INTRODUCTION
In their recent article in the World Journal of Gastrointestinal Surgery, Qiu et al[1] evaluated the clinical utility of early ultrasound (US)-guided percutaneous catheter drainage (CD) in pyogenic liver abscesses (PLA). They demonstrated that early CD is both safe and effective, even in non-fully liquefied abscesses, supporting its use in early-stage PLA[1]. We concur with their conclusions but, based on extensive clinical experience, wish to emphasize additional considerations relevant to comprehensive PLA management[2,3].
PLA is a clinically significant condition with notable morbidity and mortality, particularly among patients with biliary pathology, diabetes, or immunosuppression[2-4]. Although relatively rare, it presents a diagnostic and therapeutic challenge requiring prompt recognition and a coordinated, multidisciplinary approach. Optimal outcomes depend on early administration of targeted antibiotics alongside timely and effective abscess drainage to achieve source control[5,6].
Traditionally, clinical management of PLA favored delayed intervention, deferring drainage until complete liquefaction due to concerns over incomplete evacuation, iatrogenic spread, and hemorrhagic complications in partially liquefied or solid lesions[7-9]. However, accumulating evidence supports the safety and efficacy of early US-guided drainage, even in non-liquefied abscesses[10,11]. Early intervention facilitates sepsis control, prevents abscess rupture or extension, and accelerates clinical recovery. It is also associated with faster resolution of systemic disease manifestations, reduced hospital stay, and lower rates of reintervention or more invasive procedures[1,10-12].
This paradigm shift reflects a broader trend toward personalized, risk-adapted infectious disease management, emphasizing early source control to mitigate systemic complications[1]. In this context, current PLA treatment algorithms warrant critical reassessment. This editorial reviewed available therapeutic options, emerging data, and clinical implications of early, minimally invasive US-guided strategies.
US-GUIDED INTERVENTIONS IN PYOGENIC LIVER ABSCESS
The treatment of PLA has improved significantly with the development of minimally invasive techniques, especially those guided by real-time imaging. Among them, US-guided interventions have emerged as the method of choice due to their precision, safety, and bedside applicability. US facilitates precise localization of the abscess, minimizes the risk of vascular or organ damage, and allows interventions to be performed without exposure to ionizing radiation - a particularly important advantage in critically ill or unstable patients[13]. The two main minimally invasive techniques are needle aspiration (NA) and CD, each with specific indications advantages, and limitations.
NA
US-guided NA of PLA involves the percutaneous insertion of a fine needle into the abscess cavity to aspirate purulent material. This method is minimally invasive, technically straightforward, and particularly suitable for small (< 5 cm), superficial, or multiloculated abscesses. It is also appropriate for early-stage abscesses that are partially liquefied. Recent studies indicate that in selected patients - especially those with early-stage, partially liquefied collections - aspiration alone may lead to complete resolution, thereby avoiding the need for catheter placement or surgical intervention[14,15]. Additionally, NA is associated with shorter procedure times and fewer post-procedural complications in appropriately selected cases[7,16].
CD
CD typically involves the placement of a pigtail catheter under US or computed tomography (CT) guidance for continuous evacuation of larger or more complex abscesses. Although more invasive than aspiration, CD is generally preferred for larger abscesses (> 5 cm), those refractory to NA, or in cases of significant reaccumulation[17,18]. CD provides a continuous route for drainage, facilitates the resolution of complex or thick purulent material, and reduces the risk of reaccumulation.
Advantages of US guidance
US guidance offers several advantages over blind or CT-guided approaches: (1) Real-time visualization of vascular structures, reducing the risk of hemorrhage; (2) Enables bedside procedures - crucial for hemodynamically unstable or intensive care unit-bound patients[19]; (3) Absence of radiation exposure, making it safer for repeated or prolonged interventions; and (4) Superior for accessing deep-seated or multiloculated liver abscesses, where careful planning of the needle or catheter path is essential to avoid injury to adjacent vital structures[20,21].
Evidence and outcomes
Meta-analyses and randomized studies have shown that both NA and CD are effective, with comparable abscess resolution rates. NA has the advantage of being less invasive and associated with fewer periprocedural complications, but it may lead to a higher recurrence rate or require secondary interventions, particularly in larger or more complex abscesses. In contrast, CD is generally more effective in treating PLA but may be associated with longer hospital stays and catheter-related complications[2,12,16,18].
In summary, US-guided interventions represent the cornerstone of modern PLA management, offering a balance of efficacy, safety, and patient comfort. The choice between NA and CD should be guided by abscess size, location, complexity, and patient-specific characteristics, with US serving as the enabling modality for both approaches. Also, US-guided interventions are particularly well suited for implementation in resource-limited settings. Compared to CT-guided procedures or surgery, US-guided interventions are significantly more cost-effective, portable, and do not require specialized infrastructure or radiation shielding[22-24]. Even in hospitals with limited access to high-end imaging systems, basic real-time US equipment - paired with proper operator training - can enable safe and effective percutaneous interventions. In this regard, early US-guided procedure represents not only a clinically advantageous but also an economically feasible option, especially in low-and middle-income countries, where untimely referral and restricted surgical capacity often worsen outcomes[25-27].
OPTIMAL TIME FOR PYOGENIC LIVER ABSCESS EVACUATION: WHY EARLIER MAY BE BETTER
The conventional management of PLA has typically followed a conservative approach, deferring intervention until the abscess has fully liquefied. This practice, rooted in concerns about incomplete drainage and potential iatrogenic complications during the early, semi-solid phase of abscess maturation, is now being increasingly questioned. Emerging clinical evidence suggests that early image-guided intervention - particularly US-guided drainage - can be both safe and effective, especially when performed by experienced clinicians. Such early interventions may positively influence clinical outcomes by preventing progression and reducing complication rates[2,28].
There is growing evidence that early percutaneous drainage - even in cases with incomplete liquefaction - can accelerate clinical improvement, shorten hospitalization, and reduce the risk of complications. In a recent prospective cohort study, Qiu et al[1] (2025) demonstrated that early US-guided aspiration or CD in selected patients significantly hastened the resolution of systemic manifestations of the disease and reduced the rate of treatment failure compared to delayed intervention[17,28]. Similarly, data from randomized controlled trials have shown that early drainage decreases abscess progression and shortens the duration of antibiotic therapy without increasing the risk of procedural complications[2].
One of the key insights emerging from recent research is the reassessment of procedural safety during early-stage intervention for PLA. Contrary to previous concerns, early decompression of the abscess cavity may in fact mitigate the systemic inflammatory response, reduce microbial burden, and improve antibiotic penetration into the infected tissue[21,28]. In contrast, delaying drainage may permit further abscess enlargement, thereby increasing the risk of rupture, peritonitis, and systemic septic spread.
NA is particularly recommended during the early stages of abscess development. This technique is especially advantageous in patients with borderline coagulation profiles or those at increased risk of bleeding complications. In contrast to CD - which is typically reserved for large, mature, or refractory abscesses - NA is minimally invasive and generally well tolerated, even in patients with coagulopathies or elevated procedural risk[2,3,15,21,28-30].
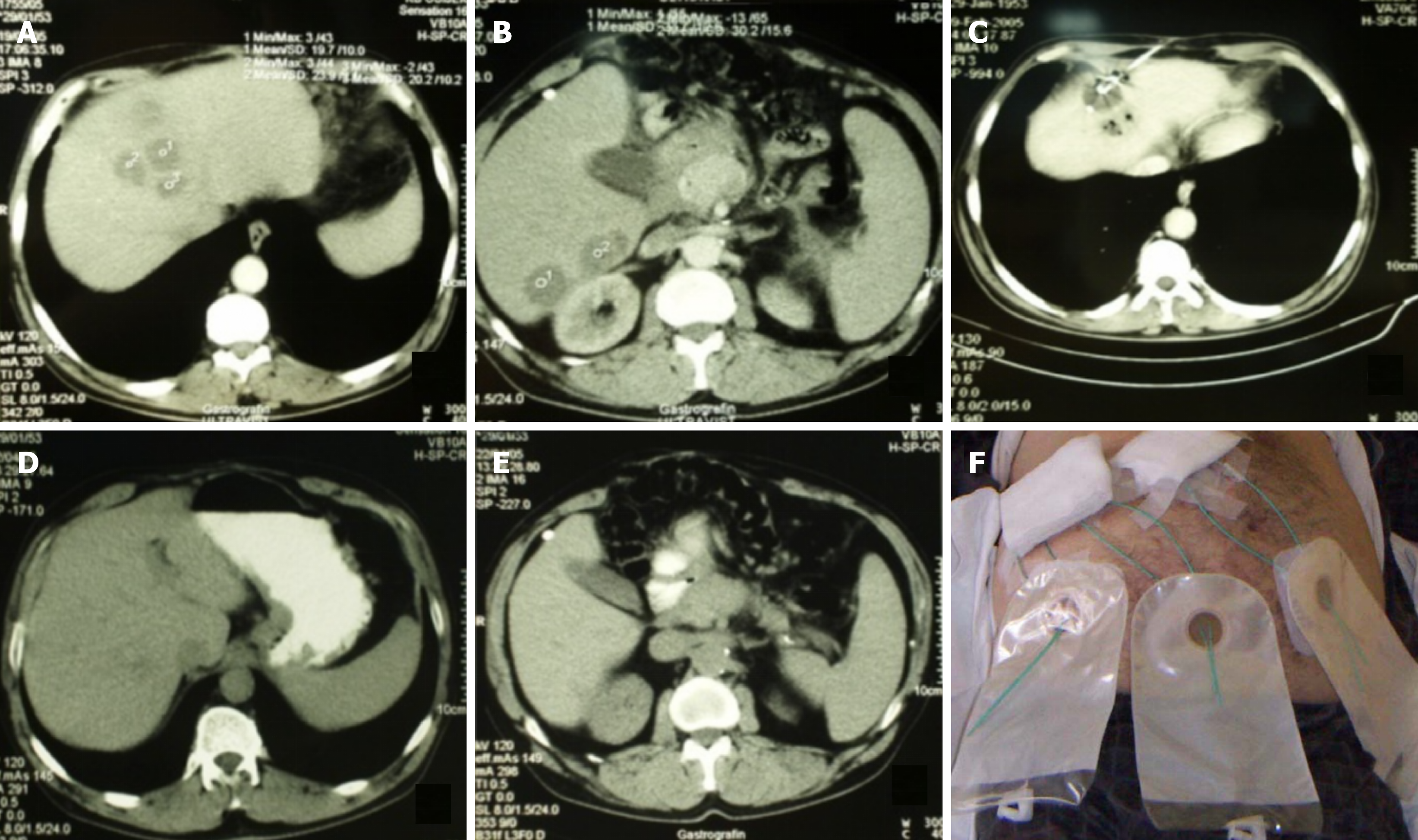
Microbiological etiology can also influence both the indication and success of early intervention. Anaerobes - often underdiagnosed due to culture limitations - are associated with polymicrobial infections and potential resistance to empiric therapy, which requires percutaneous intervention for diagnostic and therapeutic reasons. Early percutaneous intervention provides high-quality samples for culture, enabling timely adjustment of antibiotics[31,32]. Notably, some PLA, particularly multiple ones (Figure 1), may follow prior intra-abdominal interventions and be preceded by sepsis of unclear origin, making early intervention essential for pathogen identification and guiding targeted therapy[33,34]. Persistent bacteremia or poor response to therapy may indicate resistant strains, justifying intervention even in minimally liquefied collections[4-6,34,35].
Figure 1 Percutaneous treatment of multiple liver abscesses (a total of 10) using needle aspiration for those < 30 mm and catheter drainage for those > 30 mm.
A and B: Abscess collections prior to the intervention; C: Catheter placed in one of the abscess collections; D and E: The same liver region six weeks after the intervention; F: Same patient as in panels A-E, with six catheters visible shortly after the procedure.
Recent clinical evidence supports a paradigm shift in the management of pyogenic liver abscess, favoring early intervention over delayed drainage based solely on radiological maturation. When guided by clinical indicators (e.g., persistent fever, sepsis), laboratory findings (elevated inflammatory markers, leukocytosis), and sonographic features (early liquefaction, favorable location), early US-guided interventions have proven both safe and effective. This strategy promotes faster resolution and lowers the risk of complications such as rupture or systemic spread. Although assessment of specific patient characteristics remains essential, early intervention should be seriously considered in appropriately selected cases[2,3,21,28,29].
Nonetheless, early intervention should not be applied indiscriminately. Incompletely liquefied or poorly demarcated abscesses may result in suboptimal drainage, requiring repeat procedures or conversion to more aggressive interventions[12,36]. Additionally, increased vascularity in early-stage collections may slightly elevate the risk of bleeding during intervention[3,12,37]. Some studies also emphasize that in selected cases, antibiotic therapy alone may suffice, and unnecessary invasive procedures should be avoided[2]. These considerations reinforce the importance of individualized, image-guided decision-making when adopting early intervention strategies.
NA IN EARLY-STAGE PYOGENIC LIVER ABSCESS
US-guided NA is a valuable modality in the management of early-stage PLA. In contrast to CD, which requires prolonged drainage via an indwelling catheter, NA is a single-session, minimally invasive procedure associated with shorter duration, reduced patient discomfort, and fewer complications such as bleeding, bile leakage, or secondary infection[2,29]. Moreover, this approach may eliminate the need for prolonged CD, thereby reducing nursing demands and minimizing the risks of catheter dislodgement and reinsertion[2,12,16]. In its early stages, PLA often presents as an edematous, non-encapsulated lesion with partial liquefaction. In such cases, CD may cause tissue injury and result in suboptimal evacuation. NA offers a less invasive alternative, particularly suited for smaller abscesses (< 5 cm) or those with mild septations, especially when located in anatomically challenging regions of the liver[21,30].
Clinical outcomes of NA in early-stage PLA are encouraging, with many patients needing only one or two sessions for complete abscess healing. In randomized controlled trials, NA has demonstrated comparable success rates to CD, while being associated with significantly fewer complications and shorter recovery times in selected patient groups[2,12]. Selection criteria for NA include: (1) Abscess diameter ≤ 5 cm; (2) Uniloculated or minimally septated cavity; (3) Early or partial liquefaction; (4) Absence of rupture, multiloculation, or sepsis-related organ dysfunction; and (5) Contraindications to catheter placement (e.g., coagulopathy).
US-guided NA offers advantages such as shorter procedure time and avoidance of catheter-related issues like prolonged drainage, displacement, or secondary infection. Additionally, several studies report comparable efficacy and low complication rates for both NA and CD, with no consistent differences between the two[7,12,15-17,21]. However, multiple studies suggest that CD is superior for treating larger (> 5 cm), multiloculated, or poorly liquefied abscesses, due to its ability to provide continuous drainage and access to complex collections. They suggest that NA should be reserved for selected cases with small unilocular abscesses, where single-session aspiration is likely to be effective[2,3,13,14,36-39]. Given the variability in study outcomes, further multicenter and randomized controlled trials are needed to determine the optimal treatment strategy. Ultimately, the choice between NA and CD should be individualized, based on abscess morphology, expected efficacy, and guided by clinical, radiological, and microbiological findings.
INDIVIDUALIZED APPROACHES: MATCHING TECHNIQUE TO PATIENT AND DISEASE
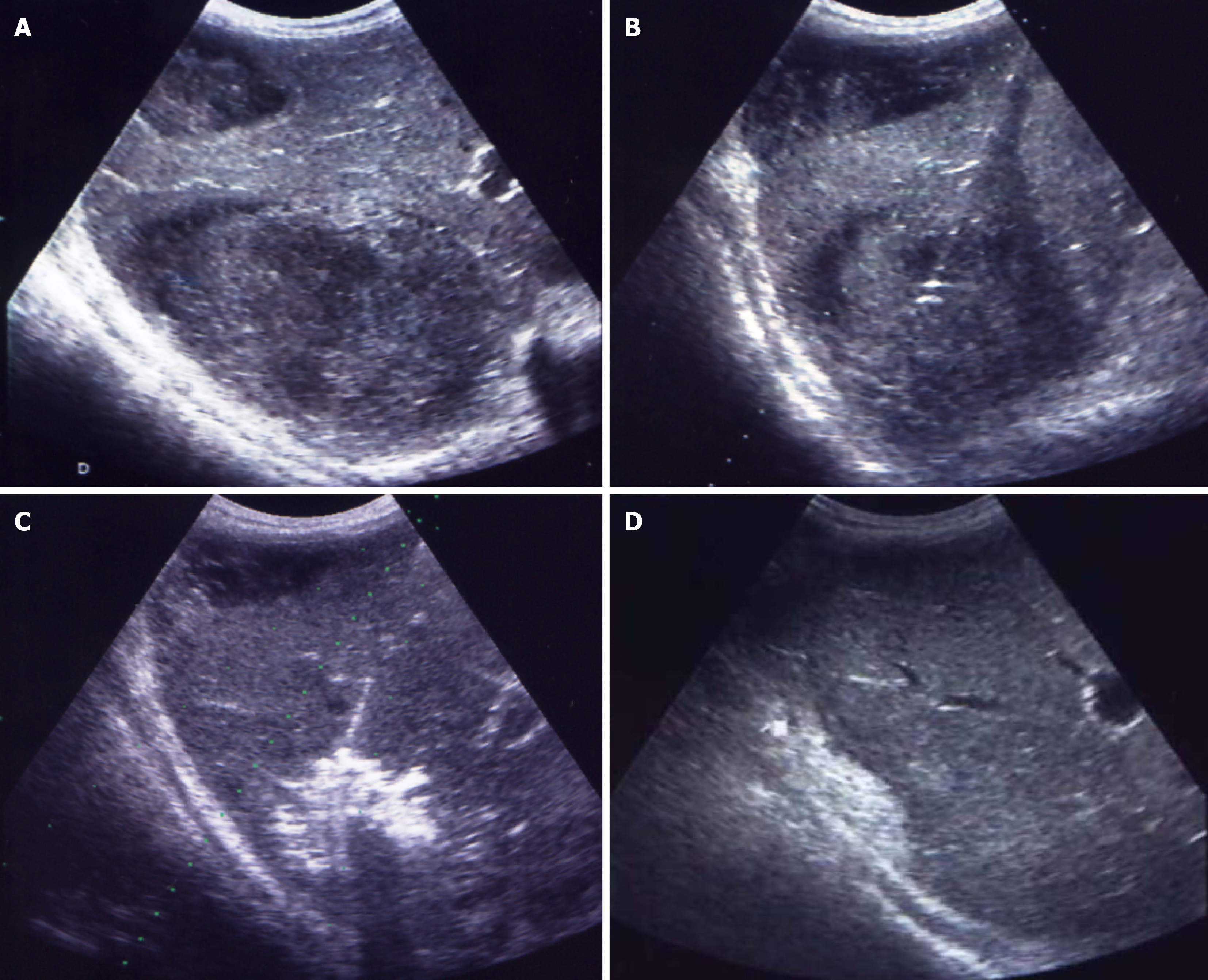
There is no standardized approach to the treatment of PLA, as the clinical and radiological heterogeneity among patients necessitates individualized therapeutic strategies tailored to both the characteristics of the abscess and the patient’s overall clinical condition. The choice between NA, CD, or surgical intervention should be based on a comprehensive assessment of abscess features - such as size, location, number, degree of encapsulation, and liquefaction - as well as the presence of patient comorbidities (Figures 1 and 2)[36,38,40].
Figure 2 Percutaneous drainage of a double abscess collection in the right liver lobe, formed two weeks after appendectomy.
A: Two abscess collections in the right liver lobe; B: Transhepatic access to the abscess collection using the trocar technique; C: Vigorous irrigation of the abscess cavity with a 50/50 mixture of iodine and saline through a catheter; D: Scar formation in liver tissue three weeks post-intervention.
Among these factors, abscess size is one of the most widely recognized determinants in selecting the appropriate intervention. In general, abscesses measuring ≤ 5 cm are considered suitable for NA, whereas larger lesions (> 5 cm), or those showing incomplete resolution following NA, typically require CD[2,12]. However, anatomical location is also a crucial factor, as deeply situated abscesses in the posterior hepatic segments or near the hepatic hilum may pose technical challenges for percutaneous access and catheter placement (Figures 1 and 2)[2,29].
The stage of abscess maturation-ranging from solid to partially or fully liquefied-plays a critical role in determining the optimal percutaneous management strategy. Early-stage abscesses may appear poorly defined and heterogeneous on imaging; in such cases, aggressive catheter manipulation can increase the risk of parenchymal injury or bleeding, and less invasive US-guided NA is often preferred to minimize complications. Conversely, in well-formed, encapsulated, and liquefied abscesses, CD is considered both safe and more effective in achieving complete evacuation (Figures 1 and 2)[2,7,12,19].
Optimal management of PLA requires an individualized approach that balances therapeutic efficacy with procedural safety. In patients with coagulopathy or thrombocytopenia, prolonged CD increases the risk of bleeding, making single-time aspiration a potentially safer alternative[30,41,42]. Anatomical variants - such as aberrant vasculature or biliary dilatation - require a detailed pre-procedural analysis to minimize the risk of iatrogenic injury during intervention[4,42]. Moreover, comorbidities such as diabetes mellitus, immunosuppression, or a history of previous biliary interventions may increase the risk of recurrence, underscoring the need for a personalized treatment plan and a multidisciplinary approach[43-46].
FUTURE PERSPECTIVES
Despite the growing body of evidence supporting early US-guided intervention in PLA, current data are derived primarily from single-center studies and retrospective analyses. Future research should focus on well-designed, prospective multicenter trials comparing early vs delayed intervention strategies across different clinical subgroups, including variations in abscess size, microbial etiology, degree of liquefaction, and host immune status. Standardized criteria for patient selection and intervention timing should also be developed. These efforts will be critical to establishing new evidence-based guidelines, improving procedural safety, and optimizing clinical outcomes in diverse practice settings.
CONCLUSION
Recent evidence supports a paradigm shift in the management of PLA, favoring early, US-guided intervention over delayed drainage. Early procedures - particularly NA in small or immature abscesses - can reduce complications, shorten hospital stays, and improve clinical outcomes. Central to this approach is individualized decision-making based on abscess characteristics and patient condition, with US guidance enhancing both procedural safety and precision. These findings underscore the need to revise clinical protocols by integrating early, image-guided strategies as the standard of care in appropriately selected PLA cases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Bosnia and Herzegovina
Peer-review report’s classification
Scientific Quality: Grade A, Grade C
Novelty: Grade A, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade C
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Kanthlal S, Chief, Full Professor, India; Lomeli SM, PhD, Adjunct Associate Professor, Associate Research Scientist, Researcher, Mexico S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH