Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.111975
Revised: August 4, 2025
Accepted: August 15, 2025
Published online: October 27, 2025
Processing time: 101 Days and 5.1 Hours
Mixed adenoneuroendocrine carcinoma (MANEC) is a rare malignancy that can be effectively treated with surgery in its early stages. However, there is currently no established treatment protocol for metastatic cases.
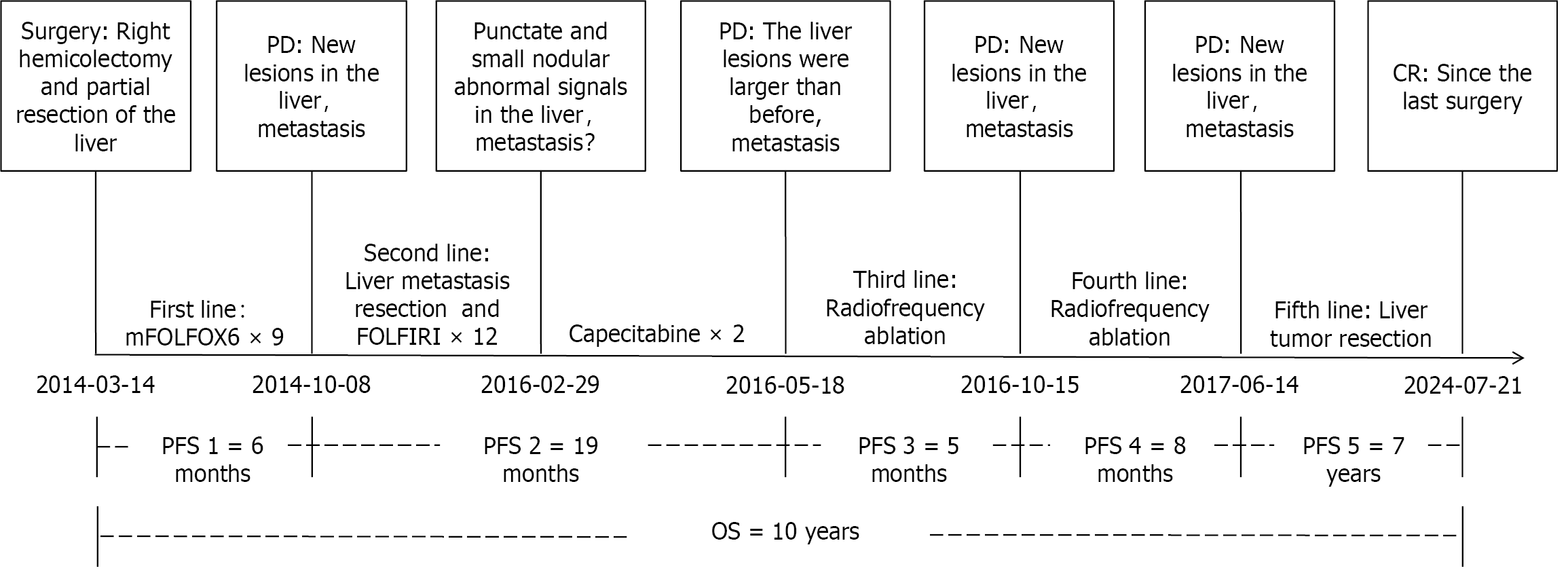
We present a case of a patient diagnosed with liver metastasis (LM) from ascending colon MANEC. The patient is a 40-year-old male who was diagnosed to have ascending colon MANEC with LM in March 2014. Following surgical resection of both the primary tumor and the LM, the patient received first-line chemotherapy with the mFOLFOX6 regimen. After six months of the initial treatment, the patient experienced multiple recurrences of LM. These LM were successfully treated by a multidisciplinary approach including chemotherapy, surgical resection, and radiofrequency ablation (RFA). Remarkably, in June 2017, the patient achieved a complete response, which has been maintained to date, resulting in overall long-term survival of ten years. Advanced colorectal MANEC is often associated with LM, and multiple intrahepatic recurrences may be characteristic of this disease.
The use of local treatment modalities, such as surgery and RFA, in conjunction with chemotherapy, has demon
Core Tip: Mixed adenoneuroendocrine carcinoma (MANEC) is a rare type of malignant tumor, but there is no standard treatment protocol. This article reviews a case of a patient diagnosed with liver metastasis from ascending colon MANEC. The patient experienced multiple recurrent liver metastases. Through a multidisciplinary treatment approach including chemotherapy, surgical resection, and radiofrequency ablation, the patient achieved complete response and a long-term survival of ten years. The comprehensive treatment approach has shown encouraging results for MANEC and is worthy of further study.
- Citation: Pan J, Zhang RS, Chen Y, Cao XH, Yang ZH, Lu L, Chen YT, Chu XY. Long-term survival after multimodality treatment of metastatic mixed adenoneuroendocrine carcinoma of colon: A case report. World J Gastrointest Surg 2025; 17(10): 111975
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/111975.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.111975
Neuroendocrine neoplasm (NEN) represents a heterogeneous group of tumors that originate from peptidergic neurons and neuroendocrine cells. These neoplasms are commonly found in various organs, including the stomach, intestine, pancreas, liver, biliary system, lungs, and thyroid. Among these, gastroenteropancreatic NEN is the most prevalent[1,2]. According to the WHO classification for digestive system tumors, NEN can be categorized into neuroendocrine tumor (NET), neuroendocrine carcinoma (NEC), and mixed neuroendocrine-non-NENs (MiNEN)[3]. MiNEN refers to a mixed epithelial neoplasm characterized by the presence of both neuroendocrine and non-neuroendocrine components, each of which is distinguishable through histological morphology and immunohistochemistry (IHC), with each component accounting for at least 30%. Consequently, the diagnostic criteria for mixed adenoneuroendocrine carcinoma (MANEC) are applicable only to a subset of patients with mixed neoplasms[4]. Although the incidence of colorectal MANEC is low, it is associated with high malignancy potential and rapid disease progression, with 29% of patients having advanced stage at the time of diagnosis. The prognosis is generally poor, and no standardized treatment protocols exist for this condition[5,6]. In this report, we describe the management of a patient with metastatic ascending colon MANEC, treated successfully by multidisciplinary approach involving surgery, radiofrequency ablation (RFA), and chemotherapy, resulting in a long-term survival of 10 years.
A 40-year-old male was admitted to our hospital for abdominal pain lasting for three days in March 2014.
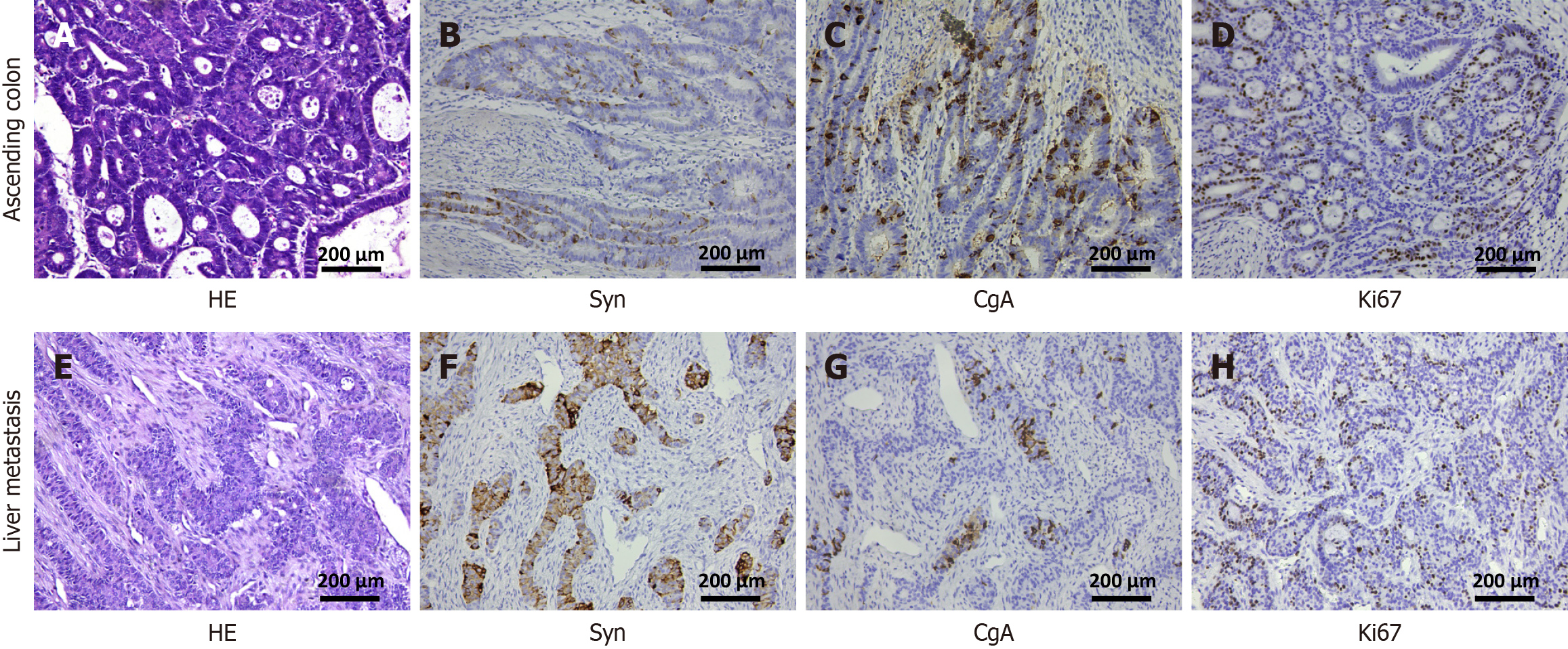
An abdominal contrast enhanced computed tomography (CECT) revealed a space-occupying lesion in both the ascending colon and the segment Ⅵ of right lobe of the liver measuring 2.0 cm × 2.0 cm. A colonoscopy identified a cauliflower-like mass measuring 5.0 cm × 6.0 cm located at the ileocecal junction. The mass was characterized by ulceration and erosion on its surface, fragile texture, and a tendency to bleed. The biopsy indicated moderately differentiated adenocarcinoma. Following a discussion at the tumor board meeting, the decision was made to proceed with surgical treatment. On March 14, 2014, the patient underwent open radical right hemicolectomy and non-anatomical resection of the right lobe hepatic metastasis. The surgery lasted for 4.5 hours and was completed without complications. The postoperative hospital stay was 15 days. The postoperative pathology revealed the following findings: (1) Ascending colon: Moderately differentiated adenocarcinoma with a protuberant type, measuring approximately 5.0 cm × 4.5 cm × 1.8 cm. The cancer tissue invaded the full thickness of the intestinal wall (T4). No metastasis was detected in any of the 22 Lymph nodes surrounding the tumor (N0). The resection margins, as well as the appendix, showed no involvement by the cancerous tissue; and (2) Liver metastasis (LM): Adenocarcinoma infiltration was observed in the liver tissue, consistent with metastasis from colon adenocarcinoma (M1). The tumor size was approximately 2.2 cm × 2.0 cm × 1.0 cm with resection margins free of tumor. The results of IHC were as follows: (1) Ascending colon: Synaptophysin (Syn) (++), chromogranin (CgA) (+), Ckpan (+++), p53 (+), COX-2 (+), CDX-2 (++), TopoII (+), ERCC1 (+), TS (-), Ki67 (90%), supporting the diagnosis of moderately differentiated adenocarcinoma of the colon with neuroendocrine expression; and (2) LM: Syn (++), CgA (+), CK20 (-), CK7 (-), Villin (++), NapsinA (-), Ki67 (90%), supporting the diagnosis of LM of colon adenocarcinoma with neuroendocrine differentiation (Figure 1). As both the adenocarcinoma and NEC components in the patient exceed 30%, the diagnostic criteria for MANEC was fulfilled and the final diagnosis of metastatic MANEC originating from the colon was made.
The patient was diagnosed with diabetes based on the presence of polydipsia and abnormal blood glucose concentrations.
He had no family history of MANEC. The patient's father is alive, while his mother died of glioblastoma in 2009.
All vital signs were stable and physical examination revealed no notable abnormalities.
Laboratory findings showed slightly decreased hemoglobin (110 g/L) and otherwise normal blood counts.
Preoperative CECT revealed a mass located in both the liver and the ascending colon of the patient. A colonoscopy confirmed moderately differentiated adenocarcinoma.
The presence of both adenocarcinoma and NEC components (each exceeding 30%) met the diagnostic criteria for MANEC, confirming a final diagnosis of metastatic MANEC of colonic origin.
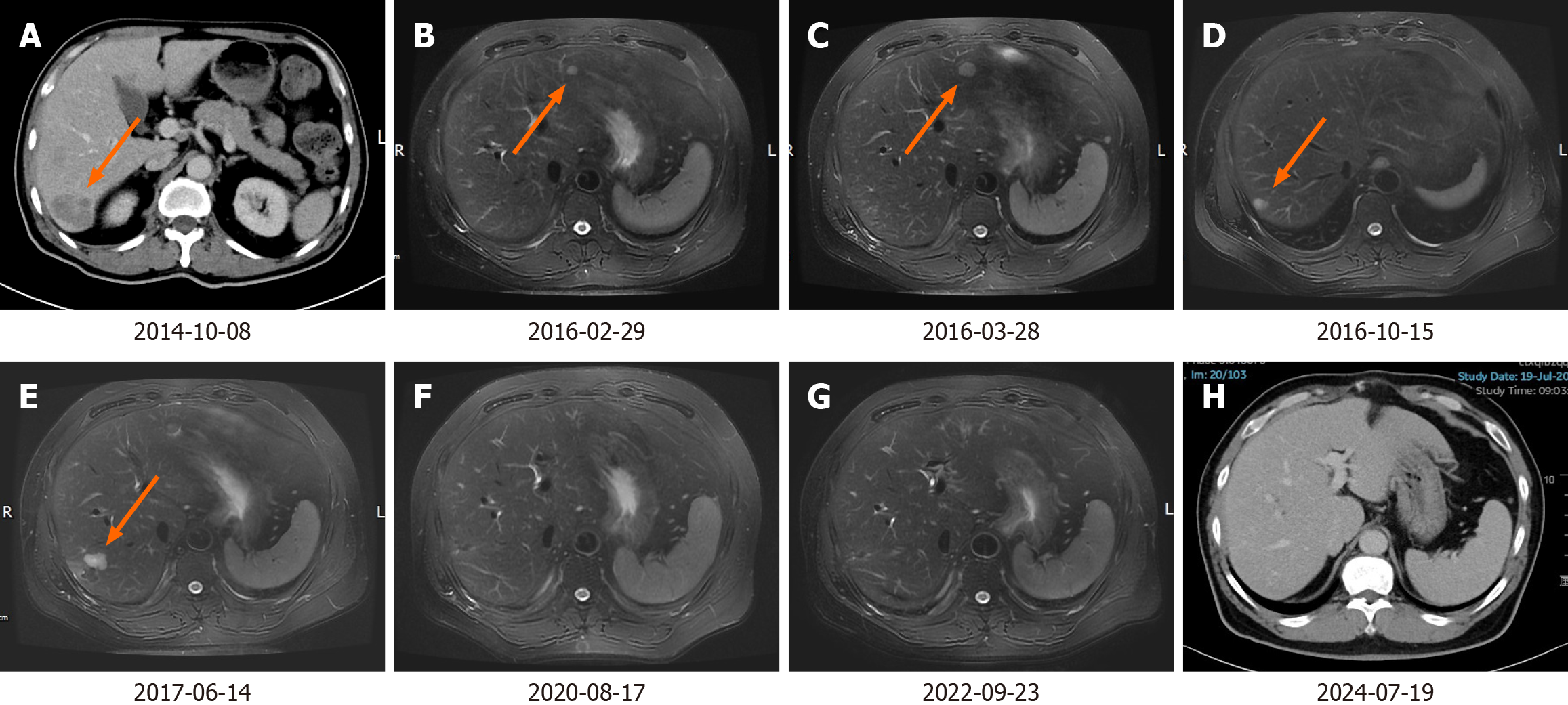
According to the National Comprehensive Cancer Network (NCCN) guidelines, PET-CT is not routinely recommended for baseline assessment prior to chemotherapy. Consequently, we performed only CECT of the chest and abdomen and no definitive new lesions were identified. From April 12, 2014, to September 16, 2014, the patient received nine cycles of chemotherapy as per mFOLFOX6 regimen (oxaliplatin, 100 mg/m2, day 1; leucovorin, 400 mg/m2, day 1-2; fluorouracil, 2400 mg/m2, once over 46 hours, day 1-3). On October 8, 2014, repeat CECT abdomen revealed a nodular lesion in the segment Ⅶ of right lobe of the liver, measuring approximately 1.9 cm, which was deemed a metastasis (Figure 2A). We did not perform a preoperative biopsy or fine needle aspiration cytology (FNAC). On October 23, 2014, the patient underwent open non-anatomical R0 resection of the LM. The surgery lasted for 3 h and 25 min, and there were no complications. The duration of the hospital stay was 11 days. The postoperative pathology confirmed LM from colon adenocarcinoma. Between November 28, 2014, and July 24, 2015, the patient underwent 12 cycles of chemotherapy using the FOLFIRI regimen (irinotecan, 200 mg/m2, day 1; leucovorin, 400 mg/m2, day 1-2; fluorouracil, 2400 mg/m2, once over 46 hours, day 1-3). On February 29, 2016, an abdominal magnetic resonance imaging (MRI) revealed punctate and small nodular abnormal signals in both the left and right lobes of the liver (Figure 2B). New metastatic foci could not be ruled out, and disease progression was suspected. The patient did not undergo biopsy, FNAC, or PET-CT. According to the NCCN guidelines, for advanced colorectal cancer, maintenance therapy with oral 5-FU agents, such as capecitabine, may be considered following the achievement of optimal efficacy with 5-FU-based mFOLFOX and FOLFIRI regimens. From March 10 to April 1, 2016, the patient received two cycles of capecitabine treatment (capecitabine, 1000 mg/m2, twice daily on days 1-14). On May 28, 2016, the MRI indicated a small nodular area exhibiting increased signal intensity in the left lobe of the liver, alongside an abnormal punctate lesion in the right lobe. The larger lesion, situated in segment IV, measures approximately 1.4 cm × 1.7 cm (Figure 2C). On May 30, 2016, RFA of left lateral lobe LM was performed. On October 15, 2016, the MRI demonstrated that the size of the left lateral lobe lesion had slightly decreased, with no internal enhancement. However, the lesion in the right lobe had increased in size compared to previous assessments (Figure 2D). On October 17, 2016, RFA of the right lobe LM was performed. On June 14, 2017, the MRI indicated that the lesions in the left lateral lobe and right posterior lobe had decreased in size, while several new nodules were identified in the segment Ⅵ and Ⅶ of the right lobe, suggestive of new metastatic foci (Figure 2E). Subsequently, on June 22, 2017, the patient underwent open non-anatomical resection of the right lobe LM with R0 margins. The duration of the operation was 3 hours and 5 minutes, and it proceeded without complications. The patient was discharged after a hospital stay of 10 days. The postoperative pathology indicated that the liver resection specimen measured approximately 3 cm × 2.5 cm × 4 cm. Histopathological examination revealed extensive infiltration of moderately differentiated adenocarcinoma within the liver tissue. Additionally, vascular tumor thrombi were present. Subsequently, the patient underwent follow-up examinations every six months to one year. Each examination includes routine blood tests, biochemical tests, tumor marker assessments, and CECT of the chest and abdomen. Multiple follow-up examinations have indicated no new lesions (Figure 2F and G). The most recent follow-up examination occurred on July 19, 2024 (Figure 2H).
Currently, the patient remains under observation, and his quality of life is reported to be good. The case timeline is presented in Figure 3.
MANEC is a rare subtype of NET characterized by the presence of both adenocarcinoma and neuroendocrine cells. In its early stages, inconsistent nomenclature resulted in confusion among clinicians and pathologists, complicating the summarization of the disease. This confusion contributed to a limited understanding of MANEC, ultimately leading to insufficient attention given to it. Furthermore, due to the disease's low incidence, its demographic, clinical, and prognostic characteristics remain poorly defined, which hinders clinicians' ability to effectively treat affected patients. A retrospective analysis from the SEER database conducted by Wang et al[7] encompassing a total of 581 patients with MANEC indicated that the incidence of gastrointestinal MANEC rose from 0.23 per million in 2000 to 1.16 per million in 2016, reflecting an annual percentage change (APC) of 8.0%. Additionally, the mortality rate exhibited a consistent upward trend, with an APC of 12.9%. The Cox regression analysis identified age at the time of diagnosis, tumor grade and stage, lymph node metastasis, surgical intervention, and tumor size as independent factors associated with mortality. The median survival time for patients was found to be 75 months.
The diagnosis of MANEC presents significant challenges, particularly when both the adenocarcinoma and NET components are poorly differentiated because of similar tumor morphology on routine histology. This often leads to misdiagnosis as poorly differentiated adenocarcinoma. A study identified 47 patients with MANEC, of which 20 patients (42.6%) were misdiagnosed as poorly differentiated adenocarcinoma during endoscopic biopsy[8]. Hence, it is important to incorporate IHC of NETs for accurate diagnosis in patients with poorly differentiated adenocarcinoma. Syn and CgA are widely utilized indicators for NEN. In the present case, the hematoxylin and eosin staining of a patient following the initial surgery was misinterpreted as poorly differentiated adenocarcinoma. However, IHC revealed strong positive expression of both Syn and CgA, with the tissue components of adenocarcinoma and NETs each exceeding 30%. This led to a diagnosis of LM from ascending colon MANEC. In addition to these primary markers, other diagnostic indicators such as CD56 and neuron-specific enolase may also be beneficial. However, their specificity and sensitivity are inferior to those of Syn and CgA[9,10].
Currently, it is widely accepted that resectable colorectal LM can be treated by upfront surgical resection if complete resection can be performed. Hence, we opted for simultaneous resection of the primary lesion and the liver metastatic lesion in this patient. Although the initial operation resulted in R0 resection of both the primary and metastatic lesions, the patient had stage IV disease at that time, indicating a high risk of recurrence and metastasis; thus, systemic chemotherapy was administered. The objective was to minimize the likelihood of recurrence and metastasis, aiming for a potential cure. Given the low incidence of colorectal MANEC, there is a notable absence of standardized and guideline-recommended chemotherapy regimens[11]. Some studies have reported that without systemic intervention, such as chemotherapy, the median overall survival (OS) for these patients is approximately 7 to 10 months[12]. As MANEC encompasses both adenocarcinoma and NET components, the chemotherapy regimens of both these tumors have been used for the treatment of MANEC[13]. Hence, the FOLFOX/XELOX and FOLFIRI regimens have been used for colorectal MANEC[14,15]. Recent studies have indicated that the EP regimen (etoposide with cisplatin) and the IP regimen (irinotecan with cisplatin), may be more effective in addressing NETs while treating adenocarcinoma, thus representing viable treatment options[16,17]. Vanacker et al[18] reported a patient with colon MANEC having LM who received the EP regimen following the resection of the primary tumor. Notably, the LM exhibited significant shrinkage, achieving partial remission after four cycles and complete response after six cycles, with survival exceeding three years. In the present case, the initial postoperative pathology revealed that both the primary tumor and the LM predominantly consisted of adenocarcinoma, suggesting that the adenocarcinoma component played a critical role in the tumor's development. Consequently, we implemented the FOLFOX regimen as the first-line chemotherapy and the FOLFIRI regimen as the second-line to primarily focus on the adenocarcinoma component. This approach resulted in a progression-free survival of approximately six months. Throughout the treatment, the patient demonstrated good tolerance, with the main adverse reactions being grade 2 Leukopenia, grade 2 neutropenia, grade 1 nausea, and grade 1 fatigue. Importantly, the patient did not experience nausea and vomiting, hand and foot numbness, or diarrhea.
As previously mentioned, surgical resection is an effective treatment modality that may cure patients with liver lesions[19]. However, the occurrence of intrahepatic recurrence following surgical resection has become increasingly common. Current evidence suggests that neoadjuvant therapy prior to surgery may reduce the recurrence rate[20]. For patients who develop intrahepatic recurrence, secondary or even multiple surgical resections may still lead to a cure[21-23]. Therefore, when feasible, surgical resection remains the preferred treatment option. Alternatively, local treatment methods such as RFA, resection combined with RFA, and stereotactic body radiation therapy can also be considered. In the current case, after the initial surgery, there were a total of four intrahepatic recurrences. Characteristically, these metastatic lesions were small (all measuring less than 5 cm), were isolated lesions, with no extensive or diffuse intrahepatic metastases away from the major vessels, and no extrahepatic disease. As there are no established guidelines for treating colorectal MANEC with LM, we treated this patient similar to those with colorectal adenocarcinoma. Hence, after obtaining informed consent, we employed surgical resection and RFA in this patient due to the absence of extrahepatic disease. Systemic chemotherapy was administered only after the first and second recurrences, while local treatment methods were utilized for the third and fourth recurrences. Since the last operation, the patient has remained disease free, achieving OS of 10 years without any significant adverse reactions or complications.
In conclusion, colorectal MANEC is a clinically rare colorectal malignant tumor with lack of specific clinical manifestations. It is diagnosed mainly on histopathology when both the tissue components are more than 30%. IHC is necessary to detect the neuroendocrine component of these tumors. Advanced colorectal MANEC is prone to develop LM, and multiple intrahepatic recurrences may be one of its characteristics. In such cases, multimodality approach using surgery, RFA and chemotherapy can help to achieve good survival and is worthy of further exploration in clinical practice.
| 1. | Ito T, Masui T, Komoto I, Doi R, Osamura RY, Sakurai A, Ikeda M, Takano K, Igarashi H, Shimatsu A, Nakamura K, Nakamoto Y, Hijioka S, Morita K, Ishikawa Y, Ohike N, Kasajima A, Kushima R, Kojima M, Sasano H, Hirano S, Mizuno N, Aoki T, Aoki T, Ohtsuka T, Okumura T, Kimura Y, Kudo A, Konishi T, Matsumoto I, Kobayashi N, Fujimori N, Honma Y, Morizane C, Uchino S, Horiuchi K, Yamasaki M, Matsubayashi J, Sato Y, Sekiguchi M, Abe S, Okusaka T, Kida M, Kimura W, Tanaka M, Majima Y, Jensen RT, Hirata K, Imamura M, Uemoto S. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: diagnosis, treatment, and follow-up: a synopsis. J Gastroenterol. 2021;56:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 2. | Zhang XB, Fan YB, Jing R, Getu MA, Chen WY, Zhang W, Dong HX, Dakal TC, Hayat A, Cai HJ, Ashrafizadeh M, Abd El-Aty AM, Hacimuftuoglu A, Liu P, Li TF, Sethi G, Ahn KS, Ertas YN, Chen MJ, Ji JS, Ma L, Gong P. Gastroenteropancreatic neuroendocrine neoplasms: current development, challenges, and clinical perspectives. Mil Med Res. 2024;11:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Rindi G, Wiedenmann B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: towards precision medicine. Nat Rev Endocrinol. 2020;16:590-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Cattaneo L, Centonze G, Sabella G, Lagano V, Angerilli V, Pardo C, Bertani E, Spada F, Prinzi N, Pusceddu S, Fassan M, Fazio N, Milione M. Digestive MiNENs: Could histological classification and molecular characterization drive clinical outcome and therapeutic approach? Crit Rev Oncol Hematol. 2023;188:104044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Dulskas A, Pilvelis A. Oncologic outcome of mixed adenoneuroendocrine carcinoma (MANEC): A single center case series. Eur J Surg Oncol. 2020;46:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Grossi U, Bonis A, Carrington EV, Mazzobel E, Santoro GA, Cattaneo L, Centonze G, Gallo G, Kazemi Nava A, Romano M, Di Tanna GL, Zanus G. Mixed adenoneuroendocrine carcinoma (MANEC) of the lower gastrointestinal tract: A systematic review with Bayesian hierarchical survival analysis. Eur J Surg Oncol. 2021;47:2893-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Wang J, He A, Feng Q, Hou P, Wu J, Huang Z, Xiao Z, Sun C, Liao W, Wu L. Gastrointestinal mixed adenoneuroendocrine carcinoma: a population level analysis of epidemiological trends. J Transl Med. 2020;18:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | van der Veen A, Seesing MFJ, Wijnhoven BPL, de Steur WO, van Berge Henegouwen MI, Rosman C, van Sandick JW, Mook S, Haj Mohammad N, Ruurda JP, Brosens LAA, van Hillegersberg R; (MA)NEC group. Management of resectable esophageal and gastric (mixed adeno)neuroendocrine carcinoma: A nationwide cohort study. Eur J Surg Oncol. 2018;44:1955-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Elpek GO. Mixed neuroendocrine-nonneuroendocrine neoplasms of the gastrointestinal system: An update. World J Gastroenterol. 2022;28:794-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 10. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 623] [Article Influence: 155.8] [Reference Citation Analysis (2)] |
| 11. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 794] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 12. | Lin JP, Zhao YJ, He QL, Hao HK, Tian YT, Zou BB, Jiang LX, Lin W, Zhou YB, Li Z, Xu YC, Zhao G, Xue FQ, Li SL, Fu WH, Li YX, Zhou XJ, Li Y, Zhu ZG, Chen JP, Xu ZK, Cai LH, Li E, Li HL, Xie JW, Huang CM, Li P, Lin JX, Zheng CH. Adjuvant chemotherapy for patients with gastric neuroendocrine carcinomas or mixed adenoneuroendocrine carcinomas. Br J Surg. 2020;107:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Zhou K, Li ZW, Wu Y, Wang ZJ, Wang LQ, Zhou LX, Jia L, Ji K, Yang XS, Zhang J, Wu XJ, Wang AQ, Bu ZD. Lymph node metastatic patterns of gastric carcinoma with a combination of adenocarcinoma and neuroendocrine carcinoma components. World J Gastroenterol. 2025;31:102347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Michael A, Nath DK. Neoadjuvant and Adjuvant Chemotherapeutic Strategy of Colorectal Mixed Adeno-Neuroendocrine Carcinomas. Cureus. 2021;13:e16645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Tanaka T, Kaneko M, Nozawa H, Emoto S, Murono K, Otani K, Sasaki K, Nishikawa T, Kiyomatsu T, Hata K, Morikawa T, Kawai K, Watanabe T. Diagnosis, Assessment, and Therapeutic Strategy for Colorectal Mixed Adenoneuroendocrine Carcinoma. Neuroendocrinology. 2017;105:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Zhang P, Li J, Li J, Zhang X, Zhou J, Wang X, Peng Z, Shen L, Lu M. Etoposide and cisplatin versus irinotecan and cisplatin as the first-line therapy for patients with advanced, poorly differentiated gastroenteropancreatic neuroendocrine carcinoma: A randomized phase 2 study. Cancer. 2020;126 Suppl 9:2086-2092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J; Japan Clinical Oncology Group (JCOG). Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022;8:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 18. | Vanacker L, Smeets D, Hoorens A, Teugels E, Algaba R, Dehou MF, De Becker A, Lambrechts D, De Greve J. Mixed adenoneuroendocrine carcinoma of the colon: molecular pathogenesis and treatment. Anticancer Res. 2014;34:5517-5521. [PubMed] |
| 19. | Wu Y, Mao A, Wang H, Fang G, Zhou J, He X, Cai S, Wang L. Association of Simultaneous vs Delayed Resection of Liver Metastasis With Complications and Survival Among Adults With Colorectal Cancer. JAMA Netw Open. 2022;5:e2231956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Kanani A, Veen T, Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg. 2021;108:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Giuliante F, Viganò L, De Rose AM, Mirza DF, Lapointe R, Kaiser G, Barroso E, Ferrero A, Isoniemi H, Lopez-Ben S, Popescu I, Ouellet JF, Hubert C, Regimbeau JM, Lin JK, Skipenko OG, Ardito F, Adam R. Liver-First Approach for Synchronous Colorectal Metastases: Analysis of 7360 Patients from the LiverMetSurvey Registry. Ann Surg Oncol. 2021;28:8198-8208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Tian Y, Wang Y, Wen N, Wang S, Li B, Liu G. Prognostic factors associated with early recurrence following liver resection for colorectal liver metastases: a systematic review and meta-analysis. BMC Cancer. 2024;24:426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 23. | Buisman FE, Filipe WF, Kemeny NE, Narayan RR, Srouji RM, Balachandran VP, Boerner T, Drebin JA, Jarnagin WR, Kingham TP, Wei AC, Grünhagen DJ, Verhoef C, Koerkamp BG, D'Angelica MI. Recurrence After Liver Resection of Colorectal Liver Metastases: Repeat Resection or Ablation Followed by Hepatic Arterial Infusion Pump Chemotherapy. Ann Surg Oncol. 2021;28:808-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/