Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.109869
Revised: June 21, 2025
Accepted: October 9, 2025
Published online: November 27, 2025
Processing time: 182 Days and 3.8 Hours
Acute pancreatitis (AP) is a frequent gastrointestinal emergency characterized by inflammation. It has the potential to progress to organ failure. Fluid therapy plays a critical role in early AP management, mitigating hypovolemia-induced ischemia and systemic inflammatory response syndrome (SIRS).
To evaluate dextran 40 + Ringer’s lactate solution (RLS) vs RLS alone for fluid therapy in mild to moderate AP.
We conducted a single-center, single-blind, randomized controlled trial involving 108 patients with mild to moderate AP. Participants were randomized to receive either dextran 40 + RLS (1:3 ratio) or RLS alone. All patients underwent stan
The dextran 40 + RLS group demonstrated significantly lower CRP levels. No differences were observed in SIRS changes, fluid overload, refractory status mortality, local complications, or organ failure rates. Hospitalization tended to be shorter in the dextran 40 + RLS group (5 days vs 6 days) although not to a statistically significant level (P = 0.1). Adverse events were mild and comparable in both groups.
Dextran 40 + RLS improved the early CRP response in patients with AP without added complications. Although medium-term outcomes were similar, early benefits support its use in initial management.
Core Tip: This randomized controlled trial investigated the effects of fluid resuscitation in patients with mild to moderate acute pancreatitis. The combination therapy of dextran 40 and Ringer’s lactate solution significantly improved early inflammatory markers and accelerated systemic inflammatory response syndrome resolution without increasing complication rates compared with Ringer’s lactate solution alone. Medium-term outcomes were comparable between groups. These findings support the potential benefit of early colloid-crystalloid fluid strategies in acute pancreatitis management and highlight the need for larger, multicenter studies to confirm long-term efficacy and safety.
- Citation: Costea NC, Vesa S, Toma M, Pojoga C, Seicean A. Fluid therapy strategies in acute pancreatitis: Randomized controlled trial comparing dextran and Ringer’s lactate. World J Gastrointest Surg 2025; 17(11): 109869
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/109869.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.109869
Fluid resuscitation, nutritional support, and pain management are the cornerstones of acute pancreatitis (AP) ma
Some studies have explored the combination of crystalloid and colloid administration for fluid resuscitation in AP[13]. A review of seven studies found that early, aggressive crystalloid infusion may be beneficial[14]. However, rapid administration later in the disease course was associated with increased morbidity and mortality. Two studies with crystalloid and colloid administration were associated with lower morbidity and reduced fluid requirements[14]. Du et al[15] compared crystalloids alone with a combined colloid/crystalloid strategy. They found decreased intraabdominal pressure, need for respiratory ventilation, and overall fluid requirements. Similarly, another study found that patients in the combination group required less fluid to achieve resuscitation targets and experienced fewer abdominal and respiratory infections[16]. Although specific data were not reported, both intensive care unit (ICU) stay and total length of the hospital stay were shorter in the combination group. Interestingly, almost twice as much liquid was drained from the crystalloid (normal saline) group when compared with the two combination colloid/crystalloid groups[14].
One study investigated the use of isovolumic hemodilution with dextran 60 in a small number of patients with severe AP. They found that 15% of the patients experienced progression due to necrosis and required surgery[17]. However, there are no studies evaluating the combination of crystalloids and the colloid dextran 40. Therefore, we designed a single-blind, randomized controlled trial to assess the effect of dextran 40 + RLS for resuscitation fluid therapy in AP. The primary objective of the study was resolution of systemic inflammation, and the secondary objectives were to assess the progression of AP severity and the mortality rate.
This was a single-blind, randomized controlled trial, in which patients were effectively blinded to their treatment allocation, conducted at Satu Mare County Hospital, Romania. This study was approved by the Ethics Committee of the Satu Mare County Hospital (Approval No. 27/11.10.2021). It was also registered on ClinicalTrials.gov (NCT06835023). The protocol followed the CONSORT guidelines for reporting randomized trials. All authors had full access to the study data and approved the final manuscript following a comprehensive review.
This study included consecutive patients admitted to our hospital for AP. The inclusion criteria were ages 18 years or older, initial presentation to the emergency department, and subsequent admission with a diagnosis of mild to moderate AP. At the initial assessment in the emergency department, the patients signed the informed consent form before enrollment.
Exclusion criteria were severe AP, pancreatitis after endoscopic retrograde cholangiopancreatography, history of severe cardiovascular disease (New York Heart Association class II heart failure, active myocardial ischemia, cardiovascular intervention within the previous 60 days), severe respiratory disease (chronic obstructive pulmonary disease with a requirement for home oxygen), renal disease (creatinine clearance < 40 mL/minute), cirrhosis, severe hematological diseases, metastatic malignancy, autoimmune disease, chronic infectious disease, systemic inflammation due to human immunodeficiency virus, tuberculosis, acute cholangitis, acute cholecystitis, or bowel ischemia. Pregnant patients or those unable to provide informed consent were also excluded.
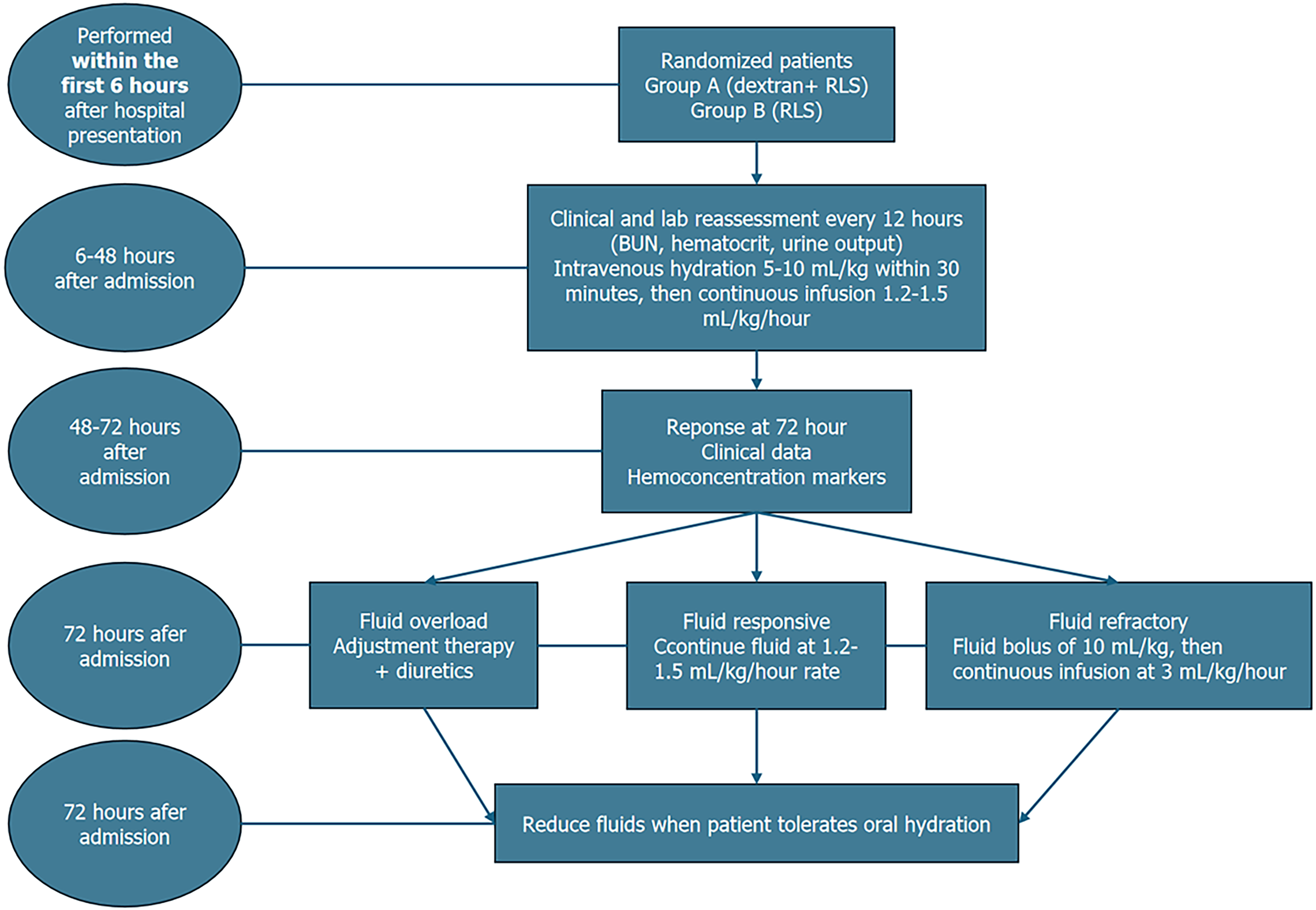
The included patients were randomly assigned to study groups in a 1:1 ratio, but they were unaware of the solution being administered. Clinical staff involved in administering fluids were necessarily aware of group assignments, due to the visual differences between dextran 40 and RLS. The intervention group received intravenous hydration with dextran 40 in 0.9% NaCl + RLS in a 1:3 ratio (i.e. for every 500 mL of dextran 40, the patient received 1500 mL of RLS). The control group received intravenous hydration with RLS alone. Each participant received fluid resuscitation in accordance with a goal-directed protocol (Figure 1). Fluid resuscitation was administered through the peripheral vein in all patients. However, if patients developed organ failure and were transferred to the ICU, then fluid resuscitation was given via venous central access.
The primary outcomes were: (1) Resolution of systemic inflammatory response syndrome (SIRS) by 72 hours; and (2) Reduction in serum C-reactive protein (CRP) levels from baseline to 72 hours. Secondary outcomes included duration of hospital and ICU stay, incidence of organ failure, local complications (e.g., necrosis, pseudocysts), 30-day and 90-day mortality, and treatment-related adverse events. Outcomes were assessed clinically and through laboratory and imaging data at predefined time points during hospitalization and follow-up. The parameters for follow-up were: (1) Clinical data (assessed every 24 hours until discharge) including heart rate, mean arterial pressure, signs of fluid overload such as pulmonary rales, jugular vein distension, peripheral edema, ascites, and pain intensity on a 1 to 10 numeric scale; (2) Hemoconcentration markers (assessed every 12 hours for the first 72 hours and then every 24 hours until discharge) including blood urea nitrogen (BUN), hematocrit, and creatinine; (3) Inflammatory markers (measured every 24 hours until discharge except procalcitonin, which was collected again after 72 hours) including CRP, Erythrocyte sedimentation rate, fibrinogen, ferritin, procalcitonin, and white blood cell count; (4) Blood glucose levels (monitored every 12 hours; when necessary, a bolus of 1-2 vials of 10 mL 33% glucose were administered to correct hypoglycemia); (5) SIRS (assessed at 24 hours, 48 hours, and 72 hours after admission); and (6) Computed tomography (CT) scan (performed 3 days after admission or when the patient exhibited clinical deterioration; also performed at the 1-month follow-up).
To minimize potential bias, outcome assessors and data analysts remained blinded to treatment allocation throughout the data collection and analysis process. Clinical data were collected and abdominal ultrasound (or CT scan in cases of local complications) were performed 3 months after admission. Length of hospitalization and ICU stay, in-hospital mortality and 3-month mortality were recorded for each case. The cases that developed severe AP were admitted to the ICU, and invasive percutaneous, endoscopic, and surgical treatments were recorded.
AP diagnosis was established according to the revised Atlanta classification[18]. Diagnosis required at least two of the following three criteria: (1) Characteristic abdominal pain; (2) Serum amylase and/or lipase levels greater than three times the upper limit of normal; and (3) Imaging findings consistent with AP on contrast-enhanced CT or magnetic resonance imaging. SIRS was defined as the presence of two of the following criteria: Pulse > 90 beats/minute, respirations > 20/ minute or PaCO2 < 32 mmHg; temperature 38 °C; and white blood cell count 12000 cells/mm3 or > 10% band forms[19]. Patients were considered to have SIRS at baseline if two of the four criteria were met at the time of randomization. Organ failure as a systemic complication was defined as an arterial oxygen partial pressure/fraction of inspired oxygen ratio ≤ 300, creatinine ≥ 1.9 mg/dL, and systolic blood pressure < 90 mmHg after fluid resuscitation. Local complications were determined according to the revised Atlanta classification[20].
An independent data safety committee consisting of two independent gastroenterologists and one gastroenterologist monitored patient safety while the study was in progress. The committee evaluated all serious adverse events during the study in 12-24 hours. All patients were monitored for potential adverse events during the first 72 hours of hospitalization. Safety assessments included vital signs, physical examination, and laboratory testing. Daily coagulation profiles (prothrombin time, activated partial thromboplastin time, international normalized ratio, platelet count) and complete blood counts were obtained. Patients in the intervention group were additionally observed for signs of hypersensitivity reactions or volume overload during and after dextran 40 administration. All adverse events were recorded and graded according to the common terminology criteria for adverse events version 5.0.
Statistical analyses were performed using STATA version 18.0 (Stata Corp LLC, College Station, TX, United States). Sampe size and study power calculations were based on 10 patients from the intervention group and 10 patients from the control group. We observed a mean CRP at 72 hours of 84 ± 87 mg/L in the intervention group and 145 ± 117 mg/L in the control group, with an absolute difference of 60 ± 103 mg/L. Using a two-sided test with α = 0.05 and 80% power, the required sample size was 48 patients per group.
Qualitative data were expressed as frequencies and percentages. Quantitative data were presented as mean ± SD for normally distributed variables or as median and interquartile range for non-normally distributed variables as assessed by the Shapiro-Wilk test. Differences between groups were assessed by the Student’s t-test, Mann-Whitney U test, and χ2 test where appropriate. A P-value of less than 0.05 indicated a statistically significant difference. A two-way repeat measures analysis of variance was performed to evaluate the effects of the treatment and time on CRP after logarithmic transformation. The analysis also assessed the interaction between treatment and time. The generalized estimating equation was used to analyze repeated measurements and account for within-subject correlations over time for SIRS. The model included the treatment group as a between-subjects factor and time as a within-subject factor. An exchangeable correlation structure was assumed. For binary outcomes, a logistic link function was used. To account for baseline differences in CRP, an ANCOVA model was used with CRP at 72 hours as the dependent variable and baseline CRP as a covariate. We applied a Bonferroni correction across all comparisons (CRP, SIRS, and procalcitonin at baseline and 72 hours). A corrected threshold of P < 0.005 was considered statistically significant. The randomization was performed using permuted block randomization with variable block sizes to ensure balanced allocation.
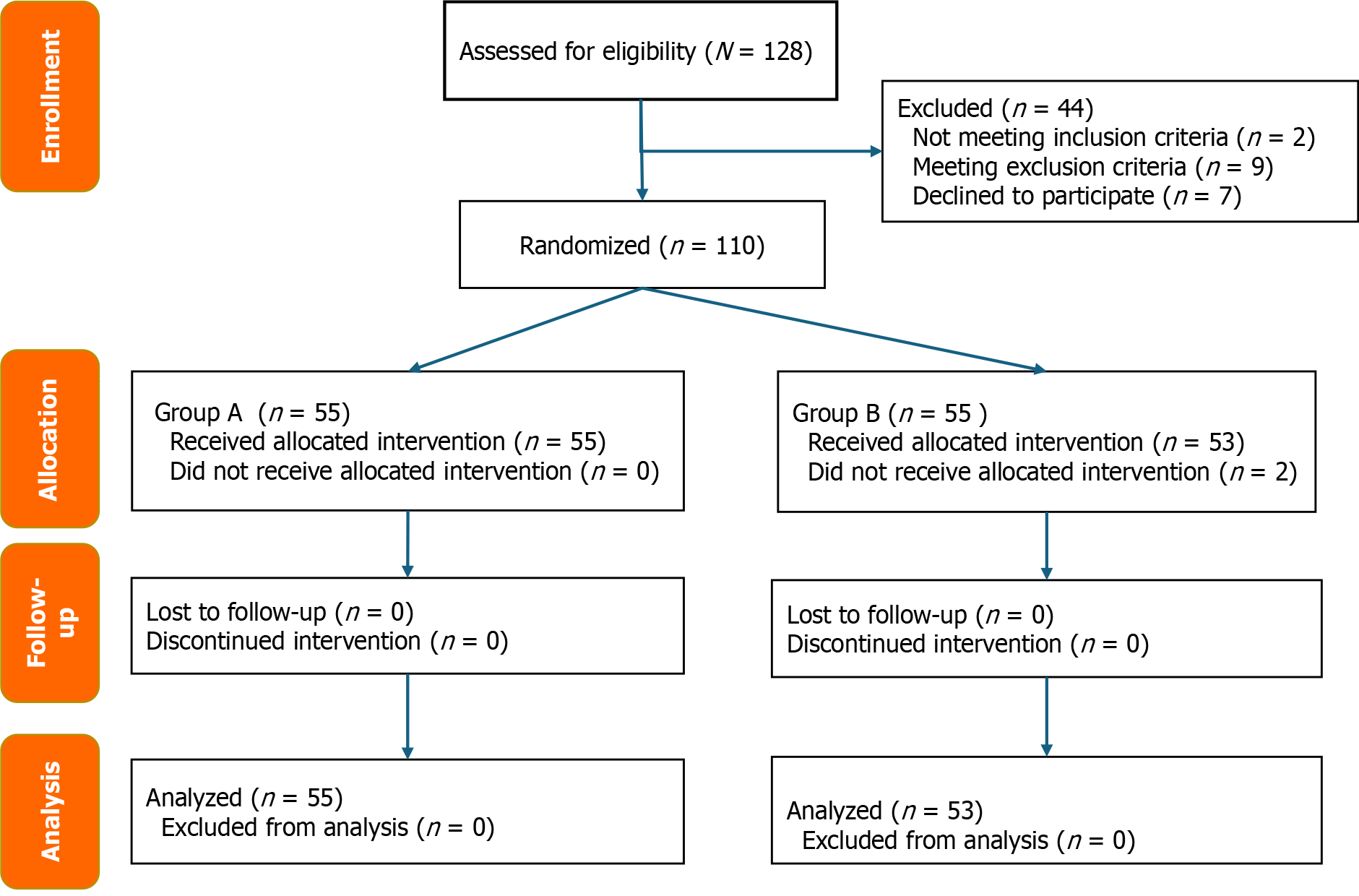
From January 2022 to March 2025, 128 patients were assessed for eligibility. In total 110 patients were randomized into treatment groups. However, 2 patients developed acute cholecystitis and were excluded. The 3-month follow-up was conducted for the remaining 108 patients. The participant flow chart is displayed in Figure 2.
The intervention group (n = 55) and control group (n = 53) were similar in terms of age and sex distributions, symptom duration, and primary etiologies of AP. Alcohol was the most common cause in both groups. The prevalence of diabetes and history of smoking did not differ significantly between the groups. Baseline laboratory values, including hematocrit, serum creatinine, BUN, and albumin, were comparable. Although not statistically significant, the intervention group showed slightly higher BUN and creatinine levels at admission, with a trend toward improved renal markers over the first 72 hours. Clinical severity scores, including the Bedside Index of Severity in AP, Sequential Organ Failure Assessment, Marshall, and CT severity, at 72 hours, were also similar.
The intervention group received marginally more fluid at 24 hours and 48 hours, though these differences were not statistically significant. At admission, respiratory dysfunction (arterial oxygen partial pressure/fraction of inspired oxygen ratio < 300) was observed in 2 patients (3.63%) in the intervention group and 1 patient (1.89%) in the control group. Renal dysfunction (serum creatinine > 1.9 mg/dL) was present in 1 patient (1.81%) in the intervention group and 2 patients (3.77%) in the control group. No cardiovascular organ failure was documented in either group at baseline (Table 1).
| Type of solution | Intervention group, n = 55 | Control group, n = 53 | P value |
| Age in years | 50.7 ± 13.2 | 51.9 ± 13.6 | 0.6 |
| Male sex | 42 (76.36) | 41 (77.36) | 1.0 |
| Etiology | 0.4 | ||
| Alcohol | 30 (54.55) | 28 (52.83) | |
| Gallstone | 8 (14.55) | 10 (18.87) | |
| Metabolic | 14 (25.45) | 10 (18.87) | |
| Others | 3 (5.45) | 5 (9.43) | |
| Previous diabetes | 7 (12.73) | 8 (15.09) | 0.9 |
| History of smoking | 25 (45.45) | 18 (33.96) | 0.3 |
| Hematocrit as % | 39.6 ± 6.0 | 39.6 ± 4.8 | 0.9 |
| BUN in mg/dL | 18 (13-25) | 16 (12-21) | 0.1 |
| Serum creatinine in mg/dL | 0.77 (0.60-0.90) | 0.70 (0.58-0.89) | 0.1 |
| Albumin in g/dL | 3.60 (3.34-4.00) | 3.65 (3.50-4.10) | 0.4 |
| CTSI at 72 hours | 3 (2-3) | 3 (2-4) | 0.2 |
| BISAP score > 2 | 1 (0-1) | 1 (0-1) | 0.2 |
| Marshal score | 1 (0-1) | 1 (0-1) | 0.2 |
| SOFA score | 1 (1-3) | 1 (1-3) | 0.5 |
| Severity of acute pancreatitis | 0.7 | ||
| Mild | 12 (21.80) | 14 (26.40) | |
| Moderate | 43 (78.20) | 39 (73.60) | |
| Volume of fluid resuscitation | 0.4 | ||
| At 24 hours | 2500 (2000-2500) | 2000 (2000-2500) | |
| At 48 hours | 4500 (4000-5000) | 4000 (4000-4500) | |
| At 72 hours | 6500 (6000-7000) | 6500 (6000-6500) | |
| Total hospitalization costs (Euro) | 1050 (853-1395) | 1064 (885-1516) | 0.2 |
In both groups, most patients were fluid-responsive during the early resuscitation phase. Although the differences did not reach statistical significance, the intervention group exhibited a higher proportion of fluid-responsive cases and fewer instances of fluid-refractory cases compared with the control group, suggesting a trend toward more favorable early hemodynamic response. Additionally, fluid overload was observed less frequently in the intervention group (Table 2).
| Type of solution | Intervention group, n = 55 | Control group, n = 53 | P value |
| Fluid responsive | 49 (89.10) | 42 (79.20) | 0.2 |
| Fluid refractory | 4 (7.30) | 6 (11.30) | 0.6 |
| Fluid overload | 2 (3.60) | 5 (9.40) | 0.4 |
Baseline CRP levels were similar between the two groups. In the intervention group, CRP increased sharply at 24 hours (from 23.0 mg/L to 97.0 mg/L) followed by a steady decline to 43.0 mg/L by 72 hours. In contrast, the control group showed a progressive increase, peaking at 176.0 mg/L at 48 hours and remaining elevated at 171.0 mg/L at 72 hours. These differences were statistically significant (P < 0.001). After adjusting for baseline CRP, the CRP level at 72 hours remained significantly lower in the intervention group (P = 0.001; Table 3).
| Parameter | Intervention group, n = 55 | Control group, n = 53 | P value |
| CRP in mg/L | |||
| At admission | 23.0 (11.8-62.0) | 27.0 (9.2-59.0) | 0.7 |
| At 24 hours | 97.0 (30.5-149.0) | 75.0 (35.0-153.0) | 0.7 |
| At 48 hours | 80.0 (43.5-266.0) | 176.0 (73.0-295.0) | 0.06 |
| At 72 hours | 43.0 (20.0-104.5) | 171.0 (66.0-300.0) | < 0.001 |
| SIRS ≥ 2 criteria | |||
| At admission | 47 (83.93) | 41 (77.36) | 0.2 |
| At 24 hours | 29 (51.79) | 33 (62.26) | 0.3 |
| At 48 hours | 14 (25.00) | 18 (33.96) | 0.3 |
| At 72 hours | 2 (3.57) | 11 (20.75) | 0.008 |
| PCT in ng/mL | |||
| At admission | 0.1 (0.0-0.1) | 0.1 (0.0-0.5) | 0.05 |
| At 72 hours | 0.1 (0.0-0.2) | 0.2 (0.0-0.3) | 0.06 |
The proportion of patients meeting ≥ 2 SIRS criteria at admission was comparable in the two groups (83.93% in the intervention group vs 77.36% in the control group). During follow-up the intervention group demonstrated a quicker resolution of systemic inflammation, with lower SIRS positivity at 24 hours, 48 hours, and 72 hours. The overall group difference across the observation period was not statistically significant (P = 0.008; Table 3). Procalcitonin levels remained low in both groups throughout the observation period, with no significant intergroup differences at admission or 72 hours (P = 0.06; Table 3).
At 3 months, local complications (pancreatic necrosis and pseudocyst formation) were less frequent in the intervention group than in the control group (7.27% vs 9.43% and 12.73% vs 18.87%, respectively), though these differences were not statistically significant (P > 0.4). Infection local complications were also slightly lower in the intervention group (9.09% vs 13.21%, P = 0.7; Table 4). By 72 hours, organ failure occurred in 3.64% of patients in the intervention group and 12.2% of patients in the control group, primarily due to respiratory dysfunction (1.82% vs 9.43%, respectively). Cardiovascular dysfunction was rare in both groups (1.82% vs 3.77%, respectively), and no renal failure was observed (Table 4). The intervention group had a shorter median hospital stay (5 days vs 6 days, P = 0.1). ICU admission and 30-day mortality occurred only in the control group (3.77% and 1.89%, respectively). The 3-month mortality rate was similar (1.82% in the intervention group vs 1.89% in the control group), with the death in the intervention group attributed to a cardiovascular event unrelated to pancreatitis (Table 4). Adverse events were mild and included 2 cases of rash in the intervention group. The control group experienced hypoglycemia more frequently than the intervention group (5 patients vs 1 patient, respectively). No severe treatment-related events were reported (Table 4).
| Parameter | Intervention group A, n = 55 | Control group, n = 53 | P value |
| Local complications at 3 months | 11 (20.00) | 15 (28.30) | 0.4 |
| Pancreatic necrosis at 3 months | 4 (7.27) | 5 (9.43) | 0.7 |
| Pseudocysts at 3 months | 7 (12.73) | 10 (18.87) | 0.5 |
| Infected local complications | 5 (9.09) | 7 (13.21) | 0.7 |
| Organ failure at 72 hours | 2 (3.64) | 7 (12.20) | NA |
| OF respiratory (PaO2/FiO2 < 300) at 72 hours | 1 (1.82) | 5 (9.43) | NA |
| OF creatinine (> 1.9 mg/dL) at 72 hours | 0 (0) | 0 (0) | NA |
| OF cardiovascular at 72 hours | 1 (1.82) | 2 (3.77) | NA |
| Total hospital stays in days | 5 (4-7) | 6 (5-7) | 0.1 |
| ICU stay in days | 0.0 ± 0.0 | 0.0 ± 0.2 | |
| OF at 3 months | 1 (1.82)1 | 2 (3.77)1 | NA |
| 30-day mortality rate | 0 (0) | 1 (1.89) | NA |
| 3-month mortality rate | 1 (1.82) | 1 (1.89) | NA |
The present randomized controlled trial demonstrated the non-superiority of dextran 40 + RLS over RLS alone for fluid resuscitation in AP. The inflammatory status was significantly lower in the dextran 40 + RLS group, but only the reduction in CRP at 72 hours remained statistically significant after Bonferroni correction. Although baseline CRP was slightly higher in the control group, this difference was not statistically significant. Furthermore, adjustment using ANCOVA confirmed that the reduction in CRP at 72 hours remained significantly greater in the intervention group, supporting the robustness of this finding. These patients also had shorter hospital stays. However, the medium-term variables such as organ failure, mortality rate, and local complications were comparable between the two groups.
The goal of fluid resuscitation in AP is to decrease pancreatic blood flow and prevent pancreatic cellular death, necrosis, and the continuous release of pancreatic enzymes. Fluid resuscitation can limit the progression of moderate AP to severe AP[11]. Starting fluid resuscitation shortly after admission (within the first 24 hours) may reduce the risk of SIRS, organ failure, and mortality[2]. Generally, crystalloids or colloids can be used for fluid resuscitation, but isotonic crystalloids are recommended in the latest guidelines, because they improve hemodynamics and limit over-resuscitation[2]. Colloid use is related to possible adverse effects including volume overload, hyperoncotic renal impairment, coagulopathy, and anaphylactic reaction[11,21]. However, none of these adverse effects were encountered in our study. The crystalloid hydroxyethyl starch (HES) should be avoided due to renal impairment in patients with sepsis[22,23]. The renal impairment is likely related to downregulation of proinflammatory cytokines, but further studies are required to confirm[22]. One meta-analysis revealed that the rate of organ failure and adverse events was lower for normal saline than for HES resuscitation[13]. The mortality in the patients who received HES was higher, indicating that HES should be avoided.
RLS produces anti-inflammatory effects and is widely used for AP resuscitation. However, dextran may enhance pancreatic microcirculation, potentially reduce fluid sequestration and limit the progression of pancreatic necrosis. At 72 hours, the lower CRP level, without decreasing the SIRS frequency, was observed in the intervention group, demonstrating a potential enhanced anti-inflammatory effect of dextran 40 in the context of increased local blood flow. Another colloid used for fluid resuscitation in AP is albumin. It is thought to have a protective effect against aggressive hydration while minimizing the risk of fluid overload. A retrospective study demonstrated that administrating exogenous albumin during the first 28 days after the onset of AP alleviated symptoms and improved prognosis[24]. However, when it was used for pancreatitis after endoscopic retrograde cholangiopancreatography, its efficacy did not remain[25]. A study with a small sample size used a combination of albumin diluted with dextran, and the investigators observed a 7.7% reduction in mortality rates and a 15.0% slowing of pancreatic necrosis progression[17]. However, it may be more useful in patients with hypoalbuminemia.
Dextran 40 was selected for this study due to its capacity to improve capillary perfusion and reduce erythrocyte aggregation, thereby enhancing pancreatic microcirculation. In contrast to HES[26], which has been associated with renal impairment and increased mortality in critically ill or septic patients, dextran 40 has a more favorable safety profile at standard doses. Albumin, while physiologically beneficial, is significantly more expensive and is generally reserved for specific indications such as hypoalbuminemia or cirrhosis, rather than routine fluid therapy in AP.
This was the first study that combined dextran 40 with RLS and demonstrated that there was no significant difference in clinical outcomes compared with RLS alone. While dextran may improve capillary perfusion and reduce vascular leakage, the rate of fluid overload was comparable in the two groups. These potential benefits did not translate into measurable improvements in inflammation markers. Although the CRP level was lower at 72 hours in the intervention group compared with the control group, the procalcitonin level and SIRS at 72 hours was similar in both groups. Although the median hospital stay was shorter in duration for the intervention group, the difference was not statistically significant and should not be interpreted as clinically meaningful. In addition, organ dysfunction and mortality were similar in both groups. These observations indicate that the combination of dextran 40 and RLS is non-inferior to RLS alone. The combination treatment tended to diminish the early inflammatory status after admission and did not have a clinical impact nor induce side effects during the medium-term follow-up.
Although dextran 40 has a higher unit cost (4.6 Euro per vial vs 1 Euro for Ringer’s lactate), the total hospitalization costs were comparable and slightly lower in the dextran 40 and RLS group (Table 4), likely reflecting the shorter hospital stay duration. These findings suggest that colloid-based fluid therapy did not increase overall costs in our cohort. Nevertheless, future studies should include formal cost-effectiveness analyses to fully assess the economic impact of different resuscitation strategies in AP. Dextran 40 was well tolerated in our study without nephrotoxicity, which has been reported previously particularly in older or hypovolemic patients[26,27]. The proposed mechanism involves osmotic nephrosis and tubular injury[27]. In our study, renal function was monitored using daily BUN and serum creatinine measurements, and no cases of acute kidney injury were observed. Nevertheless, these reports highlight the importance of cautious use and appropriate renal monitoring.
The strengths of our study included its randomized, blinded design, standardized fluid administration protocol, close monitoring of inflammatory markers over 72 hours, and medium-term follow-up of 3 months. However, as with previous trials, our study focused on inflammation as a surrogate marker rather than direct clinical endpoints. While CRP is widely accepted as a marker of severe AP, larger multicenter randomized controlled trials with robust clinical outcomes are needed to determine whether fluid resuscitation strategies incorporating colloids like dextran or albumin provide meaningful advantages over RLS alone.
Our findings should be interpreted in light of current international guidelines and recent meta-analyses on fluid resuscitation in AP. Nearly all major societies recommend the early use of isotonic or balanced crystalloids, with RLS generally preferred over normal saline[1,7]. Resuscitation strategies have shifted toward moderate, goal-directed fluid therapy, typically in the range of 5-10 mL/kg/hour, to improve organ perfusion while avoiding over-resuscitation[6]. A 2025 systematic review concluded that isotonic crystalloids - particularly RLS - are superior to saline in reducing SIRS, organ failure, and ICU length of stay, without increasing mortality[1]. Additionally, a 2023 meta-analysis found that aggressive hydration (≥ 20 mL/kg/hour) increased the risk of fluid overload and mortality, especially in severe AP, while controlled strategies-maintained efficacy with fewer complications[28]. Our protocol was consistent with these recommendations, and the anti-inflammatory effects observed in the dextran 40 + RLS group align with the overarching goal of minimizing systemic inflammation and microcirculatory impairment without inducing volume-related adverse events.
These data may influence clinical decision-making regarding fluid therapy in AP; however, studies with a larger patient sample are needed to strengthen the evidence base. In mild to moderate AP with elevated CRP at admission, and in the absence of renal disease, acute cholecystitis, or cholangitis, our data suggest that dextran 40 (administered in a 1:3 ratio with RLS) may enhance plasma expansion and reduce systemic inflammation without increasing adverse events or overall costs. At 72 hours post-admission, fluid responsiveness should be reassessed in all patients. However, the use of dextran-based fluid therapy in patients with pre-existing renal disease or severe AP has not been evaluated and cannot currently be recommended. In such cases, goal-directed crystalloid therapy remains the standard of care, potentially supplemented by vasopressors or other organ support as clinically indicated.
Meanwhile, the study still has several limitations. The main limitations of this study were the single-center design, the limited number of patients included, the exclusion of severe AP, and the limited follow-up which may have missed late complications. Firstly, this study was conducted at a single secondary care center, which may limit the generalizability of the findings. Differences in institutional protocols, patient populations, and healthcare infrastructure may influence the applicability of our results to other clinical settings. A multicenter design would have increased the external validity of the study; however, this was not feasible due to logistical constraints and the need for uniform data collection during a period of significant strain on the healthcare system. Secondly, the statistical power was calculated for the primary objective of the study, considered as the resolution of systemic inflammation. In contrast, the secondary outcomes, including the progression of AP severity and mortality rate, were underpowered due to the lower incidence of these events. As a result, although the observed difference in organ failure rates between the two groups (3.64% vs 12.2%) may be clinically relevant, these results should be interpreted with caution, representing another limitation of the present study. These findings should be interpreted as exploratory and warrant further investigation in larger trials. Thirdly, although patients with severe AP could potentially benefit from optimized fluid resuscitation with dextran, this was not the focus of our study. Therefore, our conclusions cannot be generalized to all severities of AP, which constitutes an additional limitation of the present work.
The combination of dextran 40 and RLS was associated with a more favorable early inflammatory response in patients with AP. The combination treatment had no systemic adverse effects, potentially due to the anti-inflammatory effects of lactate and the microcirculatory benefits of dextran. There was a decrease in the CRP level without SIRS decreasing at 72 hours in the intervention group, but the systemic complication rate and mortality were similar in both groups, indicating the lack of benefits in the medium-term follow-up. Larger prospective studies using the combination of colloid-crystalloid for fluid resuscitation in AP are needed.
We would like to thank all the patients who participated in this study, as well as the clinical and nursing staff whose collaboration made this research possible. Special appreciation goes to the emergency department and intensive care teams for their dedication and support during patient enrollment and data collection. We are also grateful to the research assistants and data analysts for their valuable contributions. Finally, we thank the institutional review board for their guidance and approval of this trial.
| 1. | Costea CN, Pojoga C, Seicean A. Advances in the Management of Fluid Resuscitation in Acute Pancreatitis: A Systematic Review. Diagnostics (Basel). 2025;15:810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 195] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 3. | Takada T, Isaji S, Mayumi T, Yoshida M, Takeyama Y, Itoi T, Sano K, Iizawa Y, Masamune A, Hirota M, Okamoto K, Inoue D, Kitamura N, Mori Y, Mukai S, Kiriyama S, Shirai K, Tsuchiya A, Higuchi R, Hirashita T. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2022;29:1057-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 4. | Arvanitakis M, Ockenga J, Bezmarevic M, Gianotti L, Krznarić Ž, Lobo DN, Löser C, Madl C, Meier R, Phillips M, Rasmussen HH, Van Hooft JE, Bischoff SC. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin Nutr. 2020;39:612-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 5. | Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, Thrower EC, Gorelick FS. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology. 2009;137:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | de-Madaria E, Buxbaum JL, Maisonneuve P, García García de Paredes A, Zapater P, Guilabert L, Vaillo-Rocamora A, Rodríguez-Gandía MÁ, Donate-Ortega J, Lozada-Hernández EE, Collazo Moreno AJR, Lira-Aguilar A, Llovet LP, Mehta R, Tandel R, Navarro P, Sánchez-Pardo AM, Sánchez-Marin C, Cobreros M, Fernández-Cabrera I, Casals-Seoane F, Casas Deza D, Lauret-Braña E, Martí-Marqués E, Camacho-Montaño LM, Ubieto V, Ganuza M, Bolado F; ERICA Consortium. Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. N Engl J Med. 2022;387:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 7. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 8. | Yang R, Uchiyama T, Alber SM, Han X, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate ameliorates distant organ injury in a murine model of acute necrotizing pancreatitis. Crit Care Med. 2004;32:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Coelho AM, Jukemura J, Sampietre SN, Martins JO, Molan NA, Patzina RA, Lindkvist B, Jancar S, Cunha JE, D'Albuquerque LA, Machado MC. Mechanisms of the beneficial effect of hypertonic saline solution in acute pancreatitis. Shock. 2010;34:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas. 2000;20:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Yaowmaneerat T, Sirinawasatien A. Update on the strategy for intravenous fluid treatment in acute pancreatitis. World J Gastrointest Pharmacol Ther. 2023;14:22-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (3)] |
| 12. | Haydock MD, Mittal A, van den Heever M, Rossaak JI, Connor S, Rodgers M, Petrov MS, Windsor JA; Pancreas Network of New Zealand. National survey of fluid therapy in acute pancreatitis: current practice lacks a sound evidence base. World J Surg. 2013;37:2428-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Di Martino M, Van Laarhoven S, Ielpo B, Ramia JM, Manuel-Vázquez A, Martínez-Pérez A, Pavel M, Beltran Miranda P, Orti-Rodríguez R, de la Serna S, Ortega Rabbione GJ, Sanz-Garcia A, Martín-Pérez E. Systematic review and meta-analysis of fluid therapy protocols in acute pancreatitis: type, rate and route. HPB (Oxford). 2021;23:1629-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Thomson A. Intravenous fluid therapy in acute pancreatitis: a critical review of the randomized trials. ANZ J Surg. 2018;88:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Du XJ, Hu WM, Xia Q, Huang ZW, Chen GY, Jin XD, Xue P, Lu HM, Ke NW, Zhang ZD, Li QS. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas. 2011;40:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Zhao G, Zhang JG, Wu HS, Tao J, Qin Q, Deng SC, Liu Y, Liu L, Wang B, Tian K, Li X, Zhu S, Wang CY. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Klar E, Foitzik T, Buhr H, Messmer K, Herfarth C. Isovolemic hemodilution with dextran 60 as treatment of pancreatic ischemia in acute pancreatitis. Clinical practicability of an experimental concept. Ann Surg. 1993;217:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Colvin SD, Smith EN, Morgan DE, Porter KK. Acute pancreatitis: an update on the revised Atlanta classification. Abdom Radiol (NY). 2020;45:1222-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Baddam S, Burns B. Systemic Inflammatory Response Syndrome. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 20. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4672] [Article Influence: 359.4] [Reference Citation Analysis (48)] |
| 21. | Trikudanathan G, Navaneethan U, Vege SS. Current controversies in fluid resuscitation in acute pancreatitis: a systematic review. Pancreas. 2012;41:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Chen QJ, Yang ZY, Wang CY, Dong LM, Zhang YS, Xie C, Chen CZ, Zhu SK, Yang HJ, Wu HS, Yang C. Hydroxyethyl starch resuscitation downregulate pro-inflammatory cytokines in the early phase of severe acute pancreatitis: A retrospective study. Exp Ther Med. 2016;12:3213-3220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Serpa Neto A, Veelo DP, Peireira VG, de Assunção MS, Manetta JA, Espósito DC, Schultz MJ. Fluid resuscitation with hydroxyethyl starches in patients with sepsis is associated with an increased incidence of acute kidney injury and use of renal replacement therapy: a systematic review and meta-analysis of the literature. J Crit Care. 2014;29:185.e1-185.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Ni T, Wen Y, Wang Y, Jiang W, Sheng H, Chen E, Mao E, Lan Z, Huang Y, Zhou Y. Association between albumin or prealbumin levels at different stages and prognosis in severe acute pancreatitis: a 5-year retrospective study. Sci Rep. 2022;12:16792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Shatsnimitkul E, Laopeamthong I, Tansawet A, Techapongsatorn S, Kasetsermwiriya W, Leungon P, Sukhvibul P. High-volume lactated Ringer's solution with human albumin versus standard-volume infusion as a prophylactic treatment for post-endoscopic retrograde cholangiopancreatography pancreatitis: randomized clinical trial. BJS Open. 2024;9:zrae149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Vos SC, Hage JJ, Woerdeman LA, Noordanus R. Acute renal failure during dextran-40 antithrombotic prophylaxis: report of two microsurgical cases. Ann Plast Surg. 2002;48:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Biesenbach G, Kaiser W, Zazgornik J. Incidence of acute oligoanuric renal failure in dextran 40 treated patients with acute ischemic stroke stage III or IV. Ren Fail. 1997;19:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Li XW, Wang CH, Dai JW, Tsao SH, Wang PH, Tai CC, Chien RN, Shao SC, Lai EC. Comparison of clinical outcomes between aggressive and non-aggressive intravenous hydration for acute pancreatitis: a systematic review and meta-analysis. Crit Care. 2023;27:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |