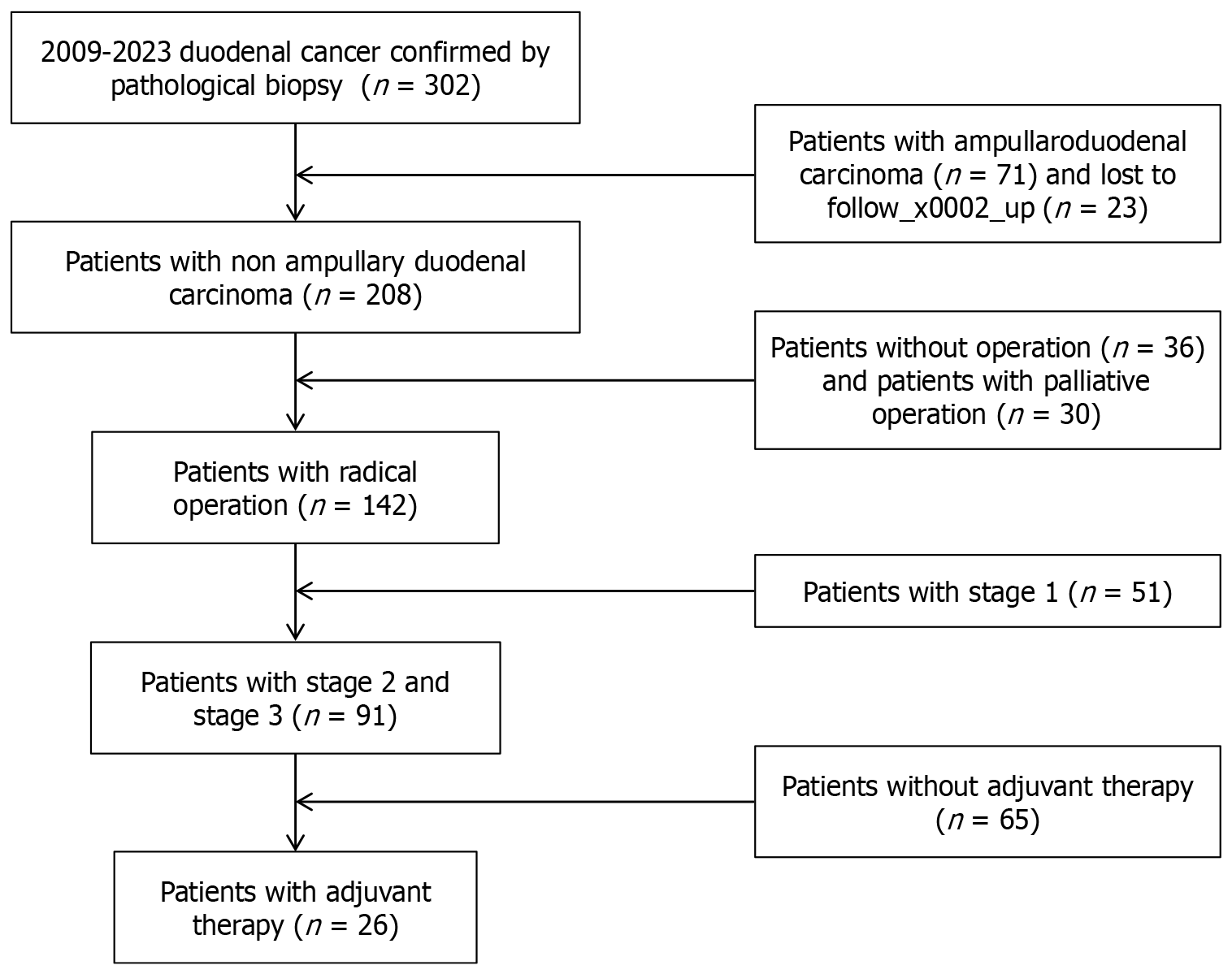
The patients included in this study were drawn from a hospital-based cohort and included cases diagnosed and treated at the facility. Inclusion criteria were histologically confirmed non-ampulla DA, including all stages of disease at diagnosis. Patients were excluded if they had ampulla cancer or other forms of gastrointestinal cancer, had incomplete medical records, or lost follow-up shortly after diagnosis. This rigorous selection process ensured the homogeneity of the study population and the relevance of the findings to non-ampulla DA.
Comprehensive data collection was a cornerstone of this study, ensuring a broad and detailed dataset for analysis. Information was gathered retrospectively from the electronic medical records, pathology reports, and radiological images. The data included demographic characteristics [age, gender, body mass index (BMI)], clinical presentation (symptoms, tumor location, stage at diagnosis), treatment modalities (surgical intervention, adjuvant therapy), and survival outcomes (length of survival, recurrence).
Key variables were meticulously recorded, including preoperative laboratory values (e.g., CEA, CA19-9 levels), details of the surgical procedure (extent of resection, lymph node dissection), pathological findings (tumor differentiation, lymphovascular invasion, perineural invasion), and genetic markers when available (KRAS mutation, dMMR/MSI-H). This comprehensive dataset allowed for a nuanced analysis of factors influencing patient outcomes.
The treatment modalities employed for managing non-ampullary DA were detailed in this study to understand their impact on survival. Radical operation means that the tumor specimen after surgical resection is confirmed by pathological examination that the surgical margin is clean and no tumor remains. Radical surgical methods include radical PD and endoscopic mucosal resection, and palliative surgical methods include gastrojejunostomy and endoscopic stent implantation. Radical operation was also recorded in detail including the extent of excision and the mode of lymph node dissection. Information was also recorded on adjuvant therapy, which is generally based on fluorouracil and platinum-based drugs and lasts 4-6 cycles. This information is critical because the study was designed to evaluate the effectiveness of different treatment strategies in improving long-term survival in patients with non-ampulla DA.
This study used SPSS 26.0 software to make a comprehensive statistical analysis of all the collected data. Survival outcomes, including OS and DFS, were calculated from the date of diagnosis to the date of death or last follow-up for OS and from the date of diagnosis to the date of disease recurrence or last follow-up for DFS. Kaplan-Meier survival curves were generated to illustrate survival trends over time, and log-rank tests were used to compare survival distributions among different patient subgroups[16]. The Cox proportional hazards model was utilized to identify the independent prognostic factors affecting survival. Variables considered in the model included demographic factors (age, gender), clinical characteristics (BMI, jaundice, hemorrhage, anemia), preoperative laboratory values, treatment details (radical operation, adjuvant therapy), and pathological findings (AJCC stage, tumor differentiation, lymph node involvement). Hazard ratios (HRs) and 95% confidence intervals (95%CIs) were calculated to estimate the strength of association between each variable and survival outcomes[17]. The study has been reported in line with the STROCSS criteria[18].
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethical committee of the related institution (NFEC-2024-171). The study was registered at ClinicalTrials.gov (NCT06443086).
A total of 208 patients pathologically diagnosed with non-ampullary DA between 2009 and 2023 were included in present study (Figure 1). The cohort's demographic and clinical characteristics indicate a median age of 60.67 years old. Notably, the gender distribution showed a slight male predominance, consistent with broader trends in gastrointestinal cancers. Radical resection was performed in 82.56% of the cases, Among 142 patients who underwent radical operation, 46 patients (32.21%) had postoperative complications, 32 patients (69.57%) were tested positive for lymph node resection, the main complications were incisional infection, abdominal abscess and pancreatic fistula. Lymph node metastasis was present in 51 (39.92%) patients. Pathological results showed that 111 patients (53.37%) had advanced tumors, with tumor stages of III and IV. After radical operation, 26 patients (18.31%) received adjuvant chemotherapy. The main adjuvant chemotherapy methods were capox regimen and folfox regimen based on 5-fluorouracil and oxaliplatin. Several of the patients with MSI-H received immunotherapy as part of subsequent treatment. In our study, only a small number of DA patients underwent genetic testing for KRAS, Her-2, and MSI, so there was no discussion of genetic markers in our results. The other surgery-related conditions and pathological variables are shown in (Table 1).
| Variable | n (%) |
| Age, mean (SD) | 60.7 (12.5) |
| Female gender, n (%) | 85 (40.9) |
| BMI (kg/m2), mean (SD) | 22.9 (3.8) |
| Preoperative lab, n (%) | |
| CA199 (U/mL) > 37 | 50 (24.0) |
| CEA (ug/L) > 5 | 64 (30.8) |
| Operational types, n (%) | |
| Pancreatoduodenectomy | 123 (71.5) |
| Local resection | 19 (11.1) |
| Palliative operation | 30 (17.4) |
| Radical operation, n (%) | 142 (82.6) |
| Total number of harvested lymph nodes, median (IQR) | 12 (8-16) |
| Positive lymph nodes, n (%) | 51 (35.9) |
| Tumor stage, n (%) | |
| Stage I | 54 (26.0) |
| Stage II | 43 (20.7) |
| Stage III | 57 (27.4) |
| Stage IV | 54 (26.0) |
| Tumor location, n (%) | |
| 1st portion | 99 (47.6) |
| 1st-2nd portion | 64 (30.8) |
| 2nd portion | 13 (6.3) |
| 3rd portion | 5 (2.4) |
| 4th portion | 27(13.0) |
| Postoperative complications, n (%) | 46 (32.2) |
| Hospital stays (days), mean (SD) | 22.5 (5.1) |
| Pathological type1, n (%) | |
| Adenocarcinoma | 147 (85.5) |
| Non-adenocarcinoma | 25 (14.5) |
| Pathological differentiation2, n (%) | |
| Poorly differentiation | 41 (28.9) |
| Medium-high differentiation | 101 (71.1) |
| Pancreatic invasion, n (%) | 68 (47.7) |
| Vascular invasion, n (%) | 50 (35.5) |
| Nerve invasion, n (%) | 69 (48.3) |
| Lymphatic vessel invasion3, n (%) | 51(35.9) |
| KRAS mutation, n (%) | 6 (15.38) |
| DMMR/MSI-H, n (%) | 7 (7.22) |
| Her-2 positive, n (%) | 56 (49.12) |
| Ki-67 mutation, n (%) | 7 (20.59) |
| Adjuvant therapy, n (%) | 26 (18.3) |
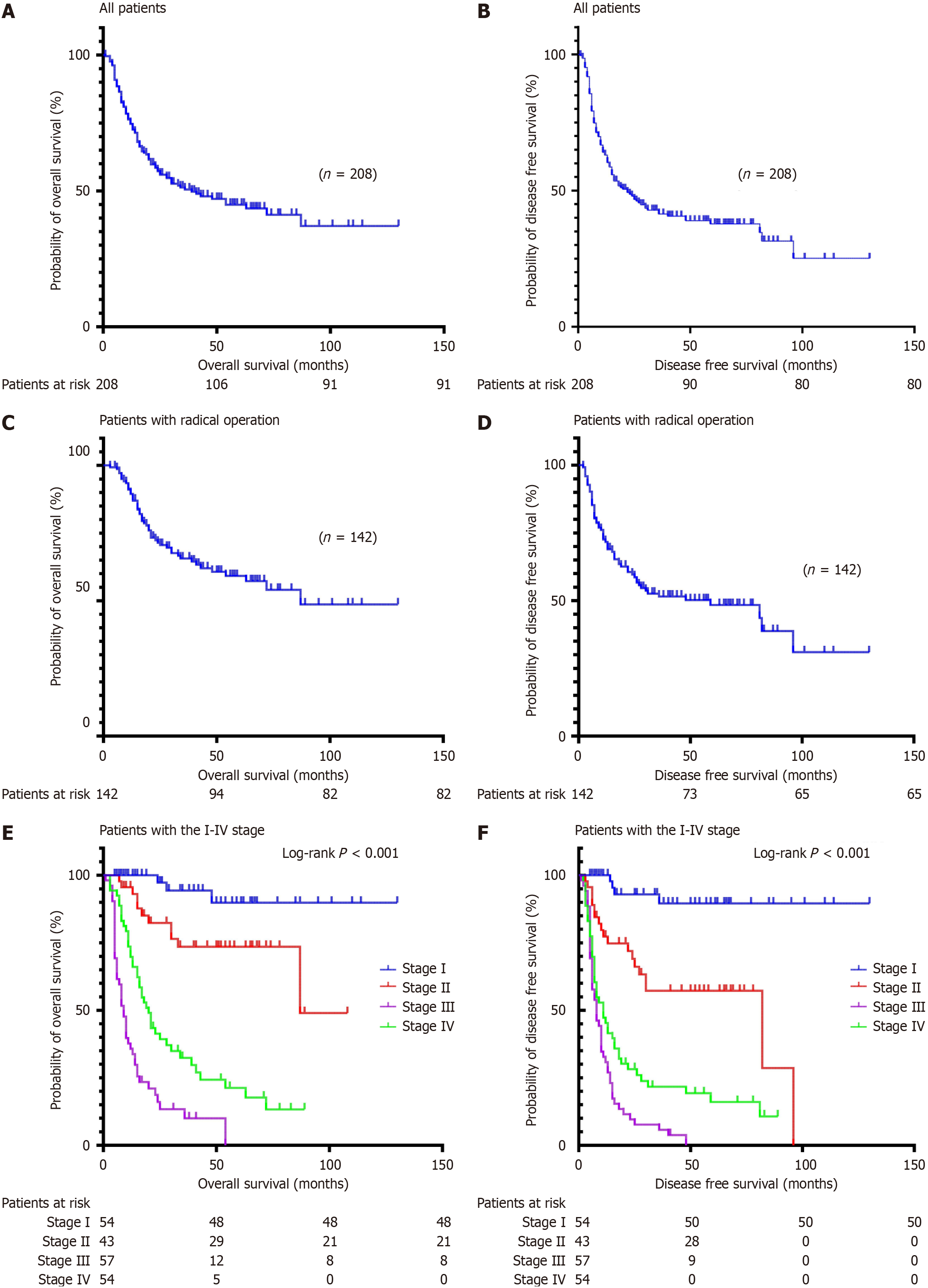
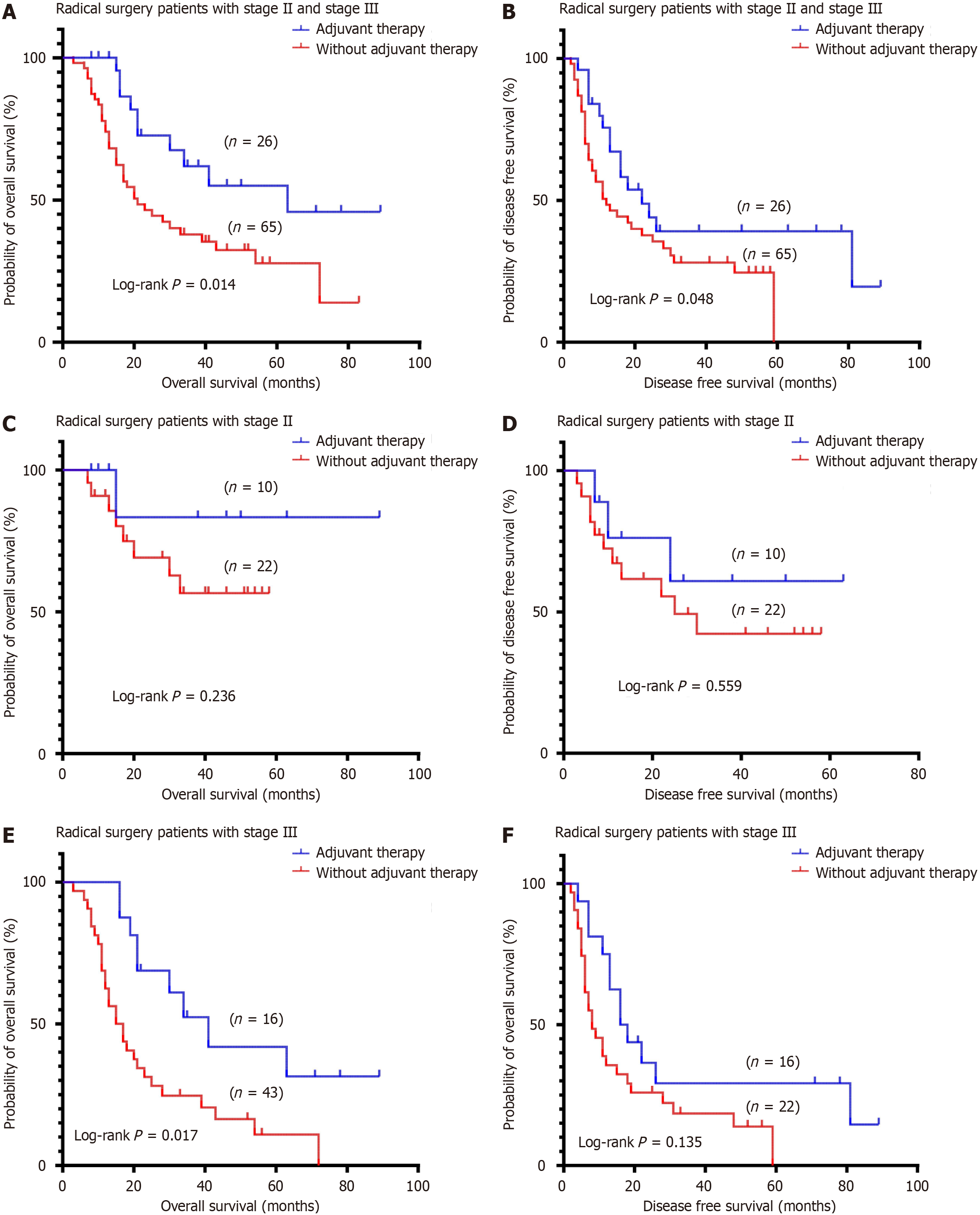
Among the 208 patients diagnosed with DA, the median OS of all patients was 39 months; the 3- and 5-year OS rates were 51.2% and 43.6%, respectively (Figure 2A); The median DFS was 22 months; and the 3-year and 5-year DFS rates were 43.2% and 38.7%, respectively (Figure 2B). The median OS of patients undergoing radical operation was 87 months; the 3- and 5-year OS rates were 66.5% and 57.8% (Figure 2C); the median DFS rates were 59 months; and the 3- and 5-year DFS rates were 51.2% and 45.6%, respectively (Figure 2D). Stage I patients did not achieve a median OS of 87 months at stage II, 20 months at stage III, and 9 months at stage IV (P < 0.001, Figure 2E). Stage I patients did not achieve median DFS, 82 months at stage II, 11 months at stage III, and 8 months at stage IV; P < 0.001, Figure 2F). A total of 142 patients underwent radical surgical resection, including 91 patients with stage II and III tumors, 26 patients with postoperative adjuvant therapy, and 65 patients without adjuvant therapy. There was a significant difference in the OS (P = 0.014, Figure 3A) and the DFS (P = 0.048, Figure 3B) between the adjuvant therapy and non-adjuvant therapy in stage II and III patients. There was no statistically significant difference in the OS (P = 0.236, Figure 3C) and DFS (P = 0.559, Figure 3D) between the adjuvant therapy and non-adjuvant therapy in stage II patients. There was a significant difference in OS (P = 0.017, Figure 3E) and DFS (P = 0.135, Figure 3F) between the adjuvant therapy and non-adjuvant therapy in stage III patients.
Survival outcomes varied across the cohort, with an overall median survival time of 39 months. The survival rates at different time points provide insight into the disease's trajectory, with 1-, 3-, and 5-year OS rates of 78.5%, 51.2%, and 43.6%, respectively. Multivariate analysis was conducted to identify significant predictors of survival, revealing advanced tumor stage, pancreatic invasion and adjuvant therapy as key factors. Adjuvant therapy was associated with decreased mortality (HR 0.40, 95%CI: 0.19-0.81, P = 0.008) whereas Pancreatic invasion was associated with increased mortality (HR 2.68, 95%CI: 1.41-5.11, P = 0.003; Table 2).
| Characteristics | Univariate analysis | Multivariate analysis | ||
| Hazard ratio | P value | Hazard ratio | P value | |
| Age (years; ≥ 65 vs < 65 = 1.00) | 1.01 (0.99-1.02) | 0.24 | ||
| Gender, female (vs male = 1.00) | 0.80 (0.54-1.21) | 0.30 | ||
| CA199 (U/mL; ≥ 37 vs < 37 = 1.00) | 2.92 (1.96-4.33) | < 0.001 | 1.10 (0.60-2.01) | 0.76 |
| Radical operation (vs without radical operation = 1.00) | 4.912 (2.98-8.11) | < 0.001 | ||
| AJCC stage IV (vs AJCC stage I-III = 1.00) | 3.04 (2.40-3.86) | < 0.001 | 4.75 (2.70-8.47) | < 0.001 |
| Postoperative complications (vs without postoperative complications = 1.00) | 1.22 (0.77-1.95) | 0.40 | ||
| Pancreatic invasion (vs without Pancreatic invasion = 1.00) | 5.07 (2.91-8.85) | < 0.001 | 2.68 (1.41-5.11) | 0.003 |
| Poorly differentiation (vs medium-high differentiation = 1.00) | 1.88 (1.17-3.00) | 0.009 | ||
| KRAS mutation (vs KRAS wild type = 1.00) | 1.24 (0.35-4.29) | 0.73 | ||
| dMMR/MSI-H (vs PMMR/MSS = 1.00) | 0.51 (0.12-2.11) | 0.35 | ||
| Her-2 positive (vs Her-2 negative = 1.00) | 1.87 (0.68-5.13) | 0.23 | ||
| Adjuvant therapy (vs without adjuvant therapy = 1.00) | 2.52 (1.23-5.15) | 0.01 | 2.71 (1.30-5.62) | 0.008 |
In the present study, the 5-year survival rate after curative resection of non-ampullary DA was 57.8%. The 1-, 3- and 5-year survival rates were comparable to previously published reports, although some of these reports included patients with periampullary tumors[19,20]. The crude survival curves stratified for administration of adjuvant therapy reached a significant difference, and in the multivariable analysis the adjuvant therapy was also detected to be associated with improved survival, supporting the use of capox or folfox as adjuvant therapy for advanced diseases[21,22].
Surgical resection emerges as the cornerstone of potentially curative treatment for DA. PD is currently recognized as the most radical surgical method, but PD has high complexity, high risk and high incidence of postoperative complications[23]. Compared with duodenal segectomy, the length of hospital stay is shorter and the rate of postoperative complications and mortality is lower. Adriano's study reported the efficacy of duodenal segectomy in the treatment of DA of the third and fourth parts of the duodenum, which was superior to PD[24].
However, some studies have shown that PD (Whipple surgery) is superior to duodenectomy in OS[25]. In our retrospective cohort study, the median OS after radical operation was 87 months, and the 3- and 5-year OS rates were 66.5% and 57.8%, respectively. This result is similar to the 3-and 5-year OS rates of 66.3% and 58.2% reported in a retrospective study by Jensen et al[7]. In another study of 47 patients with DA, the 5-year OS rate for patients undergoing radical surgical removal was 51%[26]. Surprisingly, lymph node status, a critical prognostic factor in many cancers, did not significantly influence survival outcomes in certain studies, suggesting a potential reevaluation of its role in DA treatment planning.
The choice of adjuvant therapy remains a contentious issue, with data indicating a nuanced benefit. For instance, patients with tumor stage II and III showed improved median OS times with adjuvant therapy compared to those without adjuvant therapy. This suggests a selective benefit of adjuvant therapy, potentially improving the outcomes in specific patient subsets. However, the effectiveness and selection criteria for adjuvant therapy, including chemotherapy, warrant further investigation to tailor approaches that maximize patient survival while considering the quality of life. A national study in Japan looked at 1083 patients with non-ampulla duodenal cancer who underwent surgery at 114 training institutions accredited by the Japanese Society for Hepatobiliary and Pancreatic Surgery between 2008 and 2017, and across the cohort, no survival benefit was observed in patients who received adjuvant therapy compared to patients who underwent surgery alone. However, in the matched cohort, there was a significant improvement in DFS at 6 months of adjuvant therapy[13] (median: 43.5 months vs 22.5 months, P = 0.016). In our cohort study, the median OS was 63 months with adjuvant therapy and 21 months without adjuvant therapy, and the 5-year OS was 43.2% with adjuvant therapy and 28.6% without adjuvant therapy (P = 0.014 ),which was more favorable than that reported previously. In a study conducted at M.D. Anderson Cancer Center, 54 patients diagnosed with radical resection of small bowel cancer were retrospectively evaluated. The results of survival analysis showed that adjuvant chemotherapy improved DFS (P = 0.05), but did not have a statistically significant benefit on OS[27]. In the present study, the median DFS with adjuvant therapy was 24 months and the median overall DFS without adjuvant therapy was 12 months (P = 0.048), adjuvant chemotherapy appears to significantly improve survival.
The present study was limited by the inherited retrospective nature with all its inherited biases, underscoring the need for the prospective studies to better understand and optimize therapy strategies[28].
In conclusion, radical resection for DA is associated with a remarkable improvement in the 5-year OS, with a rate of 57.8%. Importantly, adjuvant therapy in patients who underwent radical surgery may be associated with a survival benefit. The interplay of surgical resection, tumor characteristics, and adjuvant therapy in influencing outcomes highlights the complex nature of DA therapy. Early diagnosis, tailored surgical approaches, and a nuanced under
Duodenal adenocarcinoma currently lacks a clear natural history and treatment strategy, and we conducted a hospital-based cohort study over 15 years of DA treatment experience to investigate long-term outcomes in patients with DA. The study concluded that radical operation was associated with a significant improvement in 5-year OS, and postoperative adjuvant therapy may be associated with a survival benefit. Future research should focus on large-scale, prospective clinical studies to further explore long-term survival of duodenal adenocarcinoma.
| 1. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4691] [Article Influence: 521.2] [Reference Citation Analysis (4)] |
| 2. | Aparicio T, Svrcek M, Zaanan A, Beohou E, Laforest A, Afchain P, Mitry E, Taieb J, Di Fiore F, Gornet JM, Thirot-Bidault A, Sobhani I, Malka D, Lecomte T, Locher C, Bonnetain F, Laurent-Puig P. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer. 2013;109:3057-3066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 482] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 4. | Brueckl WM, Heinze E, Milsmann C, Wein A, Koebnick C, Jung A, Croner RS, Brabletz T, Günther K, Kirchner T, Hahn EG, Hohenberger W, Becker H, Reingruber B. Prognostic significance of microsatellite instability in curatively resected adenocarcinoma of the small intestine. Cancer Lett. 2004;203:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Cloyd JM, Norton JA, Visser BC, Poultsides GA. Does the extent of resection impact survival for duodenal adenocarcinoma? Analysis of 1,611 cases. Ann Surg Oncol. 2015;22:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 6. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 7. | Jensen KK, Storkholm JH, Chen I, Burgdorf SK, Hansen CP. Long-term results after resection of primary duodenal adenocarcinoma: A retrospective cohort study. Int J Surg. 2022;100:106599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 8. | Ecker BL, McMillan MT, Datta J, Lee MK, Karakousis GC, Vollmer CM Jr, Drebin JA, Fraker DL, Roses RE. Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: A propensity score-matched analysis of a nationwide clinical oncology database. Cancer. 2017;123:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | García-Molina FJ, Mateo-Vallejo F, Franco-Osorio Jde D, Esteban-Ramos JL, Rivero-Henández I. Surgical approach for tumours of the third and fourth part of the duodenum. Distal pancreas-sparing duodenectomy. Int J Surg. 2015;18:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Lim SY, Chung DI, Jeong HJ, Jeon HJ, Yoon SJ, Kim H, Han IW, Heo JS, Shin SH. Clinical Outcome of Resected Non-Ampullary Duodenal Adenocarcinoma: A Single Center Experience. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Matsubara A, Sekine S, Kushima R, Ogawa R, Taniguchi H, Tsuda H, Kanai Y. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol. 2013;229:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Nakagawa K, Sho M, Fujishiro M, Kakushima N, Horimatsu T, Okada KI, Iguchi M, Uraoka T, Kato M, Yamamoto Y, Aoyama T, Akahori T, Eguchi H, Kanaji S, Kanetaka K, Kuroda S, Nagakawa Y, Nunobe S, Higuchi R, Fujii T, Yamashita H, Yamada S, Narita Y, Honma Y, Muro K, Ushiku T, Ejima Y, Yamaue H, Kodera Y. Clinical practice guidelines for duodenal cancer 2021. J Gastroenterol. 2022;57:927-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Nakagawa K, Sho M, Okada KI, Akahori T, Aoyama T, Eguchi H, Fujii T, Higuchi R, Kanaji S, Kanetaka K, Kuroda S, Nagakawa Y, Nunobe S, Yamada S, Yamashita H, Yamaue H, Kodera Y; Japan Duodenal Cancer Guideline Committee. Surgical results of non-ampullary duodenal cancer: a nationwide survey in Japan. J Gastroenterol. 2022;57:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Nitta N, Ohgi K, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Sasaki K, Uesaka K. ASO Author Reflections: Pancreatic Invasion is a Crucial Independent Prognostic Factor in Duodenal Carcinoma. Ann Surg Oncol. 2020;27:4561. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Overman MJ, Kopetz S, Wen S, Hoff PM, Fogelman D, Morris J, Abbruzzese JL, Ajani JA, Wolff RA. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Poultsides GA, Huang LC, Cameron JL, Tuli R, Lan L, Hruban RH, Pawlik TM, Herman JM, Edil BH, Ahuja N, Choti MA, Wolfgang CL, Schulick RD. Duodenal adenocarcinoma: clinicopathologic analysis and implications for treatment. Ann Surg Oncol. 2012;19:1928-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Qubaiah O, Devesa SS, Platz CE, Huycke MM, Dores GM. Small intestinal cancer: a population-based study of incidence and survival patterns in the United States, 1992 to 2006. Cancer Epidemiol Biomarkers Prev. 2010;19:1908-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Mathew G, Agha R, Albrecht J, Goel P, Mukherjee I, Pai P, D'Cruz AK, Nixon IJ, Roberto K, Enam SA, Basu S, Muensterer OJ, Giordano S, Pagano D, Machado-Aranda D, Bradley PJ, Bashashati M, Thoma A, Afifi RY, Johnston M, Challacombe B, Ngu JC, Chalkoo M, Raveendran K, Hoffman JR, Kirshtein B, Lau WY, Thorat MA, Miguel D, Beamish AJ, Roy G, Healy D, Ather HM, Raja SG, Mei Z, Manning TG, Kasivisvanathan V, Rivas JG, Coppola R, Ekser B, Karanth VL, Kadioglu H, Valmasoni M, Noureldin A; STROCSS Group. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 1478] [Article Influence: 295.6] [Reference Citation Analysis (0)] |
| 19. | Sakae H, Kanzaki H, Nasu J, Akimoto Y, Matsueda K, Yoshioka M, Nakagawa M, Hori S, Inoue M, Inaba T, Imagawa A, Takatani M, Takenaka R, Suzuki S, Fujiwara T, Okada H. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer. 2017;117:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Sakamoto T, Saiura A, Ono Y, Mise Y, Inoue Y, Ishizawa T, Takahashi Y, Ito H. Optimal Lymphadenectomy for Duodenal Adenocarcinoma: Does the Number Alone Matter? Ann Surg Oncol. 2017;24:3368-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Solaini L, Jamieson NB, Metcalfe M, Abu Hilal M, Soonawalla Z, Davidson BR, McKay C, Kocher HM; UK Duodenal Cancer Study Group. Outcome after surgical resection for duodenal adenocarcinoma in the UK. Br J Surg. 2015;102:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Taliente F, Bianco G, Moschetta G, Franco A, Giovinazzo F, Agnes S, Spoletini G. From endoscopic resection to pancreatoduodenectomy: a narrative review of treatment modalities for the tumors of the ampulla of Vater. Chin Clin Oncol. 2022;11:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Tran TB, Qadan M, Dua MM, Norton JA, Poultsides GA, Visser BC. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery. 2015;158:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Tsushima T, Taguri M, Honma Y, Takahashi H, Ueda S, Nishina T, Kawai H, Kato S, Suenaga M, Tamura F, Morita S, Boku N. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist. 2012;17:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Young JI, Mongoue-Tchokote S, Wieghard N, Mori M, Vaccaro GM, Sheppard BC, Tsikitis VL. Treatment and Survival of Small-bowel Adenocarcinoma in the United States: A Comparison With Colon Cancer. Dis Colon Rectum. 2016;59:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Watari J, Mitani S, Ito C, Tozawa K, Tomita T, Oshima T, Fukui H, Kadowaki S, Natsume S, Senda Y, Tajika M, Hara K, Yatabe Y, Shimizu Y, Muro K, Morimoto T, Hirota S, Das KM, Miwa H. Molecular alterations and PD-L1 expression in non-ampullary duodenal adenocarcinoma: Associations among clinicopathological, immunophenotypic and molecular features. Sci Rep. 2019;9:10526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Zhang C, Lizalek JM, Dougherty C, Westmark DM, Klute KA, Reames BN. Neoadjuvant Therapy for Duodenal and Ampullary Adenocarcinoma: A Systematic Review. Ann Surg Oncol. 2024;31:792-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1231] [Article Influence: 175.9] [Reference Citation Analysis (0)] |
| 29. | Cloyd JM, George E, Visser BC. Duodenal adenocarcinoma: Advances in diagnosis and surgical management. World J Gastrointest Surg. 2016;8:212-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Scélo G, Boffetta P, Hemminki K, Pukkala E, Olsen JH, Andersen A, Tracey E, Brewster DH, McBride ML, Kliewer EV, Tonita JM, Pompe-Kirn V, Chia KS, Jonasson JG, Martos C, Colin D, Brennan P. Associations between small intestine cancer and other primary cancers: an international population-based study. Int J Cancer. 2006;118:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/