Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.111481
Revised: August 21, 2025
Accepted: October 27, 2025
Published online: December 27, 2025
Processing time: 177 Days and 20.9 Hours
Postoperative recurrence is common in Crohn’s disease (CD), with endoscopic lesions in a majority of patients by 12 months after surgery. Ileocolonoscopy is the reference standard but is invasive and poorly suited to frequent surveillance. Intestinal ultrasound (IUS) - including small intestine contrast ultrasound and contrast enhanced ultrasound - is a repeatable, noninvasive alternative.
To summarize the evidence on the diagnostic accuracy and prognostic value of IUS for detecting postoperative recurrence in CD.
We systematically searched PubMed and EMBASE through June 2025 for original English-language studies evaluating IUS against clinical or endoscopic outcomes in postoperative CD. This scoping review was conducted and reported in acc
Bowel wall thickness thresholds of ≥ 5 mm at the neo-terminal ileum predict endoscopic recurrence with sensitivities 81%-94% and specificities 86%-100%; lower cutoffs at the anastomosis (≥ 3-3.5 mm) also carry risk (data from singlecenter cohorts). Dualsite assessment (neo-terminal ileum + ileocolonic anastomosis) improves performance. Adding Doppler hyperemia or mesenteric lymphadenopathy increases accuracy; combining bowel wall thickness ≥ 3 mm with fecal calprotectin ≥ 50 μg/g yields high specificity (approximately 93%-100%) with a negative predictive value of nearly 95% when both are negative. Contrast enhanced ultrasound-based composite scores reach approximately 98% diagnostic accuracy in prospective cohorts. Small intestine contrast ultrasound shows similarly strong early diagnostic performance - for example, an area under the receiver operating characteristic curve up to 0.95 when using ileocolonic anastomosis wall thickness ≥ 3 mm to 3.5 mm plus lesion length, with 82%-94% sensitivity and > 90% specificity reported even within 7 days post-resection. Overall, IUS shows moderate agreement with endoscopy (κ approximately 0.5-0.8) and stronger pro
IUS can be integrated into postoperative surveillance algorithms - particularly within the first year - and can reduce routine endoscopy in selected patients. Research priorities include standardized thresholds and composite scoring, consensus training/competency, and multicenter validation including artificial intelligenceassisted interpretation.
Core Tip: Intestinal ultrasound is a practical, non-invasive tool for monitoring postoperative Crohn’s disease. Bowel wall thickness > 3-5 mm, especially when persistent or worsening, predicts recurrence. Diagnostic accuracy improves with dual-site assessment (neo-terminal ileum and ileo-colonic anastomosis), Doppler hyperemia, and lymphadenopathy. Integration with fecal calprotectin enhances specificity and negative predictive value. Advanced techniques like contrast enhanced ultrasound and small intestine contrast ultrasound further refine detection. Early assessment within 12 months post-surgery is most prognostic. Intestinal ultrasound is well-suited for repeated follow-up, and when used systematically, may reduce reliance on routine ileocolonoscopy in selected postoperative patients.
- Citation: Pal P, Kata P, Mateen MA, Gupta R, Tandan M, Duvvur NR. Intestinal ultrasound for monitoring postoperative Crohn’s disease: A systematic review and clinical implications. World J Gastrointest Surg 2025; 17(12): 111481
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/111481.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.111481
Postoperative recurrence (POR) remains a major clinical challenge in the long-term management of Crohn’s disease (CD), with endoscopic lesions developing in over 70% of patients within one year of ileocolonic resection[1]. While ileocolono
Over the past two decades, multiple studies have investigated the utility of IUS for detecting POR, with bowel wall thickness (BWT) emerging as a key sonographic marker. Enhancements such as Doppler vascularity, contrast enhanced ultrasound (CEUS), and small intestine contrast ultrasound (SICUS) have further expanded its potential[3,4]. However, current literature remains fragmented, with variability in timing, thresholds, definitions of recurrence, and operator expertise. There is no standardized algorithm integrating IUS into postoperative surveillance, and many clinicians remain uncertain about its comparative value vs fecal calprotectin (FCP) or cross-sectional imaging[5]. Additionally, questions persist regarding the optimal timing of IUS assessments and the predictive value of serial measurements or composite indices.
This systematic review aims to synthesize the literature on postoperative IUS in CD, with specific objectives to: (1) Summarize its diagnostic accuracy for recurrence detection; (2) Outline its prognostic value for clinical and surgical outcomes; and (3) Identify evidence gaps to guide future research. Unlike prior reviews, this synthesis integrates emerging evidence on CEUS, SICUS, and composite indices, thereby updating clinicians on the latest modalities.
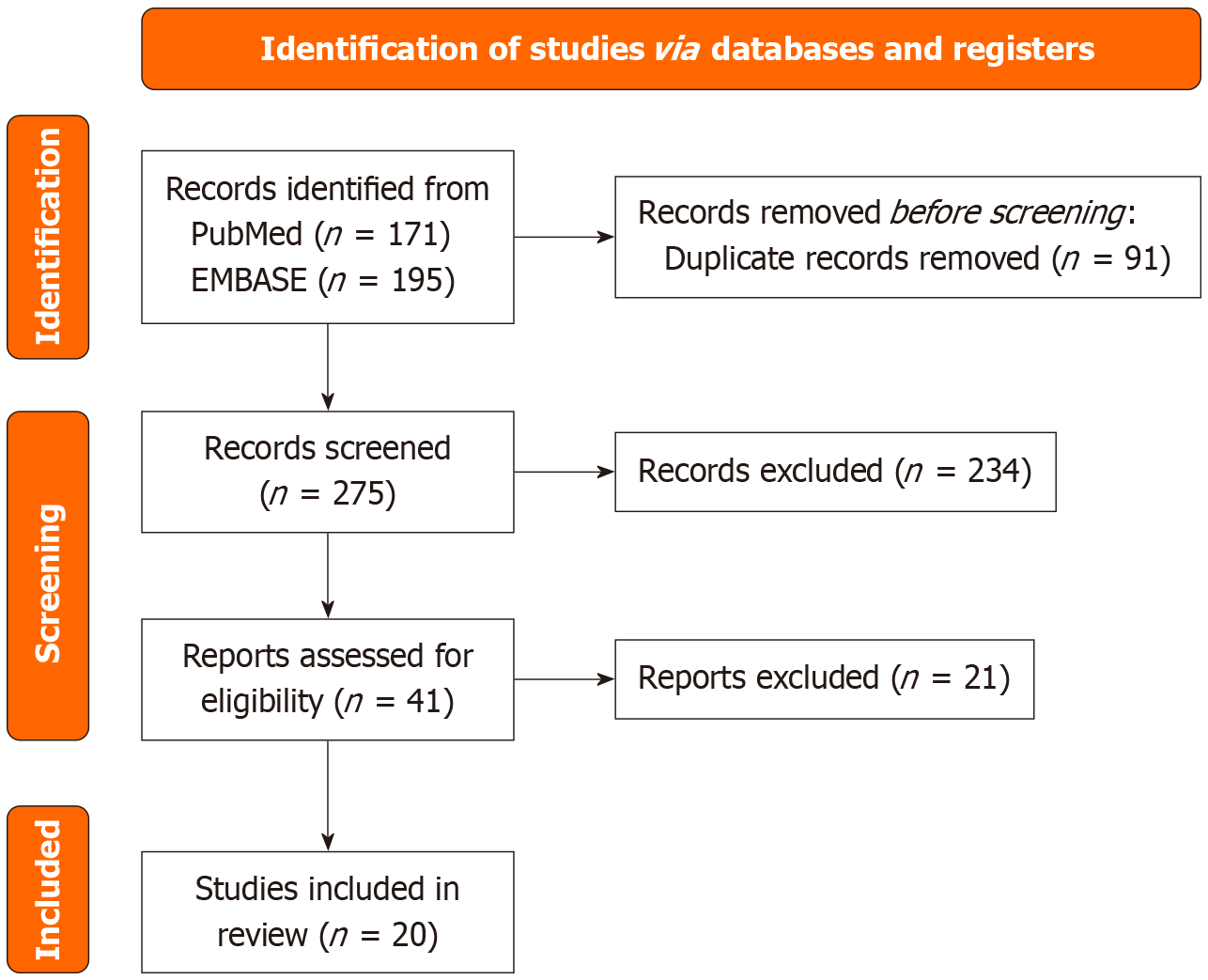
This scoping review was conducted to synthesize existing evidence on the use of IUS for detecting and monitoring POR in CD. A systematic search was performed in PubMed and EMBASE databases through June 30, 2025 using a structured strategy (“Crohn Disease” OR “Crohn’s disease”) AND (“Postoperative Complications” OR “post-surgical recurrence” OR “post-operative recurrence”) AND (“Ultrasonography” OR “ultrasonography” OR “bowel sonography”) OR (“Intestine, Small/diagnostic imaging” OR “small intestine contrast ultrasound” OR “SICUS”) OR (“contrast enhanced ultrasound” OR “CEUS”) (Figure 1). This scoping review was conducted and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses guideline. Only original research articles published in English were included; editorials, reviews, non-English publications, and conference abstracts were excluded. Two reviewers (Pal P, Kata P) independently screened titles/abstracts and full texts using a standardized form; disagreements were resolved by consensus with a third reviewer (Mateen MA). Although formal risk-of-bias tools were not applied given the scoping design, we qualitatively noted study limitations such as sample size, single-center design, and operator dependence.
A total of 366 records were retrieved (81 from PubMed, 186 from EMBASE), and after removing 8 duplicates, 259 unique articles were screened. Following title/abstract review and full-text eligibility assessment, 41 articles were selected for detailed evaluation, and 20 met the inclusion criteria for final synthesis. We used a prespecified standardized extraction template (study design, setting, sample, timing post-operative, sonographic parameters and thresholds, reference standards, diagnostic/prognostic metrics, and integration with biomarkers/imaging).
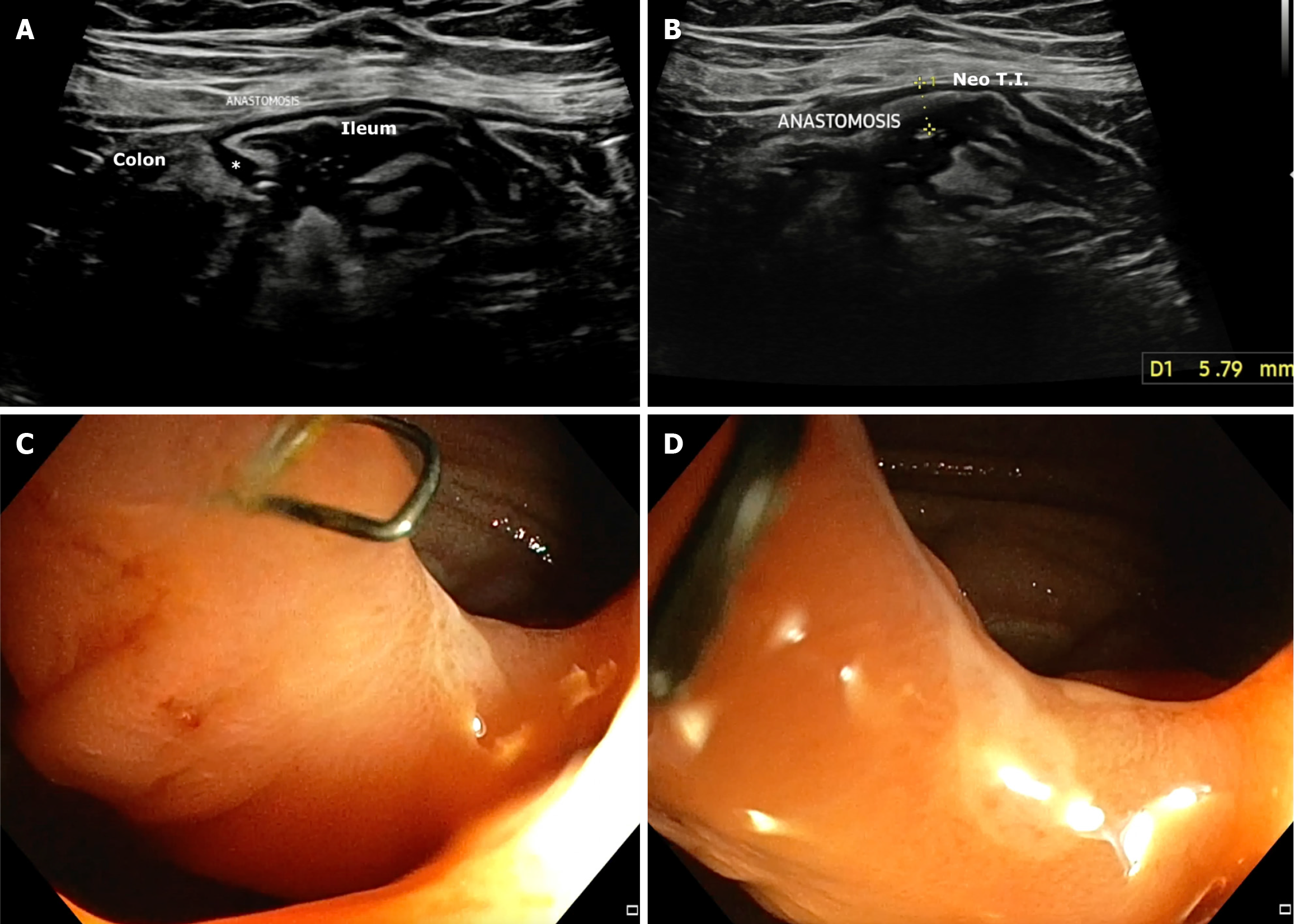
BWT: The role of IUS in predicting POR in CD has steadily evolved through a series of landmark studies, each building on prior findings (Table 1). The earliest, Andreoli et al[6], demonstrated that BWT > 5 mm in the neo-terminal ileum (NTI) strongly predicted endoscopic recurrence, with 81% sensitivity and 86% specificity (overall diagnostic accuracy 83%). This established a foundational BWT threshold and showed that IUS findings correlated well with endoscopic outcomes, even when measured within weeks of colonoscopy. Subsequently, Parente et al[1] added longitudinal perspective by showing that persistent or increased BWT (≥ 6 mm or < 40% reduction from baseline) at 12 months post-surgery was associated with a markedly elevated risk of symptomatic recurrence [hazard ratio (HR) = 8.9]. This study emphasized the value of serial monitoring over time and introduced echo pattern abnormalities (e.g., hypoechoic or mixed patterns) as independent predictors of poor outcomes. Rispo et al[7] focused on early prediction, showing that BWT > 5 mm within just 12 months postoperatively predicted severe endoscopic recurrence (Rutgeerts ≥ i3) with 94% sensitivity and 100% specificity (Figure 2). This reinforced BWT as a surrogate for endoscopic severity and highlighted its potential to replace ileocolonoscopic in high-risk patients. Cammarota et al[8] expanded the evidence to a larger retrospective cohort, showing that even modest thickening (BWT > 3 mm at the anastomotic site) was associated with an increased risk of surgical recurrence (relative risk = 2.1). Importantly, this study explored graduated BWT thresholds (> 3 mm, > 4 mm, > 5 mm, > 6 mm), demonstrating that risk increased incrementally with wall thickness, thus offering a framework for risk stratification. Across prospective studies, sensitivity for detecting endoscopic recurrence ranged from 77% to 94%, while specificity consistently exceeded 85%.
| Ref. | Year | Design | Number | Site assessed | Timing | BWT threshold | Outcome predicted | Sensitivity | Specificity | Statistic |
| Andreoli et al[6] | 1998 | Prospective | 41 | NTI | Within 2 weeks of colonoscopy | > 5 mm | Endoscopic recurrence | 81% | 86% | Accuracy = 83% |
| Parente et al[1] | 2004 | Prospective | 127 | NTI | 12 months post-operative | ≥ 6 mm or < 40% reduction | Clinical recurrence | NR | NR | HR = 8.9 |
| Rispo et al[7] | 2006 | Prospective | 45 | NTI | 12 months | > 5 mm | Endoscopic recurrence (Rutgeerts ≥ i3) | 94% | 100% | NR |
| Pallotta et al[10] | 2010 | Prospective | 58 (111 evaluations) | Anastomosis + NTI | 6-24 months post- operative | > 3.5 mm (ICA) + > 3 mm (NTI) | Endoscopic recurrence | 100% (for ICA > 3.5 mm) | NR | AUROC = 0.95 (combined) |
| Cammarota et al[8] | 2013 | Retrospective | 196 | Anastomosis | 6-15 months post- operative | > 3 mm | Surgical recurrence | NR | NR | RR = 2.1 |
Prognostic value of pre-operative and post-operative BWT monitoring by IUS in CD: Beyond its cross-sectional diagnostic role, BWT assessed via IUS also offers dynamic prognostic insights when tracked over time. In a prospective cohort, Maconi et al[9] evaluated both pre-operative and post-operative IUS findings in patients undergoing conservative surgery (strictureplasty or limited resection). They found that patients with unchanged or worsened BWT at 6 months postoperatively had significantly higher risks of both clinical recurrence (HR = 9.98) and surgical recurrence (HR = 16.15). Furthermore, a greater preoperative extent of bowel wall involvement - especially longer segments of thickened bowel - was also predictive of adverse outcomes. Several studies linked IUS findings with clinical outcomes, including reduced need for repeat endoscopy and lower rates of surgical recurrence when BWT regressed after surgery. These findings support the role of serial ultrasound monitoring in postoperative care and emphasize that not only absolute BWT thresholds but also the trajectory of BWT change can help stratify risk. Integrating early sonographic improvement into routine surveillance pathways may therefore help identify patients likely to benefit from intensified therapeutic in
Dual-site assessment of BWT: While BWT at either the ileo-colonic anastomosis (ICA) or NTI independently predicts POR in CD, combining measurements from both sites significantly improves diagnostic accuracy. In a prospective cohort study, Pallotta et al[10] demonstrated that BWT > 3.5 mm at the ICA and > 3 mm at the NTI, when assessed together, yielded an area under the receiver operating characteristics curve of 0.95 for detecting endoscopic recurrence. This combined approach outperformed assessment at either site alone and supports the use of dual-site sonographic eva
Study heterogeneity: Across included cohorts, operator experience, probe frequency, machine vendors/settings, and BWT cut-offs (≥ 3-6 mm) varied, as did timing of assessment and reference standards (endoscopy vs mixed endpoints). These factors likely contributed to betweenstudy variability and should be addressed in future standardization efforts. All included studies were conducted in adult populations; pediatric cohorts were not represented.
Integrating additional IUS features in POR detection: Beyond BWT, several ultrasound and biomarker parameters offer incremental value in identifying POR of CD (Table 2). Furfaro et al[5] demonstrated that while BWT ≥ 3 mm alone is a modest predictor (accuracy 73%), adding mesenteric lymphadenopathy (odds ratio = 15.63) or (FCP ≥ 50 μg/g; odds ratio = 8.58) significantly improves diagnostic performance. The combination of BWT and FCP enhances specificity to 93%, and adding lymph nodes further eliminates false positives (100% specificity). Complementing this, Yebra Carmona et al[11] highlighted the role of hyperemia (grade ≥ 2 by color Doppler) as a key correlate of endoscopic recurrence and FCP levels. A dual-parameter approach of BWT > 3 mm plus hyperemia achieved a diagnostic accuracy of 83%, outperforming either parameter alone. These findings reinforce the use of composite IUS indices - including vascularity, lymph nodes, and BWT - in postoperative IBD care and suggest that when FCP is incorporated, non-invasive assessment may approach the reliability of ileocolonoscopy.
| Ref. | Year | Parameter | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy | Comment |
| Furfaro et al[5] | 2023 | BWT ≥ 3 mm | 77 | 65 | 81 | 59 | 73 | Independent predictor of POR; OR = 2.43 |
| Mesenteric lymph nodes | 35 | 97 | 95 | 43 | 56 | Strong predictor; OR = 15.63 | ||
| FCP ≥ 50 μg/g | 83 | 64 | 81 | 67 | 76 | Independent predictor; OR = 8.58 | ||
| BWT ≥ 3 mm + FCP ≥ 50 μg/g | 65 | 93 | 94 | 59 | 75 | Best combined predictor | ||
| BWT ≥ 3 mm + FCP ≥ 50 μg/g + LN+ | 33 | 100 | 100 | 59 | 66 | Highest specificity; no false positives | ||
| Yebra Carmona et al[11] | 2022 | BWT > 3 mm + hyperemia | 81 | 87 | 85 | 84 | 83 | Strong correlation with endoscopy and FCP |
| Hyperemia alone (grade ≥ 2) | 67 | 74 | 70 | 72 | 71 | Associated with endoscopic recurrence and FCP |
Multiple studies have consistently validated the role of SICUS in detecting POR in CD through evaluation of BWT and other sonographic features (Table 3). Onali et al[12] demonstrated that SICUS could identify POR in all patients across 1-3 years of follow-up, though correlation with moderate-to-severe endoscopic recurrence (Rutgeerts ≥ 2) was limited. In a robust multicenter study, Pallotta et al[10] showed that BWT > 3.5 mm at the ICA predicted all cases of endoscopic recurrence, and when combined with measurement of neo-terminal ileal lesion length, it achieved an excellent discriminatory value (AUROC: 0.95). Similarly, Calabrese et al[13] reported a diagnostic accuracy of 92.5% using a BWT threshold of 3 mm. Castiglione et al[14] further reinforced the early predictive potential of SICUS, demonstrating high sensitivity (82%-94%) and specificity (> 90%) for recurrence detection within just 7 days post-resection. Finally, Onali et al[15] emphasized the long-term utility of SICUS in monitoring postoperative CD recurrence, proposing its integration into routine surveillance alongside or even in place of ileocolonoscopy. Together, these studies affirm that BWT - particularly at the anastomotic site - offers a reliable, non-invasive metric for tracking postoperative disease recurrence, with enhanced predictive accuracy when combined with lesion length and clinical correlation.
| Ref. | Year | Design | Number | Timing of SICUS | BWT threshold | Sensitivity | Specificity | Accuracy/comments | Specificity | Statistic |
| Castiglione et al[14] | 2008 | Prospective | 40 | Within 7 days post-operative | > 3 mm | 82%-94% | > 90% | Early detection with high sensitivity | NR | HR = 8.9 |
| Calabrese et al[13] | 2009 | Prospective | 40 | Within 14 days post-operative | > 3 mm | 92.5% | NR | Accurate grading of severity | NR | RR = 2.1 |
| Onali et al[12] | 2010 | Prospective | 58 | 12 months post-operative | > 3 mm | 100% | NR | SICUS detected all cases of POR | 100% | NR |
| Onali et al[15] | 2016 | Retrospective | 58 | 3-year follow-up | > 3 mm | 100% | NR | Useful long-term monitoring tool | 86% | Accuracy = 83% |
| Biancone et al[3] | 2007 | Prospective | 72 | 6-12 months post-operative | > 3 mm | NR | NR | Correlation with capsule endoscopy | NR | AUROC = 0.95 (combined) |
| Pallotta et al[10] | 2010 | Prospective | 58 | 6-24 months post-operative | > 3.5 mm (ICA) + > 3 mm (NTI) | 100% | NR | AUROC = 0.95 (combined ICA + NTI) | NR | NR |
CEUS has emerged as a powerful adjunct to conventional IUS in the postoperative setting of CD (Table 4). In a landmark prospective study, Paredes et al[16] demonstrated that CEUS significantly enhances the detection of both mild and severe endoscopic recurrence. Using parietal contrast enhancement thresholds (> 34.5% for any recurrence and > 70% for severe), the study showed that a sonographic score combining BWT > 5 mm or enhancement > 46% achieved a diagnostic accuracy of 98.3% (κ = 0.95). For identifying moderate-to-severe recurrence, a stricter composite score (BWT > 5 mm, CEUS > 70%, or presence of fistula) reached 94% sensitivity and an AUROC of 0.836. Importantly, CEUS also identified cases with normal wall thickness but abnormal enhancement, allowing early detection of recurrence. Supporting these findings, Martínez et al[4] emphasized CEUS’s utility in distinguishing inflammatory from fibrotic postoperative lesions and its additive value in comprehensive sonographic assessment. Together, these studies confirm CEUS as a reliable, non-invasive alternative to endoscopy, particularly when layered into structured sonographic scoring systems.
| Ref. | Year | Technique | Sample size | Thresholds | Key findings |
| Paredes et al[16] | 2013 | CEUS | 60 | BWT > 3 mm; CEUS > 34.5%, CEUS > 46%, CEUS > 70% | CEUS improved diagnostic accuracy for endoscopic recurrence to 98.3% with score 2 (BWT > 5 mm or enhancement > 46%). Score 3 (BWT > 5 mm, enhancement > 70%, or fistula) detected 94% of severe recurrence. AUC = 0.99 for recurrence |
| Martínez et al[4] | 2019 | CEUS | N/A | N/A | CEUS effectively differentiated inflammatory vs fibrotic lesions post-surgery. Reinforced role of CEUS in enhancing IUS precision and in early recurrence assessment |
In an innovative prospective study, Paredes et al[17] evaluated the combined performance of IUS and 99mTc-D,L-hexamethylene-propyleneamine oxime-labelled leucocyte scintigraphy (LLS) in detecting and grading POR in CD. Among 33 patients with available ileocolonoscopic data, both IUS and LLS demonstrated moderate standalone accuracy for identifying endoscopic recurrence (72.7% and 78.1%, respectively). However, when used in combination - defined as BWT > 5 mm and/or scintigraphy uptake grade 2 or grade 3 - the sensitivity and negative predictive value for diagnosing moderate-to-severe recurrence rose dramatically to 93.3% and 92.9%, respectively, with an overall accuracy of 81.8% and κ = 0.64. This synergistic approach outperformed either modality alone, especially in scenarios where colonoscopy was incomplete or declined. These findings suggest that combining IUS and LLS can provide a highly effective, non-invasive alternative for early detection and stratification of POR in CD.
Two recent cross-sectional studies have explored the diagnostic agreement between IUS and endoscopy in the po
| Ref. | Year | Sample size | IUS parameters | Reference standard | Agreement (κ) | AUROC | Sensitivity | Specificity | Key findings |
| Yebra Carmona et al[11] | 2022 | 39 | BWT > 3 mm + Limberg score > 1 | Rutgeerts ≥ i2 | 0.5 | 0.75 | 81.0% | 87.0% | IUS had higher diagnostic accuracy than clinical or lab parameters. |
| Macedo et al[18] | 2022 | 39 | BWT > 3 mm and/or Limberg score > 1 | Rutgeerts ≥ i2 | 0.5 | 0.75 | 88.9% | 61.9% | Loss of wall stratification and hyperemia were most predictive of recurrence. |
FCP and IUS are increasingly recognized as complementary tools for early detection of postoperative CD recurrence (Table 6). In a pioneering prospective study, Orlando et al[19] compared FCP and IUS performance at 3 months post-surgery, with endoscopy at 12 months as the reference standard. While IUS showed high specificity (90%) but low sensitivity (26%) using a cut-off of BWT ≥ 5 mm, FCP at > 200 mg/L offered improved sensitivity (63%) but lower specificity (75%). This suggested that a high FCP in patients with a negative IUS could justify early colonoscopy. More recently, Furfaro et al[5] reported that both FCP ≥ 50 μg/g and IUS (BWT ≥ 3 mm) independently predicted recurrence, with sensitivities of 83% and 77%, respectively. The combination of FCP and IUS further enhanced specificity and positive predictive value, while the absence of both (FCP < 50 μg/g and BWT < 3 mm) yielded a negative predictive value of 95.5%. These findings support the integrated use of IUS and FCP to guide risk-based monitoring and potentially reduce the need for immediate endoscopy in low-risk postoperative patients.
| Ref. | Year | Sample size | Timepoint | FCP cut-off | IUS threshold | FCP | IUS | Key insight |
| Orlando et al[19] | 2006 | 39 | 3 months (IUS, FCP), 12 months (endoscopy) | > 200 mg/L | BWT ≥ 5 mm | 63/75 | 26/90 | Calprotectin more sensitive; IUS more specific at 3 months. Combining both could guide early colonoscopy |
| Furfaro et al[5] | 2023 | > 100 | 3-6 months post-operative | ≥ 50 μg/g | BWT ≥ 3 mm | 83/64 | 77/65 | FCP and IUS individually useful; combination improved specificity and PPV. FCP < 50 μg/g + BWT < 3 mm had NPV 95.5% |
The study by Piaz et al[20] provides compelling evidence that the prognostic accuracy of IUS and endoscopy in postoperative CD is highly dependent on timing. In this retrospective series of 201 patients followed for a median of 7.6 years, both endoscopic and sonographic recurrence - defined by BWT ≥ 4 mm or complications - were significantly predictive of clinical relapse when assessed within 12 months of ileocolonic resection. However, this predictive value was lost when assessments were performed after 36 months. Specifically, early IUS findings were independently associated with clinical and surgical outcomes, whereas late IUS assessments showed no significant prognostic relevance[20]. These findings underscore the importance of performing IUS within the first postoperative year to guide treatment decisions and support its role in the early risk stratification of recurrence.
This scoping review synthesizes the growing body of evidence supporting IUS as a valuable, non-invasive tool for the detection and monitoring of POR in CD. Across diverse clinical settings, IUS has demonstrated consistent correlation with endoscopic and clinical recurrence, with evolving techniques enhancing both sensitivity and specificity. Most included studies are singlecenter (prospective or retrospective) cohorts with modest sample sizes; a recent multicenter prospective study strengthens external validity of composite, noninvasive strategies combining IUS with FCP[5].
BWT remains the cornerstone of sonographic evaluation, with thresholds ranging from 3 mm to 6 mm shown to predict clinical, endoscopic, and surgical recurrence[1,7,8]. Longitudinal studies reinforce that the trajectory of BWT - particularly failure to regress after surgery - is a strong prognostic marker. Incorporating serial assessments improves clinical decision-making by identifying patients at risk of early relapse. Further improvements in diagnostic accuracy are achieved by assessing both the NTI and the ICA, rather than relying on a single segment. Dual-site evaluation captures a broader spectrum of disease activity and, when combined with lesion length or enhancement, achieves near-perfect sensitivity in some studies[10].
Additional IUS features such as mesenteric lymphadenopathy, hyperemia, and wall stratification enhance the predictive power of BWT alone[5,11]. When paired with biomarkers like FCP, IUS offers a complementary and dynamic approach to non-invasive monitoring, improving both positive and negative predictive values. Notably, combining normal BWT and low FCP provides high confidence in excluding significant recurrence. Innovations such as SICUS and CEUS have further improved visualization and accuracy, particularly in patients with subtle disease or deep mural involvement[3,4,12,13,16]. CEUS, in particular, distinguishes inflammatory from fibrotic lesions, informing therapeutic choices in postoperative care. In specific scenarios, the combination of IUS with functional imaging modalities like LLS may provide additional value, particularly when colonoscopy is contraindicated or incomplete[17]. These multimodal strategies support individualized care without compromising diagnostic certainty. Finally, timing of assessment is critical. Studies consistently show that IUS has greatest prognostic value when performed within the first year following surgery. Late assessments (> 36 months) offer limited predictive utility, reinforcing the need for early incorporation of IUS into postoperative surveillance pathways[20].
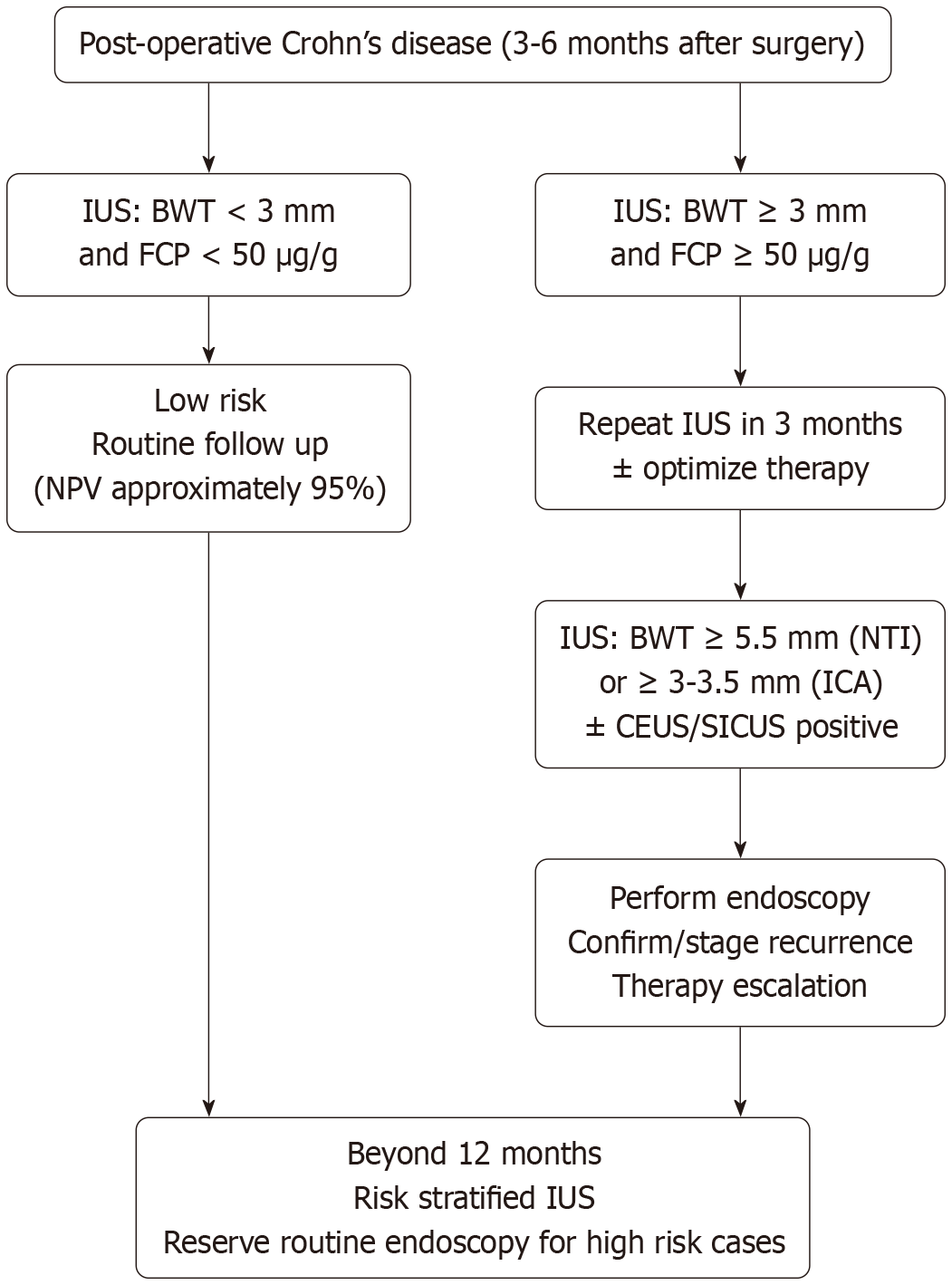
Based on the review, suggested surveillance algorithm (months 3-12 post-operative) is as follows (Figure 3). 3-6 months: IUS (NTI + ICA). If BWT < 3 mm and FCP < 50 μg/g - routine followup (negative predictive value approximately 95%). If BWT ≥ 3 mm or hyperemia/Lymph nodes - repeat IUS in 3 months ± optimize therapy; consider endoscopy based on symptoms/risk. 6-12 months: IUS ± CEUS/SICUS if equivocal. If BWT ≥ 5 mm (NTI) or ≥ 3-3.5 mm (ICA) and/or CEUS score positive - endoscopy to confirm and stage; escalate therapy. > 12 months: Continue risk-stratified IUS; reserve routine endoscopy for highrisk or discordant cases.
From a global perspective, while IUS is feasible and scalable in high-resource centers, low-resource settings may face barriers related to operator training and ultrasound infrastructure; however, its portability and low cost compared with endoscopy make it particularly attractive where access is limited.
We limited inclusion to Englishlanguage, peer-reviewed original studies and did not include grey literature, which may introduce selection/publication bias. Considerable heterogeneity existed in operator training, equipment, thresholds, timing of assessments, and reference standards, limiting metaanalytic pooling. Consistent with scoping methodology, we did not perform a formal riskofbias assessment; findings should be interpreted as an evidence map to guide practice and research rather than as graded recommendation. Potential publication bias and the lack of formal cost-effectiveness analyses remain important caveats.
Despite this progress, key unmet needs include: (1) Prospective head-to-head studies comparing IUS, CEUS/SICUS, endoscopy, and crosssectional imaging; (2) Standardized thresholds and composite indices with external validation; (3) Operator training/competency frameworks; and (4) Evaluation of artificial intelligenceassisted, automated measurements to improve reproducibility and scalability.
Collectively, the reviewed evidence supports the integration of IUS into standard care for postoperative CD, not only as a surrogate for endoscopy in selected patients but also as a real-time, repeatable tool for personalized disease monitoring. While IUS can safely defer routine endoscopy in selected lowrisk patients, it should be viewed as complementary to ileocolonoscopy until standardized scoring and multicenter validation are established.
IUS is a robust, noninvasive option for postoperative surveillance in CD, especially within the first year after surgery. Its utility is amplified when assessments are performed early post-surgery, with studies confirming that IUS can complement or, in selected cases, substitute for endoscopic evaluation. IUS, particularly when combined with biomarkers such as FCP, should be incorporated into postoperative monitoring algorithms. Standardization and international validation are urgent priorities to transition IUS from promising research to routine practice.
| 1. | Parente F, Sampietro GM, Molteni M, Greco S, Anderloni A, Sposito C, Danelli PG, Taschieri AM, Gallus S, Bianchi Porro G. Behaviour of the bowel wall during the first year after surgery is a strong predictor of symptomatic recurrence of Crohn's disease: a prospective study. Aliment Pharmacol Ther. 2004;20:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Parente F, Greco S, Molteni M, Cucino C, Maconi G, Sampietro GM, Danelli PG, Cristaldi M, Bianco R, Gallus S, Bianchi Porro G. Role of early ultrasound in detecting inflammatory intestinal disorders and identifying their anatomical location within the bowel. Aliment Pharmacol Ther. 2003;18:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Biancone L, Calabrese E, Petruzziello C, Onali S, Caruso A, Palmieri G, Sica GS, Pallone F. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn's disease. Inflamm Bowel Dis. 2007;13:1256-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Martínez MJ, Ripollés T, Paredes JM, Moreno-Osset E, Pazos JM, Blanc E. Intravenous Contrast-Enhanced Ultrasound for Assessing and Grading Postoperative Recurrence of Crohn's Disease. Dig Dis Sci. 2019;64:1640-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Furfaro F, D'Amico F, Zilli A, Craviotto V, Aratari A, Bezzio C, Spinelli A, Gilardi D, Radice S, Saibeni S, Papi C, Peyrin-Biroulet L, Danese S, Fiorino G, Allocca M. Noninvasive Assessment of Postoperative Disease Recurrence in Crohn's Disease: A Multicenter, Prospective Cohort Study on Behalf of the Italian Group for Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2023;21:3143-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Andreoli A, Cerro P, Falasco G, Giglio LA, Prantera C. Role of ultrasonography in the diagnosis of postsurgical recurrence of Crohn's disease. Am J Gastroenterol. 1998;93:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Rispo A, Bucci L, Pesce G, Sabbatini F, de Palma GD, Grassia R, Compagna A, Testa A, Castiglione F. Bowel sonography for the diagnosis and grading of postsurgical recurrence of Crohn's disease. Inflamm Bowel Dis. 2006;12:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Cammarota T, Ribaldone DG, Resegotti A, Repici A, Danese S, Fiorino G, Sarno A, Robotti D, Debani P, Bonenti G, Pellicano R, Andrealli A, Sapone N, Simondi D, Bresso F, Astegiano M. Role of bowel ultrasound as a predictor of surgical recurrence of Crohn's disease. Scand J Gastroenterol. 2013;48:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Maconi G, Sampietro GM, Cristaldi M, Danelli PG, Russo A, Bianchi Porro G, Taschieri AM. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn's disease: a prospective study. Ann Surg. 2001;233:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Pallotta N, Giovannone M, Pezzotti P, Gigliozzi A, Barberani F, Piacentino D, Hassan NA, Vincoli G, Tosoni M, Covotta A, Marcheggiano A, Di Camillo M, Corazziari E. Ultrasonographic detection and assessment of the severity of Crohn's disease recurrence after ileal resection. BMC Gastroenterol. 2010;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Yebra Carmona J, Poza Cordón J, Suárez Ferrer C, Martín Arranz E, Lucas Ramos J, Andaluz García I, Sánchez Azofra M, Rueda García JL, Martín Arranz MD. Correlation between endoscopy and intestinal ultrasound for the evaluation of postoperative recurrence of Crohn’s disease. Gastroenterol Hepatol. 2022;45:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Onali S, Calabrese E, Petruzziello C, Zorzi F, Sica GS, Lolli E, Ascolani M, Condino G, Pallone F, Biancone L. Endoscopic vs ultrasonographic findings related to Crohn's disease recurrence: a prospective longitudinal study at 3 years. J Crohns Colitis. 2010;4:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, Biancone L. Severity of postoperative recurrence in Crohn's disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Castiglione F, Bucci L, Pesce G, De Palma GD, Camera L, Cipolletta F, Testa A, Diaferia M, Rispo A. Oral contrast-enhanced sonography for the diagnosis and grading of postsurgical recurrence of Crohn's disease. Inflamm Bowel Dis. 2008;14:1240-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Onali S, Calabrese E, Petruzziello C, Lolli E, Ascolani M, Ruffa A, Sica G, Rossi A, Chiaramonte C, Pallone F, Biancone L. Post-operative recurrence of Crohn's disease: A prospective study at 5 years. Dig Liver Dis. 2016;48:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Paredes JM, Ripollés T, Cortés X, Moreno N, Martínez MJ, Bustamante-Balén M, Delgado F, Moreno-Osset E. Contrast-enhanced ultrasonography: usefulness in the assessment of postoperative recurrence of Crohn's disease. J Crohns Colitis. 2013;7:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Paredes JM, Ripollés T, Cortés X, Reyes MD, López A, Martínez MJ, Moreno-Osset E. Non-invasive diagnosis and grading of postsurgical endoscopic recurrence in Crohn's disease: usefulness of abdominal ultrasonography and (99m)Tc-hexamethylpropylene amineoxime-labelled leucocyte scintigraphy. J Crohns Colitis. 2010;4:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Macedo CP, Sarmento Costa M, Gravito-Soares E, Gravito-Soares M, Ferreira AM, Portela F, Figueiredo P. Role of Intestinal Ultrasound in the Evaluation of Postsurgical Recurrence in Crohn's Disease: Correlation with Endoscopic Findings. GE Port J Gastroenterol. 2022;29:178-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Orlando A, Modesto I, Castiglione F, Scala L, Scimeca D, Rispo A, Teresi S, Mocciaro F, Criscuoli V, Marrone C, Platania P, De Falco T, Maisano S, Nicoli N, Cottone M. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn's disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. 2006;10:17-22. [PubMed] |
| 20. | Dal Piaz G, Mendolaro M, Mineccia M, Randazzo C, Massucco P, Cosimato M, Rigazio C, Guiotto C, Morello E, Ercole E, Lavagna A, Rocca R, Ferrero A, Daperno M. Predictivity of early and late assessment for post-surgical recurrence of Crohn's disease: Data from a single-center retrospective series. Dig Liver Dis. 2021;53:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/