Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110501
Revised: June 23, 2025
Accepted: October 11, 2025
Published online: November 27, 2025
Processing time: 171 Days and 4.6 Hours
Incidental asymptomatic gallstone disease (AGD) is prevalent, but its manage
Core Tip: Asymptomatic gallstones are incidentally detected on imaging. Aside from an extremely small number of patients who eventually have issues, in a few cases, life-threatening, most never become symptomatically apparent. Our treatment strategy for such patients has evolved over the years, driven by advances in imaging, laparoscopy, artificial intelligence (AI), and precision medicine. Physicians can now weigh the risk and decide who could benefit from early intervention with more advanced diagnostic and predictive tools. This review highlights how precision tools, including genomics, AI, and decision-support systems, will cause a paradigm shift in management from a one-size-fits-all approach to an individualised, patient-centred approach.
- Citation: Sasmal PK, Singh PK, Sahoo A, Dutta T. Asymptomatic gallstone disease: Re-evaluating the threshold for surgical options in the era of precision medicine. World J Gastrointest Surg 2025; 17(11): 110501
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110501.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110501
Cholelithiasis, also known as gallstone disease (GSD), affects approximately 4% of individuals in India and 10% to 20% of individuals worldwide[1]. It occurs in women, the elderly, the obese, and individuals with hyperestrogenic states. Genetics and gut flora are also etiologic agents[2]. Of those affected, nearly 80% will be asymptomatic. However, in 1%-4%, acute cholecystitis, pancreatitis, cholangitis, or even gallbladder carcinoma may develop[3,4]. More uncommon but severe diseases, such as gallstone ileus or liver abscess, may occur in cases of delayed treatment[5]. Although infrequent, such complications may be life-threatening and distressing for patients.
Surgical interventions for gallstones have undergone significant changes over time. Open surgery has largely been replaced by laparoscopic and robotic surgery. However, indications for surgery in asymptomatic GSD (AGD) have remained relatively unchanged. However, operating indications in AGD have stayed conservative and are still controversial as well. The current international recommendations advise that such patients be closely monitored unless they belong to high-risk categories, such as those with sickle cell disease or immunocompromised conditions. An Indian multicenter prospective observational study reported a 30-day morbidity of 11.1%, 30-day mortality of 0.2%, rate of conversion to open of 1.4%, and rate of bile duct injury (BDI) of 0.3%[6]. In symptomatic ones, laparoscopic chole
The research gap lies in the fact that, although the natural history and surgical risk of AGD are well described, there is a lack of knowledge about integrating newer paradigms of precision medicine, genomics, and artificial intelligence (AI) into decision-making for patients with AGD. Few reviews exist that focus on conventional risk factors, and none explain how predictive analytics, decision-support systems, and genomic markers can optimally select patients for prophylactic surgery. With the increasing incidence of incidental diagnoses and the availability of high-resolution imaging, clinicians are confronted more frequently with the question of whether and when to intervene early. This mini-review is therefore timely: It connects epidemiology, natural history, outcomes of surgery, and, for the first time, the emerging role of AI-driven risk models and precision medicine in AGD. By delineating where guidelines are lacking and how new instruments may fill the gap, it should guide surgeons toward patient-specific, evidence-based solutions instead of a single solution. However, the risk of BDI, currently very low at 0.01%-0.04%, remains an issue[9].
Treatment of AGD is more complex in India. Certain areas are endemic for such diseases as gallbladder cancer and sickle cell disease, and these may necessitate surgery earlier in such areas. Obesity and diabetes are also higher in these areas, so there are more individuals at risk. Variations in healthcare access, expense, and surgeons' abilities from one area to another create challenges in decision-making. It raises several important questions: Is AGD's natural history variable? Does untreated gallstone stress? Moreover, do the harms of surgery outweigh the advantages in some instances? These are the key questions that this review proposes to address.
This mini-review addresses a relevant question: What are the best current practices for managing AGD, and how can precision medicine and AI strategies be incorporated into surgical decision-making? More precisely, the review is directed toward (1) outcomes of conservative vs surgical intervention; (2) paradigms for decision-making that consider surgical safety and cost-effectiveness; and (3) the use of evolving technologies, including genomics, AI, and decision-support systems, in directing patient-centred care. By making the objectives explicit, this current review aims to provide surgeons with a defined platform for evidence-based and precision-guided treatment of AGD.
We did a comprehensive literature search of PubMed, PubMed Central, and Google Scholar for studies published between 1994 and 2024. The MeSH terms used for searching included: Cholelithiasis, gallstones, AGD, laparoscopic cholecystectomy, complications of gallstones, BDI, gallbladder cancer, day-care surgery, precision medicine, and artificial intelligence. Additional studies were also screened from the lists of references of the retrieved articles.
We included peer-reviewed descriptions of the natural history, clinical outcomes, surgical indications, or management plans for asymptomatic gallstones in adult patients. We included observational and intervention studies, systematic reviews, and meta-analyses.
We excluded studies of the pediatric population, case reports involving fewer than five patients, animal models, and non-English language publications due to feasibility limitations.
Due to the magnitude of a mini-review, a systematic application of official tools for risk-of-bias was not undertaken; however, high-quality evidence, including prospective cohorts, randomised controlled trials (RCTs), and population-based large studies, was prioritized. Narrative reviews, as well as expert opinions, were included only when they provided individual insights or contextual clues that enhanced the understanding of the topic.
Two reviewers independently reviewed all articles found to be relevant, and in case of disagreement, consensus was reached. The studies were synthesised qualitatively, with particular focus laid on epidemiology, risk stratification, surgical safety, quality of life (QoL), cost-effectiveness, and new approaches using precision medicine and AI.
All the authors unanimously shortlisted 87 articles that met the criteria and were also able to utilise them to conclude. The most relevant publications from the last three decades were review articles, with a few notable exceptions, including good-quality prospective cohort studies and RCTs. All the authors independently reviewed the data collected from the selected articles.
GSD has a heterogeneous distribution and risk patterns worldwide. It is crucial to understand the epidemiological trends and demographic risk factors to develop region-specific and patient-specific management strategies for AGD.
GSD is prevalent but is extremely geographically, ethnically, sex, and socioeconomically variable. The worldwide prevalence is 10%-20% in adults, with a significant increase in women above 40 years of age[1,10,11].
Epidemiological evidence always reports increased prevalence in North India and Latin America, alongside increased risk of gallbladder cancer, and decreased prevalence in East Asian countries. These global variations suggest that control measures, as observed in low-prevalence regions, may not apply to high-risk populations[12-14].
Habitual diet and culture add much to the prevalence of gallstones. Urban Latin Americans and Indians with diets rich in saturated fats and refined carbohydrates but poor in fibre have a higher prevalence. A reverse correlation is observed among high-fibre and unsaturated fat-consuming mediterranean populations, with a lower prevalence. Environmental exposures, such as endemic waterborne infections and long-standing inflammatory diseases, also contribute to a preponderance towards gallstone formation in specific areas. The excessive occurrence of AGD, particularly in northern women, has been linked to a mix of dietary, cultural, and environmental causes. The diets high in saturated fats, refined car
Female gender, advancing age, obesity, excessive weight reduction, pregnancy, diabetes mellitus, and metabolic syndrome are established risk factors for AGD. Obesity is associated with increased hepatic cholesterol secretion, a primary factor contributing to the formation of cholesterol gallstones. Likewise, insulin resistance and hypertriglyceridemia, features of metabolic syndrome, change bile composition and gallbladder contractility[16]. Diabetic autonomic neuropathy can impair gallbladder emptying, contributing to stasis of bile. These risk factors are especially applicable in the urban Indian population, where diet modification and lack of physical activity are leading to increasing obesity and type 2 diabetes prevalence[17].
Metabolic risk factors need more attention in AGD. Obesity, insulin resistance, and metabolic syndrome increase biliary cholesterol saturation and decrease gallbladder contractility, thereby promoting the formation of gallstones. Diabetic patients not only develop stones more often, but also more serious complications like gangrenous cholecystitis or pancreatitis. Concomitant conditions decrease the threshold for surgical referral, even for asymptomatic disease, because the potential effect of delayed intervention may be more severe. Such comorbidities can be considered within the risk scoring to optimise decision-making and prioritise early treatment of high-risk patients. Although the majority of European and North American series adopt conservative management, Chilean and North Indian evidence indicates prophylactic surgery because of the high rates of gallbladder cancer.
Risk stratification thus has to incorporate both demographic and metabolic factors. A middle-aged obese diabetic patient from North India, a region where gallbladder cancer is endemic, would be counselled differently than a lean asymptomatic patient from Western Europe. These geographic variations highlight that management algorithms cannot be one-size-fits-all but must conform to population-based risk patterns.
Key takeaway: Epidemiology and risk stratification should extend beyond prevalence information to encompass cultural, dietary, and metabolic factors that influence the development of GSD, thereby enabling management recommendations to be tailored to the population's specific risk environment.
The natural history of asymptomatic gallstones is usually innocuous, and most patients can remain symptom-free for decades. However, detecting the minority of patients at risk of developing complications is crucial to intelligent decision-making and operative planning.
Most asymptomatic gallstones are benign. About 10% will become symptomatic over 9 years, and the risk of complications decreases with increasing patient age[1,18,19]. The Group for Epidemiology and Prevention of Cholelithiasis experience in Rome, too, echoes figures supporting this claim, with a cumulative probability of developing complications after 10 years being 3.0% ± 1.8% in the initially asymptomatic group and 6.5% ± 4.4% in the symptomatic group[20]. A randomised trial studying the natural history of gallstones followed patients for 14 years after randomising them to observation vs cholecystectomy. It found no significant difference in symptomatic events or major complications between the two groups[21]. A recent systematic review found that a majority of the evidence favours conservative treatment (clinical follow-up) for asymptomatic cholelithiasis as safe and feasible[22]. Nevertheless, a substantial proportion of patients going under the knife still belong to this subset of asymptomatic stones[23].
Some authors have also experimented with oral dissolution therapy for gallstones in asymptomatic/mildly symptomatic patients using salts containing magnesium trihydrate of ursodeoxycholic acid and chenodeoxycholic acid, claiming to achieve good results[24]. The same, however, has not been reproducible or widely practised in the clinical field. Watchful waiting continues to be practised by many centres for silent stones[25]. However, there is a lack of studies directly comparing surgical and conservative approaches in patients with asymptomatic gallstones, and high-quality studies are needed to explore geographic/ethnic differences in the natural history of asymptomatic gallstones and to predict better the progression of the disease in specific patient subgroups[26].
Key takeaway: The asymptomatic natural history of gallstones mandates expectant management in most cases. To recognise at-risk subgroups and implement appropriate, timely interventions, a patient-specific, data-driven strategy must be adopted.
The successful management of AGD depends on accurately identifying patients at risk for developing complications. This section discusses clinical risk predictors, decision-support tools for patient stratification, and evidence-based management strategy guidance.
Literature suggests that fewer than one in five individuals with gallstones will develop clinical consequences, with fewer than half experiencing complications. Common predictors for the same from cohort studies include younger age, female sex, and having multiple, older, larger stones[27-29]. The PREOP gallstone risk predictor score was used in a few studies at the first presentation as a starting point for customised care and informed decision-making in high-risk patients with gallstones[30]. It is highly variable and determined by personal risk factors. Risk factors include stones greater than 1 cm, multiple small stones, or sludge/microlithiasis filling more than 50% of the gallbladder volume, all of which are likely to produce colic, cholecystitis, or pancreatitis[31]. Furthermore, hemolytic disease patients, immunosuppressed patients, and spinal cord injury patients are high-risk special subgroups.
Major societies, such as the American College of Gastroenterology, the World Gastroenterology Organisation, and the National Institute for Health and Care Excellence, recommend observing AGD unless high-risk features are present. The basis is the low incidence of symptom conversion and complications of unnecessary surgery. In reality, local practice and surgeon preference generally guide decision-making. In nations such as India or Chile, where the risk of gallbladder cancer is elevated, it could be less challenging for a person to need to have surgery[15].
Key takeaway: Although the majority of patients with AGD are asymptomatic, clinical practice guidelines and risk models offer an evidence-based method for the systematic identification of those at increased risk. A personalised assessment based on predictors in the clinical setting and local epidemiology is necessary to weigh the benefits and risks of early surgery.
Selecting whether to perform a cholecystectomy in AGD is a clinical challenge. While most guidelines advocate expectant management, innovative clinical scenarios, such as comorbid disease or elective surgery, have broadened the indications for prophylactic cholecystectomy. This section describes patient subgroups in whom anticipatory treatment may be indicated.
There are no robust data on meta-analyses comparing surgery and observation of asymptomatic gallstones. A Cochrane review noted that the evidence was insufficient to support or exclude surgery in this setting[32]. Nevertheless, over time, cholecystectomy rates have risen and mirror expanding indications, particularly in transplant candidates waiting for transplants, diabetic individuals, younger individuals, or larger gallstones[33,34]. Some other surgical indications include calcification of the gallbladder, hemolytic disease, and a dysfunctional gallbladder[35].
The management of asymptomatic gallstones in an inpatient-free clinic was deemed effective[36]. Surgery is conventionally favoured in people with diabetes when there is a significant risk of complications or emergency surgery[37,38]. Likewise, sickle cell patients have been advantaged by elective cholecystectomy to prevent serious complications and emergency admissions[39].
In organ transplant recipients, varying practices are followed. Cardiac transplant patients can be advantaged by prophylactic surgery, while renal transplant patients are generally managed conservatively[40-43].
Routine removal of the gallbladder during bariatric surgery in patients who are morbidly obese is controversial[44,45]. Meta-analysis revealed low rates of postoperative complications (5.3% for biliary colic, 1% for cholecystitis, and 0.2% for pancreatitis) and no deaths[46,47]. Routine cholecystectomy is, therefore, not required unless symptoms occur.
Key takeaway: While routine surgery is not recommended for all asymptomatic gallstone patients, specific subgroups, such as individuals with diabetes, sickle cell disease, organ transplant candidates, or large solitary stones, may benefit from prophylactic cholecystectomy. Decision-making should be individualised based on comorbidity, anticipated future procedures, and risk of complications, favouring a selective and evidence-informed approach.
The question of how and why asymptomatic patients should be operated upon is more pertinent as laparoscopic cholecystectomy becomes safer and more prevalent. The pros and cons of elective vs emergent surgery in AGD are discussed in this section, along with the importance of perioperative preparation and technical safety.
The risk of developing symptomatic stone disease is more marked in young patients with pain syndrome and silent stones before surgery[48,49]. The incidence of stone-related complications is higher in patients with gallstones preoperatively and after gastric bypass and lower in cases of gastroplasty[45]. Ursodeoxycholic acid has also been proposed to have a protective effect in those not opting for concomitant cholecystectomy.
Presence of microliths (Gallstones < 4 mm) and biliary sludge. Microliths, owing to their size, may lead to all possible complications of cholelithiasis, especially biliary pancreatitis. The management options to obtain long-lasting relief include cholecystectomy and endoscopic sphincterotomy. Chemical dissolution through ursodeoxycholic acid can be considered in old age and patients with high surgical risk[2,40]. Although there is a low level of recommendation, the management of biliary sludge remains the same as that of microliths[3].
A porcelain gallbladder has been considered nefarious for harbouring malignancy. Nonetheless, a porcelain gallbladder in asymptomatic individuals does not always warrant surgery. Cholecystectomy is advocated for symptomatic and selective mucosal calcifications with low surgical risk[49].
Tenets of safe cholecystectomy, as in every case, become paramount while operating for asymptomatic gallstones[50]. On the other hand, it is difficult to foretell which patients presented will develop severe complications like pancreatitis or cancer later on. Emergency surgery is likely to be technically demanding, with increased rates of conversion and poorer outcomes, especially in the elderly and comorbid patients[51,52]. There may be substantial apprehension on the part of the patient regarding GSD and its possible complications. Therefore, proper counselling is required from the treating physician, considering the various risk factors while advocating expectant management.
Key takeaway: Although AGD is not generally an emergent condition requiring acute treatment, elective laparoscopic cholecystectomy in a high-risk environment has safer and lower complication rates compared to emergency operations. The safety record of current surgical equipment also justifies a selectively aggressive, proactive approach in patients with an estimated risk of complications.
New insights into the human gut microbiota have revealed fascinating correlations between GSD and outcomes of cholecystectomy. Especially in asymptomatic patients, identifying the microbiome's function in evaluating long-term risk and refining surgical indications is increasingly necessary.
New evidence connects gut microbiota with the pathophysiology of gallstones. Post-cholecystectomy alterations, like continuous bile flow and motility changes, can cause dysbiosis, increasing the risk of colorectal cancer. Discrepancies in the microbiota of gallstone carriers were not identified by the population-based Study of Health in Pomerania (SHIP) study. However, patients after cholecystectomy showed a decrease in Faecalibacterium and an increase in Escherichia/Shigella[53]. A meta-analysis of ten cohort studies also implicated cholecystectomy in colon cancer in 2017[54]. The results emphasise the role of the gut microbiome and warn against unnecessary cholecystectomy in asymptomatic gallstones.
However, a recently conducted study on faecal microbiota (SHIP) concluded that a large population long-term study is needed to establish the association between increased morbidity and risk of colorectal cancer in post-cholecystectomy patients[53].
Key takeaway: New data are making it more probable that cholecystectomy causes gut microbiota dysbiosis, which could lead to a heightened risk of colorectal cancer. It also suggests caution against the overuse of surgery for asymptomatic gallstones, given the absence of indications until more definitive long-term data on the microbiome's effect are determined.
Although laparoscopic cholecystectomy has been deemed safe and routine, its effect on patient-reported QoL, particularly in asymptomatic gallstone patients, should be adequately assessed. This section examines whether surgery in such patients is associated with significant improvements in daily functioning and symptom reduction.
QoL outcomes need to be given a higher priority in decision-making, especially since asymptomatic patients may not perceive any direct benefit from surgery. The evidence suggests that the gain in QoL is significantly more impressive in symptomatic patients than in those treated for silent stones, as the advantages are often arbitrary and, in some cases, offset by new gastrointestinal symptoms. These results indicate that QoL outcomes need to be used directly in decision-making models. In the future, AI-based decision-support systems may incorporate QoL scores (e.g., SF-36, GIQLI) that have been validated against clinical risk scores to quantify patient-centred outcomes better. It will enable surgeons to balance hard endpoints, such as the risk of complications, with softer yet highly useful indicators of patient well-being[55-57]. QoL improvement is variable, with improvement noted in only 53% of asymptomatic patients but in 94% of symptomatic patients[58]. The prospective study also shows higher health-related QoL gains for symptomatic, low-surgical-risk patients compared to asymptomatic and high-surgical-risk patients[59].
In comparison with the control population, though, post-cholecystectomy patients may experience an increase in the frequency of other gastrointestinal symptoms[60]. Needless to say, the occurrence of BDI, although more marked in complicated stone disease, is an unfortunate complication following cholecystectomy, which renders a substantial economic burden and significantly hinders the QoL, showing no improvement up to as long as 5 years post-surgery[61-64]. Split stones during cholecystectomy can also impose a significant challenge, with the majority leading to post
Although the majority of patients opting for prophylactic surgery should ideally be treated laparoscopically, those with risk factors such as elderly age, males, previous upper abdominal surgery, radiologically thickened gall bladder and comorbidities or unexpected intra-operative difficulty must be informed regarding the possibility of conversion[66-68].
Key takeaway: Asymptomatic gallstone surgery is not invariably followed by important quality-of-life improvement and can sometimes be accompanied by new gastrointestinal symptoms or complications. Therefore, shared decision-making, honest discussion of outcomes, and prudent patient selection are important before recommending elective cholecystectomy.
While uncommon, gallbladder cancer is a biologically aggressive tumour. In some asymptomatic patients, the repeated suggestion that large or solitary gallstones bear some relation to gallbladder malignancy presents a frighteningly compelling argument in favour of prophylactic cholecystectomy. This section examines the existing evidence and clinical implications of this correlation.
Gallbladder cancer is a rare but lethal cancer with marked ethnic and geographical variations[69,70]. The association between gallstones and gallbladder carcinoma has long been speculated and is perhaps the foremost cause of apprehension among asymptomatic patients. Gallbladder cancer is rarely diagnosed at an early stage, and few patients are candidates for curative resection. Such scepticism fuels the controversy regarding waiting or eliminating 'asymptomatic stones'. Multiple studies have investigated this association, with some even attributing gallstones to biliary tract cancers in general and gallbladder cancers in particular[12,71]. Cholesterol stones, in particular, have been noted to be a risk factor for carcinoma gall bladder, reflecting its association with lifestyle factors such as diet, obesity, etc.[71]. The coexistence of gallstones with polyps further increases the risk of malignancy, especially for single, large ones in the Indian population above 60 years of age[72]. With the advances in ultrasonography, a more detailed evaluation of the gallbladder wall and its lesions has made it possible to select patients with gallstones who might also have a suspicious wall thickening. Gallbladder reporting and data system is a risk stratification tool that helps identify malignant lesions accurately, aiding appropriate management[73]. Literature has also described a proportionate increase in the incidence of carcinoma with increasing size of stones, with some documenting the risk to be higher in symptomatic patients. It has been attributed to some extent to the carcinogenicity of bile, which is more tumour-promoting in symptomatic than asymptomatic patients. Opinions remain split between expectant management vs prophylactic cholecystectomy for silent stones. Factors such as operative morbidity, mortality, economic consequences, disease burden and resource availability are imperative in decision-making[74].
Key takeaway: The linkage of gallstones and gallbladder carcinoma, especially in endemic populations and regions, warrants preventive cholecystectomy in a select group of asymptomatic patients. Strictly individualised risk stratification according to stone features, age, family history, and imaging is required to achieve prevention without undue surgical risk.
In regions where typhoid is endemic, AGD has a particular public health implication due to its role in Salmonella typhi (S. typhi) carriage. This section addresses the relevance of cholecystectomy in breaking the infection cycle in inapparent carriers.
Studies from regions endemic for typhoid have proven that cholecystectomy specimens and gallstones from asymptomatic patients showed evidence of colonisation by S. typhi, predominantly as a dense bacterial biofilm over the gallstones[75]. This clinical data brings into the picture the concept of biofilm-mediated chronic carriage of S. typhi on gallstones and highlights the importance of identifying and treating this subset of healthy carriers in the endemic regions.
Key takeaway: In endemic regions, asymptomatic gallstones can act as reservoirs for S. typhi, perpetuating the chronic carrier state and continuous transmission. In these patients, cholecystectomy is not only of clinical but also epidemiologic benefit, backing active surgical management of diagnosed carriers.
Although gallstones are normally a benign condition, new evidence indicates an association with higher long-term mortality, including cardiovascular and cancer mortality. The remainder of this section discusses the overall effect of GSD on survival in patients.
Multiple large prospective cohort studies have concluded that gallstone patients had increased overall mortality, as well as deaths due to cardiovascular and cancer causes[76,77]. It points towards the possibility of gallstones being a cardiometabolic risk factor[78]. However, the numbers did not vary between those diagnosed on ultrasound or those who underwent cholecystectomy, suggesting that the cause would lie somewhere in the mechanism of the formation of gallstones and not the surgery. Of specific mention is the United States National Health and Nutrition Examination Survey-based cohort study published in 2011 that included many asymptomatic gallstone patients and drew similar conclusions[76]. Another study evaluating mortality associated with gallstone treatment showed that the majority of deaths were among elderly comorbid patients who were being managed conservatively[79].
Key takeaway: GSD could be an expression of an underlying metabolic or inflammatory illness with an increased risk of mortality. While cholecystectomy is not proven to reduce mortality, identification of gallstones as a possible marker of systemic illness can lead to more comprehensive patient assessment and risk stratification.
Precision medicine and AI technologies provide new opportunities for risk stratification and personalised treatment of AGD. This section of the article discusses the future and potential role of genomics, predictive models, and decision-support systems in changing the management of AGD.
Precision medicine in AGD entails patient-specific decision-making according to risk profiles derived from genetic, environmental, and lifestyle elements. This is clinically manifested through using instruments to risk-stratify according to radiological parameters (e.g., stone size, gallbladder wall thickness), biochemical tests (e.g., liver enzymes, inflammatory markers), and patient demographics to estimate the risk of progression. AGD treatment plans have historically been population-level and based on broad clinical guidelines. With the increased availability of big data analytics, genomics, and AI, a more patient-specific and nuanced strategy is now feasible[80].
In clinical practice, high-risk patients with giant solitary gallstones (> 2 cm), gallbladder polyps, or a family history of gallbladder cancer can be identified and provided with elective cholecystectomy. In contrast, low-risk patients can be safely followed[10]. Precision models will avoid unnecessary surgery in low-risk patients but provide timely treatment in risky patients with impending complications or malignancy.
Genetic polymorphisms ABCG8 D19H and ABCB4 mutations are involved in GSD and supersaturation of cholesterol. Such variants may be identifiable in high-prevalence populations (e.g., Chile, North India) to allow more precise surgical thresholds and reserve surgery in those genetically predisposed. Family history and inherited syndromes can also be used to counsel for earlier intervention. As the cost of genetic testing decreases, including such markers in risk scores has the potential to identify surgical candidates prior to symptom onset[81].
The concept of AI is also emerging rapidly in the present era. For example, machine learning models trained on big clinical datasets can predict the course of symptoms from radiological, clinical, and laboratory information. The AI nomogram based on gallstone diameter, wall thickness, body mass index (BMI), liver enzymes, and even faecal microbiota signature can be utilised to estimate an individualised risk score. Machine learning models based on imaging and electronic health record (EHR) information can also stratify subjects into low, intermediate and high-risk groups for the development of complications. Convolutional neural networks based on ultrasound, for instance, can identify mild microlithiasis and gallbladder wall thickening. In contrast, algorithms based on demographic and metabolic information have been used to predict the probability of symptomatic conversion. A pilot study recently demonstrated an AI platform with > 85% sensitivity to forecast the onset of symptomatic disease within two years[82]. Clinical decision support systems are also being incorporated into hospital EHRs to automatically conduct risk scoring for AGD using input parameters like stone size, patient age, BMI, comorbidities, and laboratory tests. These systems can provide real-time alerts suggesting elective referral or continuous observation, which enables busy physicians to make evidence-based decisions on whom to prioritise first, particularly in primary care[83].
Some pilot trials have already evaluated the clinical and feasibility effects of precision-guided care. One European trial comparing guideline-based care with algorithm-based care led by AI found that the latter decreased unnecessary cholecystectomies by 22% but enhanced the early intervention rate among high-risk patients. Similarly, patient self-reporting results and lifestyle monitoring using mobile health applications have rendered dynamic care pathways responsive to an unsteady clinical profile of a patient[84]. AI-assisted image recognition was employed to differentiate malignant from benign gallbladder wall changes on ultrasound with excellent sensitivity, possibly to enable early intervention in high-risk cases[82].
As the field continues to mature, potential futures include the creation of comprehensive platforms that integrate genomics, imaging, and real-time health monitoring to provide real-time, adaptive advice. Such technologies represent a fundamental departure from the dichotomous "operate or observe" approach and instead unlock the potential for sophisticated, data-informed, patient-specific decision-making in AGD. The application of precision medicine to other gastrointestinal diseases demonstrates how these technologies can be applied in AGD. In colorectal cancer, genomics profiling informs the choice of adjuvant therapy; in inflammatory bowel disease, pharmacogenomics and biomarkers inform response to biologics.
Although promising, the use of AI and genomics-based precision medicine tools is greeted with a major set of ethical and operational challenges in low-resource settings. There are dangers to data privacy, algorithmic bias, and explainability, particularly when Western data is mostly used for training AI platforms. Furthermore, few rural and semi-urban areas possess digital infrastructure, trained staff, and interoperable EHRs, which retarded implementation in practice. The pragmatic barriers include patient literacy, trust in AI-based recommendations, and genomic testing costs. For instance, while genomic profiling is conceivable within urban hospitals, it is still limited within the government sector or tribal medical facilities without access to subsidies.
The treatment of AGD is being revolutionised by the movement to personalise medical decisions based on various factors in the patient. Models of age, sex, comorbidities, stone size, and imaging findings-based risk stratification are increasingly guiding clinicians. Massive data sets are trained with machine learning algorithms to identify patients likely to be candidates for a cholecystectomy. Moreover, even at this stage, genetic testing is capable of finding individuals with a hereditary risk of severe GSD[82].
Key takeaway: In AGD, precision medicine may have the potential to enable combined risk scores consisting of clinical factors (age, BMI, comorbidities) and genomic biomarkers (such as ABCG8, ABCB4 variants) and microbiome signatures. These multi-modal models would determine those most likely to be asymptomatic. They can be safely observed, as opposed to those with increased risk of complication or gallbladder cancer who would require prophylactic cholecystectomy. Therefore, AGD precision medicine is more a matter of personalising management into observation or cholecystectomy, based on the individual genetic, metabolic, and lifestyle phenotype of the patient, with guidance by AI-supported clinical decision support tools.
Surgical timing in AGD has broad implications for patient outcomes. Due to advances in laparoscopic and robotic technology, elective surgery is safer and more convenient. This section explores where the timing of surgery overlaps with risk reduction and resource optimisation in managing AGD.
Laparoscopic cholecystectomy is nowadays an extremely safe procedure and is considered a routine operative procedure across most tertiary care centres. Its less invasive character, less postoperative pain, and faster recovery have rendered it the standard procedure for gallbladder removal. Earlier single-centre observational series had indicated higher complication rates with emergency surgery. However, subsequent multicenter prospective data[6] reassured the safety of laparoscopic elective cholecystectomy in well-selected high-risk patients. The rate of severe complications, especially BDI, has decreased with better standardisation of procedures like the critical view of safety, better imaging, and surgeon training.
Elective cholecystectomy in high-risk AGD patients (such as large solitary stones, calcified gallbladders, gallbladder polyps, or positive family history of gallbladder cancer) has improved perioperative results and fewer complications than occur with urgent surgery, which is commonly carried out in the context of acute inflammation like mucocele, empyema, or pancreatitis. Urgent cholecystectomies are more likely to have higher rates of conversion to open surgery, bleeding during surgery, longer hospital stays, and increased morbidity. Further adding to the advantage is the rapid development of surgical technologies, including AI-assisted image-guided platforms, robotic technology, 3D, 4K, 8K laparoscopy, and energy devices with enhanced precision and efficacy, improving operative safety and efficacy, especially in high-risk patients[85].
Key takeaway: Elective routine laparoscopic cholecystectomy in meticulously selected high-risk AGD patients provides a considerably safer and more controlled operating scenario than urgent surgery. Advanced surgical technology helps to increase this margin of safety and renders time and planning dominant considerations for successful outcomes.
Cost considerations can be just as relevant in choosing to practice within resource-constrained settings as clinical information. A review of the cost-effectiveness of conservative vs elective treatment of AGD is presented in this section, as well as policy and resource implications.
Cost-effectiveness is a key determinant of surgical decision-making for AGD in low and middle-income countries (LMICs). In a prospective Indian study, early definitive laparoscopic cholecystectomy in patients of acute cholecystitis decreased mean hospital stay (6.5 days vs 10.8 days) and overall cost of treatment (approximately INR 23800 vs INR 29800) compared with delayed/interval cholecystectomy[8]. A systematic review by Bagepally et al[86] in 2022 implies that early laparoscopic cholecystectomy is a cost-effective option compared with delayed laparoscopic cholecystectomy in the treatment of GSD or acute cholecystitis in high-income countries.
Markov decision models have established that risk-stratified elective cholecystectomy in patients at high risk is cost-effective in the long term by avoiding expensive complications such as acute pancreatitis or gallbladder cancer, particularly in patients aged > 60 years or with large stones[87]. Such models include disease progression probabilities, patient age, operative risk, and local treatment costs. The net monetary benefit favours early surgery in older patients with high-risk factors, whereas expectant management remains more feasible in younger, low-risk patients (Table 1).
| Parameter | Prophylactic surgery | Expectant management |
| Risk of complications | Low when elective; increases in elderly/comorbid patients | Low initially; increases with time in high-risk patients |
| Clinical risk | Large solitary stone; ≥ 10 mm polyp/rapid growth, calcified non-functioning gall bladder, haemolysis, immunosuppression | Small stones without high-risk features; low comorbidity; reliable access to urgent care |
| Quality of life impact | Can be improved in symptomatic or high-risk patients | Stable; may decrease if complications |
| Risk of gallbladder cancer | Lower in selected high-risk groups (e.g., solitary large stones) | Higher in endemic areas with large stones or familial risk |
| Cost-effectiveness | More cost-effective in high-risk, elderly patients | More cost-saving in young, low-risk patients with very good follow-up |
| Postoperative complications | Bile duct injury, diarrhoea, dyspepsia (1%-5%), possible | None at first, but the danger of emergency surgery complications |
In LMICs, with impaired surgical access and frequently postponed emergency care, cost-effectiveness is measured differently. Elective surgery, while expensive in the short term, could be less expensive than managing life-threatening complications that involve intensive care unit, endoscopic retrograde cholangiopancreatography (ERCP), or tertiary care transfer. Policymakers are tasked with balancing short-term surgical cost with long-term system cost savings.
Cost-effectiveness analyses must be incorporated into national health policy to guide AGD management. The stratification of patients into "observe" and "elective surgery" categories using age, comorbidities, and regional cancer incidence allows governments to allocate surgical resources best. By way of illustration, an incentivised early elective surgery payment program for high-risk gallbladder cancer regions (e.g., North India, Chile, and Northern Pakistan) will decrease delayed presentation and long-term costs[87].
Key takeaway: Elective surgery is also cost-effective for high-risk AGD patients by preventing the more costly emergency surgery. Economic modelling favours introducing risk-based decision tools into national policy, especially in resource-poor systems with an agenda to deliver efficient, evidence-based surgical care. Lastly, reconciling cost-effectiveness with health equity is required to ensure that AGD management policy not only achieves savings in resources but also mitigates disparities to enable timely and safe treatment of vulnerable groups.
Technologies can redefine AGD management by advancing proactive, personalised, and non-invasive approaches. This section acknowledges the promise of wearable technology, digital biomarkers, and clinical integration of the platform to revolutionise AGD monitoring and treatment.
With the rapid evolution of digital health technologies, the management and monitoring of AGD are poised for a radical change. In the future, AGD management will be expected to employ wearable health devices, AI mobile apps, and digital biomarkers, revolutionising AGD management from reactive intervention to proactive surveillance and prevention.
Some early examples of AI applications in GSD are convolutional neural networks used to evaluate ultrasound scans and identify microlithiasis or correctly predict common bile duct stones. Clinical and biochemical variables have been used to derive machine-learning algorithms that have been studied to determine which asymptomatic patients are likely to develop biliary colic or pancreatitis. AGD-specific models are limited to date, but these methods show the potential for AI to improve risk stratification beyond traditional parameters. Wearable devices like smart belts, patch biosensors, and accelerometers may be utilised to observe gallbladder motility, identify meal-induced abdominal distension, and measure local temperature change as the initial indicators of biliary inflammation. Mobile health applications will also become increasingly popular in AGD care. They would allow patients to track symptoms, monitor eating habits, avoid foods that can lead to gallstones, and communicate with their care team[88].
AI algorithms in hepatology have been demonstrated to predict the risk of hepatocellular carcinoma in cirrhosis, as well as in pancreatology to predict the severity of acute pancreatitis. The same models would be transferable to AGD, with symptomatic conversion or prediction of cancer determining the extent of prophylactic surgery. Digital biomarkers in the form of quantifiable physiological signals from smart devices, such as heart rate variability and distension indices, will enable individual risk profiling. Meanwhile, AI algorithms in EHR systems will someday alert to incidental gallstone cases and cross-match patient data with validated risk models.
Wearable sensors and smartphones are being used to monitor diet, activity, and biliary symptoms in order to facilitate dynamic risk adjustment and the detection of early symptoms. Integration with hospital systems could alert physicians to intervene prior to complications arising. The systems will allow clinicians to provide timely, accurate, and non-invasive decisions, establishing a smarter, preventive health paradigm where preventive treatment may be imparted[88].
Key takeaway: AGD care's future is a preventive, technology-driven model combining wearables, AI, and digital health technologies to identify risk early and direct intervention. These emerging technologies promise more safety, avoiding unnecessary surgery, and transforming care delivery to proactive and personal disease management. Therefore, although AI, genomics, and digital health technologies promise to revolutionise the management of AGD, their utility in practice will be contingent upon demonstration of validity in multiple populations and equitable, ethical implementation in routine surgical practice.
The choice for surgery in asymptomatic gallstones is still uncertain. Given new developments in perioperative surgical safety and individualised risk stratification, this section reassesses the indication and optimal timing of elective cholecystectomy for asymptomatic gallstones.
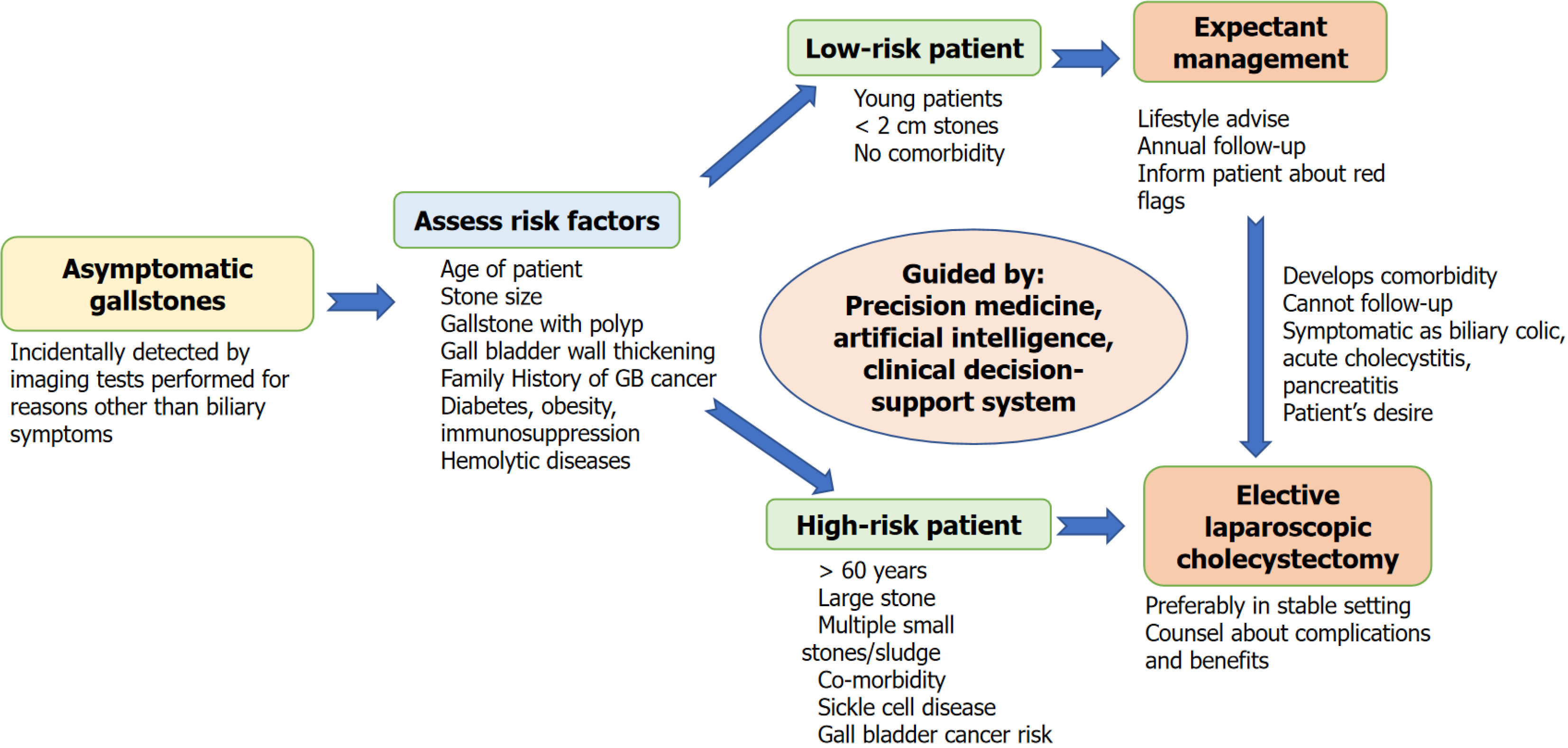
Laparoscopic cholecystectomy is the treatment of choice in symptomatic cholelithiasis. In the asymptomatic, however, there is agreement to accept expectant management because of surgical and anaesthetic risk, extra healthcare expenditure, and adverse cost-to-benefit ratio. Prophylactic surgery is indicated in special conditions only. However, most data supporting these statements are old and from observational studies. A balanced critique of expectant management over prophylactic cholecystectomy has to put the natural history risk of developing AGD against the non-zero risk of complications in surgery, particularly where there is comorbidity or limited access to follow-up (Figure 1). There is observational evidence that supports conservative treatment in the majority. However, clinical experience assures us that some develop complications such as pancreatitis or acute cholecystitis and require emergency surgery with increased rates of conversion, morbidity, and expense[2]. The surgical procedure for gallstones has evolved substantially compared to the past, with many technical advancements. The standardisation of laparoscopic surgery, better training and a "critical view of safety" approach have drastically reduced the risks and complications associated with cholecystectomy[52].
To summarise, the advantages of expectant management are avoiding immediate surgical/anaesthetic risk, maintaining QoL in individuals who will never experience symptoms and resource-sparing in resource-constrained nations.
On the other hand, prophylactic surgery is augmented by elective timing, controlled environment, and preoperative optimisation, particularly in high-risk situations like giant solitary stones, gallbladder polyps, or familial history of carcinoma (Table 1). However, it risks bile duct damage, postoperative dyspepsia, or even persistent diarrhoea in a small minority of cases, worsening long-term QoL in some[60-63]. Long-term outcome comparative studies have shown that 10%-20% of symptom-free patients only develop symptoms in 10 years, and less than 5% need emergency surgery. Gurusamy et al[32] meta-analysis, though, introduced the question of whether complications are more frequent with emergency cholecystectomy (2.7), indicating perhaps an advantage to elective surgery in appropriately chosen high-risk conditions. Patients with symptomatic cholelithiasis may end up with complications, making surgery not only difficult but also increasing the chances of biliary tract injury, conversion to open, and added procedures like ERCP. It not only adds to morbidity and mortality but also results in a substantial increase in healthcare costs that may outweigh the benefit of not doing surgery in asymptomatic cases[62,63]. On the other hand, a 10-year follow-up study by Halldestam et al[19] could not demonstrate any discrepancy in total survival or severe complications between operated and watched patients in asymptomatic patients, supporting the argument for conservative treatment in low-risk patients.
To summarise, the potential advantages of a prophylactic laparoscopic cholecystectomy are: Avoiding the risk of future biliary events, getting time to optimise anaesthetic risk and availability of expert skills, preventing increased morbidity of emergency operation, and may be necessary where the risk of gallbladder cancer or recurrent access obstacles is high.
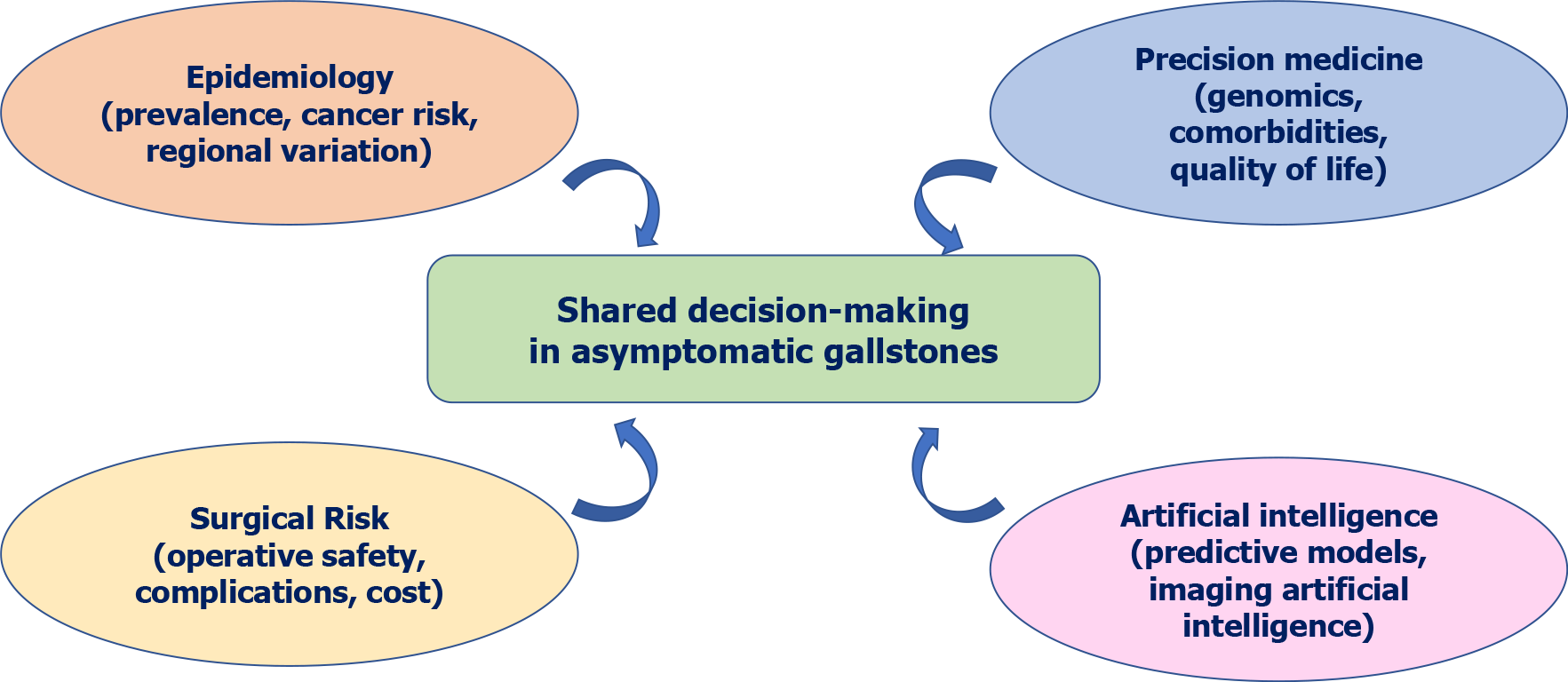
Key takeaway: Elective surgery for AGD can never be discouraged en masse, and various factors need to be followed for managing asymptomatic gallstones (Figure 2). Improved laparoscopic safety and risk stratification preoperatively enable selective therapeutic intervention in high-risk individuals, with a preference for patient-focused, individualised over universal expectant management. For the majority of truly asymptomatic patients in low-risk populations with assured access, watchful waiting is still indicated. Preventive surgery is most fair when specific risk markers and situational variables (e.g., geographic cancer risk and system limitations) tip the scale in favour of intervention. Cumulatively, these differences imply why the decisions need to shift away from the dichotomous “operate or observe” discussion towards risk and context-sensitive decisions, informed by patient preference and underpinned by predictive modelling and QoL evidence.
Addressing AGD in LMICs, as in India, must have scientific merit, ethical rationale, and regional relevance. All of the challenges outlined in India, unequal access, heterogeneity of surgical capacity, lack of digital infrastructure, and sociocultural barriers are the same and applicable to all LMICs across Asia, Africa, and Latin America. This section addresses the ethical requirement for informed decision-making and region-specific guidelines, tabulated according to locality-based epidemiological and healthcare infrastructure variation.
Even though promising, deploying AI and genomics-based precision technology ethically and practically is extremely challenging, particularly for low-resource communities. Ethical issues also arise with the proposal of prophylactic surgery for a condition that may never cause symptoms. Informed consent procedures need to be solid, with patients comprehending both the possible benefits of complication avoidance and the risks of unwarranted surgery. Patient autonomy must be respected in recommending forceful therapy when there are no symptoms. Shared decision-making aids using clinical, genetic, and patient-reported information are important in facilitating ethical and clear communication[89].
Treatment of asymptomatic gallstones must be tailored; it is no longer a “one-size-fits-all” approach. AGD poses a classic clinical dilemma, particularly in our modern age of minimally invasive surgery and precision medicine. The majority of patients are symptom-free, but a select few carry a risk of severe complications. Precision medicine technologies now enable more advanced risk stratification to inform decision-making. Attention must be turned to optimising the balance of patient preference, individual risk, and efficient healthcare resource utilisation.
Global series evidence indicates heterogeneity of outcomes based on geography, healthcare environment, and patient populations. Treatment is varied between geographical regions, and the majority apply conservative management, except for complications or the presence of features such as polyps. In high-risk countries with regions such as Chile, asymptomatic individuals may be operated on, given the high prevalence of gallbladder cancer[15]. It underscores regionalised recommendations based on regional epidemiology in addition to infrastructure availability.
In India, AGD management needs to consider the double public-private healthcare systems, geographical patterns of cancer, and access gradients. The condition is more prevalent in women (> 20%) and has a greater risk of gallbladder cancer, particularly in patients with large solitary gallstones[4,15,26] in Northern India. Surgeons who work in high-risk environments like Northern India or Chile, where gallbladder carcinoma has been widely prevalent, will prefer prophylactic cholecystectomy even in asymptomatic individuals. In these high-risk patient groups, e.g., older adults and patients with a family history of gallbladder polyps or cancer, elective cholecystectomy can be warranted based on cost-effectiveness as well as surgical safety. On the other hand, a South Indian male (incidence of gallbladder malignancy is less), aged 30 years with multiple small stones but without comorbidities, can be treated safely with annual follow-up ultrasonography and dietary counselling.
Health equity is still a major concern in the treatment of AGD. Urban and private health centres usually provide day-care laparoscopic cholecystectomy, while rural, tribal, and public health centres do not have trained laparoscopic surgeons, anaesthesia personnel, and imaging centres. Similar barriers have been described elsewhere in LMICs: e.g., restricted access to day-care laparoscopic surgery in sub-Saharan Africa, delayed cancer diagnosis in Latin America, and a digital divide to the application of AI-based decision tools in Southeast Asia[90]. These results are in line with what we have seen in India and confirm the argument that precision medicine and AI-driven decision models need to be adapted to LMIC environments with deliberate design for equity, infrastructure, and workforce ability. Women belonging to low-income families, although at greater risk of gallstones and gallbladder carcinoma, postpone or abstain from surgery due to gendered access barriers, illiteracy in health, and economic subordination[15]. Public sector programs and outreach surgery services must be designed to meet this access deficit. While Indian surgical societies are acting aggressively to narrow disparities with training and outreach, the same regional capacity development must occur across all LMICs if precision-guided AGD management is to be applied evenly. An expansion of this emphasis beyond India enhances this review's relevance globally[91].
Key takeaway: A sophisticated, case-sensitive strategy, accounting for the regional risk of cancer, availability of elective surgery, comorbidity, and patient values, is needed to conclude at the end of the argument on undertaking prophylactic cholecystectomy or watchful waiting. Data must guide it, as well as context and individualised counselling.
The future of AGD management is a balanced, risk-adjusted approach based on technological progress, ethical prudence, and population-oriented strategies. This section outlines a future vision integrating clinical risk modelling, socioeconomic considerations, and individualised treatment.
Technological, ethical, and inequities vision: Future AGD management will increasingly be informed by clinical risk modelling, real-world evidence, and shared decision-making tools. As personalised treatment becomes the new norm, ethical controls will be required to guarantee that precision will not be bought at the price of patient rights, justice, or equity[88]. As highlighted by Obermeyer and Emanuel[89] and Vayena et al[92], some of the fundamental ethical and infrastructural issues, including algorithmic bias, global representativeness of the AI algorithms, infrastructural deficits, and patient literacy constraints, need to be resolved in order to achieve equitable access to precision tools among various and underserved groups (Table 2). For example, while genomic profiling may be feasible in metropolitan hospitals, its utility remains restricted in the government sector or tribal healthcare setups without subsidised access.
| Technology | Application | Benefits |
| Risk models using AI | Predict transition from asymptomatic to symptomatic disease | Reduces unnecessary surgery, focuses on early intervention |
| Genomic markers | Detect genetic predisposition to gallstones | Facilitates early diagnosis and tailored management |
| Mobile health apps | Track symptoms and allow remote monitoring | Enhances patient activation and self-triage |
| Wearables | Detect motility changes and early inflammation | Allows proactive, non-invasive surveillance |
| CDSS with EHR | Automated risk scoring and clinical decision support | Facilitates evidence-based, equitable delivery of care |
Regional epidemiology & risk-based intervention: Most AGD cases are benign, but a significant proportion will develop life-threatening complications. With better awareness of risk factors and changing technology, clinicians can identify patients who benefit from early treatment. Public concern lies with data privacy, discriminatory algorithms, and transparency, especially as AI systems are largely trained on Western databases and cannot be developed for generalised deployment, ignoring the geographical realities. A lifestyle diet of high saturated fats and processed carbohydrates, and rising obesity and metabolic syndrome, underlie the increasing incidence of AGD. It necessitates the shift from random diagnosis to structured, risk-stratified management recommendations, particularly in resource-limited practices.
Economic impact & behavioural barriers: Economic factors also influence decision-making. While elective laparoscopic cholecystectomy costs upfront, it is more cost-effective in the long term by avoiding emergency surgery and minimising morbidity. Finally, health-seeking behaviour, literacy, and cultural beliefs control early intervention. Public awareness measures tailored to individual needs and multilingual decision aids must be available to facilitate informed, shared decision-making. India can lead toward establishing a precision-guided, resource-directed AGD management model with universal applicability.
Finally, we need to introspect whether we would opt for early intervention or observation should we encounter an inadvertent gallstone diagnosis, an ethical and practical dilemma facing our patients.
Key takeaway: The convergence of AI, genomics, and digital health technologies can transform AGD management. Local adaptation, ethical transparency, and readiness of health systems are essential for successful implementation, particularly in multi-regional and resource-limited environments such as India. To provide equal benefit, a step-by-step strategy involving low-grade ultrasound, strategic panels of biomarkers, and mobile AI solutions may serve as a bridge towards upper-grade digital decision technologies in underprivileged populations.
This review, although extensive, has some limitations. Most of the studies included were case series, retrospective in design, or observational and contained very few directly comparable high-quality RCTs in AGD, comparing directly expectant management with prophylactic cholecystectomy. Most had short follow-up periods and small numbers, which limited the evaluation of longer-term outcomes such as mortality or complications. The majority of quoted evidence is from high-risk cancer areas for gallbladder cancer, like North India and Chile. Although very pertinent to those populations, they are not always instantly transferable to low-risk cancer areas like much of Europe or East Asia. It reduces global generalisability for prophylactic cholecystectomy recommendations.
Methodological variability among the studies, e.g., differences in patient selection, risk factor definition, and regional practice, also reduces the generalizability of results. Most of the studies did not stratify patients by contemporary risk prediction models or adjust for comorbid conditions like diabetes, obesity, and metabolic syndrome that are more pertinent in treating AGD.
Also, high-risk region prospective data from North India, Chile, and regions of Pakistan, where the risk of gallbladder carcinoma might warrant the initial application of surgical intervention, are lacking. Without this kind of information translatable to context, one cannot formulate recommendations that can be used worldwide.
The other limitation is the excessive dependence of today's AI-based risk models on databases that are predominantly Western populations in origin. It is a source of algorithmic bias, underestimation when extended to heterogeneous LMIC populations, and risk of further embedding inequities if such resources are scaled up without local pilot-testing.
Lastly, possible publication bias should not be forgotten, wherein unfavourable or neutral findings on conservative management are under-published, overemphasising the benefits perceived of surgery. These gaps underline the importance of multi-centre, prospective trials, especially in LMICs, to establish validated, risk-based treatment plans for AGD.
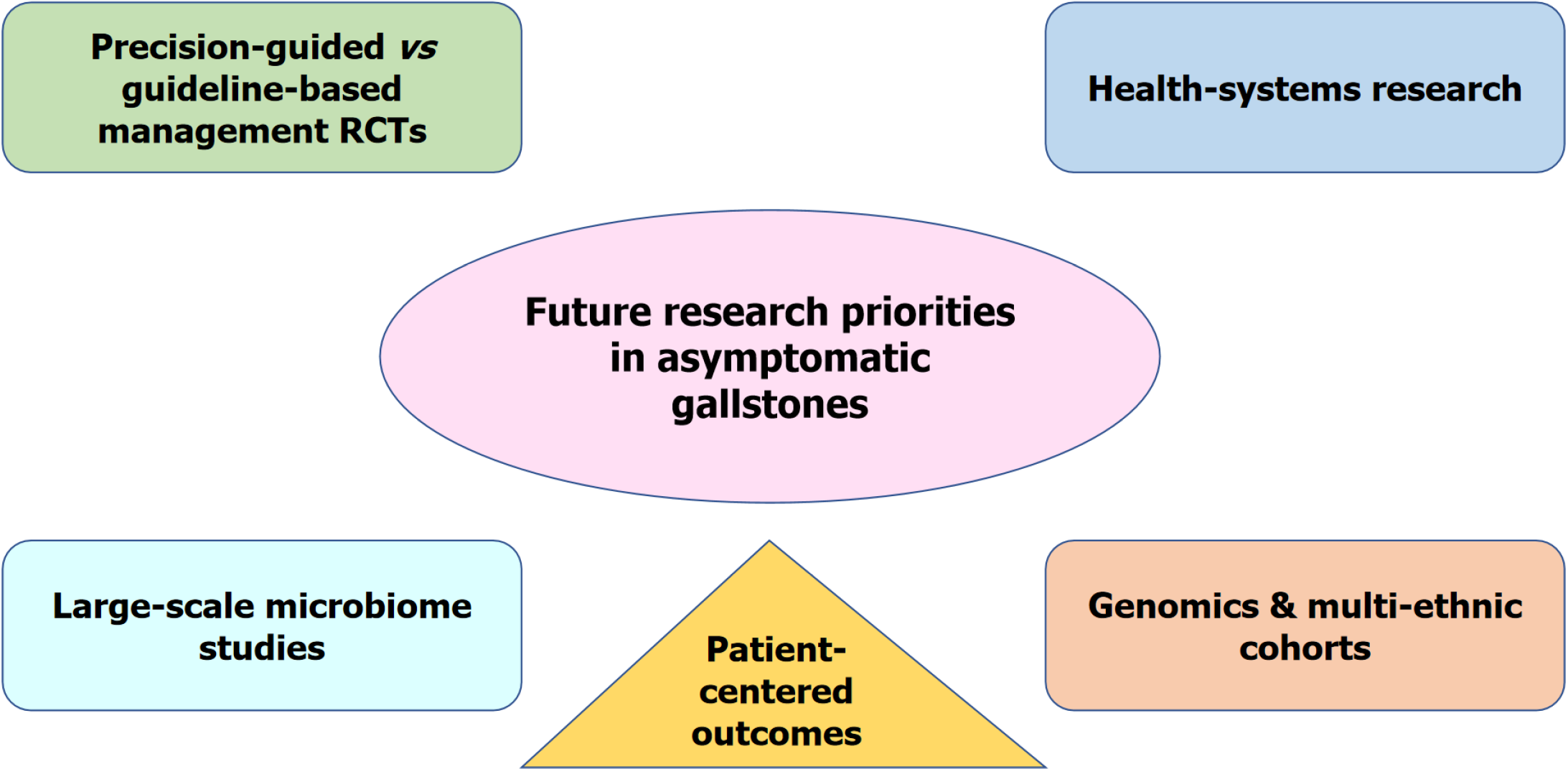
Future research should have the following highest priority (Figure 3): Prospective RCTs comparing precision-guided vs standard guideline-based therapy (AI/biomarker-based risk stratification) to assess whether surgery by personalised thresholds of surgery improves outcomes and cost-effectiveness.
Multi-centre, long-term microbiome research needs to be undertaken to determine whether post-cholecystectomy dysbiosis is indeed an increased causative factor in colorectal cancer, changing practice in asymptomatic cases. Subsequent genomic and biomarker analyses need to specifically enroll non-Western, high-burden countries in order to develop legitimate models of risk and prevent algorithmic bias.
Implementation studies in LMICs will need to decide how decision-support tools in digital formats can be optimised for settings that involve constraints, inequalities, and uneven surgical abilities.
Long-term cost and QoL comparisons for specific prophylactic surgery vs observation planned are not available, particularly beyond high-incidence areas. System-level costs and patient-reported outcomes from prospective registries and pragmatic trials need to establish thresholds that enhance the actual lived patient experience.
In summary, while this review reflects on the latest developments, stringently critical research—most importantly, multi-regional RCTs and large-scale translation studies- is what the field desperately requires to confirm precision strategies, correct biases in AI tools, and advance fair, patient-focused methods that are actionable globally.
AGD is typically benign and remains amenable to conservative management in the majority of patients. The paradigm is changing, though, from universal policy to precision-based, patient-specific care. Using the combination of genomics, AI-based risk stratification, and patient-reported outcomes, surgeons can more accurately select those who will truly benefit from early cholecystectomy without undertreating others. The future is building precision tools into shared decision-making to provide safe, cost-effective, and equitable care globally.
| 1. | Patel AM, Yeola M, Mahakalkar C. Demographic and Risk Factor Profile in Patients of Gallstone Disease in Central India. Cureus. 2022;14:e24993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Saraswat VA, Sharma BC, Agarwal DK, Kumar R, Negi TS, Tandon RK. Biliary microlithiasis in patients with idiopathic acute pancreatitis and unexplained biliary pain: response to therapy. J Gastroenterol Hepatol. 2004;19:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Kim SB, Kim KH, Kim TN, Heo J, Jung MK, Cho CM, Lee YS, Cho KB, Lee DW, Han JM, Kim HG, Kim HS. Sex differences in prevalence and risk factors of asymptomatic cholelithiasis in Korean health screening examinee: A retrospective analysis of a multicenter study. Medicine (Baltimore). 2017;96:e6477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Virk S, Arora H, Patil P, Sarang B, Khajanchi M, Bains L, Kizhakke DV, Jain S, Nathani P, Dev Y, Gadgil A, Roy N. An Indian surgeon's perspective on management of asymptomatic gallstones. Asian J Endosc Surg. 2024;17:e13297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Mayumi T, Okamoto K, Takada T, Strasberg SM, Solomkin JS, Schlossberg D, Pitt HA, Yoshida M, Gomi H, Miura F, Garden OJ, Kiriyama S, Yokoe M, Endo I, Asbun HJ, Iwashita Y, Hibi T, Umezawa A, Suzuki K, Itoi T, Hata J, Han HS, Hwang TL, Dervenis C, Asai K, Mori Y, Huang WS, Belli G, Mukai S, Jagannath P, Cherqui D, Kozaka K, Baron TH, de Santibañes E, Higuchi R, Wada K, Gouma DJ, Deziel DJ, Liau KH, Wakabayashi G, Padbury R, Jonas E, Supe AN, Singh H, Gabata T, Chan ACW, Lau WY, Fan ST, Chen MF, Ker CG, Yoon YS, Choi IS, Kim MH, Yoon DS, Kitano S, Inomata M, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Thapar VB, Thapar PM, Goel R, Agarwalla R, Salvi PH, Nasta AM, Mahawar K; IAGES Research Collaborative Group. Evaluation of 30-day morbidity and mortality of laparoscopic cholecystectomy: a multicenter prospective observational Indian Association of Gastrointestinal Endoscopic Surgeons (IAGES) Study. Surg Endosc. 2023;37:2611-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Gamo GO, Reichardt GS, Guetter CR, Pimentel SK. Risk factors for surgical wound infection after elective laparoscopic cholecystectomy. Arq Bras Cir Dig. 2022;35:e1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Singh G, Singh RR, Bansal D. A comparative study of interval cholecystectomy and early cholecystectomy in acute cholecystitis. Int Surg J. 2020;7:1419-1423. [DOI] [Full Text] |
| 9. | Li ZZ, Guan LJ, Ouyang R, Chen ZX, Ouyang GQ, Jiang HX. Global, regional, and national burden of gallbladder and biliary diseases from 1990 to 2019. World J Gastrointest Surg. 2023;15:2564-2578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 10. | Lammert F, Gurusamy K, Ko CW, Miquel JF, Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 566] [Article Influence: 56.6] [Reference Citation Analysis (1)] |
| 11. | Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 255] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Hamdani NH, Qadri SK, Aggarwalla R, Bhartia VK, Chaudhuri S, Debakshi S, Baig SJ, Pal NK. Clinicopathological study of gall bladder carcinoma with special reference to gallstones: our 8-year experience from eastern India. Asian Pac J Cancer Prev. 2012;13:5613-5617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 14. | Mathur AV. Need for Prophylactic Cholecystectomy in Silent Gall Stones in North India. Indian J Surg Oncol. 2015;6:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Chin Clin Oncol. 2019;8:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 16. | Bray F, Balcaen T, Baro E, Gandon A, Ficheur G, Chazard E. Increased incidence of cholecystectomy related to gallbladder disease in France: Analysis of 807,307 cholecystectomy procedures over a period of seven years. J Visc Surg. 2019;156:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Donovan JM. Physical and metabolic factors in gallstone pathogenesis. Gastroenterol Clin North Am. 1999;28:75-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Festi D, Reggiani ML, Attili AF, Loria P, Pazzi P, Scaioli E, Capodicasa S, Romano F, Roda E, Colecchia A. Natural history of gallstone disease: Expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol. 2010;25:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg. 2004;91:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Attili AF, De Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology. 1995;21:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Schmidt M, Søndenaa K, Vetrhus M, Berhane T, Eide GE. A randomized controlled study of uncomplicated gallstone disease with a 14-year follow-up showed that operation was the preferred treatment. Dig Surg. 2011;28:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Alves JR, Klock DM, Ronzani FG, Santos SLD, Amico EC. Asymptomatic cholelithiasis: expectant or cholecystectomy. A systematic review. Arq Bras Cir Dig. 2023;36:e1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Portincasa P, Di Ciaula A, de Bari O, Garruti G, Palmieri VO, Wang DQ. Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol. 2016;10:93-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Hyun JJ, Lee HS, Kim CD, Dong SH, Lee SO, Ryu JK, Lee DH, Jeong S, Kim TN, Lee J, Koh DH, Park ET, Lee IS, Yoo BM, Kim JH. Efficacy of Magnesium Trihydrate of Ursodeoxycholic Acid and Chenodeoxycholic Acid for Gallstone Dissolution: A Prospective Multicenter Trial. Gut Liver. 2015;9:547-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Ibrahim M, Sarvepalli S, Morris-Stiff G, Rizk M, Bhatt A, Walsh RM, Hayat U, Garber A, Vargo J, Burke CA. Gallstones: Watch and wait, or intervene? Cleve Clin J Med. 2018;85:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Behari A, Kapoor VK. Asymptomatic Gallstones (AsGS) - To Treat or Not to? Indian J Surg. 2012;74:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Shabanzadeh DM, Sørensen LT, Jørgensen T. A Prediction Rule for Risk Stratification of Incidentally Discovered Gallstones: Results From a Large Cohort Study. Gastroenterology. 2016;150:156-167.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Williams TP, Dimou FM, Adhikari D, Kimbrough TD, Riall TS. Hospital readmission after emergency room visit for cholelithiasis. J Surg Res. 2015;197:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Sodhi JS, Zargar SA, Khateeb S, Showkat A, Javid G, Laway BA, Parveen S, Khan BA, Yattoo GN, Shah A, Gulzar GM, Khan MA. Prevalence of gallstone disease in patients with type 2 diabetes and the risk factors in North Indian population: a case control study. Indian J Gastroenterol. 2014;33:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Riall TS, Adhikari D, Parmar AD, Linder SK, Dimou FM, Crowell W, Tamirisa NP, Townsend CM Jr, Goodwin JS. The risk paradox: use of elective cholecystectomy in older patients is independent of their risk of developing complications. J Am Coll Surg. 2015;220:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Pineda O, Maydón HG, Amado M, Sepúlveda EM, Guilbert L, Espinosa O, Zerrweck C. A Prospective Study of the Conservative Management of Asymptomatic Preoperative and Postoperative Gallbladder Disease in Bariatric Surgery. Obes Surg. 2017;27:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Gurusamy KS, Samraj K. Cholecystectomy versus no cholecystectomy in patients with silent gallstones. Cochrane Database Syst Rev. 2007;2007:CD006230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Schwesinger WH, Diehl AK. Changing indications for laparoscopic cholecystectomy. Stones without symptoms and symptoms without stones. Surg Clin North Am. 1996;76:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Goswami AG, Basu S. Cracking the silent gallstone code: Wait or operate? World J Clin Cases. 2024;12:2692-2697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (3)] |
| 35. | Abraham S, Rivero HG, Erlikh IV, Griffith LF, Kondamudi VK. Surgical and nonsurgical management of gallstones. Am Fam Physician. 2014;89:795-802. [PubMed] |
| 36. | Sakai Y, Tsuyuguchi T, Ohyama H, Kumagai J, Kaiho T, Ohtsuka M, Kato N, Sakai T. Natural history of asymptomatic gallbladder stones in clinic without beds: A long-term prognosis over 10 years. World J Clin Cases. 2024;12:42-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (4)] |
| 37. | Currò G, Meo A, Ippolito D, Pusiol A, Cucinotta E. Asymptomatic cholelithiasis in children with sickle cell disease: early or delayed cholecystectomy? Ann Surg. 2007;245:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Luthra A, Behura A, Behera CR, Mishra A, Mohanty S, Panda B. Intraoperative Findings of Elective Laparoscopic Cholecystectomy in Diabetics Versus Nondiabetics: A Comparative Study. Cureus. 2022;14:e20886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Muroni M, Loi V, Lionnet F, Girot R, Houry S. Prophylactic laparoscopic cholecystectomy in adult sickle cell disease patients with cholelithiasis: A prospective cohort study. Int J Surg. 2015;22:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Banli O, Guvence N, Altun H. Laparoscopic cholecystectomy for renal transplants. Transplant Proc. 2005;37:2127-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Kao LS, Flowers C, Flum DR. Prophylactic cholecystectomy in transplant patients: a decision analysis. J Gastrointest Surg. 2005;9:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Richardson WS, Surowiec WJ, Carter KM, Howell TP, Mehra MR, Bowen JC. Gallstone disease in heart transplant recipients. Ann Surg. 2003;237:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | DiBrito SR, Haugen CE, Holscher CM, Olorundare IO, Alimi Y, Segev DL, Garonzik-Wang J. Complications, length of stay, and cost of cholecystectomy in kidney transplant recipients. Am J Surg. 2018;216:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Şen O, Türkçapar AG. Risk of Asymptomatic Gallstones Becoming Symptomatic After Laparoscopic Sleeve Gastrectomy. Am Surg. 2023;89:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Desbeaux A, Hec F, Andrieux S, Fayard A, Bresson R, Pruvot MH, Mulliez E. Risk of biliary complications in bariatric surgery. J Visc Surg. 2010;147:e217-e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Plecka Östlund M, Wenger U, Mattsson F, Ebrahim F, Botha A, Lagergren J. Population-based study of the need for cholecystectomy after obesity surgery. Br J Surg. 2012;99:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Warschkow R, Tarantino I, Ukegjini K, Beutner U, Güller U, Schmied BM, Müller SA, Schultes B, Thurnheer M. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg. 2013;23:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 48. | Haal S, Guman MSS, Bruin S, Schouten R, van Veen RN, Fockens P, Dijkgraaf MGW, Hutten BA, Gerdes VEA, Voermans RP. Risk Factors for Symptomatic Gallstone Disease and Gallstone Formation After Bariatric Surgery. Obes Surg. 2022;32:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Morimoto M, Matsuo T, Mori N. Management of Porcelain Gallbladder, Its Risk Factors, and Complications: A Review. Diagnostics (Basel). 2021;11:1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Pucher PH, Brunt LM, Fanelli RD, Asbun HJ, Aggarwal R. SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc. 2015;29:3074-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci. 2007;52:1313-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Trindade EN, Difante LDS, Wendt LRR, Trindade MRM. Expectant management or cholecystectomy in asymptomatic cholelithiasis. Arq Bras Cir Dig. 2024;37:e1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Frost F, Kacprowski T, Rühlemann M, Weiss S, Bang C, Franke A, Pietzner M, Aghdassi AA, Sendler M, Völker U, Völzke H, Mayerle J, Weiss FU, Homuth G, Lerch MM. Carrying asymptomatic gallstones is not associated with changes in intestinal microbiota composition and diversity but cholecystectomy with significant dysbiosis. Sci Rep. 2021;11:6677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Zhang Y, Liu H, Li L, Ai M, Gong Z, He Y, Dong Y, Xu S, Wang J, Jin B, Liu J, Teng Z. Cholecystectomy can increase the risk of colorectal cancer: A meta-analysis of 10 cohort studies. PLoS One. 2017;12:e0181852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Tan CH, Pang TC, Woon WW, Low JK, Junnarkar SP. Analysis of actual healthcare costs of early versus interval cholecystectomy in acute cholecystitis. J Hepatobiliary Pancreat Sci. 2015;22:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Amirthalingam V, Low JK, Woon W, Shelat V. Tokyo Guidelines 2013 may be too restrictive and patients with moderate and severe acute cholecystitis can be managed by early cholecystectomy too. Surg Endosc. 2017;31:2892-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 57. | Doherty G, Manktelow M, Skelly B, Gillespie P, Bjourson AJ, Watterson S. The Need for Standardizing Diagnosis, Treatment and Clinical Care of Cholecystitis and Biliary Colic in Gallbladder Disease. Medicina (Kaunas). 2022;58:388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 58. | Gach T, Bogacki P, Markowska B, Bonior J, Paplaczyk M, Szura M. Quality of life in patients after laparoscopic cholecystectomy due to gallstone disease - evaluation of long-term postoperative results. Pol Przegl Chir. 2021;93:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Quintana JM, Aróstegui I, Cabriada J, López de Tejada I, Perdigo L. Predictors of improvement in health-related quality of life in patients undergoing cholecystectomy. Br J Surg. 2003;90:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Wanjura V, Sandblom G. How Do Quality-of-Life and Gastrointestinal Symptoms Differ Between Post-cholecystectomy Patients and the Background Population? World J Surg. 2016;40:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | de Reuver PR, Sprangers MA, Rauws EA, Lameris JS, Busch OR, van Gulik TM, Gouma DJ. Impact of bile duct injury after laparoscopic cholecystectomy on quality of life: a longitudinal study after multidisciplinary treatment. Endoscopy. 2008;40:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Törnqvist B, Waage A, Zheng Z, Ye W, Nilsson M. Severity of Acute Cholecystitis and Risk of Iatrogenic Bile Duct Injury During Cholecystectomy, a Population-Based Case-Control Study. World J Surg. 2016;40:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Rice CP, Vaishnavi KB, Chao C, Jupiter D, Schaeffer AB, Jenson WR, Griffin LW, Mileski WJ. Operative complications and economic outcomes of cholecystectomy for acute cholecystitis. World J Gastroenterol. 2019;25:6916-6927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (3)] |
| 64. | Andersson R, Eriksson K, Blind PJ, Tingstedt B. Iatrogenic bile duct injury--a cost analysis. HPB (Oxford). 2008;10:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Gavriilidis P, Catena F, de'Angelis G, de'Angelis N. Consequences of the spilled gallstones during laparoscopic cholecystectomy: a systematic review. World J Emerg Surg. 2022;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Kama NA, Kologlu M, Doganay M, Reis E, Atli M, Dolapci M. A risk score for conversion from laparoscopic to open cholecystectomy. Am J Surg. 2001;181:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Licciardello A, Arena M, Nicosia A, Di Stefano B, Calì G, Arena G, Minutolo V. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy. Eur Rev Med Pharmacol Sci. 2014;18:60-68. [PubMed] |
| 68. | Iwashita Y, Ohyama T, Honda G, Hibi T, Yoshida M, Miura F, Takada T, Han HS, Hwang TL, Shinya S, Suzuki K, Umezawa A, Yoon YS, Choi IS, Huang WS, Chen KH, Watanabe M, Abe Y, Misawa T, Nagakawa Y, Yoon DS, Jang JY, Yu HC, Ahn KS, Kim SC, Song IS, Kim JH, Yun SS, Choi SH, Jan YY, Sheen-Chen SM, Shan YS, Ker CG, Chan DC, Lee KT, Toyota N, Higuchi R, Nakamura Y, Mizuguchi Y, Takeda Y, Ito M, Norimizu S, Yamada S, Matsumura N, Shindoh J, Sunagawa H, Hasegawa H, Rikiyama T, Sata N, Kano N, Kitano S, Tokumura H, Yamashita Y, Watanabe G, Nakagawa K, Kimura T, Yamakawa T, Wakabayashi G, Endo I, Miyazaki M, Yamamoto M. What are the appropriate indicators of surgical difficulty during laparoscopic cholecystectomy? Results from a Japan-Korea-Taiwan multinational survey. J Hepatobiliary Pancreat Sci. 2016;23:533-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 770] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 70. | Jung SJ, Kim JS, Hong SG, Joo MK, Lee BJ, Kim JH, Yeon JE, Park JJ, Byun KS, Bak YT, Kim WB, Choi SY. [Critical reappraisal of cholecystectomy in patients with asymptomatic gallstones for early diagnosis and removal of dysplasia and cancer]. Korean J Gastroenterol. 2010;55:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, Fraumeni JF Jr. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Elmasry M, Lindop D, Dunne DF, Malik H, Poston GJ, Fenwick SW. The risk of malignancy in ultrasound detected gallbladder polyps: A systematic review. Int J Surg. 2016;33 Pt A:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Gupta P, Dutta U, Rana P, Singhal M, Gulati A, Kalra N, Soundararajan R, Kalage D, Chhabra M, Sharma V, Gupta V, Yadav TD, Kaman L, Irrinki S, Singh H, Sakaray Y, Das CK, Saikia U, Nada R, Srinivasan R, Sandhu MS, Sharma R, Shetty N, Eapen A, Kaur H, Kambadakone A, de Haas R, Kapoor VK, Barreto SG, Sharma AK, Patel A, Garg P, Pal SK, Goel M, Patkar S, Behari A, Agarwal AK, Sirohi B, Javle M, Garcea G, Nervi F, Adsay V, Roa JC, Han HS. Gallbladder reporting and data system (GB-RADS) for risk stratification of gallbladder wall thickening on ultrasonography: an international expert consensus. Abdom Radiol (NY). 2022;47:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 74. | Wang JK, Foster SM, Wolff BG. Incidental gallstones. Perm J. 2009;13:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol. 2011;9:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 76. | Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140:508-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 77. | Zheng Y, Xu M, Heianza Y, Ma W, Wang T, Sun D, Albert CM, Hu FB, Rexrode KM, Manson JE, Qi L. Gallstone disease and increased risk of mortality: Two large prospective studies in US men and women. J Gastroenterol Hepatol. 2018;33:1925-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 78. | Shabanzadeh DM, Sørensen LT, Jørgensen T. Gallstone disease and mortality: a cohort study. Int J Public Health. 2017;62:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Scollay JM, Mullen R, McPhillips G, Thompson AM. Mortality associated with the treatment of gallstone disease: a 10-year contemporary national experience. World J Surg. 2011;35:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Bajwa J, Munir U, Nori A, Williams B. Artificial intelligence in healthcare: transforming the practice of medicine. Future Healthc J. 2021;8:e188-e194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 541] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 81. | Joshi AD, Andersson C, Buch S, Stender S, Noordam R, Weng LC, Weeke PE, Auer PL, Boehm B, Chen C, Choi H, Curhan G, Denny JC, De Vivo I, Eicher JD, Ellinghaus D, Folsom AR, Fuchs C, Gala M, Haessler J, Hofman A, Hu F, Hunter DJ, Janssen HL, Kang JH, Kooperberg C, Kraft P, Kratzer W, Lieb W, Lutsey PL, Darwish Murad S, Nordestgaard BG, Pasquale LR, Reiner AP, Ridker PM, Rimm E, Rose LM, Shaffer CM, Schafmayer C, Tamimi RM, Uitterlinden AG, Völker U, Völzke H, Wakabayashi Y, Wiggs JL, Zhu J, Roden DM, Stricker BH, Tang W, Teumer A, Hampe J, Tybjærg-Hansen A, Chasman DI, Chan AT, Johnson AD. Four Susceptibility Loci for Gallstone Disease Identified in a Meta-analysis of Genome-Wide Association Studies. Gastroenterology. 2016;151:351-363.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Ferkingstad E, Oddsson A, Gretarsdottir S, Benonisdottir S, Thorleifsson G, Deaton AM, Jonsson S, Stefansson OA, Norddahl GL, Zink F, Arnadottir GA, Gunnarsson B, Halldorsson GH, Helgadottir A, Jensson BO, Kristjansson RP, Sveinbjornsson G, Sverrisson DA, Masson G, Olafsson I, Eyjolfsson GI, Sigurdardottir O, Holm H, Jonsdottir I, Olafsson S, Steingrimsdottir T, Rafnar T, Bjornsson ES, Thorsteinsdottir U, Gudbjartsson DF, Sulem P, Stefansson K. Genome-wide association meta-analysis yields 20 loci associated with gallstone disease. Nat Commun. 2018;9:5101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 83. | Latenstein CSS, Hannink G, van der Bilt JDW, Donkervoort SC, Eijsbouts QAJ, Heisterkamp J, Nieuwenhuijs VB, Schreinemakers JMJ, Wiering B, Boermeester MA, Drenth JPH, van Laarhoven CJHM, Dijkgraaf MGW, de Reuver PR; SECURE trial collaborators. A Clinical Decision Tool for Selection of Patients With Symptomatic Cholelithiasis for Cholecystectomy Based on Reduction of Pain and a Pain-Free State Following Surgery. JAMA Surg. 2021;156:e213706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Wu S, Tang M, Liu J, Qin D, Wang Y, Zhai S, Bi E, Li Y, Wang C, Xiong Y, Li G, Gao F, Cai Y, Gao P, Wu Z, Cai H, Liu J, Chen Y, Fang C, Yao L, Jiang J, Peng B, Wu H, Li A, Wang X. Impact of an AI-based laparoscopic cholecystectomy coaching program on the surgical performance: a randomized controlled trial. Int J Surg. 2024;110:7816-7823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 85. | Nam C, Lee JS, Kim JS, Lee TY, Yoon YC. Evolution of minimally invasive cholecystectomy: a narrative review. BMC Surg. 2024;24:378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Bagepally BS, Sajith Kumar S, Natarajan M, Sasidharan A. Incremental net benefit of cholecystectomy compared with alternative treatments in people with gallstones or cholecystitis: a systematic review and meta-analysis of cost-utility studies. BMJ Open Gastroenterol. 2022;9:e000779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Lee BJH, Yap QV, Low JK, Chan YH, Shelat VG. Cholecystectomy for asymptomatic gallstones: Markov decision tree analysis. World J Clin Cases. 2022;10:10399-10412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (7)] |
| 88. | Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 3465] [Article Influence: 495.0] [Reference Citation Analysis (5)] |
| 89. | Obermeyer Z, Emanuel EJ. Predicting the Future - Big Data, Machine Learning, and Clinical Medicine. N Engl J Med. 2016;375:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1744] [Article Influence: 174.4] [Reference Citation Analysis (2)] |
| 90. | Yi S, Yam ELY, Cheruvettolil K, Linos E, Gupta A, Palaniappan L, Rajeshuni N, Vaska KG, Schulman K, Eggleston KN. Perspectives of Digital Health Innovations in Low- and Middle-Income Health Care Systems From South and Southeast Asia. J Med Internet Res. 2024;26:e57612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 91. | Patil P, Nathani P, Bakker JM, van Duinen AJ, Bhushan P, Shukla M, Chalise S, Roy N, Gadgil A. Are LMICs Achieving the Lancet Commission Global Benchmark for Surgical Volumes? A Systematic Review. World J Surg. 2023;47:1930-1939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 92. | Vayena E, Dzenowagis J, Brownstein JS, Sheikh A. Policy implications of big data in the health sector. Bull World Health Organ. 2018;96:66-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (1)] |