Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.109002
Revised: July 22, 2025
Accepted: September 18, 2025
Published online: November 27, 2025
Processing time: 168 Days and 3 Hours
The current study was to assess the application effects of conventional surgical techniques and ultrasound-guided precise localization technology for early gastric cancer (EGC), with an emphasis on long-term survival, postoperative complica
To evaluate perioperative results, postoperative complications, and long-term survival in order to conduct a thorough comparison between conventional sur
Of 100 EGC patients were gathered, and they were subsequently divided into two groups based on the surgical technique used: The observation group (n = 52) received surgery assisted by ultrasound-guided precise localization technology, whereas the control group (n = 48) received traditional surgical treatment. The baseline characteristics were similar between the groups. Operation time, intrao
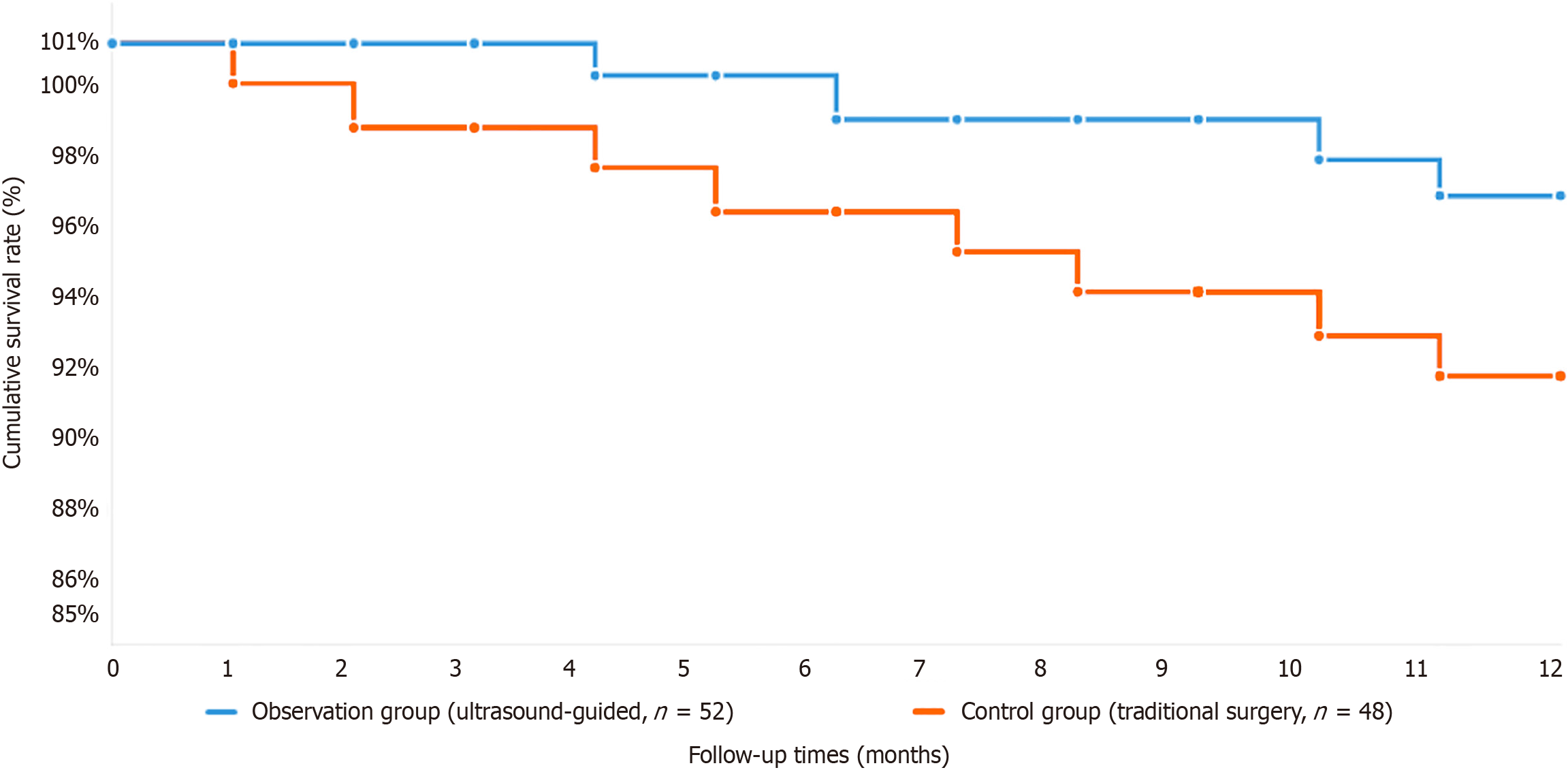
Compared with the control group, the observation group had significantly less intraoperative blood loss (80 mL vs 120 mL, P < 0.05) and more dissected lymph nodes (28 vs 22, P < 0.05). There were fewer postoperative complications in the observation group than in the routine group (8% vs 16%, P < 0.05), hospitalization after surgery was shorter, and gastrointestinal function returned sooner. The long-term survival rates at 5 years and 3 years were significantly greater in the observation group than in the control group: 82% and 88% vs 70% and 78%, respectively (P < 0.05).
It is possible that ultrasound-guided accurate localization technology might be utilized more widely in clinical practice because it could significantly enhance the results of surgery for EGC, including reduced blood loss, better lymphadenectomy, lower complication rates, and improved survival rates. Further studies should aim to refine this technology and consider its utility in other types of oncologic surgery.
Core Tip: The ultrasound-guided accurate localization technique greatly increases the surgical effect on patients with early gastric cancer, including a clearer tumor margin, shorter operation time, reduced intraoperative blood loss, greater degree of lymph node dissection, and fewer postoperative complications. This was a retrospective study that revealed that ultrasound surgery results in better 3- and 5-year survival rates than traditional surgery does. In vivo imaging allows for improved anatomical guidance to enhance accurate tumor resection and careful lymphadenectomy. These findings indicate that ultrasound guidance should be considered a routine procedure in clinical practice to increase surgical accuracy, accelerate recovery, and increase the long-term survival of patients with early gastric cancer.
- Citation: Chen SY, Hu M, Feng ZB, Xu Q, Wang Y. Application of ultrasound-guided localization technology in early gastric cancer surgery and prognostic analysis. World J Gastrointest Surg 2025; 17(11): 109002
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/109002.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.109002
Gastric cancer is one of the most important oncological challenges worldwide and is one of the leading causes of cancer-related death worldwide. In this spectrum, early gastric cancer (EGC) is strictly referred to as a malignant lesion restricted to the mucosa or submucosa without lymph node involvement[1-3]. The use of endoscopic screening and growing awareness of preventive healthcare are primarily responsible for the notable increase in the rate of EGC diagnoses in recent decades.
Surgery is still the mainstay of curative treatment for EGC and aims to obtain optimal oncologic results while maintaining gastric function to improve the quality of life of patients. Nevertheless, surgical outcomes can still depend crucially on the precision of tumor localization and the accuracy of margin assessment during open surgery. Modern surgical methods for treating gastric cancer suffer from serious restrictions in the exact demarcation of the tumor. The lack of accurate intraoperative localization techniques affects clinical therapeutics remarkably, such as insufficient resection margins or unnecessary resection of healthy stomach tissue[4-6]. Since underresection may result in poor oncological outcomes with remaining malignant tissue, local recurrence or metastasis, and a lack of appropriate immunotherapy, the strength of resection is crucial. Reduced postoperative quality of life, dietary problems, and functional disability might result from excessive extensive resection. Additionally, the systematic removal of lymph nodes by conventional surgery is often ineffective, leading to inadequate staging and a lack of regional disease management.
One possible way to get around these inherent drawbacks of gastric cancer surgery is through the development of ultrasound-guided precise localization technology[7,8]. By improving vision through the use of live ultrasound guidance, this novel method helps to clearly define tumor boundaries as well as anatomical and crucial features. This method provides real-time intraoperative information on the amount and depth of penetration of tumors by mapping and controlling margins. Furthermore, ultrasound guidance eliminates the quality and completeness gains in lymph node dissection that come from real-time anatomical presentations, particularly when it comes to identifying small and deeply situated lymph nodes that others might overlook[9-11].
Similarly, the hemostatic impact of real-time ultrasound guidance is impressive because, with this technology, vascular structures and bleeding sources may be recognized early, leading to decreased intraoperative blood loss and a decreased rate of hemorrhagic complications. Although the advantages of ultrasound-guided approaches in EGC surgery are theoretical and the amount of clinical evidence is increasing, to date, there are no large-scale comparative studies to prove its clinical efficacy. The evidence that is currently available is primarily made up of case series, small studies, and preliminary reports. While these are encouraging, they are not very strong in terms of our capacity to evaluate the technology’s effects on important endpoints, such as quality of life, local recurrence, and long-term overall survival.
The current study conducted a comprehensive comparison between hand-assisted split localization and conventional operation for the treatment of EGC in order to close this notable knowledge gap. Our study can serve as an assessment of perioperative and postoperative morbidity and oncological outcomes on a long-term basis by directly comparing ileal conduits and orthotopically and neobladder conduits to aid in clinical decision-making. By recording the clinical efficacy of ultrasound-guided EGC surgery and its safety profile, this study seeks to contribute significant evidence to the surgical oncology literature and establish new guidelines for care for patients with EGC.
The Medical Ethics Committee of Affiliated Yueqing Hospital of Wenzhou Medical University gave its approval to this study, No. YQYY202400091. This study included 100 patients with EGC who underwent surgical treatment at our hospital from January 2020 to December 2022. EGC was defined as cancer confined to the gastric mucosa or submucosa, regardless of the degree of lymph node metastasis. Patients were included if they had pathologically confirmed EGC via endoscopic biopsy, were aged between 18 and 75 years, had no distant metastasis, and had not received prior antitumor treatment. Additionally, patients with no severe dysfunction of the heart, lungs, liver, or kidneys and who provided signed informed consent were eligible. Patients were excluded if they had distant metastasis before surgery, other concurrent malignant tumors, a history of gastric surgery or radiotherapy, or if the patient or their family refused to participate in the study.
Patient allocation was performed on the basis of the surgical methodology employed during the study period. The observation group included 52 patients who underwent surgical resection with ultrasound-guided precise localization technology assistance, whereas the control group included 48 patients who received conventional surgical treatment without ultrasound guidance. For all ultrasound-guided procedures, we utilized high-frequency linear ultrasound probes (7.5-12 MHz) integrated with a dedicated intraoperative ultrasound system (SonoSite Edge II, FUJIFILM SonoSite, Inc., Bothell, WA, United States). Standardized imaging parameters were consistently applied across all the examinations, including optimized depth settings (2-6 cm), gain adjustment (60%-80%), and color Doppler parameters (pulse repetition frequency: 750-1500 Hz, wall filter: Low, color gain: 50%-70%). The ultrasound system was calibrated before each procedure to ensure optimal image quality and measurement accuracy. Comprehensive statistical analysis revealed no significant differences between the two groups in terms of baseline demographic or clinical characteristics, including age distribution, sex ratio, tumor anatomical location, tumor size, histological differentiation, and comorbidity profiles (all P > 0.05). This demographic equilibrium ensures the validity of the comparative analysis and minimizes the potential impact of confounding variables on the study outcomes. While preserving the validity and generalizability of our results, the retrospective grouping technique used in this study successfully accounts for selection bias (Figure 1). Board-certified radiologists who had at least five years of intraoperative ultrasound imaging experience and specialized training in gastric cancer localization techniques carried out all ultrasound-guided surgeries. For all patients in the observation group, a systematic procedure was put in place to guarantee uniformity in image collection, tumor border detection, and surgical guidance.
Surgical procedure for the control group: The control group underwent traditional radical gastrectomy. The surgical steps were as follows: The patient was placed in the supine position, and the abdomen was disinfected routinely and draped. An upper midline abdominal incision was made, and the skin, subcutaneous tissue, and anterior sheath of the rectus abdominis muscle were successively incised to enter the abdominal cavity. The abdominal cavity was explored to confirm the absence of distant metastasis, and then the gastric tumor and surrounding tissues were resected according to the standard range of radical gastrectomy. Lymph node dissection was performed, covering lymph node groups 1 to 6. Finally, the digestive tract was reconstructed, the abdominal cavity was closed, and a drainage tube was placed.
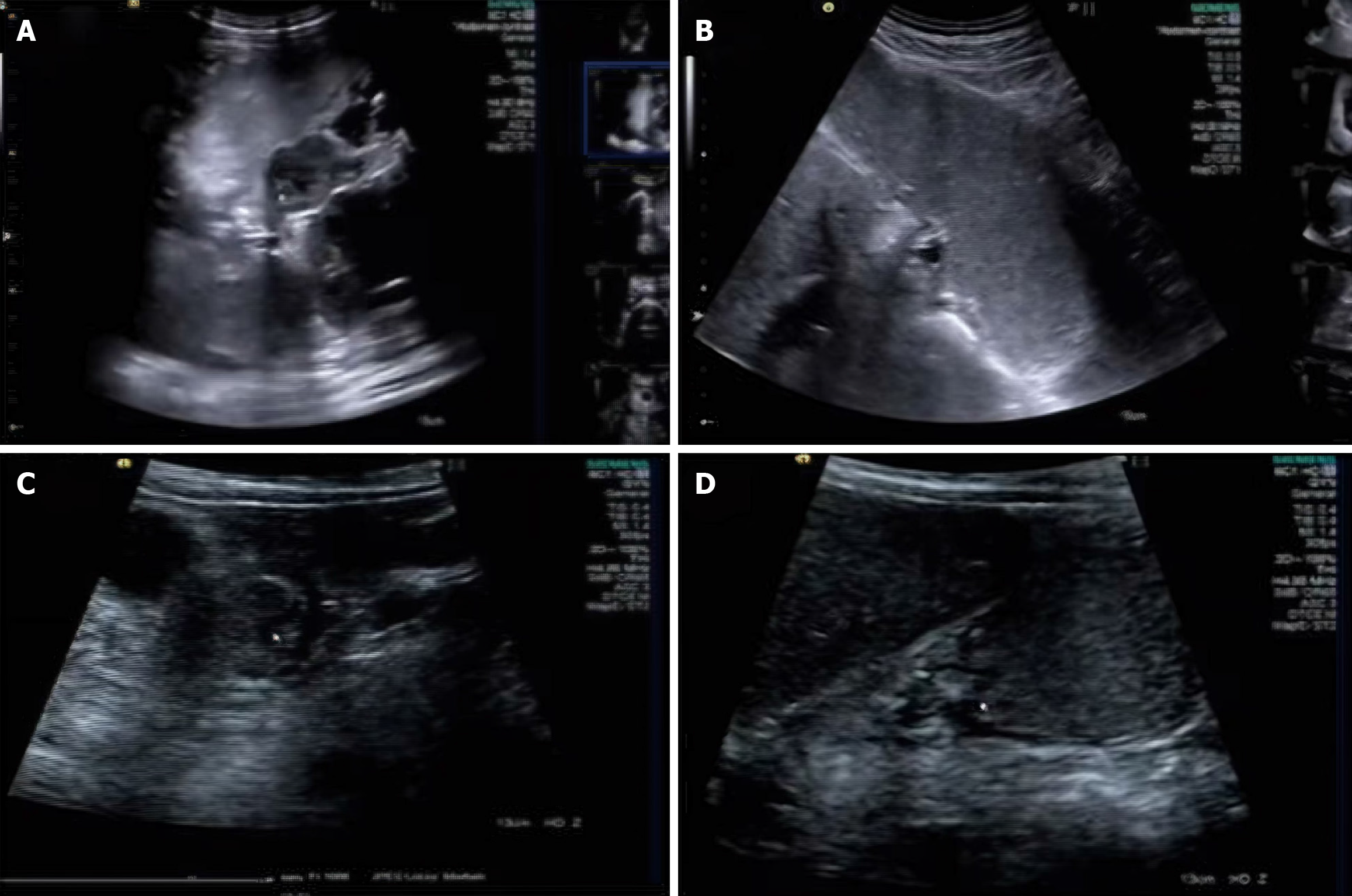
Surgical procedure for the observation group: The observation group underwent precise localization surgery under ultrasound guidance. The surgical steps were as follows: The patient was placed in the supine position, and the abdomen was disinfected routinely and draped. An upper midline abdominal incision was made, and the skin, subcutaneous tissue, and anterior sheath of the rectus abdominis muscle were successively incised to enter the abdominal cavity. The stomach was scanned in real time with an ultrasound probe to determine the size, location, and relationship of the tumor with surrounding tissues. The core advantage of ultrasound-guided technology is its precise visualization, which can clearly display the relationship between the tumor and surrounding blood vessels and nerves, avoiding injury. Under ultrasound guidance, markers (such as methylene blue stain or metal clips) were used to mark the tumor boundary. This marking method helps surgeons more accurately identify the tumor boundary during surgery, reducing the removal of normal tissues. The tumor and surrounding tissues were precisely resected according to the ultrasound-guided markings, ensuring negative surgical margins. Lymph node dissection was performed under ultrasound guidance, ensuring that the dissection range covered lymph node groups 1 to 6. Ultrasound-guided technology can monitor the lymph node dissection process in real time, ensuring thorough dissection. Finally, the digestive tract was reconstructed, the abdominal cavity was closed, and a drainage tube was placed[3,12,13].
The observation indicators included surgery-related indicators and postoperative follow-up indicators. Surgical-related indicators included operation time, intraoperative blood loss, and the number of lymph nodes dissected. The operation time was defined as the total time from skin incision to abdominal closure. Intraoperative blood loss was measured by the volume of blood collected by the aspirator. The number of lymph nodes dissected referred to the actual number of lymph nodes removed during surgery. Postoperative follow-up indicators included the incidence of postoperative complications (such as infection, bleeding, and anastomotic leakage), as well as the 1-year, 3-year, and 5-year survival rates. Pos
All the data were analyzed via SPSS 22.0 statistical software. Continuous data are expressed as the means ± SD, and intergroup comparisons were performed via independent sample t tests. Categorical data are expressed as percentages (%), and intergroup comparisons were performed via χ2 tests. A P value of less than 0.05 was considered statistically significant.
A total of 100 patients with early-stage stomach cancer were recruited for this trial; 52 were placed in the observation group and 48 in the control group. Comparability was guaranteed because there were no appreciable variations between the two groups in terms of age, sex, tumor location, tumor size, or other baseline characteristics (P > 0.05). The specific data are as follows: The average age of the observation group was 55 years, with 30 males and 20 females. With an average tumor diameter of 1.2 cm, the tumors were mostly found in the stomach’s antrum (25 patients), body (15 patients), and fundus (10 patients). The control group consisted of 22 girls and 28 males, with an average age of 54. With an average tumor diameter of 1.3 cm, the tumor distribution resembled that of the observation group. These baseline features’ similarities offered a solid foundation for comparing the findings of later research. The study’s scientific and rigorous nature was further supported by the fact that there were no appreciable differences between the two groups in terms of preoperative body mass index, comorbidities, or pathological tumor types (such as well-differentiated, mo
| Characteristic | Observation group (n = 52) | Control group (n = 48) | P value |
| Age (years) | 55 ± 3.5 | 54 ± 3.8 | > 0.05 |
| Gender (male/female) | 30/22 | 28/20 | > 0.05 |
| Antrum | 25 | 24 | > 0.05 |
| Body | 15 | 16 | > 0.05 |
| Fundus | 10 | 10 | > 0.05 |
| Tumor size (cm) | 1.2 ± 0.3 | 1.3 ± 0.4 | > 0.05 |
| Body mass index | 23.5 ± 2.1 | 23.8 ± 2.3 | > 0.05 |
| None | 40 | 38 | > 0.05 |
| Present | 12 | 10 | > 0.05 |
| Well-differentiated adenocarcinoma | 15 | 14 | > 0.05 |
| Moderately differentiated adenocarcinoma | 20 | 22 | > 0.05 |
| Poorly differentiated adenocarcinoma | 15 | 14 | > 0.05 |
| Tumor depth (mucosa/submucosa) | 26/26 | 24/24 | > 0.05 |
| Lymph node metastasis (yes/no) | 10/42 | 12/36 | > 0.05 |
| Smoking history (yes/no) | 20/32 | 18/30 | > 0.05 |
| Alcohol consumption (yes/no) | 15/37 | 14/34 | > 0.05 |
| Family history of cancer (yes/no) | 10/42 | 12/36 | > 0.05 |
| Helicobacter pylori infection (positive/negative) | 18/34 | 16/32 | > 0.05 |
| Chronic gastritis (yes/no) | 22/30 | 20/28 | > 0.05 |
| Previous endoscopic history (yes/no) | 25/27 | 23/25 | > 0.05 |
| Diabetes mellitus (yes/no) | 5/47 | 4/44 | > 0.05 |
| Hypertension (yes/no) | 10/42 | 12/36 | > 0.05 |
From skin incision to abdominal closure, the entire duration is referred to as the operation time. The observation group’s operating time was 120 minutes, while the control group’s was 115 minutes. The results showed that there was no significant difference between the two groups (P > 0.05). This result suggests that surgical efficiency was unaffected and that the use of ultrasound-guided precise localization technology did not considerably lengthen the operation time. Ultrasound-guided technology adds steps for real-time intraoperative monitoring and preoperative localization, but it has little effect on the total operation time. This might be the result of the technology’s better surgical precision, which reduced needless procedures and somewhat made up for the longer localization time. Because it demonstrates that ultrasound-guided technology can perform more precise surgical procedures on patients without lengthening the operating time, this conclusion is crucial for clinical practice (Table 2).
| Characteristic | Observation group (n = 52) | Control group (n = 48) | P value |
| Operation time (minutes) | 120 ± 10 | 115 ± 12 | > 0.05 |
| Intraoperative blood loss (mL) | 80 ± 15 | 120 ± 20 | < 0.05 |
| Number of lymph nodes dissected | 28 ± 4 | 22 ± 3 | < 0.05 |
| Postoperative hospital stays (days) | 7 ± 1 | 8 ± 1 | < 0.05 |
| Time to flatus after surgery (hours) | 48 ± 6 | 60 ± 8 | < 0.05 |
| Time to first meal after surgery (hours) | 72 ± 10 | 96 ± 12 | < 0.05 |
| Postoperative pain score (VAS) | 3 ± 1 | 5 ± 1 | < 0.05 |
| Incidence of postoperative complications | 8 | 16 | < 0.05 |
| 1-year survival rate | 96 | 92 | > 0.05 |
| 3-year survival rate | 88 | 78 | < 0.05 |
| 5-year survival rate | 82 | 70 | < 0.05 |
| Recurrence rate | 10 | 20 | < 0.05 |
The aspirator’s volume of blood was used to calculate the amount of blood lost during surgery. The intraoperative blood loss in the observation group was 80 mL, considerably less than the control group’s 120 mL (P < 0.05). Through accurate localization, ultrasound-guided technology can minimize needless tissue injury, which will increase surgical safety and effectively lower intraoperative blood loss. The link between the tumor and surrounding blood arteries can be readily seen during surgery with ultrasound-guided equipment, which helps surgeons avoid major blood vessels and minimize intraoperative hemorrhage. Furthermore, the system can continuously track the direction and position of surgical instruments, which significantly lowers the chance of unintentional harm. This finding suggests that ultrasonography-guided technology offers a notable benefit in lowering intraoperative blood loss, which lowers surgical risks and the likelihood of postoperative problems, particularly those related to bleeding that may arise during surgery (Table 3).
| Characteristic | Observation group (n = 52) | Control group (n = 48) | P value |
| Intraoperative blood loss (mL) | 80 ± 15 | 120 ± 20 | < 0.05 |
| Operation time (minutes) | 120 ± 10 | 115 ± 12 | > 0.05 |
| Number of lymph nodes dissected | 28 ± 4 | 22 ± 3 | < 0.05 |
| Postoperative hospital stays (days) | 7 ± 1 | 8 ± 1 | < 0.05 |
| Time to flatus after surgery (hours) | 48 ± 6 | 60 ± 8 | < 0.05 |
| Time to first meal after surgery (hours) | 72 ± 10 | 96 ± 12 | < 0.05 |
| Postoperative pain score (VAS) | 3 ± 1 | 5 ± 1 | < 0.05 |
| Incidence of postoperative complications | 8 | 16 | < 0.05 |
| 1-year survival rate | 96 | 92 | > 0.05 |
The records of postoperative complications included but were not limited to common complications such as post-operative infection, bleeding, and anastomotic leakage. The observation group’ complication rate was 8% (4/50), significantly lower than the control group’ reported rate of 16% (8/50) (P < 0.05). By precisely localizing the damage to surrounding tissues during surgery, ultrasound-guided technology lowers the risk of postoperative problems. Patients in the observation group recovered more quickly than those in the control group during the postoperative follow-up, and there was a notable decrease in the frequency of bleeding and postoperative infections. According to these results, ultrasound-guided technology not only increases surgical accuracy but also greatly enhances patients’ postoperative recuperation, lowering the risk of complications and enhancing their quality of life. Additionally, patients’ hospital stays are shortened and medical expenses are decreased when postoperative complications are reduced (Table 4).
| Item | Observation group (n = 52) | Control group (n = 48) | P value |
| Total postoperative complications | 8 (4/50) | 16 (8/50) | < 0.05 |
| Postoperative infection | 4 (2/50) | 10 (5/50) | < 0.05 |
| Wound infection | 2 (1/50) | 6 (3/50) | < 0.05 |
| Pulmonary infection | 2 (1/50) | 4 (2/50) | > 0.05 |
| Urinary tract infection | 0 (0/50) | 2 (1/50) | > 0.05 |
| Postoperative bleeding | 2 (1/50) | 6 (3/50) | < 0.05 |
| Minor bleeding | 2 (1/50) | 4 (2/50) | > 0.05 |
| Major bleeding | 0 (0/50) | 2 (1/50) | > 0.05 |
| Anastomotic leakage | 2 (1/50) | 4 (2/50) | > 0.05 |
| Postoperative pain (VAS), mean ± SD | 3 ± 1 | 5 ± 1 | < 0.05 |
| Postoperative nausea and vomiting | 2 (1/50) | 6 (3/50) | > 0.05 |
| Postoperative ileus | 0 (0/50) | 2 (1/50) | > 0.05 |
| Deep vein thrombosis | 0 (0/50) | 2 (1/50) | > 0.05 |
| Other complications | 2 (1/50) | 4 (2/50) | > 0.05 |
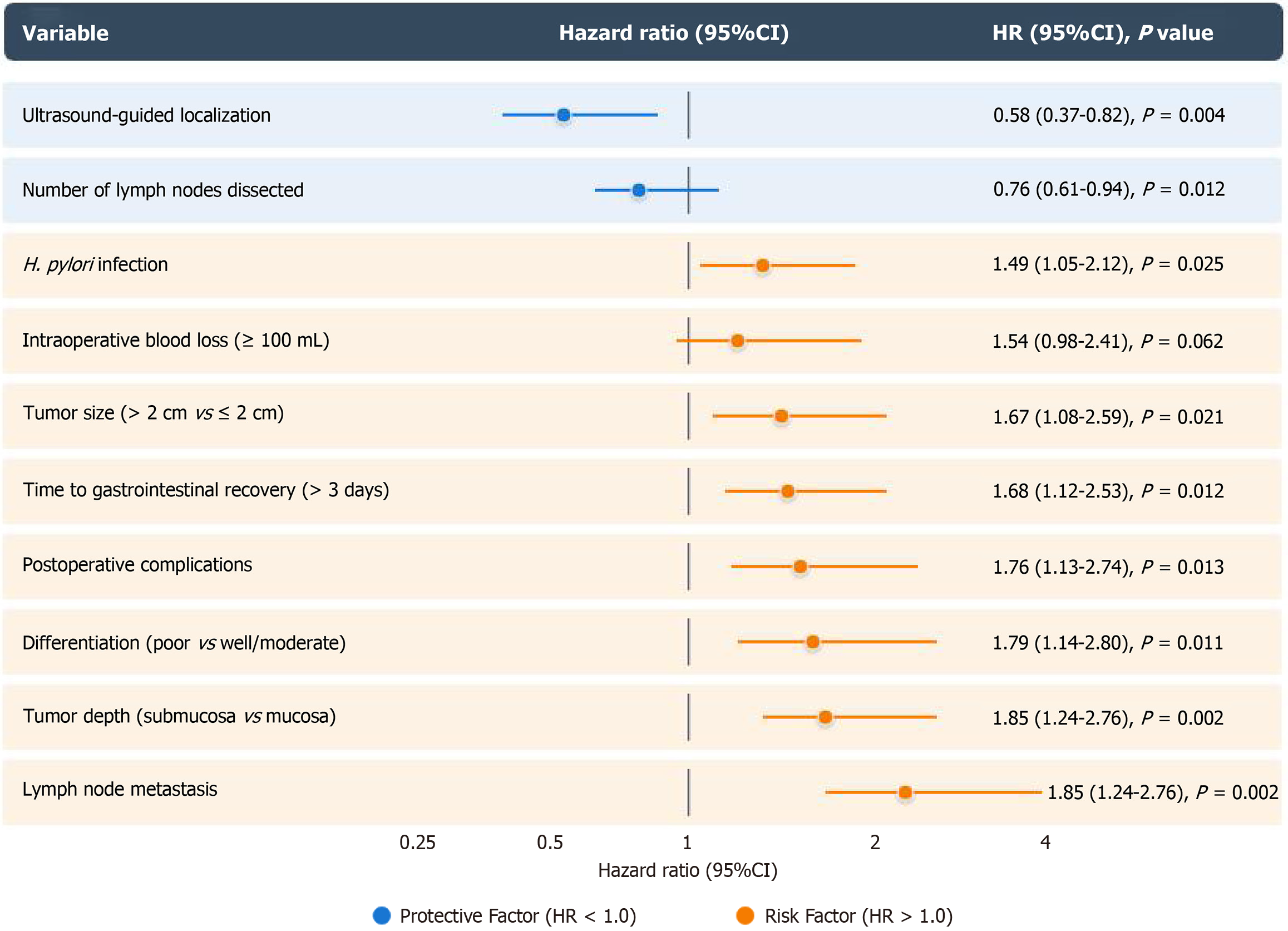
Cox proportional hazards regression was performed to analyze factors influencing the 5-year survival rate. The results revealed that ultrasound-guided precise localization [hazard ratio (HR) = 0.58, 95%CI: 0.37-0.82, P = 0.004] was an independent protective factor for long-term survival after adjusting for age, sex, tumor location, tumor size, and pathological differentiation. Other significant independent prognostic factors included lymph node metastasis (HR = 2.46, 95%CI: 1.72-3.51, P < 0.001), tumor depth (HR = 1.85, 95%CI: 1.24-2.76, P = 0.002), and the number of lymph nodes dissected (HR = 0.76, 95%CI: 0.61-0.94, P = 0.012; Figure 3).
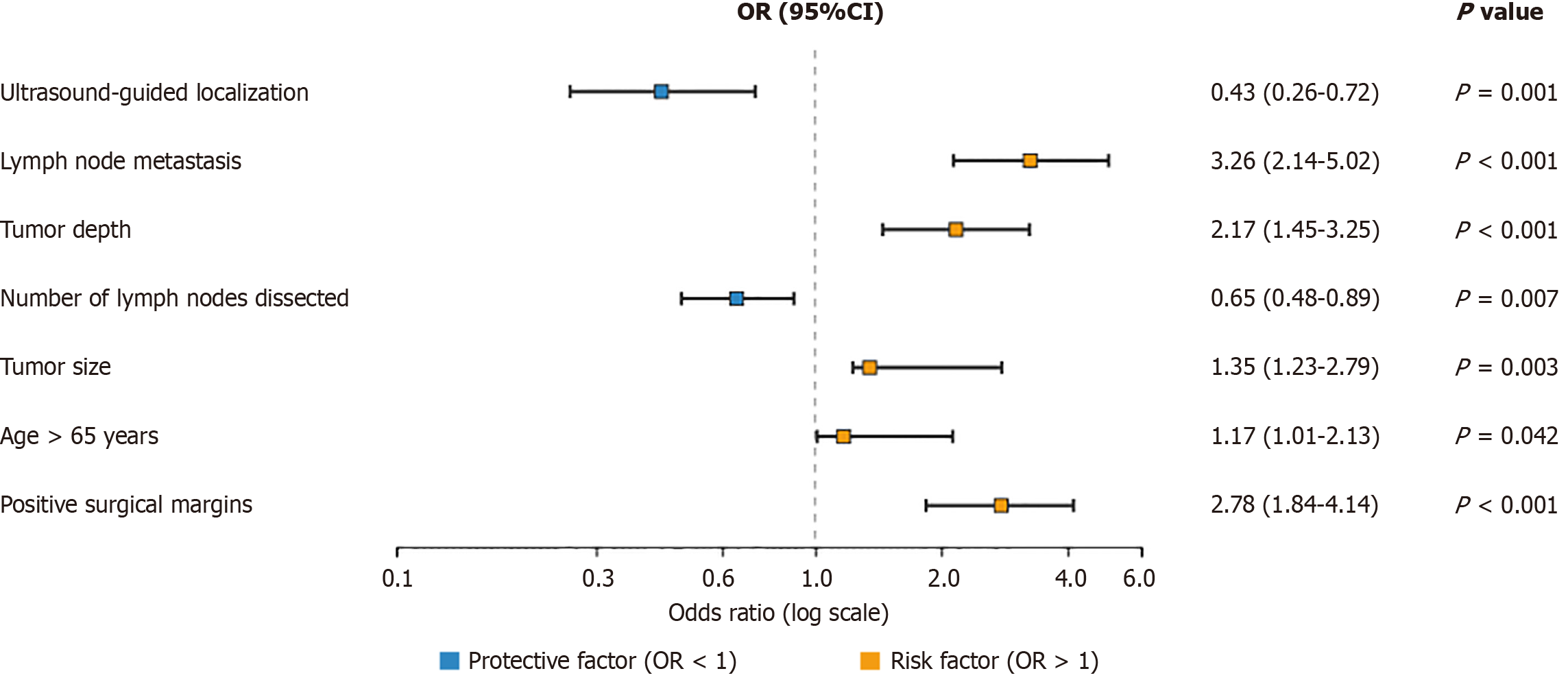
Logistic regression analysis was conducted to identify factors associated with cancer recurrence. Ultrasound-guided precise localization significantly reduced the risk of recurrence [odds ratio (OR) = 0.43, 95%CI: 0.26-0.72; P = 0.001]. Other significant factors included lymph node metastasis (OR = 3.28, 95%CI: 2.14-5.02; P < 0.001), tumor depth (OR = 2.17, 95%CI: 1.45-3.25; P < 0.001), and the number of lymph nodes dissected (OR = 0.65, 95%CI: 0.48-0.89; P = 0.007; Figure 4).
In this study, the impact of ultrasound-guided localization techniques on recovery outcomes during EGC procedures was evaluated. According to these results, ultrasonography guidance significantly boosts surgical precision, minimizes bleeding during surgery, improves the evacuation of lymphatic tissue, lowers postoperative problems, and improves long-term patient survival. These findings demonstrate the potential for ultrasound technology to transform medical practices and enhance patient recovery during EGC treatment[14-16]. Gastric cancer is one of the leading causes of cancer death globally, and early detection and treatment are essential to improving patient outcomes[17-19]. Traditional surgical methods for EGC sometimes aren’t precise enough to reach and accurately outline tumor margins, which can lead to problems and poor outcomes[20-22]. By providing real-time visualization during surgeries, ultrasound-guided localization overcomes these drawbacks. This method allows for better lymph node extraction, less tissue damage, and more accurate tumor removal, all of which improve surgical outcomes[23-25].
According to significant perioperative and oncological endpoints, the current study demonstrates a number of distinct advantages of ultrasound-guided lesion localization approaches. These benefits can be separated into three categories: Long-term survival benefits, postoperative recovery indices, and acute surgical end points. The most obvious advantage of ultrasound guidance was a substantial decrease in intraoperative blood loss, and the amount of hemorrhage in the observation group was much lower than that in the control group (80 mL vs 120 mL, P < 0.05). Real-time visualization helps with the ability to clearly visualize vessels and to accurately control hemostasis during the procedure, which can help contribute to a 33% reduction in blood loss compared with the 65-μ and 1-Nd:YAG (neodymium:yttrium-aluminum-garnet) laser treatments. Real-time anatomical information enables deliberate tissue dissection and selective vascular preservation to avoid inadvertent sources of bleeding in circumstances. Additionally, the improved lymphadenectomy realized by ultrasonic guidance was equally important since the number of lymph nodes retrieved was greater in the observation group (28 nodes vs 22 nodes, P < 0.05). This 27% increase in lymph node yield is an essential oncological benefit, given that thorough nodal sampling is the cornerstone for staging and optimal regional disease management. Better long-term oncological outcomes and a lower chance of occult metastatic illness follow the thorough lymphadenectomy performed with ultrasound assistance. In addition to being beneficial during the procedure, intraoperative ultrasonography guidance regarding the tumor margin also led to a significantly reduced rate of postoperative morbidity in the observation group.
Veterinary surgeons reported a lower rate of surgical complications (wound infection and other complications) in patients who underwent sonography-guided procedures. This development may be a manifestation of the technology’s capacity to reduce inflammatory response, improve wound healing, and minimize iatrogenic tissue damage through precise surgical planning and execution. In addition, patients in the observation group experienced faster recovery, shorter postoperative hospital stays, and faster recovery of gastrointestinal functions. These enhanced recovery trends could indicate that the ultrasound-guided surgery not only enhances their immediate surgical outcomes but also allows for a more rapid recovery at the physiological level, which may provide additional efficiency in the use of healthcare resources and patient satisfaction. The strongest argument in favor of ultrasound-guided surgery is the significant improvement in long-term survival. The 3-year and 5-year survival rates were higher in the observation group (88% and 82%, respectively) than in the control group (78% and 70%, respectively) (P < 0.05). These differences are statistically significant, meaningful from a clinical point of view, and translate into real patient advantages. The higher surgical precision made achievable by ultrasound guiding may be the cause of the better survival advantages. Reduced local recurrence and distant metastasis could be the outcome of more accurate tumor margin evaluation and thorough lymph node dissection. Since it provides the opportunity for radical resection as well as preservation of normal tissues, te
Based on these promising results, future research should broaden the scope of ultrasound-guided technologies for different patient demographics, stages, and subtypes in order to support the ongoing development of clinical evidence[26-28]. Artificial intelligence algorithms and sophisticated imaging analytics are examples of emerging technology that can be used to increase the precision and reliability of ultrasound-guided treatments. This convergence of technology may result in even better patient outcomes, less reliance on individuals, and a way to learn from other centers’ ex
Through enhanced lymphadenectomy, decreased complications, improved long-term survival rates, and decreased blood loss, this study showed that ultrasound-guided precision localization technology greatly improved EGC surgical results. For patients with EGC, this technology should be widely used in clinical practice to offer safer and more effective treatment alternatives.
| 1. | Ni WJ, Xi YX, Zhou YC. Efficacy of combined psychological and physical nursing in preventing peripherally inserted central catheter-related thrombosis in gastric cancer patients. World J Gastrointest Surg. 2025;17:100430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Wang Y, Fan X, Luo Z, Wang Q, Fang Y, Han C, Qiu Z, Wang H, Huang C. A comprehensive study on the radiomic score derived from perineural invasion in gastric cancer and its correlation with the overall survival of patients. Radiol Med. 2025;130:865-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Zhai Y, Chen Z, Luo X, Zheng Z, Zhang H, Wang X, Yan X, Liu X, Yin J, Wang J, Zhang J. Generation of surgical reports for lymph node dissection during laparoscopic gastric cancer surgery based on artificial intelligence. Int J Comput Assist Radiol Surg. 2025;20:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Jiang W, Wang H, Zhang X, Dou X, Zhu K, Xu L, Huang Y, Yu J, Yue J. Patterns of Recurrence After D2 Radical Surgery for Gastric Cancer: Implications for Postoperative Radiation Therapy. Pract Radiat Oncol. 2025;15:485-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Zhou B, Song Y, Chen C, Chen X, Tao T. Preoperative Prediction of Sarcopenia in Patients Scheduled for Gastric and Colorectal Cancer Surgery. J Gastrointest Cancer. 2025;56:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Power FR, Ryan AG, Murphy MN, Cleary MS. Extradigital glomus tumor of the elbow with preoperative ultrasound-guided wire localization: a case report. J Shoulder Elbow Surg. 2017;26:e352-e356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10:2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 9. | Chapellier P, Pache B, Haefliger L, Lelièvre L, Mathevet P, Hajri R. Contribution of preoperative ultrasound-guided implantation of a magnetic seed for optimal localization and resection of vulvar angiosarcoma: A case report. Int J Surg Case Rep. 2024;114:109107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (1)] |
| 11. | Staniszewski T, Buckley N, Hanna AS. Lateral Femoral Cutaneous Nerve Transposition With Ultrasound-Guided Wire Localization: 2-Dimensional Operative Video. Oper Neurosurg. 2024;26:104-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11:1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 13. | Zhu LL, Shen RZ. Follow-up of elderly gastric cancer post-radical surgery: Trauma, complications, and prognosis. World J Gastrointest Surg. 2025;17:100143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Morito A, Eto K, Iwatsuki M, Toihata T, Kosumi K, Iwagami S, Baba Y, Miyamoto Y, Yoshida N, Baba H. Clinical impact of very early recurrence after conversion surgery for stage IV gastric cancer. Ann Gastroenterol Surg. 2024;8:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Zhou J, Li R, Zhao S, Sun L, Wang J, Fu Y, Wang D. Sentinel Node Navigation Surgery for Early Gastric Cancer: A Narrative Review. Am J Clin Oncol. 2024;47:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Liang R, An R, Yun D. Letter to the editor on "Impact of postoperative complications on gastric cancer survival". Surgery. 2025;181:109125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Liu C, Li Y, Xu Y, Hou H. The impact of preoperative skeletal muscle mass index-defined sarcopenia on postoperative complications and survival in gastric cancer: An updated meta-analysis. Eur J Surg Oncol. 2025;51:109569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 20. | Futawatari N, Fukuyama T, Akimoto Y, Maehara J, Hihara D, Okamoto Y, Yokouchi Y, Takahashi K, Watanabe M, Saida Y. Association Between Kita-kyushu Lung Cancer Antigen-1 Expression in Gastric Cancer and Helicobacter pylori-infection Status. Anticancer Res. 2025;45:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Kuroda K, Toyokawa T, Tsujio G, Miki Y, Yoshii M, Kasashima H, Fukuoka T, Tamura T, Shibutani M, Lee S, Yashiro M, Maeda K. Significance of Systemic Inflammatory Markers as Prognostic Predictors in Stage II/III Gastric Cancer Among Older Patients. Anticancer Res. 2025;45:1681-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 23. | Finkelstein ER, Buitrago J, Jose J, Levi AD, Xu KY, Burks SS. Lower extremity peripheral nerve pathology: Utility of preoperative ultrasound-guided needle localization before operative intervention. Skeletal Radiol. 2023;52:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Kim H, Ko EY, Han BK, Ko ES, Choi JS, Lee JE, Cho SY. Feasibility of Ultrasound-Guided Localization for Clipped Metastatic Axillary Lymph Nodes After Neoadjuvant Chemotherapy in Breast Cancer Patients: A Pilot Study. J Breast Cancer. 2023;26:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 26. | Wu L, Yang L, Qian X, Hu W, Wang S, Yan J. Mannan-Decorated Lipid Calcium Phosphate Nanoparticle Vaccine Increased the Antitumor Immune Response by Modulating the Tumor Microenvironment. J Funct Biomater. 2024;15:229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 27. | Lei C, Sun W, Wang K, Weng R, Kan X, Li R. Artificial intelligence-assisted diagnosis of early gastric cancer: present practice and future prospects. Ann Med. 2025;57:2461679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Liao Y, Chen X, Hu S, Chen B, Zhuo X, Xu H, Wu X, Zeng X, Zeng H, Zhang D, Zhi Y, Zhao L. Artificial Intelligence for Predicting HER2 Status of Gastric Cancer Based on Whole-Slide Histopathology Images: A Retrospective Multicenter Study. Adv Sci (Weinh). 2025;12:e2408451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |