Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.112520
Revised: September 26, 2025
Accepted: October 27, 2025
Published online: December 27, 2025
Processing time: 147 Days and 21.4 Hours
Neoadjuvant therapy prior to surgery plays a critical role in improving the prog
To develop an ML model to predict Clavien-Dindo grade ≥ II complications in patients with GC after neoadjuvant therapy and laparoscopic gastrectomy.
Clinical data were collected from 455 patients with GC who underwent neoad
A total of 455 patients were included of whom 69 (15.16%) developed Clavien-Dindo grade ≥ II complications. The predictive model was constructed using seven variables, including smoking status, Nutritional Risk Screening-2002 score, American Society of Anesthesiologists classification, neoadjuvant therapy, surgical approach, operating time, and intraoperative blood loss. Among the six models the NNE model outperformed the others, achieving the highest area under the receiver operating characteristic curve (0.789, 0.739-0.840) and demonstrating superior discrimination, clinical utility, and calibration.
The NNE-based prediction model effectively identified patients with GC at high risk of Clavien-Dindo grade ≥ II complications after neoadjuvant therapy and laparoscopic gastrectomy.
Core Tip: Addressing data scarcity in gastric cancer neoadjuvant therapy, this study used a large patient cohort (n = 455) to pioneer a machine learning model for predicting Clavien-Dindo grade ≥ II complications post-neoadjuvant therapy and laparoscopic gastrectomy. Key predictors included smoking status, Nutritional Risk Screening-2002 score, American Society of Anesthesiologists classification, neoadjuvant therapy, surgical approach, operating time, and intraoperative blood loss. The neural network ensemble model demonstrated superior performance with optimal discrimination, calibration, and clinical utility, potentially offering a tool for perioperative risk stratification and management optimization.
- Citation: Li RY, Zhao ZR, Yu T, Yu JC. Machine-learning-based prediction model for Clavien-Dindo grade ≥ II complications after neoadjuvant therapy and laparoscopic gastrectomy in gastric cancer. World J Gastrointest Surg 2025; 17(12): 112520
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/112520.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.112520
Gastric cancer (GC) is a common malignant tumor of the digestive system, ranking fifth in incidence and third in mortality among all cancers worldwide[1]. Its development is associated with multiple risk factors, including family history, smoking, alcohol consumption, dietary habits, Helicobacter pylori infection, and Epstein-Barr virus infection[2,3]. Notably, > 95% of GCs are adenocarcinomas[4]. The diagnosis of GC mainly relies on clinical symptoms, enhanced CT, positron emission tomography/CT, tumor markers, and gastroscopic biopsy[3]. Despite significant advancements in diagnostic techniques in recent years, most patients are diagnosed at an advanced stage due to the late onset of symptoms[5,6]. Currently, surgery remains the cornerstone of treatment for advanced GC[3]. However, with the evolution of multidisciplinary treatment approaches, neoadjuvant therapy has emerged as an increasingly important component of comprehensive management.
Neoadjuvant therapy, administered prior to surgery, encompasses chemotherapy, radiotherapy, targeted therapy, and immunotherapy[7,8]. Leading clinical guidelines from Japan, United States, Europe, and China recommend preoperative neoadjuvant therapy for patients with locally advanced or potentially resectable GC to improve prognosis[6,8-10]. Recently, research on novel neoadjuvant therapy regimens has become a key focus in GC treatment[11,12].
Despite significant advances in systemic treatment, postoperative complications remain a major challenge in GC. Based on the Clavien-Dindo classification system, 30-day postoperative complications are categorized into five grades: I (minor complications requiring no treatment); II (requiring pharmacological intervention); III (needing surgical/endoscopic/radiological intervention); IV (life-threatening complications requiring intensive care); and V (death). Grade ≥ II complications adversely affect patient recovery, prolong hospitalization time, and escalate healthcare costs and readmission rates. These impacts collectively impose significant burdens on patients and healthcare systems, emphasizing the necessity of effective complication prediction and prevention strategies to optimize outcomes and resource allocation.
Machine learning (ML), a rapidly advancing branch of artificial intelligence, has been increasingly applied in medical research, particularly in oncology for disease prediction and risk stratification[13,14]. While several ML-based studies have investigated postoperative complications in patients with GC[15,16], most have excluded those receiving neoadju
This study aimed to bridge this critical research gap by identifying influential preoperative and intraoperative factors associated with Clavien-Dindo grade ≥ II complications in patients with neoadjuvant-treated GC and developing an ML-based predictive model. The proposed model has significant clinical implications as it can stratify the risk of perioperative complications in patients with GC undergoing neoadjuvant therapy. It assists clinicians in optimizing treatment strategies to achieve precision medicine, thereby preventing complications and improving patient outcomes.
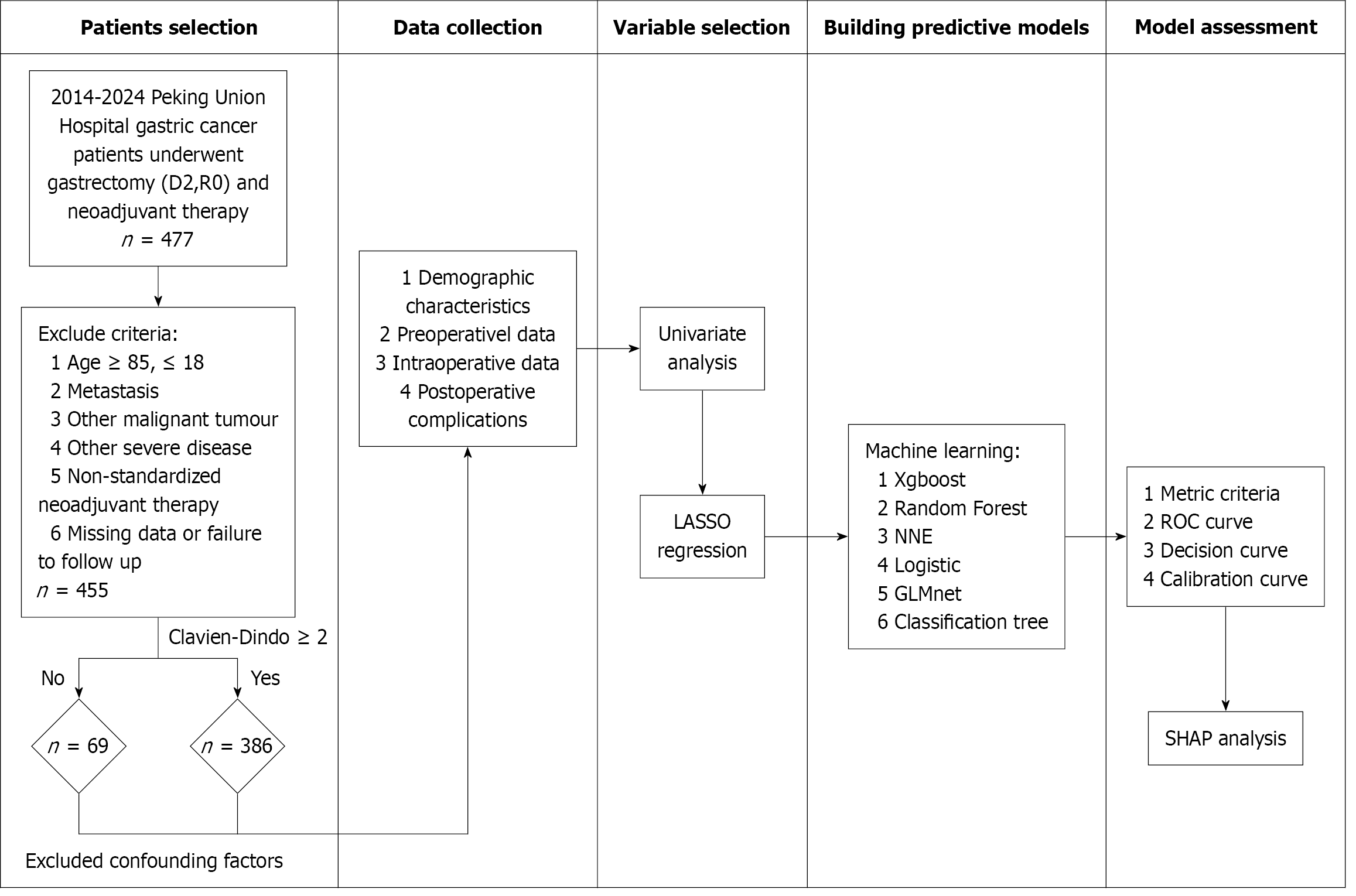
This study retrospectively analyzed patients who underwent laparoscopic radical gastrectomy following neoadjuvant therapy at Peking Union Medical College Hospital from January 2014 to December 2024. Clinical data were systematically collected for all eligible cases. Inclusion criteria included: (1) Completion of preoperative neoadjuvant therapy; (2) Radical gastrectomy (D2 lymphadenectomy with R0 resection); (3) Histopathological confirmation of gastric adenocarcinoma; and (4) Procedures performed by experienced surgeons (minimum 50 prior cases of the same procedure). Exclusion criteria included: (1) Age < 18 or > 85 years; (2) Intraoperative findings of peritoneal metastasis, adjacent organ invasion, or distant metastasis; (3) Other malignancies; (4) Severe comorbidities (e.g., cardiovascular or cerebrovascular diseases); (5) Non-standardized chemotherapy regimen (e.g., < 2 treatment cycles); and (6) Loss to follow-up. The primary outcome was the occurrence of Clavien-Dindo grade ≥ II complications.
Comprehensive patient data were systematically collected, encompassing demographic characteristics, preoperative clinical data, intraoperative data, and postoperative complications. Following adjustment for potential confounders, 34 clinically relevant factors were analyzed, including age, sex, preoperative hospital stay, smoking, alcohol consumption, hypertension, diabetes, gastroesophageal reflux disease, gastric outlet obstruction, psychological disorders, history of abdominal surgery, thyroid function, Helicobacter pylori infection, combination of other disease, family history of tumors, neoadjuvant therapy, Nutritional Risk Screening-2002 (NRS-2002) score, tumor location, clinical stage at initial diagnosis, American Society of Anesthesiologists (ASA) status, cardiac function, body mass index, preoperative WBC count, hemoglobin, glucose, albumin, albumin-to-globulin ratio, surgical approach, operating time, blood loss, intraoperative endoscopy use, implantation of nutritional tube, blood transfusion, and postoperative complication occurrence and classification.
In this study variables with missing values exceeding 30% were excluded from analysis. For variables with missing values not exceeding 30%, we used multiple imputation for data completion. Specifically, we utilized the bootstrap method for resampling and applied the predictive mean matching algorithm to generate imputed values. All analytical variables were simultaneously incorporated into the imputation model to maintain intervariable relationships. Finally, we generated and pooled results from five imputed datasets to obtain the final estimates.
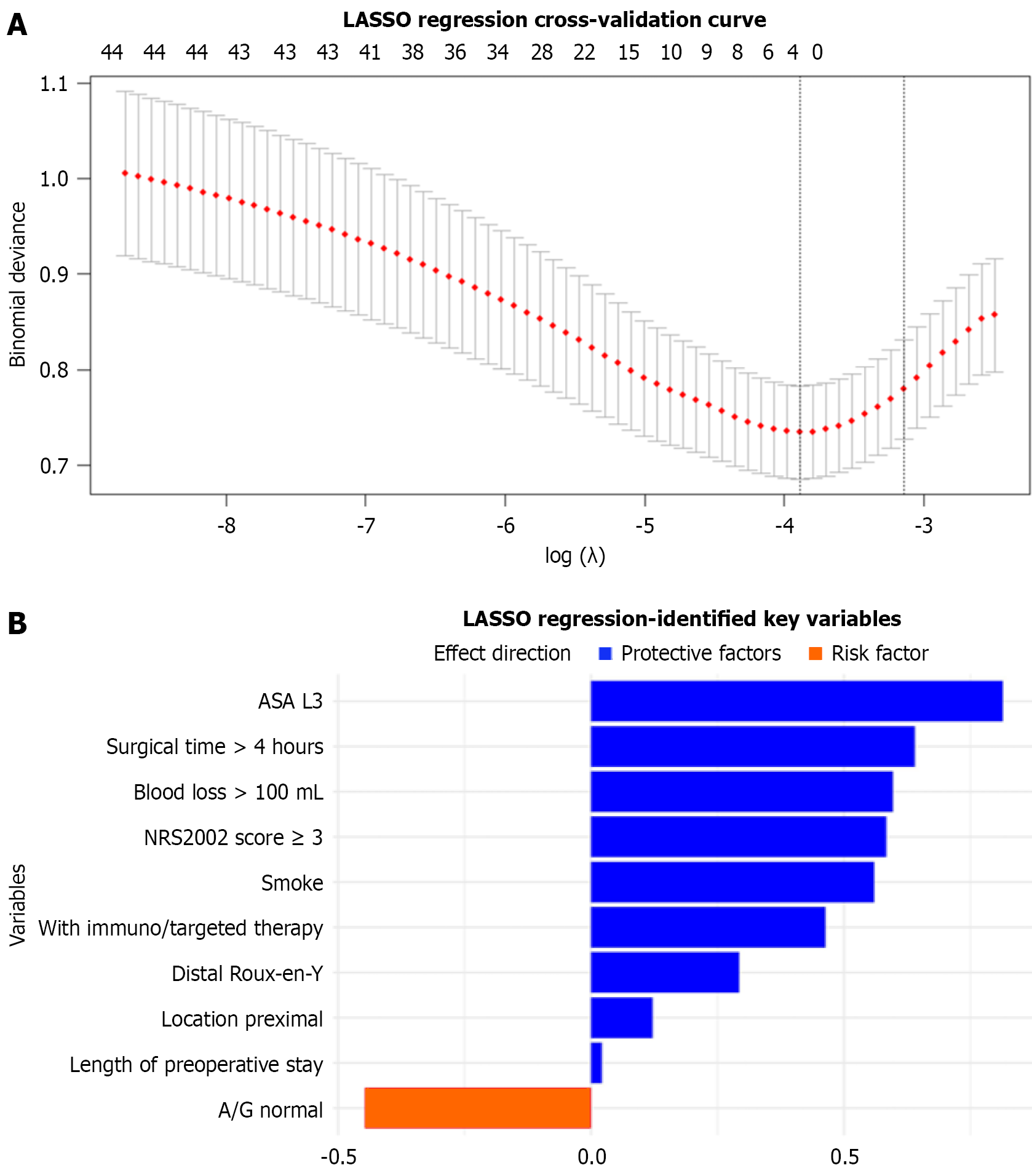
Univariate analyses were first performed with categorical variables analyzed using χ2 or Fisher’s exact tests, normally distributed continuous variables compared using t tests (reported as mean ± SD), and non-normally distributed variables assessed using Wilcoxon rank-sum tests (reported as median; interquartile range). Variables with P < 0.05 were considered statistically significant. Subsequently, least absolute shrinkage and selection operator (LASSO) regression with 10-fold cross-validation was used to identify predictive features in which the optimal regularization parameter (λ) was determined through minimum criteria and variables with non-zero coefficients were retained. Finally, the intersection of significant variables from both univariate analysis and LASSO regression was used to construct the ML prediction models.
Within the mlr3 framework utilizing nested cross-validation (fivefold inner loop and fivefold outer loop), we imple
SHapley Additive exPlanations (SHAP) analysis was used to quantify and rank the relative contributions of predictive features in the optimal ML model, thereby facilitating identification of key risk factors for Clavien-Dindo grade ≥ II complications following neoadjuvant therapy in patients with GC. All statistical analyses were performed using R software (version 4.4.3). The workflow of this study is illustrated in Figure 1.
This study included 455 patients with GC who received neoadjuvant therapy prior to radical gastrectomy, comprising 342 males (75.2%) and 113 females (24.8%) with a median age of 61 (52-68) years. The baseline characteristics, including demographic data, preoperative clinical data, surgical details, and postoperative complications are presented in Table 1. Clavien-Dindo grade ≥ II complications occurred in 69 patients (15.2%). The spectrum of complications included: Anastomotic leaks (n = 16, 3.5%), pulmonary infection (n = 16, 3.5%), hemorrhage (n = 12, 2.6%), gastroparesis (n = 12, 2.6%), anastomotic stricture (n = 4, 0.9%), intestinal obstruction (n = 3, 0.7%), venous thrombosis, incision infection, and lymphatic leaks (n = 2 each, 0.4%), pancreatic fistulas, bloodstream infections, myocardial infarction, postoperative adrenal crisis, atrial fibrillation, urinary tract infection, and pulmonary embolism (n = 1 each, 0.2%).
| All (N = 455) | No (n = 386) | Yes (n = 69) | Univariate analysis | N | ||
| OR | P value | |||||
| Age, year | 61.0 (52.0-68.0) | 61.0 (52.2-67.0) | 62.0 (51.0-68.0) | 1.0 (0.98-1.02) | 0.809 | 455 |
| Sex | 0.045a | 455 | ||||
| Female | 113 (24.8) | 103 (26.7) | 10 (14.5) | Reference | ||
| Male | 342 (75.2) | 283 (73.3) | 59 (85.5) | 2.12 (1.09-4.57) | ||
| Smoking | < 0.001a | 455 | ||||
| No | 230 (50.5) | 209 (54.1) | 21 (30.4) | Reference | ||
| Yes | 225 (49.5) | 177 (45.9) | 48 (69.6) | 2.68 (1.56-4.75) | ||
| ASA status | < 0.001a | 455 | ||||
| L1 | 54 (11.9) | 48 (12.4) | 6 (8.70) | Reference | ||
| L2 | 314 (69.0) | 279 (72.3) | 35 (50.7) | 0.98 (0.42-2.75) | ||
| L3 | 87 (19.1) | 59 (15.3) | 28 (40.6) | 3.70 (1.49-10.7) | ||
| Neoadjuvant therapy | 0.008a | 455 | ||||
| Chemotherapy | 344 (75.6) | 300 (77.7) | 44 (63.8) | Reference | ||
| Radiochemotherapy | 60 (13.2) | 43 (11.1) | 17 (24.6) | 2.70 (1.38-5.10) | ||
| Combined immuno/targeted therapy | 51 (11.2) | 43 (11.1) | 8 (11.6) | 1.28 (0.53-2.80) | ||
| NRS-2002 ≥ 3 | < 0.001a | 455 | ||||
| No | 294 (64.6) | 265 (68.7) | 29 (42.0) | Reference | ||
| Yes | 161 (35.4) | 121 (31.3) | 40 (58.0) | 3.01 (1.78-5.13) | ||
| Surgical approach | < 0.001a | 455 | ||||
| Distal-Billroth 1 | 155 (34.1) | 146 (37.8) | 9 (13.0) | Reference | ||
| Distal-Billroth 2 | 6 (1.32) | 4 (1.04) | 2 (2.90) | 8.11 (0.9-50.9) | ||
| Distal-Roux-en-Y | 68 (14.9) | 50 (13.0) | 18 (26.1) | 5.74 (2.46-14.3) | ||
| Proximal | 8 (1.76) | 7 (1.81) | 1 (1.45) | 2.54 (0.09-17.4) | ||
| Total-Roux-en-Y | 218 (47.9) | 179 (46.4) | 39 (56.5) | 3.48 (1.70-7.93) | ||
| Implantation of nutrition tubes | 0.025a | 455 | ||||
| No | 237 (52.1) | 192 (49.7) | 45 (65.2) | Reference | ||
| Yes | 218 (47.9) | 194 (50.3) | 24 (34.8) | 0.53 (0.31-0.90) | ||
| Operating time > 4 h | < 0.001a | 455 | ||||
| No | 254 (55.8) | 231 (59.8) | 23 (33.3) | Reference | ||
| Yes | 201 (44.2) | 155 (40.2) | 46 (66.7) | 2.96 (1.74-5.17) | ||
| Blood loss > 100 mL | < 0.001a | 455 | ||||
| No | 321 (70.5) | 289 (74.9) | 32 (46.4) | Reference | ||
| Yes | 134 (29.5) | 97 (25.1) | 37 (53.6) | 3.43 (2.03-5.85) | ||
Univariate analysis demonstrated significant differences between patients with Clavien-Dindo grade ≥ II complications and those without in the following variables: Sex (P = 0.045); smoking status (P < 0.001); ASA status (P < 0.001); neoadjuvant therapy (P = 0.008); NRS-2002 score ≥ 3 (P < 0.001); surgical approach (P < 0.001); nutritional tube placement (P = 0.025); operating time > 4 h (P < 0.001); and blood loss > 100 mL (P < 0.001).
Figure 2 presents the LASSO regression results at the optimal λ value, identifying several clinically significant predictors of Clavien-Dindo grade ≥ II complications: Higher ASA status (grade 3, coefficient = 0.814); prolonged operating time (> 4 h, coefficient = 0.640); increased blood loss (> 100 mL, coefficient = 0.597); elevated nutritional risk (NRS-2002 score ≥ 3, coefficient = 0.584); current smoking status (coefficient = 0.560); combined neoadjuvant chemotherapy with target/immunotherapy (coefficient = 0.463); Roux-en-Y reconstruction following distal gastrectomy (coefficient = 0.293); tumor location (proximal, coefficient = 0.122); and longer preoperative hospitalization (coefficient = 0.021). Normal albumin-to-globulin ratio demonstrated a protective effect (coefficient = -0.447).
Based on the combined results of univariate and LASSO analyses, the following variables were selected for ML model construction: Smoking; NRS-2002 score ≥ 3; ASA status; neoadjuvant therapy; surgical strategy; operating time > 4 h; and blood loss > 100 mL. No significant multicollinearity was detected among these predictors. The correlation heatmap illustrating the relationships between these variables is provided in Supplementary Figure 1. The sample size in this study was determined in accordance with established guidelines for predictive model research. Based on the events per variable criterion, 455 patients were included, among whom 69 experienced postoperative complications (positive events). Following feature selection, the model incorporated seven predictor variables. The resulting events per variable ratio was approximately 9.86:1, which was close to the commonly recommended threshold of 10:1, indicating that the current sample size is sufficient to ensure the stability and reliability of the model construction.
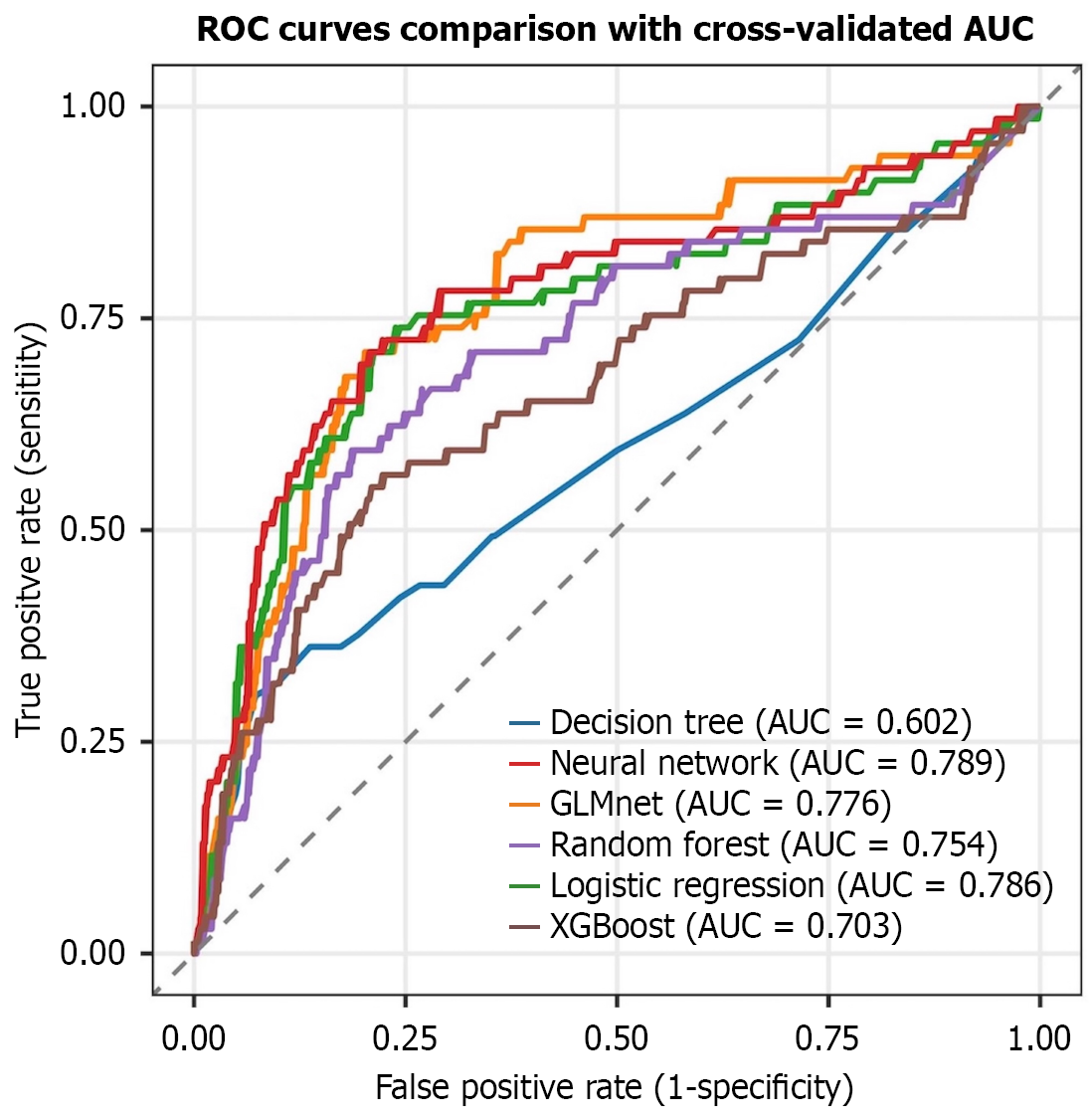
Using seven clinically significant predictors (smoking status, NRS-2002 score ≥ 3, ASA status, neoadjuvant therapy, surgical approach, operating time > 4 h, and blood loss > 100 mL), we developed and validated six ML models (XGBoost, random forest, NNE, logistic regression, GLMnet, and decision tree). NNE demonstrated superior performance, achieving the highest area under the curve (AUC = 0.789) and a high recall value (0.731) (Table 2). Logistic regression also showed competitive predictive accuracy with an AUC of 0.786.
| Model | AUC | Accuracy | Recall | Sensitivity | Specificity |
| NNE | 0.789 (0.739-0.840) | 0.732 (0.657-0.807) | 0.731 (0.610-0.851) | 0.731 (0.610-0.851) | 0.733 (0.625-0.841) |
| Logistic regression | 0.786 (0.606-0.966) | 0.752 (0.697-0.807) | 0.763 (0.525-0.824) | 0.763 (0.525-0.824) | 0.756 (0.704-0.807) |
| GLMnet | 0.776 (0.668-0.884 | 0.67 (0.555-0.786) | 0.739 (0.570-0.908) | 0.739 (0.570-0.908) | 0.656 (0.508-0.803) |
| Random forest | 0.754 (0.648-0.861) | 0.666 (0.641-0.691) | 0.735 (0.505-0.965) | 0.735 (0.505-0.965) | 0.659 (0.598-0.720) |
| XGBoost | 0.703 (0.571-0.835) | 0.679 (0.567-0.791) | 0.606 (0.400-0.813) | 0.606 (0.400-0.813) | 0.693 (0.577-0.810) |
| Decision tree | 0.602 (0.544-0.660) | 0.756 (0.670-0.842) | 0.371 (0.259-0.482) | 0.371 (0.259-0.482) | 0.827 (0.725-0.928) |
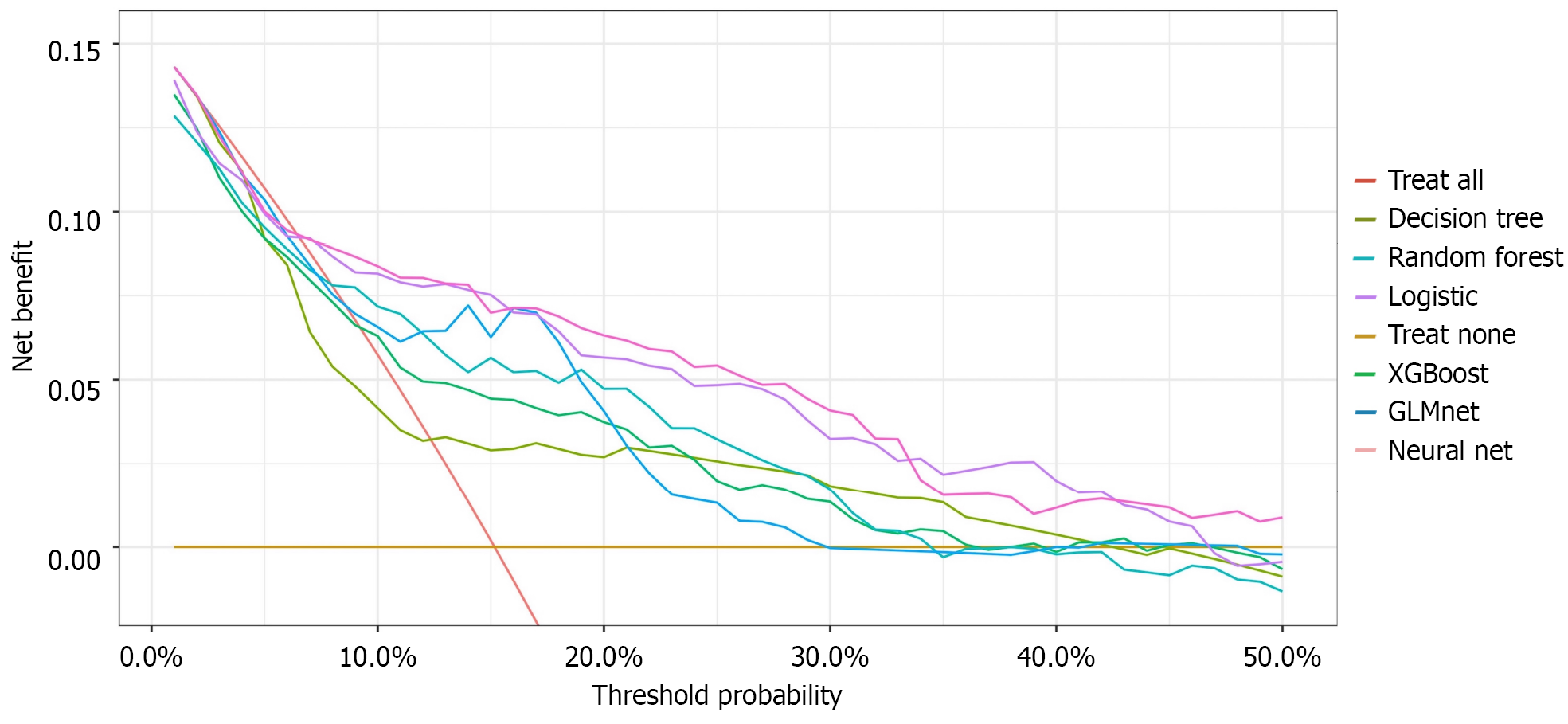
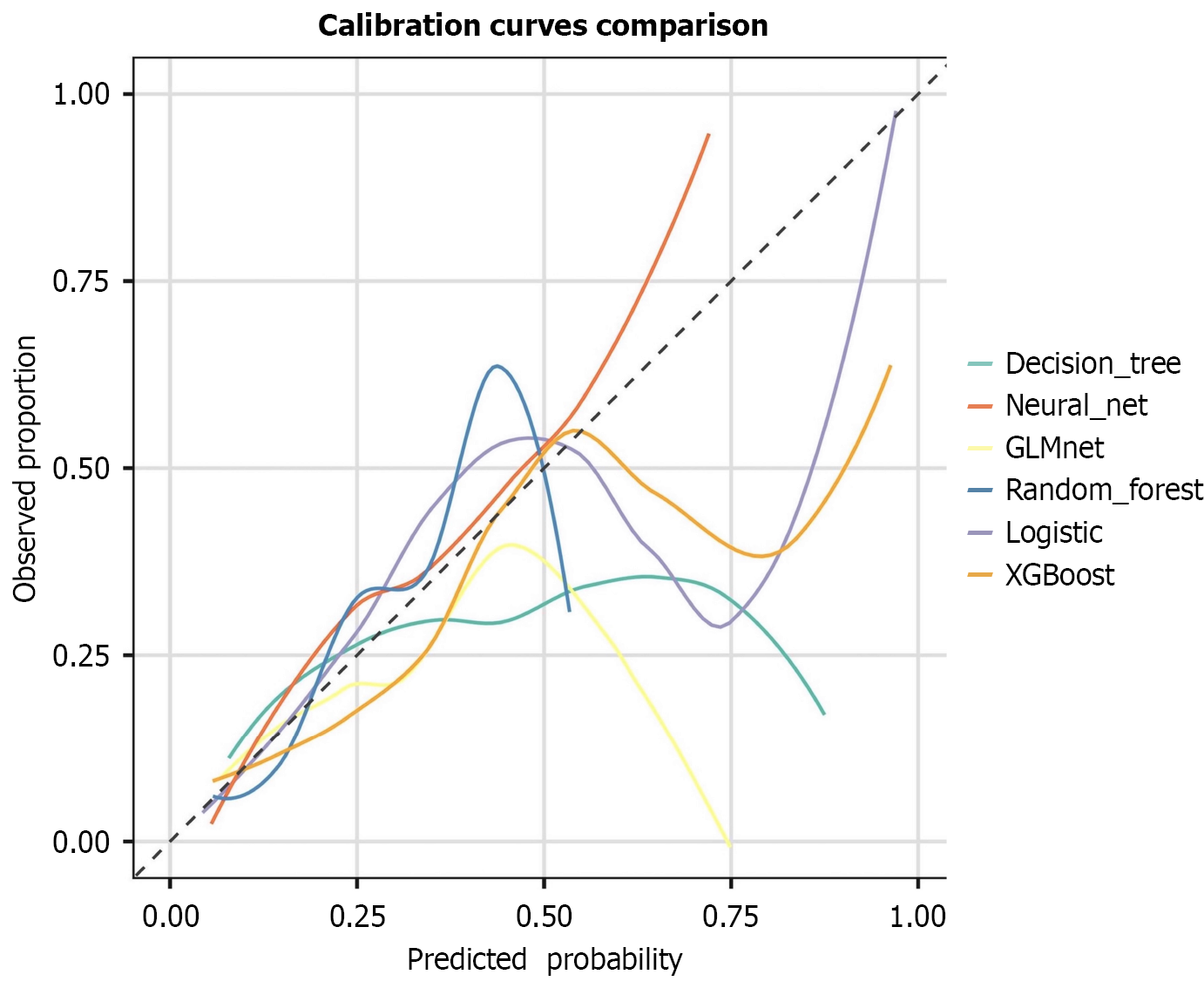
ROC curve analysis confirmed the optimal discriminative ability of NNE (AUC = 0.789), followed by logistic regression (AUC = 0.786) and GLMnet (AUC = 0.776) (Figure 3). DCA revealed that NNE provided the greatest clinical net benefit across threshold probabilities, outperforming logistic regression and GLMnet (Figure 4). Calibration plots indicated that the predictions of NNE best approximated the ideal diagonal, demonstrating excellent calibration accuracy (Figure 5). In summary, NNE emerged as the most robust predictive model for Clavien-Dindo grade ≥ II complications following neoadjuvant therapy in GC patients, combining high discriminative power (AUC), clinical utility (DCA), and calibration accuracy.
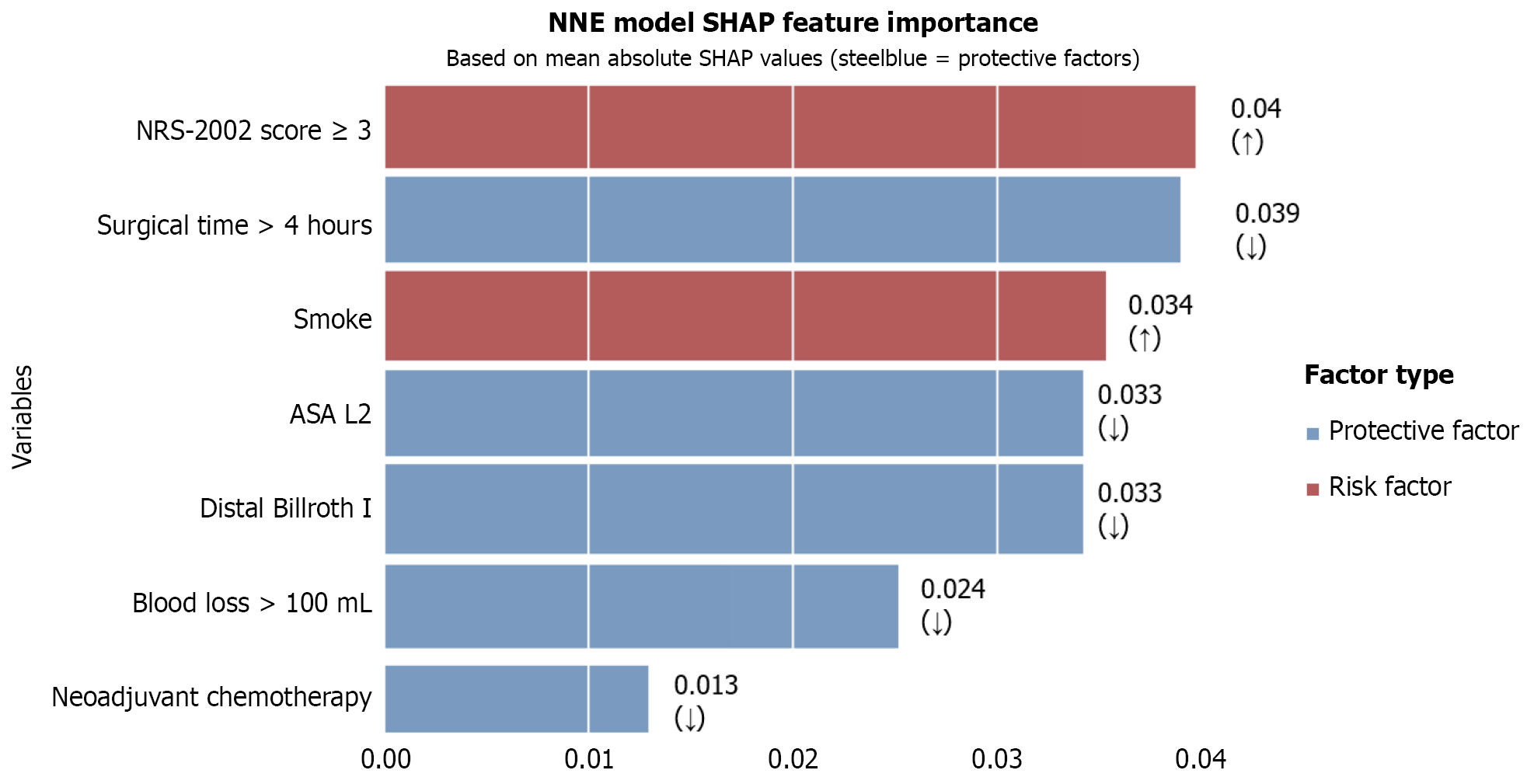
SHAP analysis of the optimal NNE model (Figure 6) revealed the following ranked order of predictive features for Clavien-Dindo grade ≥ II complications: (1) NRS-2002 score > 3 (risk factor); (2) Operating time ≤ 4 h (protective factor); (3) Smoking (risk factor); (4) ASA-L2 (protective factor); (5) Distal gastrectomy with Billroth I anastomosis (protective factor); (6) Blood loss ≤ 100 mL (protective factor); and (7) Neoadjuvant chemotherapy (protective factor).
Based on the aforementioned SHAP analysis results, we performed subgroup analysis focusing on the type of neoadjuvant therapy. The cohort was stratified into three subgroups: Neoadjuvant radiotherapy alone, neoadjuvant chemoradiotherapy, and neoadjuvant chemotherapy combined with immunotherapy or targeted therapy. Propensity score matching was applied to compare these subgroups in a pairwise manner. The baseline characteristics after matching are presented in Supplementary Tables 1-3. χ2 tests conducted on the matched data revealed no significant differences in the incidence of postoperative complications among the different neoadjuvant therapy regimens (Supplementary Table 4).
Neoadjuvant therapy has become an established treatment for locally advanced GC, demonstrating efficacy in tumor downstaging and improved prognosis[17,18]. However, research on postoperative complications following neoadjuvant-treated resection remain limited. Given ML proven utility in predicting oncological outcomes[19,20], this study leveraged six ML algorithms within the mlr3 framework utilizing nested cross-validation for enhanced stability, rigorous leakage prevention, and robust evaluation in limited sample sizes[21] to analyze Clavien-Dindo grade ≥ II complications based on preoperative/intraoperative variables. The developed predictive models identified key risk factors, offering clinical utility for preoperative risk stratification, intraoperative decision-making, and early postoperative management to mitigate complications and optimize recovery.
This study identified smoking status, NRS-2002 score ≥ 3, ASA status, neoadjuvant therapy, surgical approach, operating time > 4 h, and blood loss > 100 mL as significant predictors of Clavien-Dindo grade ≥ II complications following neoadjuvant therapy for GC. Among the six ML models, the NNE model demonstrated superior predictive performance, achieving the highest AUC (0.789) compared with conventional logistic regression (AUC = 0.786). The NNE model also exhibited optimal clinical utility on DCA and maintained excellent calibration accuracy. SHAP value analysis further revealed the relative importance of predictive features in the NNE model ranked in descending order: NRS-2002 score; operating time; smoking status; ASA status; surgical approach; blood loss; and neoadjuvant therapy. It is easy to find that surgical factors represent the most significant determinants of Clavien-Dindo grade ≥ II complications in patients with GC patients following neoadjuvant therapy.
Our findings demonstrate that both distal and total gastrectomy with Roux-en-Y reconstruction are associated with significantly higher rates of Clavien-Dindo grade ≥ II complications compared with distal gastrectomy with Billroth I reconstruction, a result that aligns with the 2024 multicenter study (n = 2508) evaluating complication risk factors in GC surgery[22]. This association may be explained by the increased technical complexity and greater tissue trauma associated with this anastomotic technique. The study further demonstrated that intraoperative blood loss > 100 mL serves as a risk factor, corroborating results reported by Yu et al[22]. The underlying pathophysiology likely involves impaired gastrointestinal perfusion during significant hemorrhage, potentially increasing the risk of anastomotic leakage and related complications[23].
Additionally, operating time > 4 h was significantly associated with postoperative complications in this study. These findings align with previous research demonstrating that surgical procedures > 4 h correlated with higher complication rates and infection rates following radical gastrectomy[24,25]. The cumulative evidence suggests that surgical manage
The NRS-2002 score is a commonly used clinical tool for nutritional screening in patients with GC with a score ≥ 3 indicating nutritional risk[26]. This paper demonstrated that an NRS-2002 score ≥ 3 is a risk factor for postoperative complications in patients with GC receiving neoadjuvant therapy. Previous research has shown that an NRS-2002 score ≥ 5 is an independent risk factor for postoperative infections in patients with GC[27] while malnutrition is associated with increased complications and poor prognosis following various oncological operations[28,29]. These findings align with the results of the present study, underscoring the necessity of perioperative nutritional assessment. For patients with GC undergoing neoadjuvant therapy who are at nutritional risk, nutritional support therapy should be implemented to prevent postoperative complications and improve clinical outcomes.
ASA status serves as a critical preoperative risk assessment tool with existing literature demonstrating a significant correlation between higher ASA status (III/IV) and increased postoperative complication rates in GC surgery[15,30], a finding consistent with our study results. While this association may be partially mediated through elevated infection risks secondary to impaired physiological reserve, the precise mechanistic pathways warrant further investigation through prospective, pathophysiology-focused studies.
Substantial evidence has established smoking as a significant risk factor for GC development[31,32] with studies demonstrating a dose-response relationship in which prolonged smoking time increases risk while extended smoking cessation reduces susceptibility[33]. The current study further identified smoking as a risk factor for postoperative complications in patients with GC receiving neoadjuvant therapy. These findings suggest that smoking not only contributes to gastric carcinogenesis but also adversely impacts surgical outcomes although the underlying mechanisms warrant further investigation. Importantly, these results highlight the critical need for comprehensive smoking cessation counseling prior to surgery in patients who smoke and undergo neoadjuvant therapy as this intervention may significantly reduce the risk of postoperative complications.
In addition, the results of this study suggest that patients receiving neoadjuvant therapy combined with immunotherapy or targeted therapy may be at a higher risk of postoperative complications, representing a novel finding. To clarify whether the observed association between different neoadjuvant therapy and postoperative complications was attributable to the treatments themselves or to patient selection bias, we conducted a subgroup analysis based on the specific neoadjuvant therapy received. The results demonstrated that after propensity score matching there were no significant differences in postoperative complication rates among the various neoadjuvant therapy subgroups, a finding consistent with previous studies[34-36]. This suggests that the impact of neoadjuvant therapy identified by our model is primarily driven by patient selection, meaning that patients receiving neoadjuvant therapy combined with immunotherapy or targeted therapy inherently possessed higher baseline risks rather than being a direct effect of the therapies themselves. This conclusion is supported by our SHAP analysis, which indicated that the type of neoadjuvant therapy had a low overall importance ranking in the model. Although the subgroup analysis suggested that immunotherapy and targeted therapy are not independent risk factors for postoperative complications, retaining this variable in the model contributed to enhancing its overall discriminative ability. Therefore, we opted to include this feature in the final model.
The findings of this study demonstrated that in terms of overall discriminative ability (AUC) both our proposed NNE model (AUC = 0.789) and logistic regression model (AUC = 0.786) achieved superior performance, significantly outperforming the other comparative models. However, the NNE model exhibited a narrower AUC confidence interval (0.739-0.840) vs the logistic regression model (0.606-0.966), indicating stronger and more stable overall classification and discriminative capability. The NNE model (0.731) performed comparably to logistic regression (0.763), GLMnet (0.739), and random forest (0.735) but significantly better than XGBoost (0.606) and decision tree (0.371).
Concurrently, the NNE model again showed a narrower confidence interval (0.739-0.840), suggesting its excellence and stability in positive case identification. Importantly, this comparative analysis extended beyond traditional performance metrics. In terms of clinical utility, DCA revealed that the NNE model provided the highest net clinical benefit across the vast majority of threshold probability ranges. This implies that using the NNE model for clinical decision-making predictions can yield better expected outcomes for patients compared with other models, underscoring its high practical value.
Regarding predictive reliability, the calibration curve indicated that the predicted probabilities of the NNE model align most closely with the actual observed probabilities with the curve tightly following the ideal reference line, demon
To enhance the practicality of the NNE model and facilitate future validation, we have shared the model data via a stable cloud repository (https://drive.google.com/file/d/1pDo_XeJv1HsZxDzNGaBsJbPhzye0ehjN/view?usp=sharing), providing rapid and real-time clinical reference for surgeons. A screenshot of the application interface is provided in Supplementary Figure 2. Based on the SHAP analysis and NNE predictive model, surgeons can estimate the probability of Clavien-Dindo grade ≥ II complications occurring in patients during the preoperative and early postoperative periods, enabling individualized risk assessment and precision medicine. For instance, according to the predictive model outcomes, surgeons may optimize perioperative clinical decision-making, such as implementing preoperative nutritional support, smoking cessation education, or selecting superior surgical approaches, thereby potentially preventing complications and improving patient prognosis.
This study had several limitations that should be acknowledged. The retrospective design and reliance on a single high volume center dataset may impact the generalization of our findings. Additionally, continuous variables such as operative time and blood loss were subject to measurement inaccuracies inherent in retrospective data collection, which may have resulted in loss of granularity. While these constraints are currently unavoidable due to limited availability of clinical data for patients with neoadjuvant-treated GC, we are implementing two key strategies to address these limitations: (1) Initiating prospective multicenter collaborative studies to enhance sample diversity and develop more robust predictive models; and (2) Sharing the model data via an open-access repository (https://drive.google.com/file/d/1pDo_XeJv1HsZxDzNGaBsJbPhzye0ehjN/view?usp=sharing) to facilitate external validation and provide clinical reference for surgical teams worldwide. This dual approach will significantly advance the translation of our predictive models into clinical practice while ensuring continuous model optimization through global data integration. Subsequent studies will systematically compare the diagnostic efficiency of this complication prediction model with conventional clinical risk scores such as Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity and American College of Surgeons National Surgical Quality Improvement Project, representing a major focus of our future research agenda.
ML demonstrated significant potential for predicting Clavien-Dindo grade ≥ II complications in patients with GC receiving neoadjuvant therapy and laparoscopic radical gastrectomy. This study identified seven key predictive factors for Clavien-Dindo grade ≥ II complications: Smoking status; NRS-2002 score ≥ 3; ASA status; neoadjuvant therapy; surgical approach; operating time > 4 h; and blood loss > 100 mL. Among the predictive models developed using these factors, the NNE model demonstrated superior performance in predicting Clavien-Dindo grade ≥ II complications, achieving an AUC of 0.789 along with better discrimination, clinical utility, and calibration. With the implementation of the NNE prediction model web tool, it may facilitate the development of personalized precision medicine strategies to mitigate complications and optimize postoperative recovery in patients with GC.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56694] [Article Influence: 7086.8] [Reference Citation Analysis (135)] |
| 2. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 973] [Article Influence: 162.2] [Reference Citation Analysis (0)] |
| 3. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3311] [Article Influence: 551.8] [Reference Citation Analysis (6)] |
| 4. | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 756] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 5. | Tavakoli F, Khatami SS, Momeni F, Azadbakht J, Ghasemi F. Gastric Cancer Diagnosis: From Imaging Techniques to Biochemical Biomarkers. Curr Mol Med. 2021;21:355-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 916] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 7. | Fong C, Johnston E, Starling N. Neoadjuvant and Adjuvant Therapy Approaches to Gastric Cancer. Curr Treat Options Oncol. 2022;23:1247-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1174] [Article Influence: 293.5] [Reference Citation Analysis (0)] |
| 9. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 848] [Article Influence: 282.7] [Reference Citation Analysis (2)] |
| 10. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 516] [Article Influence: 103.2] [Reference Citation Analysis (2)] |
| 11. | Wang JB, Gao YX, Ye YH, Zheng QL, Luo HY, Wang SH, Zhang T, Jin QW, Zheng CH, Li P, Lin JX, Chen QY, Cao LL, Yang YH, Huang CM, Xie JW. Comprehensive multi-omics analysis of pyroptosis for optimizing neoadjuvant immunotherapy in patients with gastric cancer. Theranostics. 2024;14:2915-2933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, Wei M, Zhou W, Wang J, Zhao Z, Dai X, Xu Q, Zhang X, Huang M, Huang K, Wang J, Li J, Sheng L, Liu L. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (1)] |
| 13. | Yu Y, He Z, Ouyang J, Tan Y, Chen Y, Gu Y, Mao L, Ren W, Wang J, Lin L, Wu Z, Liu J, Ou Q, Hu Q, Li A, Chen K, Li C, Lu N, Li X, Su F, Liu Q, Xie C, Yao H. Magnetic resonance imaging radiomics predicts preoperative axillary lymph node metastasis to support surgical decisions and is associated with tumor microenvironment in invasive breast cancer: A machine learning, multicenter study. EBioMedicine. 2021;69:103460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 14. | Wang D, Li J, Sun Y, Ding X, Zhang X, Liu S, Han B, Wang H, Duan X, Sun T. A Machine Learning Model for Accurate Prediction of Sepsis in ICU Patients. Front Public Health. 2021;9:754348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Hong QQ, Yan S, Zhao YL, Fan L, Yang L, Zhang WB, Liu H, Lin HX, Zhang J, Ye ZJ, Shen X, Cai LS, Zhang GW, Zhu JM, Ji G, Chen JP, Wang W, Li ZR, Zhu JT, Li GX, You J. Machine learning identifies the risk of complications after laparoscopic radical gastrectomy for gastric cancer. World J Gastroenterol. 2024;30:79-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (5)] |
| 16. | Ji K, Shi L, Feng Y, Wang L, Guo H, Li H, Xing J, Xia S, Xu B, Liu E, Zheng Y, Li C, Liu M. Construction and interpretation of machine learning-based prognostic models for survival prediction among intestinal-type and diffuse-type gastric cancer patients. World J Surg Oncol. 2024;22:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Kang YK, Kim HD, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, Ryu MH, Rha SY, Chung IJ, Kim IH, Oh SC, Park YS, Cheong JH, Jeong O, Heo MH, Kim HK, Park C, Yoo CH, Kang SY, Zang DY, Jang YJ, Sul JY, Kim JG, Kim BS, Beom SH, Hwang JE, Ryu SW, Kook MC, Ryoo BY, Kim H, Yoo MW, Lee NS, Lee SH, Noh SH. Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer: Updated Overall Survival Outcomes From Phase III PRODIGY. J Clin Oncol. 2024;42:2961-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 18. | Kong SH, Kurokawa Y, Yook JH, Cho H, Kwon OK, Masuzawa T, Lee KH, Matsumoto S, Park YS, Honda H, Ryu SW, Ishikawa T, Kang HJ, Nabeshima K, Im SA, Shimokawa T, Kang YK, Hirota S, Yang HK, Nishida T. Long-term outcomes of a phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumors of the stomach. Gastric Cancer. 2023;26:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Skrede OJ, De Raedt S, Kleppe A, Hveem TS, Liestøl K, Maddison J, Askautrud HA, Pradhan M, Nesheim JA, Albregtsen F, Farstad IN, Domingo E, Church DN, Nesbakken A, Shepherd NA, Tomlinson I, Kerr R, Novelli M, Kerr DJ, Danielsen HE. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020;395:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 410] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 20. | Jee J, Fong C, Pichotta K, Tran TN, Luthra A, Waters M, Fu C, Altoe M, Liu SY, Maron SB, Ahmed M, Kim S, Pirun M, Chatila WK, de Bruijn I, Pasha A, Kundra R, Gross B, Mastrogiacomo B, Aprati TJ, Liu D, Gao J, Capelletti M, Pekala K, Loudon L, Perry M, Bandlamudi C, Donoghue M, Satravada BA, Martin A, Shen R, Chen Y, Brannon AR, Chang J, Braunstein L, Li A, Safonov A, Stonestrom A, Sanchez-Vela P, Wilhelm C, Robson M, Scher H, Ladanyi M, Reis-Filho JS, Solit DB, Jones DR, Gomez D, Yu H, Chakravarty D, Yaeger R, Abida W, Park W, O'Reilly EM, Garcia-Aguilar J, Socci N, Sanchez-Vega F, Carrot-Zhang J, Stetson PD, Levine R, Rudin CM, Berger MF, Shah SP, Schrag D, Razavi P, Kehl KL, Li BT, Riely GJ, Schultz N; MSK Cancer Data Science Initiative Group. Automated real-world data integration improves cancer outcome prediction. Nature. 2024;636:728-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 21. | Parvandeh S, Yeh HW, Paulus MP, McKinney BA. Consensus features nested cross-validation. Bioinformatics. 2020;36:3093-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 22. | Yu Z, Liang C, Xu Q, Li R, Gao J, Gao Y, Liang W, Li P, Zhao X, Zhou S. Analysis of postoperative complications and long term survival following radical gastrectomy for patients with gastric cancer. Sci Rep. 2024;14:23869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Renna MS, Grzeda MT, Bailey J, Hainsworth A, Ourselin S, Ebner M, Vercauteren T, Schizas A, Shapey J. Intraoperative bowel perfusion assessment methods and their effects on anastomotic leak rates: meta-analysis. Br J Surg. 2023;110:1131-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Xiao H, Quan H, Pan S, Yin B, Luo W, Huang G, Ouyang Y. Impact of peri-operative blood transfusion on post-operative infections after radical gastrectomy for gastric cancer: a propensity score matching analysis focusing on the timing, amount of transfusion and role of leukocyte depletion. J Cancer Res Clin Oncol. 2018;144:1143-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Xiao Y, Ren BC, Zhang T, Peng D, Min J. Factors influencing postoperative complications in patients with gastric cancer: A retrospective study. World J Gastrointest Surg. 2025;17:101047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Chen XY, Zhang XZ, Ma BW, Li B, Zhou DL, Liu ZC, Chen XL, Shen X, Yu Z, Zhuang CL. A comparison of four common malnutrition risk screening tools for detecting cachexia in patients with curable gastric cancer. Nutrition. 2020;70:110498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Zhang L, Wang S, Gao X, Gao T, Huang L, Lian B, Gu Y, Chen J, Guo D, Jia Z, Wang Y, Gong F, Zhou J, Xue Z, Chen Z, Xu J, Wang L, Qian J, Deng G, Hu H, Nie Y, Li G, Li M, Yang H, Zhao W, Zhou Y, Qin H, Wu X, Wang K, Chi Q, Yu J, Tang Y, Zhang P, Jin G, Ouyang B, Li G, Hang D, Wang X. Poor Pre-operative Nutritional Status Is a Risk Factor of Post-operative Infections in Patients With Gastrointestinal Cancer-A Multicenter Prospective Cohort Study. Front Nutr. 2022;9:850063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | GlobalSurg Collaborative and NIHR Global Health Unit on Global Surgery. Impact of malnutrition on early outcomes after cancer surgery: an international, multicentre, prospective cohort study. Lancet Glob Health. 2023;11:e341-e349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 29. | Wang SL, Zhang FM, Chen CB, Dong QT, Liu S, Yu Z, Shen X, Zhuang CL. Comparison between AWGC-cachexia and GLIM-malnutrition in patients with gastric cancer. Eur J Surg Oncol. 2024;50:108580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Nishibeppu K, Sakuramoto S, Matsui K, Ebara G, Fujita S, Fujihata S, Oya S, Lee S, Miyawaki Y, Sugita H, Sato H, Yamashita K. Dismal prognosis of elderly gastric cancer patients who underwent gastrectomy with American Society of Anesthesiologists (ASA) 3. Langenbecks Arch Surg. 2022;407:3413-3421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Hatta W, Koike T, Asano N, Hatayama Y, Ogata Y, Saito M, Jin X, Uno K, Imatani A, Masamune A. The Impact of Tobacco Smoking and Alcohol Consumption on the Development of Gastric Cancers. Int J Mol Sci. 2024;25:7854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Scherübl H. [Smoking tobacco and cancer risk]. Dtsch Med Wochenschr. 2021;146:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, Zhang ZF, Matsuo K, Ito H, Hu J, Johnson KC, Yu GP, Palli D, Ferraroni M, Muscat J, Lunet N, Peleteiro B, Malekzadeh R, Ye W, Song H, Zaridze D, Maximovitch D, Aragonés N, Castaño-Vinyals G, Vioque J, Navarrete-Muñoz EM, Pakseresht M, Pourfarzi F, Wolk A, Orsini N, Bellavia A, Håkansson N, Mu L, Pastorino R, Kurtz RC, Derakhshan MH, Lagiou A, Lagiou P, Boffetta P, Boccia S, Negri E, La Vecchia C. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev. 2018;27:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 34. | Li C, Duan Y, Zhou S, Tang T, Yang Y, Zhou L. Evaluating the efficacy and safety of neoadjuvant immunochemotherapy versus chemotherapy in locally advanced gastric cancer undergoing radical gastrectomy: a retrospective study. World J Surg Oncol. 2025;23:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Kang YK, Kim HD, Cho H, Park YS, Lee JS, Ryu MH. Phase 2 study of neoadjuvant durvalumab plus docetaxel, oxaliplatin, and S-1 with surgery and adjuvant durvalumab plus S-1 for resectable locally advanced gastric cancer. J Immunother Cancer. 2025;13:e010635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Zhang X, Wang J, Wang G, Zhang Y, Fan Q, Lu C, Hu C, Sun M, Wan Y, Sun S, Wang J, Zhang L, Shu Y, Luo J, Zhu D, Shen Z, Yao S, Shi Q, Yang J, Shen L; GEMSTONE-303 Investigators. First-Line Sugemalimab Plus Chemotherapy for Advanced Gastric Cancer: The GEMSTONE-303 Randomized Clinical Trial. JAMA. 2025;333:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/