Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.113490
Revised: September 15, 2025
Accepted: October 10, 2025
Published online: December 27, 2025
Processing time: 120 Days and 18.8 Hours
This letter discusses the findings of Pang et al retrospective study on omental patch repair as a balanced treatment for gastric ulcer perforation. We acknow
Core Tip: We propose a novel “multi-layer repair” concept addressing the limitations of traditional omental patch repair for gastric ulcer perforation. Utilizing three-dimensional bioprinting technology, we engineered a biologically functional patch that recapitulates native gastric layering: An inner mucosal organoid-containing region and an outer smooth muscle cell layer. Preliminary results from animal studies indicated that this approach, which aimed to restore physiological structural-functional coupling of the stomach, appears to facilitate a repair that extends beyond mere mechanical closure, showing promise for achieving true anatomical and functional recovery.
- Citation: Guan KY, Wu QZ, Ning B, Ling-Hu EQ. Exploration of a new method of a biopatch based on the central concept of the multi-layer repair. World J Gastrointest Surg 2025; 17(12): 113490
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/113490.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.113490
We have read the retrospective study by Pang et al[1] which evaluating the efficacy and postoperative complications among different surgical methods for gastric ulcer perforation. This article incorporated patients underwent surgeries like simple closure, omental patch repair and partial gastrectomy for gastric ulcer perforation, then analyzed the operative success rate and the incidence of complications. The authors indicated that omental patch repair offered the best balance of efficacy, safety, and recovery time. Omental patch repair can reduce the abnormal organ function caused by partial loss of organs, which may be most useful in large perforations with friable tissue[2], it reflects an optimal balance between effective treatment and minimal disruption of normal anatomy and physiology.
Gastric ulcer perforation is a life-threatening complication of peptic ulcer disease which is the most common indication for surgery in peptic ulcer disease[2]. Although traditional surgery is the mainstay, emerging minimally invasive techniques such as laparoscopic-assisted endoscopic overstitch technique[3], hydrogel bioadhesives for minimally invasive perforation repair[4], compressible and expandable hemostatic structural pattern[5], among others, are gaining attention.
Nevertheless, purely mechanical closure often fails to restore structural-functional integrity, which can lead to the formation of fibrous scars at the perforation site, resulting in the loss of essential functions in the mucosal layer, muscular layer, and neurovascular structures at the gastric repair site. This thereby causes abnormal gastric contraction rhythms and impairs the gastric mucosal barrier function. To address this, we propose “multilayer repair” as a regenerative strategy aimed at replicating native gastric anatomy and function. While previous studies have explored multilayer tissue constructs using organ-derived decellularized extracellular matrix hydrogels such as the heart, aorta, lung, and skin[6,7], our approach focuses on gastric-specific repair through biofabrication. This concept emphasizes the physiological layering of the gastrointestinal tract, incorporating precise anastomosis of tissue strata, preservation of neurovascular supply, and inhibition of fibroblast overactivation[8].
Our team fabricated a functional biopatch based on the central concept of the multi-layer repair. The biopatch, J@PVP-PCL via electrospinning, was a core-sheath structured wet adhesive with polyurethane as the core and polyvinylpyrro
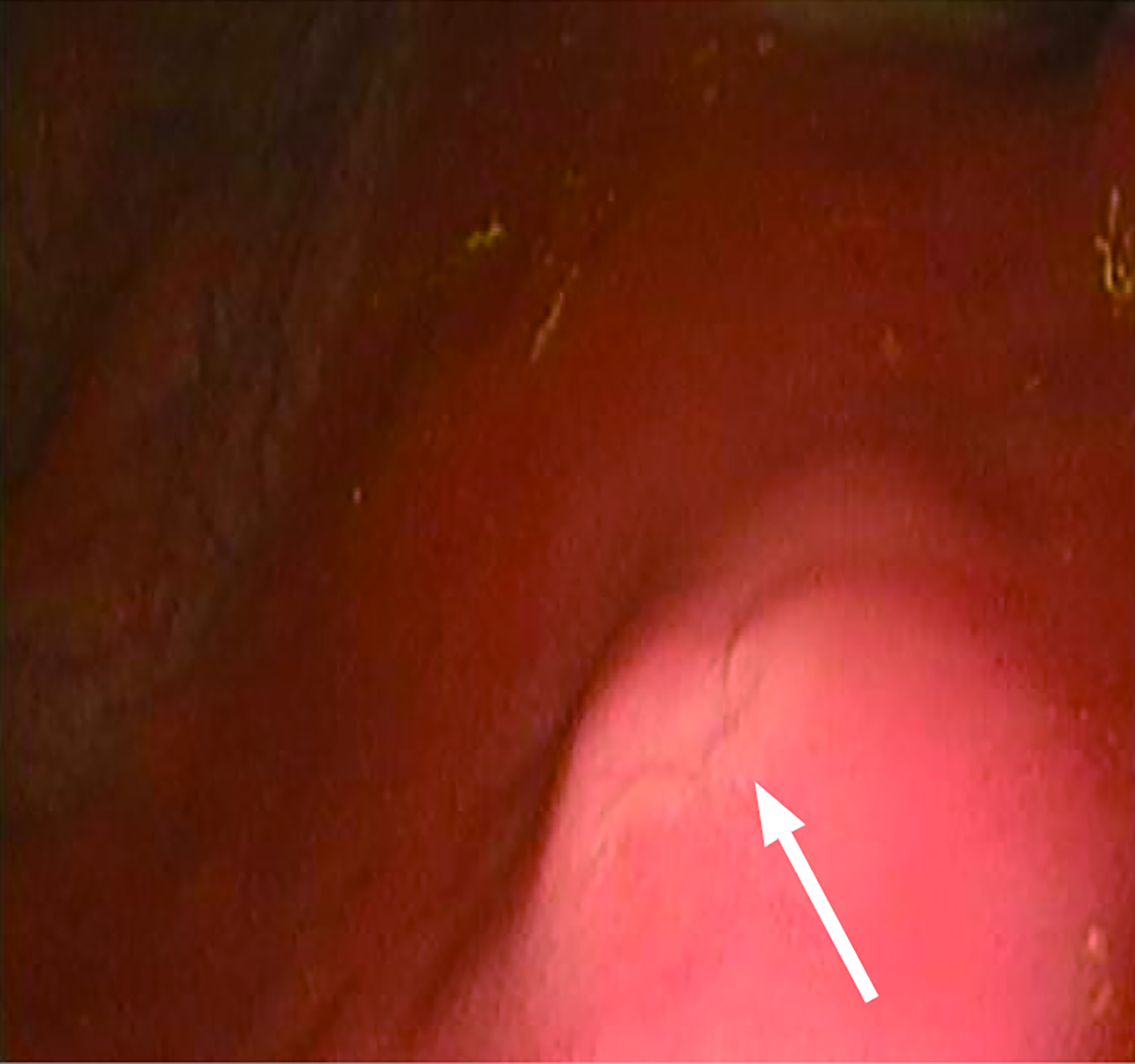
The core zone was constructed by three-dimensional bioprinting, comprising: A bottom layer of 8% gelatin methacryloyl (GelMA) infused with primary gastric smooth muscle cells (at a density of 1 × 106 cells per milliliter of GelMA); a top layer of 8% GelMA incorporated with gastric epithelial organoids (at a density of 1000 organoids per milliliter of GelMA). It has achieved certain results in animal experiments. We made a 1-cm gastric wound in a porcine model and then collected endoscopic view of the repair site at 2 weeks after applying the biopatch (Figure 2). This patch utilizes a biological mesh framework to attempt multi-layer repair of full-thickness gastric defects, which is expected to facilitate multi-layer repairs such as the repair of gastric ulcer perforation. However, larger animal models are needed to verify this, and in the future, digestive endoscopic delivery will be explored to promote wound closure and repair.
| 1. | Pang YF, Shu L, Xia CW. Retrospective comparative study of different surgical methods for gastric ulcer perforation: Efficacy and postoperative complications. World J Gastrointest Surg. 2025;17:101896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Rasslan S, Coimbra R, Rasslan R, Utiyama EM. Management of perforated peptic ulcer: What you need to know. J Trauma Acute Care Surg. 2025;99:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Huang-Pacheco A, Thomas P, Zarroug A, Shah AA. A novel combined laparoscopic-endoscopic overstitch technique for pediatric gastric ulcer perforation: an innovative approach to repair of hollow viscus perforations. J Surg Case Rep. 2025;2025:rjaf584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Wang Z, Xu J, Wu X, Han M, Peng R, Zhao R, Qin M, Li T, Yin J, Yu L, Li Y, Wu H, Lin Z, Wang L, Hu Y, Wu Y. A Sprayable Janus Hydrogel as an Effective Bioadhesive for Gastrointestinal Perforation Repair. Adv Funct Materials. 2024;34:2408479. [DOI] [Full Text] |
| 5. | Zhang M, Getova VE, Martinez-Garcia FD, Borghuis T, Burgess JK, Harmsen MC. From Macro to Micro: Comparison of Imaging Techniques to Detect Vascular Network Formation in Left Ventricle Decellularized Extracellular Matrix Hydrogels. Gels. 2022;8:729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Getova VE, Orozco-García E, Palmers S, Krenning G, Narvaez-Sanchez R, Harmsen MC. Extracellular Vesicles from Adipose Tissue-Derived Stromal Cells Stimulate Angiogenesis in a Scaffold-Dependent Fashion. Tissue Eng Regen Med. 2024;21:881-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Getova VE, Pascual A, Dijkstra R, Gładysz MZ, Ubels D, Wlodarczyk-Biegun MK, Burgess JK, Siebring J, Harmsen MC. Multilayered Tissue Assemblies Through Tuneable Biodegradable Polyhydroxyalkanoate Polymer (Mesh)-Reinforced Organ-Derived Extracellular Matrix Hydrogels. Gels. 2025;11:539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Erdogan S, Günes SN, Bulbul YE, Eskitoros-Togay ŞM, Dilsiz N. Design of multi-layer electrospun poly(ε-caprolactone)/chitosan nanofiber scaffolds loaded with tigecycline for controlled drug release and antibacterial wound healing. Int J Biol Macromol. 2025;322:146955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/