Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.109989
Revised: July 20, 2025
Accepted: October 10, 2025
Published online: November 27, 2025
Processing time: 182 Days and 14.7 Hours
Colorectal cancer is the third most common malignancy globally, with the liver being the predominant site of metastatic disease.
To evaluate safety, feasibility, and outcomes of robotic liver resection (RLR) versus laparoscopic liver resection (LLR) and open liver resection (OLR) for colorectal metastasis (CRLM).
This study followed Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Systematic searches in PubMed, EMBASE, Scopus, and Cochrane Library identified comparative and noncomparative reviews evaluating RLR versus LLR or OLR for CRLM. Two independent reviewers screened studies using predefined PICO (Population, Intervention, Comparator, Outcome) criteria, with data extraction focusing on conversion rates, operative outcomes, morbidity, mortality, and survival. Methodological quality was assessed via Assessment of Multiple Systematic Reviews 2. Pooled analyses were performed for comparative data; noncomparative studies were narratively synthesized.
Pooled evidence from two comparative systematic reviews (9792 patients) demonstrated that RLR offers distinct advantages over LLR and OLR, including significantly lower conversion rates (4.7%–6.7% vs 10.4%–12.4%, P < 0.001) and reduced intraoperative blood loss (190.8–266.8 mL vs 283.9–294.3 mL, P < 0.001) despite longer operating times (mean 304.1 vs 191.8 min). Perioperative safety and oncologic outcomes (R0 resection > 82%; 5-year overall survival: 53.1%–60.8%) were comparable across approaches. Three additional noncomparative reviews (n = 274) highlighted the technical practicability of RLR in complex cases (zero conversions in small cohorts, median 399.5 min for simultaneous resections). However, these findings were not included in pooled analyses due to the lack of comparator groups. Noncomparative data (n = 274) revealed higher upfront costs for RLR due to prolonged operating times (median 399.5 min) and the need for expensive equipment; however, no formal cost comparisons were available.
RLR is a safe and feasible alternative to LLR and OLR for CRLM, demonstrating superior technical performance and comparable short-term outcomes.
Core Tip: Robotic liver resection (RLR) for colorectal metastases offers clear advantages over laparoscopic and open approaches. This review, integrating robust comparative and noncomparative evidence, reveals significantly lower conversion rates and reduced blood loss of RLR, while maintaining comparable short-term safety and oncological outcomes. It highlights the technical feasibility of RLR in complex cases. While longer operating times and limited long-term data for major resection warrant further research, this comprehensive analysis positions RLR as a safe, feasible, and technically superior alternative, advancing precision in surgical oncology.
- Citation: Ardila CM, Zuluaga-Gómez M, González-Arroyave D. Robotic liver surgery for metastatic disease: A review of safety, feasibility, and outcomes. World J Gastrointest Surg 2025; 17(11): 109989
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/109989.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.109989
Colorectal cancer is the third most common malignancy globally, with the liver being the predominant site of metastatic disease[1,2]. Approximately 20%–25% of patients present with synchronous colorectal liver metastasis (CRLM), while up to 50% develop metachronous metastases during their disease course[3-5]. Surgical resection remains the cornerstone of curative-intent treatment for CRLM, with 5-year survival rates exceeding 50% in selected patients[6-8]. Recent evidence highlights that while liver resection improves survival for oligometastatic disease, accessibility to minimally invasive techniques such as robotic liver resection (RLR) remains limited, and long-term oncological outcomes are understudied, particularly in complex cases[9-11].
The advent of minimally invasive liver surgery, particularly laparoscopic liver resection (LLR), has demonstrated comparable oncological outcomes to open surgery, while reducing morbidity, blood loss, and hospital stay[6,10]. How
Existing systematic reviews and meta-analyses have evaluated RLR for heterogeneous indications (e.g., hepatocellular carcinoma, CRLM, benign lesions), often pooling outcomes without CRLM-specific analyses[6]. While some studies suggest RLR may facilitate technically challenging resections (e.g., simultaneous colorectal and hepatic metastasectomies), its comparative safety, feasibility, and oncological outcomes in CRLM remain debated[7,11]. For instance, RLR is associated with longer operating times but similar complication rates to LLR, although data on margin status (R0 resection) and long-term survival are sparse[6,10]. No randomized trials have compared RLR to other approaches, and existing reviews are limited by small sample sizes, selection bias, and inconsistent outcome reporting[12-14].
This review synthesizes evidence from published systematic reviews and meta-analyses to critically evaluate the safety, feasibility, and short- and long-term outcomes of RLR for CRLM, compared to LLR and open resection. Where comparative evidence was limited, findings from noncomparative RLR studies were incorporated to contextualize technical feasibility and early outcomes. By consolidating higher-level evidence, this review aims to clarify the role of RLR in metastatic liver disease and identify gaps for future research.
This review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines and registered in International Prospective Register of Systematic Reviews[15]. The study aimed to synthesize evidence from systematic reviews and meta-analyses comparing RLR with LLR or open liver resection (OLR) for CRLM. Where comparative evidence was limited, noncomparative systematic reviews of RLR were included to provide supplementary insights into technical feasibility and early outcomes. As with all systematic reviews, the conclusions were constrained by the methodological quality and heterogeneity of the included reviews, which may affect the strength and generalization of the findings.
The review primarily addressed a PICO (Population, Intervention, Comparator, Outcome) question focused on patients with CRLM or synchronous colorectal and liver metastases (Population) undergoing RLR (Intervention) compared to those treated with LLR or OLR (Comparator), evaluating safety, feasibility, and survival outcomes. A secondary analysis incorporated noncomparative RLR studies to explore technical outcomes when direct comparisons were unavailable.
A comprehensive search of PubMed/MEDLINE, EMBASE, Scopus, and the Cochrane Library was performed from inception to April 2025, without language or publication date restrictions. The search strategy combined controlled vocabulary (e.g., Medical Subject Headings terms) and free-text keywords, including “robotic hepatectomy”, “colorectal liver metastases”, and “minimally invasive liver surgery”, paired with “systematic review” or “meta-analysis”. To capture noncomparative RLR studies, additional terms such as “robotic liver resection outcomes” and “feasibility of robotic surgery” were used. Manual searches of reference lists and gray literature sources (e.g., International Prospective Register of Systematic Reviews, conference proceedings) supplemented the electronic search.
Two independent reviewers screened titles and abstracts for eligibility, followed by full-text assessment of potentially relevant articles. Discrepancies were resolved through consensus or consultation with a third reviewer. The screening process was documented using a PRISMA flow diagram to transparently report the number of studies identified, excluded, and included at each stage.
Studies were selected based on predefined criteria aligned with the objectives of the review. For the primary analysis, systematic reviews or meta-analyses were included if they directly compared RLR with LLR or OLR for CRLM (including synchronous cases) and reported at least one outcome of interest (e.g., operative metrics, complications, or survival). Noncomparative systematic reviews focusing solely on RLR were included in a secondary analysis if they provided data on technical feasibility or early outcomes. Narrative reviews, case reports, and studies addressing noncolorectal me
Data extraction was performed independently by two reviewers using a standardized form, piloted beforehand to ensure consistency. For comparative studies, extracted data included study characteristics (e.g., author, year, and sample size), patient demographics, and outcomes such as conversion rates, morbidity, and survival. Noncomparative studies contributed technical metrics like operative time and blood loss. Subgroup data (e.g., major vs minor hepatectomies) were collected where available.
The methodological quality of included reviews was critically appraised using the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) tool[16], which evaluates domains such as protocol registration, risk of bias assessment, and handling of heterogeneity. Two reviewers independently assigned confidence ratings (high, moderate, low), with discrepancies resolved through consensus.
Primary outcomes for the comparative analysis included conversion rates, 30-day morbidity and mortality, R0 resection rates, and long-term survival (3- and 5-year overall survival). Secondary outcomes encompassed operating time, intraoperative blood loss, hospital stay, cost analyses, and learning curve assessments. For noncomparative studies, outcomes focused on technical feasibility, such as procedure success rates and intraoperative challenges.
Findings were synthesized through a combination of narrative and quantitative approaches. For outcomes reported consistently across comparative meta-analyses (e.g., operating time and blood loss), pooled estimates were calculated using random-effects models to account for heterogeneity, with statistical heterogeneity quantified via I2 and χ2 tests. Sensitivity analyses were planned if substantial inconsistency (I2 > 50%) was detected. Subgroup analyses explored potential effect modifiers, such as resection complexity or timing of surgery (simultaneous vs staged). Noncomparative data were synthesized narratively to contextualize technical outcomes, with themes organized by clinical relevance (e.g., safety and feasibility).
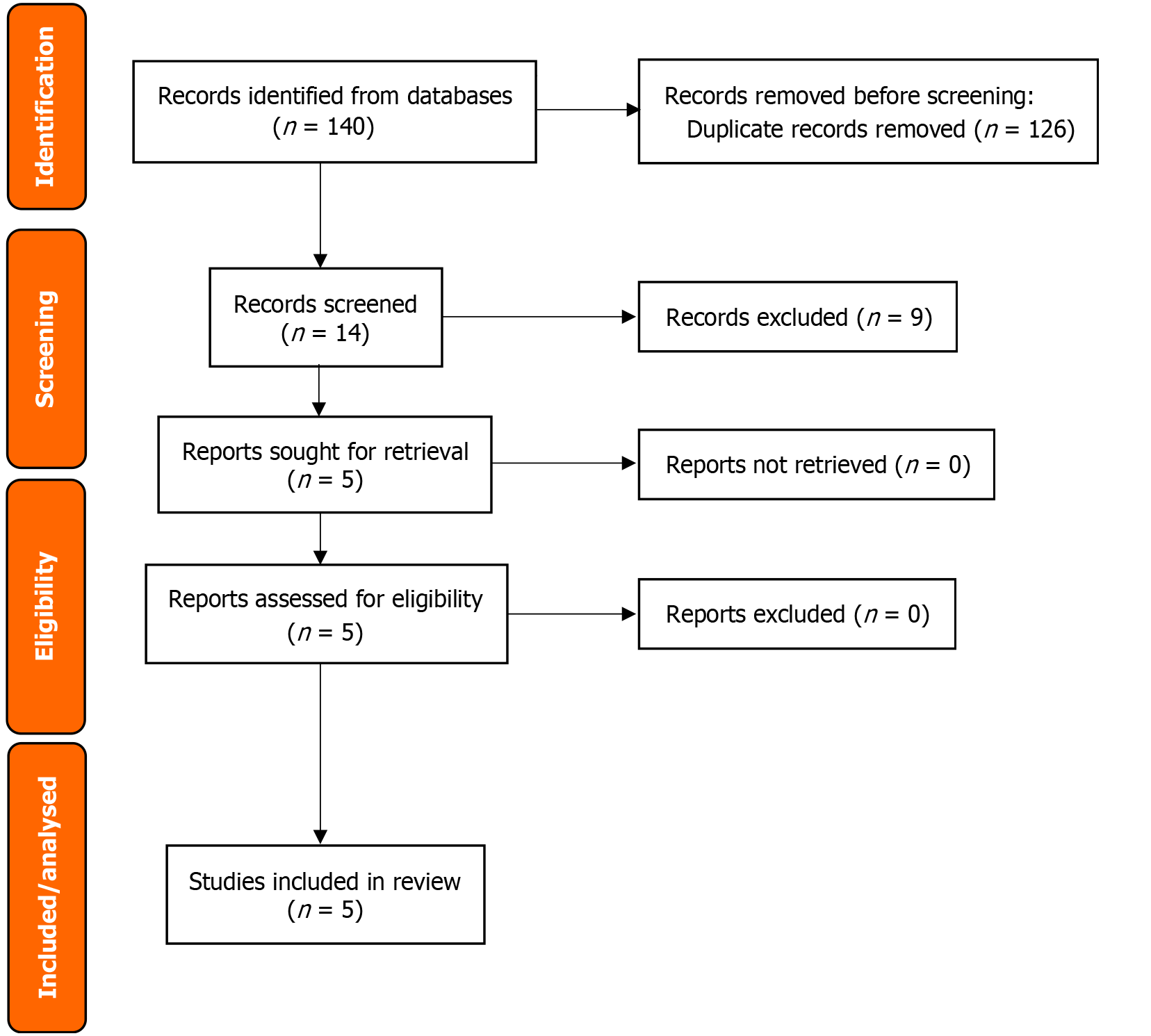
From an initial 140 records, a rigorous systematic review selection process was conducted, as depicted in the PRISMA flow diagram (Figure 1). After removing duplicates and applying predefined exclusion criteria – primarily filtering out nonsystematic review designs or those not focused on robotic liver surgery for metastatic disease – 14 studies were selected for full-text evaluation. This thorough review resulted in the inclusion of five systematic reviews and meta-analyses. Two meta-analyses provided direct comparisons of RLR with LLR and OLR[6,9], while the remaining three systematic reviews focused exclusively on RLR outcomes and were included to supplement technical feasibility data where comparative evidence was limited[7,12,13].
The review consolidated evidence from five systematic reviews evaluating RLR for CRLM. The two comparative metanalyses encompassed 9792 patients and evaluated RLR against LLR and open OLR[6,9], while the three noncomparative systematic reviews included 274 patients and provided insights into the technical performance of RLR[7,12,13]. Mkabaah et al[9] was the largest review, encompassing 13 studies with 6582 patients, including randomized controlled trials and prospective cohorts (Table 1). Safiejko et al[6] focused specifically on RLR vs LLR comparisons among 3210 patients. The noncomparative reviews were smaller in scale, with Machairas et al[7] and McGuirk et al[13] reporting on 29 and 28 RLR cases, respectively, while Ho et al[12] provided early feasibility data from 217 procedures.
| Ref. | Year | No. of studies | Patients (RLR, LLR, OLR) | Study designs | Key focus |
| Safiejko et al[6] | 2024 | 8 | 3210 (530, 2680, N/A) | 1 prospective, 6 retrospective, 1 randomized | RLR vs LLR intraoperative/postoperative |
| Machairas et al[7] | 2021 | 9 | 29 (29, N/A, N/A) | Case series | Simultaneous robotic resections |
| Mkabaah et al[9] | 2025 | 13 | 6582 (268, 3333, 2981) | 2 RCTs, 1 prospective, 10 retrospectives | Comparative outcomes of RLR, LLR, OLR |
| Ho et al[12] | 2013 | 19 | 217 (142, 43, 32) | 10 case series, 2 comparative, 7 case reports | Early RLR adoption and complications |
| McGuirk et al[13] | 2021 | 8 | 28 (28, N/A, N/A) | Case series | Technical feasibility of RLR |
Conversion rates: RLR demonstrated significantly lower conversion rates than LLR in comparative studies (4.7%–6.7% vs 10.4%–12.4%, P < 0.001)[6,9] (Table 2). The noncomparative reviews reported 0% conversion rates in their RLR cohorts, although these findings lacked statistical comparison groups[7,12,13].
| Ref. | Year | RLR conversion rate | LLR conversion rate | OLR conversion rate | Statistical comparison |
| Safiejko et al[6] | 2024 | 6.7% (NR) | 12.4% (NR) | NR | LLR vs RLR: OR = 2.18, 95%CI: 1.46-3.24 |
| Machairas et al[7] | 2021 | 0% (0/29) | NR | NR | NR |
| Mkabaah et al[9] | 2025 | 4.7% (10/214) | 10.4% (328/3166) | NR | LLR vs RLR: OR = 27.50, 95%CI: 7.73-97.48 |
| Ho et al[12]1 | 2013 | 4.6% (10/217) | NR | NR | NR |
| McGuirk et al[13] | 2021 | 0% (0/28) | NR | NR | NR |
Perioperative morbidity and mortality: Thirty-day mortality was low across all approaches (RLR: 0.5%–0.7%, LLR: 0.3%–0.7%, OLR: 0.7%), with no significant differences between RLR and LLR (P = 0.76). Postoperative complications were highest with OLR (26.4%), followed by RLR (23.6%) and LLR (16.6%) (Table 3). The noncomparative studies reported complication rates ranging from 20.3% to 37.9%, potentially reflecting more complex cases in these single-arm cohorts[7,12,13].
| Ref. | 30-day mortality | 90-day mortality | Postoperative complications |
| Safiejko et al[6] | |||
| RLR | 0.5% (2/372); RLR vs LLR: OR = 1.23, 95%CI: 0.32-4.83 | 0.0% (0/178); RLR vs LLR: OR = 1.78, 95%CI: 0.23-14.03 | 21.9% (106/482); RLR vs LLR: OR = 1.04, 95%CI: 0.81-1.32 |
| LLR | 0.7% (13/1931) | 1.3% (10/789) | 22.4% (404/1806) |
| Machairas et al[7] | 0% (0/29) | 0% (0/29) | 37.9% (11/29) |
| Mkabaah et al[9] | |||
| RLR | 0.7% (2/268); RLR vs OLR: OR = 1.24, 95%CI: 0.24-6.36 | 0.6% (1/179); RLR vs OLR: OR = 0.45, 95%CI: 0.07-3.03 | 23.6% (45/191); RLR vs OLR: OR = 0.52, 95%CI: 0.32-0.86 |
| LLR | 0.3% (5/1530); LLR vs OLR: OR = 1.01, 95%CI: 0.29-3.58 | 0.3% (1/292); LLR vs OLR: OR = 0.64, 95%CI: 0.08-5.24 | 16.6% (113/678); LLR vs OLR: OR = 0.50, 95%CI: 0.37-0.68 |
| OLR | 0.7% (6/862) | 1.4% (4/296) | 26.4% (262/992) |
| Ho et al[12] | 0% (0/217) | 0% (0/217) | 20.3% (48/236) |
| McGuirk et al[13] | 0% (0/28) | 2.8% (1/28) | 28.6% (8/28) |
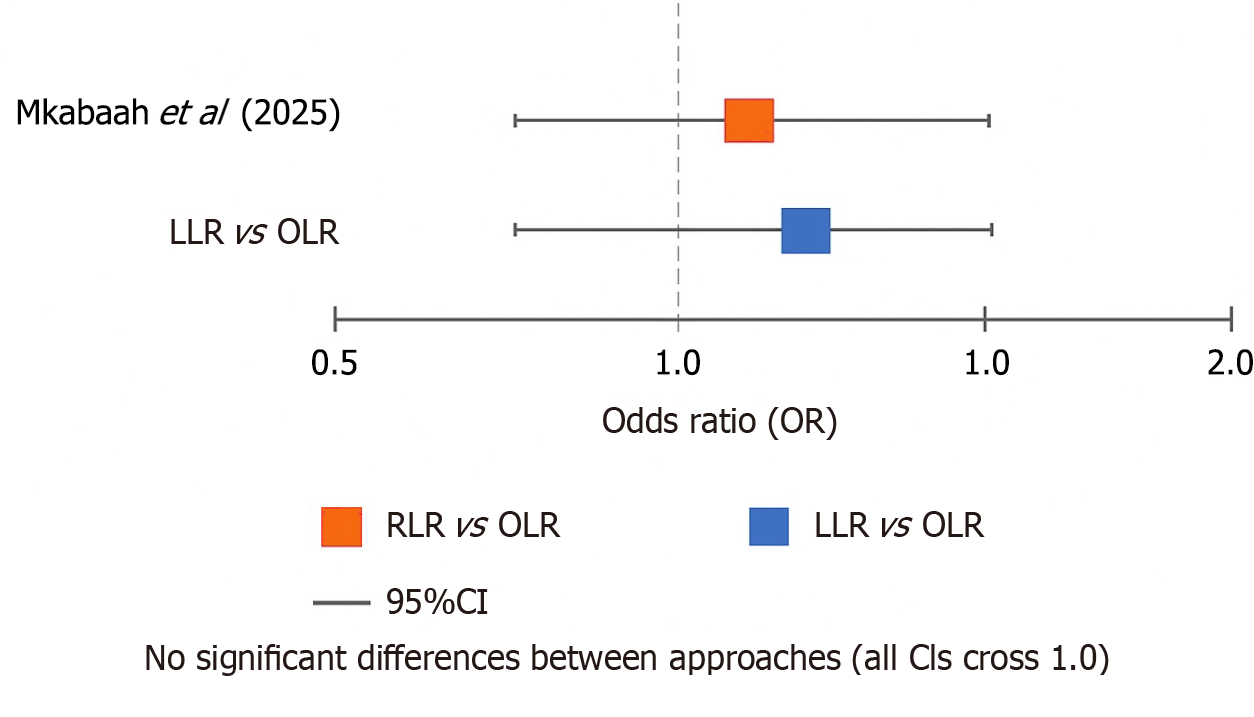
Oncological outcomes: R0 resection rates were high across all approaches (RLR > 82%, LLR > 84%, OLR > 87%), with no significant differences (Table 4). Long-term survival data were sparse, with available 5-year overall survival rates ranging from 53.1% (LLR) to 60.8% (OLR). The forest plot of R0 resection odds ratios visually confirmed the overlapping confidence intervals between approaches (Figure 2). Noncomparative studies reported 100% R0 rates in small RLR cohorts but lacked comparator data for meaningful interpretation[7,12,13].
| Ref. | R0 resection rate | 1-year OS | 3-year OS | 5-year OS | Key oncological findings |
| Safiejko et al[6] | NR | 81.8% (RLR) vs 91.8% (LLR) | 47.7% (RLR) vs 65.9% (LLR) | NR | Trend favoring LLR (NS) |
| Machairas et al[7] | 100% (29/29) | NR | NR | 75.2% (single study) | All R0 resections |
| Mkabaah et al[9] | |||||
| RLR | 82.1% (202/246) | 100% (12/12) | 41.7% (5/12) | NR | No significant differences between approaches |
| LLR | 84.6% (2501/2957) | 93.0% (213/229) | 69.4% (159/229) | 53.1% (191/360) | |
| OLR | 87.7% (2277/2597) | 96.4% (321/333) | 69.3% (231/333) | 60.8% (477/785) | |
| Ho et al[12] | NR | NR | NR | NR | Mixed malignancies included |
| McGuirk et al[13] | 100% (28/28) | NR | NR | NR | Margin status reported for all cases1 |
RLR required longer operating times than LLR (mean 304.1 vs 191.8 min) but was associated with reduced intraoperative blood loss (190.8–266.8 vs 283.9–294.3 mL, P < 0.001) and shorter hospital stays compared to OLR (6.2 vs 12.9 days) (Table 5). The noncomparative studies reported median operating times up to 399.5 min for simultaneous resections, highlighting the technical demands of complex procedures[7,12,13].
| Ref. | Operating time (min) | Blood loss (mL) | Hospital stay (d) | Key findings |
| Safiejko et al[6] | 247.9 (RLR) vs 272.9 (LLR) | 190.8 (RLR) vs 294.3 (LLR) | 5.8 (RLR) vs 5.6 (LLR) | RLR reduced blood loss vs LLR (P < 0.001) |
| Machairas et al[7] | 399.5 (median) | 274 (median) | 7 (median) | Simultaneous resections |
| Mkabaah et al[9] | ||||
| RLR | 304.1 | 266.8 | 6.2 | RLR reduced blood loss vs OLR (P < 0.001) |
| LLR | 191.8 | 283.9 | 6.7 | |
| OLR | 204.3 | 413.8 | 12.9 | |
| Ho et al12] | 200-507 (range) | 50-660 (range) | 5.5-11.7 (range) | Early RLR experience |
| McGuirk et al[13] | 420.3 | 275.6 | 8.6 | Single-arm (no comparator) |
Subgroup analyses for major versus minor hepatectomies revealed no significant differences in outcomes between approaches, although data were limited. RLR demonstrated particular feasibility in minor resections (≤ 3 segments), accounting for 83% of cases in one review. Simultaneous robotic-assisted resections, as reported in the noncomparative study of Machairas et al[7], showed a median hospital stay of 7 days and a 38% complication rate (mostly minor).
While the primary comparative reviews did not report cost data[6,9], insights from noncomparative studies highlighted key economic considerations. McGuirk et al[13] and Machairas et al[7] noted that robotic procedures incurred higher upfront costs due to equipment and maintenance fees, with median operating times exceeding 399 min contributing to prolonged operating room utilization. Ho et al[12] further contextualized these findings, observing that longer robotic procedure durations (200–507 min) offset potential savings from reduced hospital stays (5.5–11.7 days). None of the included reviews provided direct cost comparisons between RLR, LLR, and OLR, underscoring a critical evidence gap.
The methodological quality assessment using AMSTAR-2 revealed high confidence in the comparative reviews (Table 6)[6,9], but low confidence in the noncomparative studies due to small sample sizes and lack of control groups[7,12,13]. Heterogeneity was notably high (I2 > 50%) for outcomes such as operating time and blood loss, warranting cautious interpretation of pooled estimates.
| Ref. | Confidence rating | Key strengths | Key limitations |
| Safiejko et al[6] | Moderate | Direct RLR vs LLR comparison | Retrospective dominance, no RCTs |
| Machairas et al[7] | Low | Focus on SRAR | Small cohort, no comparator group |
| Mkabaah et al[9] | High | Large sample, subgroup analyses | Heterogeneity in study designs |
| Ho et al[12] | Low | Early RLR adoption data | Outdated, mixed malignancies included |
| McGuirk et al[13] | Low | Detailed technical insights | Small sample, case series bias |
This review synthesized evidence from five systematic reviews to evaluate the safety, feasibility, and outcomes of RLR for CRLM. The findings suggest that RLR offers distinct advantages over LLR and OLR, particularly in technical performance, while maintaining comparable perioperative and oncological outcomes. The lower conversion rates observed with RLR (4.7%–6.7%) compared to LLR (10.4%–12.4%) align with the conclusions of Safiejko et al[6] and Mkabaah et al[9], reinforcing the stability of the robotic approach in complex procedures[17-19]. Noncomparative studies, such as those by McGuirk et al[13] and Machairas et al[7], further support feasibility of RLR in technically demanding cases, including simultaneous resections, although these lack the statistical power of comparative analyses[6,9].
Perioperative morbidity and mortality rates were low across all approaches, with no significant differences in 30-day mortality between RLR and LLR. However, the higher complication rates reported in noncomparative RLR cohorts (20.3%–37.9%) may reflect the inclusion of more complex cases, as noted by Machairas et al[7]. Ontologically, R0 resection rates were consistently high (> 82%) and comparable to LLR and OLR, although long-term survival data remain sparse. The absence of robust 5-year survival data for RLR, particularly in major hepatectomies, underscores a critical knowledge gap, as highlighted by Safiejko et al[6] and Mkabaah et al[9].
Operative metrics revealed that RLR, while associated with longer procedure times, significantly reduced intraoperative blood loss and shortened hospital stays compared to OLR[20-22]. These findings are consistent with the observations of Safiejko et al[6] and Beard et al[23], noting the precision of the robotic platform in minimizing hemorrhage. The technical demands of RLR, particularly in simultaneous resections, were evident in the prolonged operating times reported by Machairas et al[7] and Sunil et al[24], suggesting a trade-off between procedural complexity and postoperative recovery benefits.
The economic implications of RLR remain underexplored. Reduced blood loss (190.8–266.8 mL with RLR vs 283.9–413.8 mL with LLR/OLR) and lower transfusion rates (RLR 8.8% vs LLR 20.5%) may mitigate long-term costs[6,9]; however, this remains speculative without formal economic analyses. While noncomparative studies such as those by Ho et al[12] and McGuirk et al[13] identified higher upfront costs due to required equipment and prolonged operating times, potential savings from reduced complications and shorter hospital stays were not systematically evaluated[25]. Future research should address not only direct procedural costs but also conduct formal cost-effectiveness analyses integrating clinical outcomes, including complication rates, recovery times, and long-term oncological results. Such studies are essential to determine whether the technical benefits of RLR justify its financial investment in varied healthcare settings. The lack of standardized cost analyses presents a significant limitation[26-28], as the true cost-effectiveness of RLR cannot be determined without accounting for indirect factors such as readmission rates and long-term oncological outcomes[29-31].
Several limitations must be acknowledged in interpreting these findings. The methodological heterogeneity of the included reviews, particularly the predominance of retrospective data and small sample sizes in noncomparative studies, limits the generalization of the results. The AMSTAR-2 assessment revealed high confidence in the comparative reviews by Safiejko et al[6] and Mkabaah et al[9], but low confidence in noncomparative studies due to their lack of control groups and potential selection biases[7,12,13]. These variations in quality and design contribute to different levels of risk of bias, particularly in studies with limited transparency regarding patient selection, outcome measurement, and data synthesis. For instance, unclear reporting of confounder control or heterogeneity assessments in some reviews introduces un
Future research should prioritize high-quality comparative trials with long-term follow-up to validate the observed short-term benefits of RLR. Cost-effectiveness analyses, integrating direct and indirect economic factors, are essential in determining the true value of robotic surgery in CRLM management. Standardized reporting of operating metrics, complications, and survival outcomes would enhance the comparability of future studies. Finally, investigations into the learning curve for RLR, including the number of procedures required to achieve proficiency, would provide valuable insights for surgical training and implementation.
Recent studies, such as the meta-analysis by Wang et al[33] and the network cost-effectiveness review by Koh et al[34], reinforce the growing body of comparative data supporting RLR, while highlighting the urgent need for standardized economic evaluation frameworks. These complement the consensus recommendations outlined by Hobeika et al[22], which advocate multidisciplinary integration and evidence-based implementation of robotic liver surgery.
RLR represents a safe and feasible alternative to LLR and OLR for CRLM, with superior technical performance and comparable short-term outcomes. While noncomparative studies suggest potential advantages in complex resections, the longer operating times and limited long-term data warrant cautious adoption. Addressing the gaps in economic evidence and long-term survival through rigorous comparative studies will be crucial for defining the role of RLR in the multidisciplinary management of CRLM.
| 1. | Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 711] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 2. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (3)] |
| 3. | Martin J, Petrillo A, Smyth EC, Shaida N, Khwaja S, Cheow HK, Duckworth A, Heister P, Praseedom R, Jah A, Balakrishnan A, Harper S, Liau S, Kosmoliaptsis V, Huguet E. Colorectal liver metastases: Current management and future perspectives. World J Clin Oncol. 2020;11:761-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (10)] |
| 4. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1706] [Article Influence: 341.2] [Reference Citation Analysis (1)] |
| 5. | Ye SP, Qiu H, Liao SJ, Ai JH, Shi J. Mini-invasive vs open resection of colorectal cancer and liver metastases: A meta-analysis. World J Gastroenterol. 2019;25:2819-2832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Safiejko K, Pedziwiatr M, Pruc M, Tarkowski R, Juchimiuk M, Domurat M, Smereka J, Anvarov K, Sielicki P, Kurek K, Szarpak L. Robotic versus Laparoscopic Liver Resections for Colorectal Metastases: A Systematic Review and Meta-Analysis. Cancers (Basel). 2024;16:1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Machairas N, Dorovinis P, Kykalos S, Stamopoulos P, Schizas D, Zoe G, Terra A, Nikiteas N. Simultaneous robotic-assisted resection of colorectal cancer and synchronous liver metastases: a systematic review. J Robot Surg. 2021;15:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management. Curr Gastroenterol Rep. 2019;21:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 9. | Mkabaah LB, Davey MG, Kerin EP, Ryan OK, Ryan EJ, Donnelly M, Ahmed O, McEntee GP, Conneely JB, Donlon NE. Comparing Open, Laparoscopic and Robotic Liver Resection for Metastatic Colorectal Cancer-A Systematic Review and Network Meta-Analysis. J Surg Oncol. 2025;131:262-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Alvikas J, Lo W, Tohme S, Geller DA. Outcomes and Patient Selection in Laparoscopic vs. Open Liver Resection for HCC and Colorectal Cancer Liver Metastasis. Cancers (Basel). 2023;15:1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 11. | Ivey GD, Johnston FM, Azad NS, Christenson ES, Lafaro KJ, Shubert CR. Current Surgical Management Strategies for Colorectal Cancer Liver Metastases. Cancers (Basel). 2022;14:1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Ho CM, Wakabayashi G, Nitta H, Ito N, Hasegawa Y, Takahara T. Systematic review of robotic liver resection. Surg Endosc. 2013;27:732-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | McGuirk M, Gachabayov M, Rojas A, Kajmolli A, Gogna S, Gu KW, Qiuye Q, Dong XD. Simultaneous Robot Assisted Colon and Liver Resection for Metastatic Colon Cancer. JSLS. 2021;25:e2020.00108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Lei J, Huang J, Yang X, Zhang Y, Yao K. Minimally invasive surgery versus open hepatectomy for hepatolithiasis: A systematic review and meta analysis. Int J Surg. 2018;51:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 5140] [Article Influence: 1028.0] [Reference Citation Analysis (1)] |
| 16. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 6391] [Article Influence: 710.1] [Reference Citation Analysis (0)] |
| 17. | Jin B, Wu X, Xu G, Xing J, Wang Y, Yang H, Du S, Mao Y. Evolutions of the Management of Colorectal Cancer Liver Metastasis: A Bibliometric Analysis. J Cancer. 2021;12:3660-3670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Long ZT, Li HJ, Liang H, Wu YC, Ameer S, Qu XL, Xiang ZQ, Wang Q, Dai XM, Zhu Z. Robotic versus laparoscopic liver resection for liver malignancy: a systematic review and meta-analysis of propensity score-matched studies. Surg Endosc. 2024;38:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Lo WM, Tohme ST, Geller DA. Recent Advances in Minimally Invasive Liver Resection for Colorectal Cancer Liver Metastases-A Review. Cancers (Basel). 2022;15:142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Heinrich S, Lang H. [Evidence of minimally invasive oncological surgery of the liver]. Chirurg. 2021;92:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Trastulli S, Cirocchi R, Desiderio J, Coratti A, Guarino S, Renzi C, Corsi A, Boselli C, Santoro A, Minelli L, Parisi A. Robotic versus Laparoscopic Approach in Colonic Resections for Cancer and Benign Diseases: Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0134062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Hobeika C, Pfister M, Geller D, Tsung A, Chan AC, Troisi RI, Rela M, Di Benedetto F, Sucandy I, Nagakawa Y, Walsh RM, Kooby D, Barkun J, Soubrane O, Clavien PA; ROBOT4HPB consensus group. Recommendations on Robotic Hepato-Pancreato-Biliary Surgery. The Paris Jury-Based Consensus Conference. Ann Surg. 2025;281:136-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 23. | Beard RE, Khan S, Troisi RI, Montalti R, Vanlander A, Fong Y, Kingham TP, Boerner T, Berber E, Kahramangil B, Buell JF, Martinie JB, Vrochides D, Shen C, Molinari M, Geller DA, Tsung A. Long-Term and Oncologic Outcomes of Robotic Versus Laparoscopic Liver Resection for Metastatic Colorectal Cancer: A Multicenter, Propensity Score Matching Analysis. World J Surg. 2020;44:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Sunil S, Restrepo J, Azin A, Hirpara D, Cleary S, Cleghorn MC, Wei A, Quereshy FA. Robotic simultaneous resection of rectal cancer and liver metastases. Clin Case Rep. 2017;5:1913-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Sgarbura O, Vasilescu C. The decisive role of the patient-side surgeon in robotic surgery. Surg Endosc. 2010;24:3149-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Ratti F, Fiorentini G, Cipriani F, Catena M, Paganelli M, Aldrighetti L. Laparoscopic vs Open Surgery for Colorectal Liver Metastases. JAMA Surg. 2018;153:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 509] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 28. | Shim JR, Lee SD, Park HM, Lee EC, Park B, Han SS, Kim SH, Park SJ. Outcomes of liver resection in patients with colorectal liver metastases by laparoscopic or open surgery. Ann Hepatobiliary Pancreat Surg. 2018;22:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Rahimli M, Perrakis A, Schellerer V, Gumbs A, Lorenz E, Franz M, Arend J, Negrini VR, Croner RS. Robotic and laparoscopic liver surgery for colorectal liver metastases: an experience from a German Academic Center. World J Surg Oncol. 2020;18:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Masetti M, Fallani G, Ratti F, Ferrero A, Giuliante F, Cillo U, Guglielmi A, Ettorre GM, Torzilli G, Vincenti L, Ercolani G, Cipressi C, Lombardi R, Aldrighetti L, Jovine E. Minimally invasive treatment of colorectal liver metastases: does robotic surgery provide any technical advantages over laparoscopy? A multicenter analysis from the IGoMILS (Italian Group of Minimally Invasive Liver Surgery) registry. Updates Surg. 2022;74:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Benedetti Cacciaguerra A, Görgec B, Cipriani F, Aghayan D, Borelli G, Aljaiuossi A, Dagher I, Gayet B, Fuks D, Rotellar F, D'Hondt M, Vanlander A, Troisi RI, Vivarelli M, Edwin B, Aldrighetti L, Abu Hilal M. Risk Factors of Positive Resection Margin in Laparoscopic and Open Liver Surgery for Colorectal Liver Metastases: A New Perspective in the Perioperative Assessment: A European Multicenter Study. Ann Surg. 2022;275:e213-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Corcione F, Esposito C, Cuccurullo D, Settembre A, Miranda N, Amato F, Pirozzi F, Caiazzo P. Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc. 2005;19:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Wang P, Zhang D, Huang B, Zhou WH, Wang CS, Zhao SY, Su S, Jiang XZ. Robotic versus laparoscopic hepatectomy: meta-analysis of propensity-score matched studies. BJS Open. 2025;9:zrae141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Koh YX, Zhao Y, Tan IE, Tan HL, Chua DW, Loh WL, Tan EK, Teo JY, Au MKH, Goh BKP. Comparative cost-effectiveness of open, laparoscopic, and robotic liver resection: A systematic review and network meta-analysis. Surgery. 2024;176:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |