Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.110017
Revised: July 2, 2025
Accepted: August 13, 2025
Published online: October 27, 2025
Processing time: 149 Days and 16.8 Hours
Colonic anastomotic leakage (AL) remains a feared complication of colorectal surgery. Usually, a defunctioning stoma or a proximal colostomy is performed to reduce the AL rate but cannot completely prevent AL. Moreover, defunctioning colostomy is associated with high morbidity. This study assessed the feasibility of completely preventing colonic AL using total enteric flow diversion without a defunctioning stoma in a pig model of colonic AL.
To determine the feasibility of preventing colonic AL via total enteric flow di
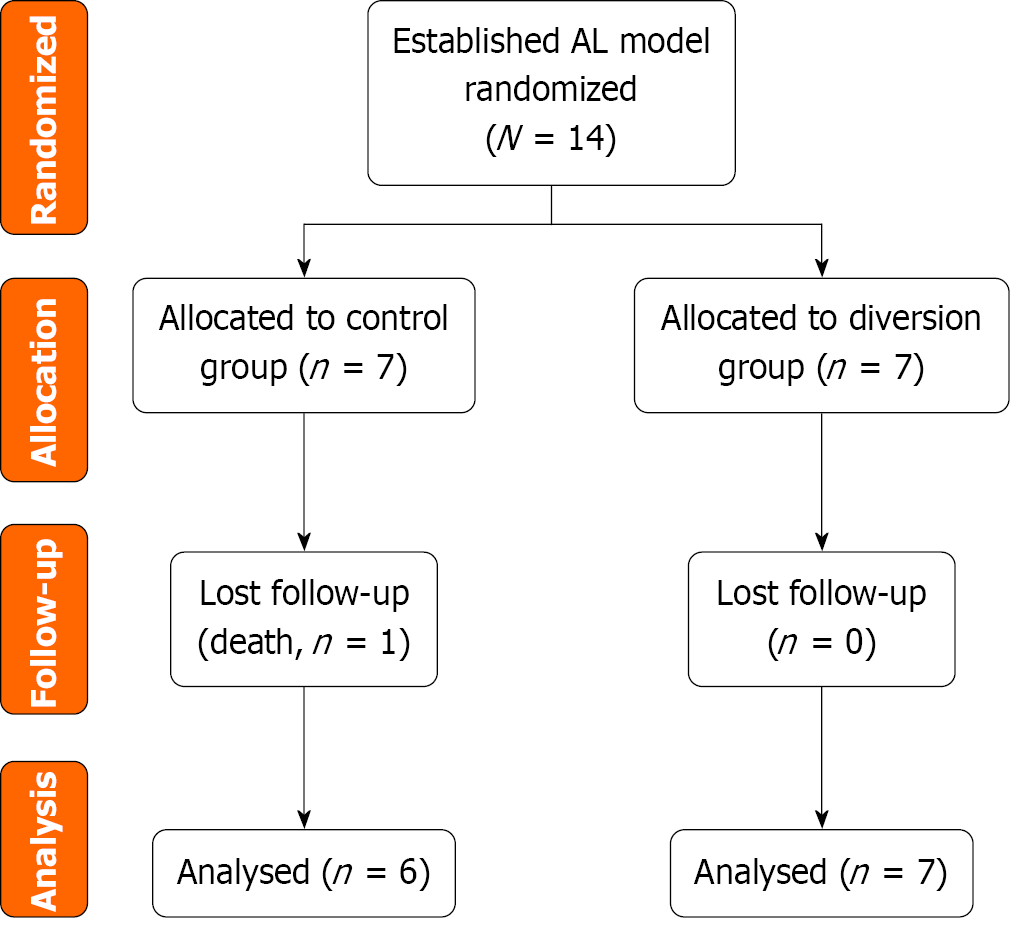
A total of 14 pigs underwent surgery to create colon anaesthesia with severe defects for establishing the AL model. The pigs were then randomized into the control group (n = 7), which received no further therapy, and a diversion group
A modified ileostomy tube with a balloon was placed and pressurized to 20 kPa at a distance of 10-20 cm proximal to the ileocecal valve, effectively obstructing the intestine without causing injury and efficiently diverting the enteric contents. In the diversion group, no cases of peritonitis or abscess were observed. In contrast, all pigs in the control group developed either abdominal abscesses or peritonitis.
Instead of ileostomy or colostomy, the total enteric flow diversion technique with the placement of a modified ileostomy tube and balloon in the ileum can effectively or completely prevent colon AL.
Core Tip: This study evaluated a novel total enteric flow diversion technique using a modified ileostomy tube with balloon inflated to 20 kPa in a pig model of colonic anastomotic leakage (AL). Compared with the control group, the diversion group showed complete prevention of AL, no peritonitis or abscesses, and mild mucosal injury without necrosis. This technique may offer an effective alternative to traditional stomas for AL prevention.
- Citation: Hu T, Wang J, Yu NH. Complete prevention of anastomotic leakage using total enteric flow diversion. World J Gastrointest Surg 2025; 17(10): 110017
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/110017.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.110017
Anastomotic leakage (AL) is a highly concerning and dreaded complication of colorectal surgery and is known for its significant morbidity, mortality, and prolonged hospital stay. In addition, AL is associated with significantly increased consumption of hospital resources and increased costs[1]. The reported incidence of AL varies from 4.3% to 15.9% in patients undergoing rectal cancer surgery[2-6]. Among the emergency colorectal procedures, colonic obstruction is associated with a 2%-16% incidence of AL and colonic peritonitis and with a 6%-19% incidence of AL[7]. Reportedly, AL considerably increases the morbidity and mortality of postoperative patients, with a mortality rate ranging from 25% to 35% in large series[8,9]. In addition, AL may result in a poorer functional outcome and increase the risk of permanent stoma formation[10].
Intraoperatively, a colostomy is often performed in high-risk patients to prevent AL and reduce its incidence. However, a colostomy cannot totally divert the material and completely prevent AL. Moreover, colostomies are associated with several complications, such as bleeding, stenosis, necrosis, poor quality of life, and mortality[7,11]. Accordingly, this study evaluated whether AL could be prevented using a modified ileostomy tube to completely divert the proximal enteric contents from entering the colon without defunctioning the colostomy in a pig model of colon anastomosis.
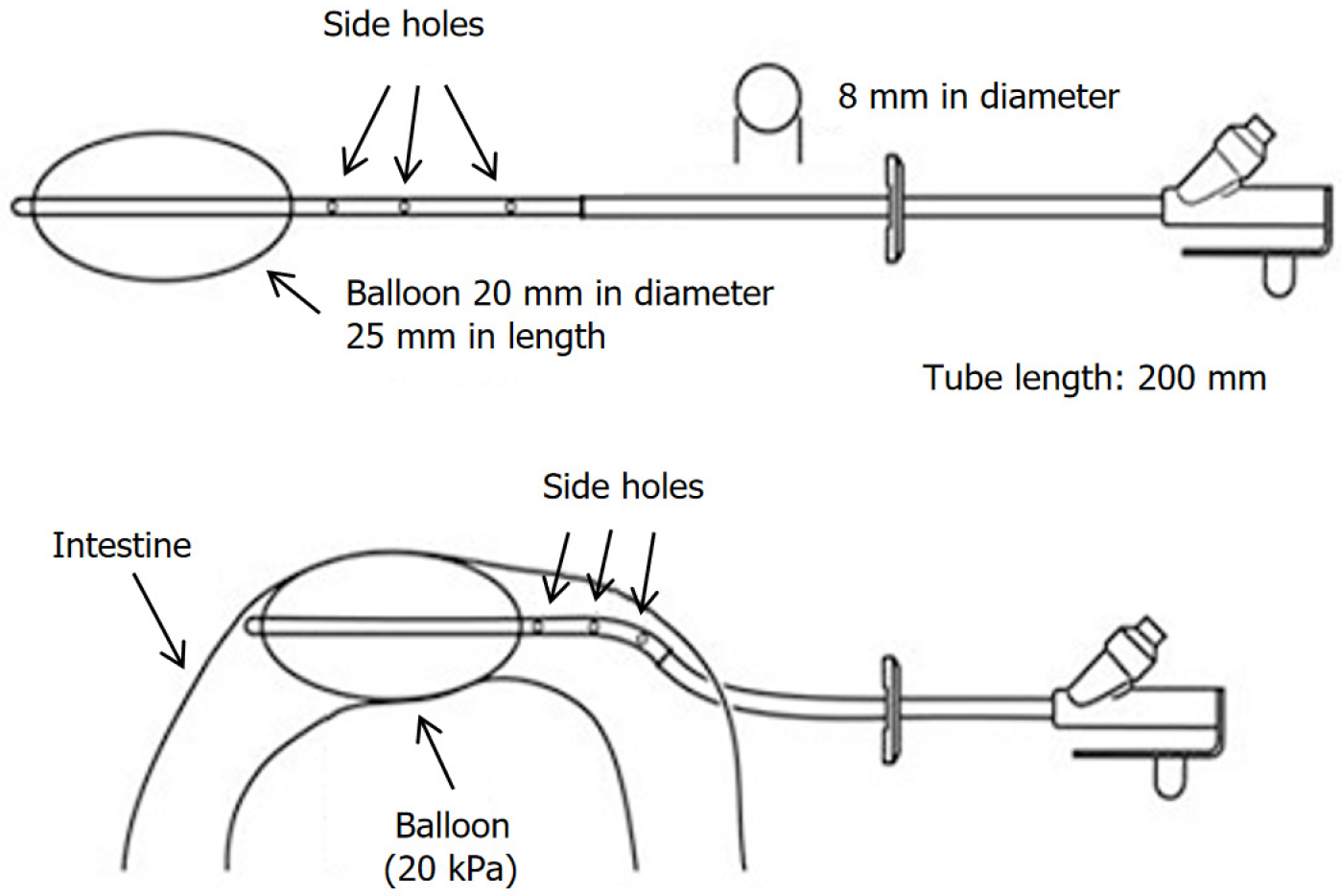
The modified ileostomy tube was procured from Zhanjiang Star Enterprise Company Limited [received Food and Drug Administration 510(k) premarket notification, No. K170233]. The tube was designed to have two channels and an obstructing balloon at one end (volume: 15 mL; Figure 1). The tube has a length of 120 cm, an outer diameter of 8 mm, an inner diameter of 5 mm, and an inflation port for the balloon. It also has three 4-mm side holes with the drainage channel spaced at 15 mm intervals.
Fourteen male pigs (4-6 months old; weighing 16-21 kg) were obtained from the Pig and Poultry Production Institute, Guangxi University, China. All animals were housed in spacious, clean facilities with balanced nutrition, appropriate temperature and humidity control, and ample lighting and ventilation at the Second Xiangya Hospital, Central South University. Preoperatively, all pigs underwent bowel preparation by fasting for 48 hours and were allowed to drink water freely until 12 hours before the procedure. The experiments were conducted in accordance with Chinese legislation on the protection of animals and the “Principles of Laboratory Animal Care” (National Institutes of Health publication No. 85-23, revised 1985). In addition, the study was approved by both the Animal Care and Use Committee and the Ethics Committee of the Second Xiangya Hospital, Central South University (Approval No. 2021934). All animals in this study were handled in accordance with the institutional and national guidelines for the ethical treatment of animals.
Preoperatively, anaesthesia was induced with intramuscular injections of ketamine (15-20 mg/kg) and chlorpromazine (6-8 mg/kg). Once the pigs were anaesthetized, they were weighed and cleaned. The marginal ear vein was accessed, and anaesthesia induction was performed with propofol (2 mg/kg), sufentanil (1-2 μg/kg), and vecuronium bromide (0.1 mg/kg). The oral cavity was opened, and surface anaesthesia was applied using a lidocaine spray. With the use of the largest adult straight laryngoscope blade, the tongue was retracted with vascular forceps, and a 6.0-6.5 cuffed endo
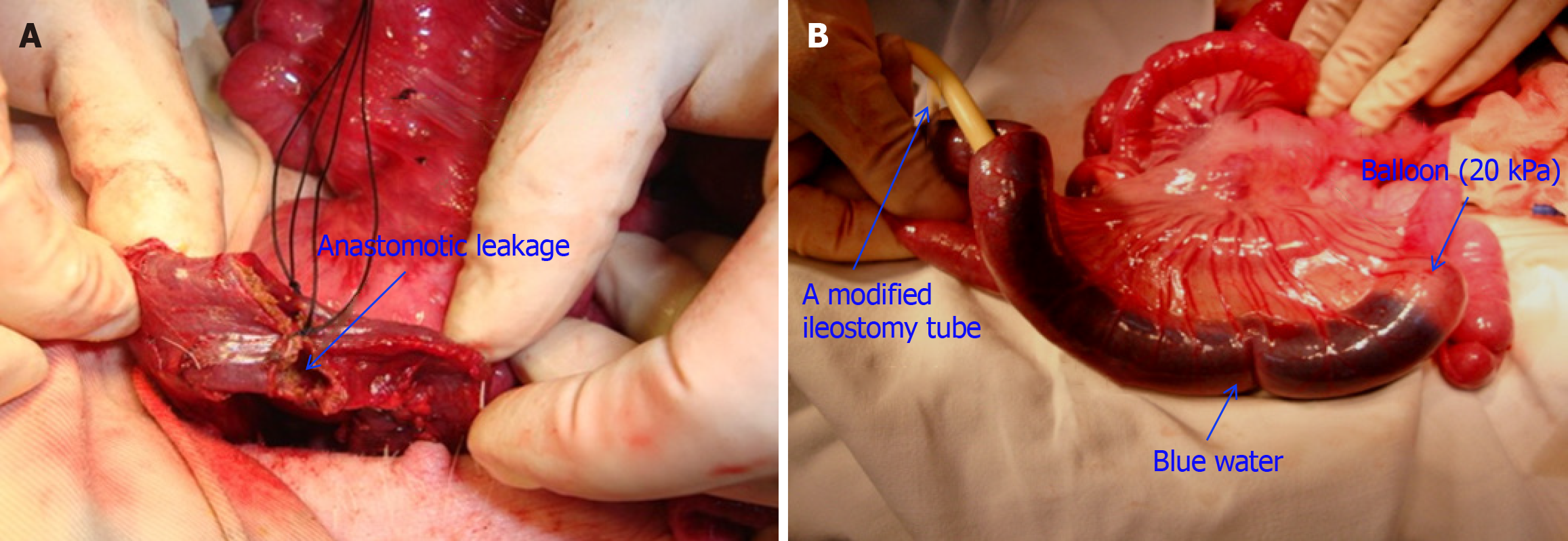
A total of 14 pigs were initially enrolled in the study and randomly assigned to two groups: The control group (n = 7) and the diversion group (n = 7). The flow diagram depicts the randomization process and the follow-up of the experimental pigs (Figure 2). All the animals were prepared under sterile conditions. A 10-cm midventral celiotomy was performed along the upper midline by using an electric scalpel, and the descending colon was transected. End-to-end, double-layer anastomosis was performed with interrupted absorbable sutures. Before completing the anastomosis, an 8 mm (25-Charr) tube was inserted into the colonic lumen. The remaining sutures were placed around the tube, which was then removed, creating a standardized defect to establish the AL model for all the experimental pigs (Figure 3A). Finally, the abdominal wall was closed.
For the diversion group (n = 7), a modified ileostomy tube with a balloon was placed with its distal end in the ileum (Figure 3B), at 10-20 cm proximal to the ileocecal valve. To optimize drainage, the part of the tube with most of the lateral side holes was placed in the proximal limb. This entrance site was closed around the tube with purse-string sutures. This tube system functioned as a proximal diverting “stoma”, completely obstructing the distal ileum using a balloon to divert all enteric contents externally. The ileostomy tube was externalized through the abdominal wall and protected from trauma and dislodgement by placing a protective garment over it. The procedure performed was not a standard feeding ileostomy but was a mechanical obstruction technique. Therefore, the balloon was inflated with normal saline from a height of 2 m so that it could inflate with approximately 20 kPa pressure without severely impairing the blood supply to the bowel wall. The tube was then fixed in the ileal loop using a purse string and brought out through the incision. Then, 140-180 mL of water containing methylene blue dye was infused into the intestine, and observers confirmed that the blue water did not pass around the balloon and into the distal ileum to the balloon.
At the end of the operation, 375 mg of penicillin was administered intramuscularly to all pigs and was readministered daily every morning until death or postoperative day 7 (POD 7). In addition, 500 mL of a glucose and saline infusion was administered intravenously until POD 3. Each pig was allowed access to a full liquid diet after POD 3. Blood sampling was performed preoperatively and on PODs 1, 3, and 5 to evaluate serum C-reactive protein levels, serum potassium levels and white blood cell counts. In addition, the general behavior, food intake, faecal production, and temperature of the pigs were recorded. Pigs that showed any signs of illness were euthanized immediately with an overdose of propofol. All other pigs were sacrificed at the end of the observation period on the seventh day post-surgery. A macroscopic examination of the abdominal cavity was subsequently conducted to assess visible leakage and local or diffuse faecal peritonitis.
After the diverting tube ileostomy was inserted into position, we set the balloon pressure at 20 kPa to obstruct the distal ileum. The selection of 20 kPa was based on a series of preliminary tests (unpublished) to ensure that this pressure would sufficiently block the intestine without causing ischaemic damage. We injected water mixed with methylene blue dye into the proximal intestine to assess the integrity of the bowel seal by evaluating whether the blue water would bypass the balloon (Figure 3B). We repeated this test immediately before sacrificing the pigs to confirm the effectiveness of intestinal obstruction. We determined 20 kPa to be the optimal pressure because it achieved complete obstruction and effectively prevented AL without causing significant damage to the intestinal wall.
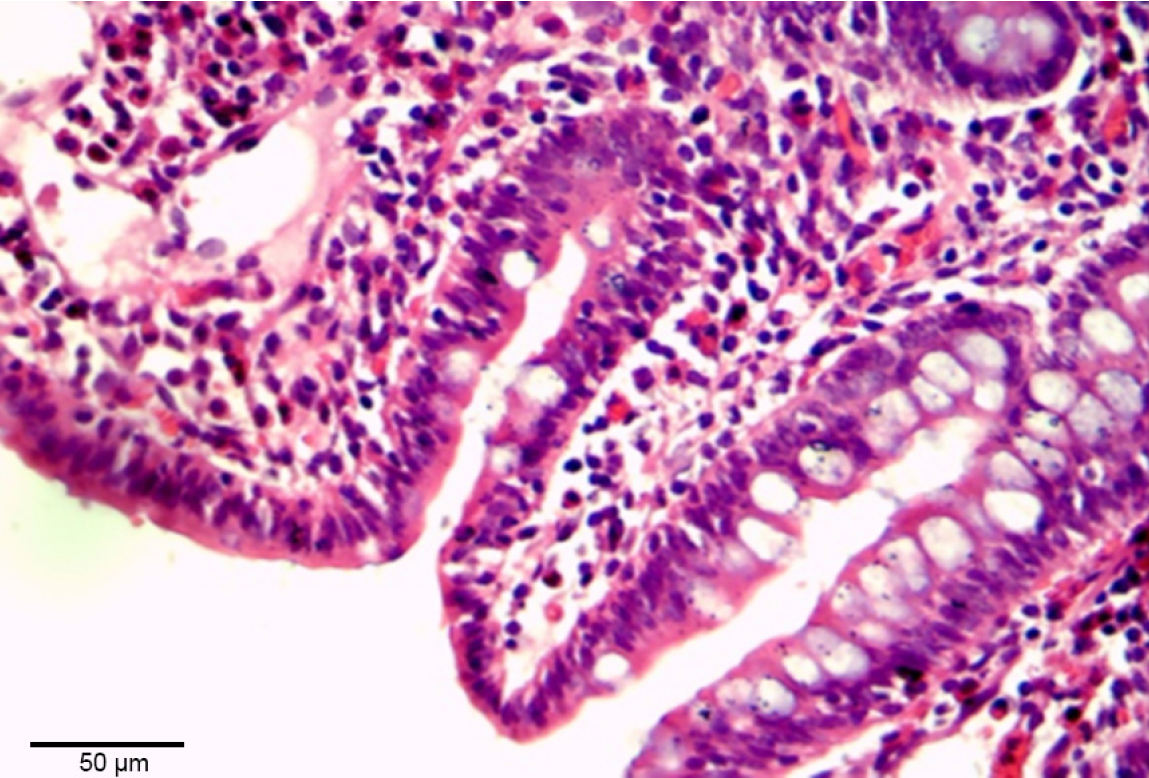
After the pigs were sacrificed, ileum tissue samples from near the balloon placement site were obtained. All the samples were then preserved in 10% formaldehyde, sectioned, and stained with haematoxylin and eosin using standard histological techniques.
Continuous variables are reported as the mean ± SD, whereas categorical variables are presented as frequencies or percentages. The Shapiro-Wilk test was used to determine the normality of distribution of continuous variables. Nonnormally distributed data were analyzed with the Mann-Whitney U test, whereas normally distributed data were assessed using the t test. For binary categorical variables, the χ2 test was applied. A P value of less than 0.05 was considered to indicate statistical significance. Statistical analysis was conducted using GraphPad Prism 9.5.0.
In the control group, all the pigs developed fever starting from POD 1, with temperatures exceeding 39 °C, whereas the temperatures in the diversion group remained below 38.5 °C. The pigs in the control group exhibited lethargy or reduced responsiveness, whereas those in the diversion group were generally in good condition. One pig in the control group developed signs of illness and was therefore euthanized before the end of the observation period. The remaining 13 pigs completed the observation period and were euthanized on POD 7. Two pigs in the control group were diagnosed with faecal peritonitis through macroscopic examination. No peritonitis or abscesses due to AL (abdominal fluid) were observed in the diversion group. Table 1 provides the baseline data for all the experimental animals, and Table 2 presents the behavioral, clinical, laboratory, and macroscopic indicators.
| | Control group (n = 6) | Diversion group (n = 7) | P value |
| Age (months) | 5.00 ± 0.63 | 4.86 ± 0.69 | 0.951 |
| Weight (kg) | 22.48 ± 2.20 | 23.07 ± 2.13 | 0.660 |
| WBCs (× 109/L) | 14.39 ± 0.45 | 13.98 ± 0.67 | 0.213 |
| Blood K+ (mmol/L) | 3.67 ± 0.29 | 3.69 ± 0.26 | 0.866 |
| Operative time (minute) | 57.50 ± 3.62 | 61.00 ± 2.83 | 0.091 |
| Control group (n = 6) | Diversion group (n = 7) | P value | |
| Behavioural indicators (%) | |||
| Lethargy | 83.33 | 0.00 | 0.002b |
| Loss of appetite | 66.67 | 0.00 | 0.009b |
| Emesis | 50.00 | 0.00 | 0.033a |
| Signs of pain | 33.33 | 0.00 | 0.097 |
| Clinical and laboratory indicators (%) | |||
| Fever (> 38.9 °C) | 100 | 0.00 | < 0.001c |
| Tachycardia (> 130 beats/minute) | 50.00 | 14.29 | 0.164 |
| Tachypnoea (> 58 breaths/minute) | 66.67 | 0.00 | 0.009b |
| Hypokalaemia (< 3.5 mmol/L) | 66.67 | 0.00 | 0.009b |
| Changes in WBC counts over time (mean ± SD) | |||
| Preoperative | 14.39 ± 0.45 | 13.98 ± 0.67 | 0.213 |
| POD 1 | 16.14 ± 2.11 | 14.50 ± 1.69 | 0.028a |
| POD 3 | 19.12 ± 3.12 | 14.91 ± 2.23 | < 0.001c |
| POD 5 | 22.18 ± 4.31 | 14.18 ± 1.01 | < 0.001c |
| Changes in CRP levels over time (mean ± SD) | |||
| Preoperative | 2.63 ± 0.39 | 2.81 ± 1.10 | 0.973 |
| POD 1 | 131.79 ± 19.34 | 108.63 ± 9.78 | 0.006b |
| POD 3 | 223.18 ± 28.21 | 87.24 ± 8.45 | < 0.001c |
| POD 5 | 256.75 ± 27.45 | 62.45 ± 7.12 | < 0.001c |
| Macroscopic indicators (%) | |||
| Enteric spillage/faecal odour | 33.33 | 0.00 | 0.097 |
| Peritonitis | 100 | 0.00 | < 0.001c |
A balloon pressure of 20 kPa completely obstructed the proximal intestine in all 14 pigs, and no blue water passed around the balloon at the time of tube insertion. Before the pigs were sacrificed, leakage of blue water was not observed around the balloon near the site of distal intestinal leakage (Figure 3B). However, autopsy findings revealed blue water leakage around the balloon, indicating that the balloon was unable to effectively obstruct the intestine.
On POD 7, the intestinal wall that was compressed by the balloon appeared normal, with no evidence of ileal necrosis or perforation. However, histological examination revealed mild mucosal injury to the balloon-compressed intestinal tissue, which was observed as an extension of the subepithelial space, and mild lifting of the epithelial layer from the lamina propria (Figure 4).
AL is the single most severe complication of colorectal surgery and is associated with significant morbidity and mortality[10,12-15]. AL is associated with poorer functional outcomes and an increased risk of permanent stoma formation[16-18]. Ileostomy or colostomy serves as a viable procedure for temporary faecal diversion after colorectal or coloanal anastomosis and is often required to mitigate conditions affecting the large intestine, thereby reducing the occurrence of clinical AL[14]. However, colostomies significantly impede patients’ quality of life, irrespective of the underlying diagnoses, and are associated with incontinence, rectal discharge, gas control issues, work-related challenges, reduced sexual activity, and hindered travel and leisure activities[14]. Moreover, colostomies require a two-stage operation. In the context of AL, the restoration of bowel continuity after stoma creation is associated with high morbidity rates of 4%-6% and mortality rates of up to 4%[19-22].
Owing to the anatomical and histological similarities between pigs and humans, pigs have become the most popular large animal experimental model for evaluating gastrointestinal anastomotic healing. Therefore, this study used pigs as experimental animals for an AL model. Animal models with anastomotic gaps or dehiscence have been developed to simulate the anatomy, physiology, and pathophysiological mechanisms of anastomotic failure in humans. Common mechanisms for the development of AL, such as local ischaemia and anastomotic dehiscence, should also be considered in the design of the model. A 21 mm AL has been reliably used to induce peritonitis in pigs[23]. In the present study, the size of the primary dehiscence was increased to 25 mm of the anastomotic circumference, as this also marked the point at which ischaemia was induced. Impaired anastomotic perfusion is known to be a major risk factor for morbidity in patients undergoing colorectal surgery[11]. All pigs in the control group developed colonic leakage and faecal peritonitis; therefore, this model was considered a high-risk AL animal model. In this study, we placed a modified ileostomy tube with a balloon at its end approximately 20-30 cm proximal to the ileocecal valve. The tube was positioned to ensure that most of the lateral side holes were in the proximal limb to optimize enteric content diversion. The balloon was inflated with normal saline in the lumen to ensure a pressure of 20 kPa; our findings revealed that this pressure did not severely impair the blood supply to the bowel wall. Furthermore, the pressure was adequate to sustain bowel obstruction, facilitating complete diversion of the enteric contents via either a stoma or an ileostomy tube. This, in turn, excluded distal anastomoses and preempted AL occurrence. Consequently, the adapted ileostomy tube has been applied in all high-risk colonic anastomosis procedures, including anus preservation surgeries for patients with low rectal cancer, patients with colonic cancer complicated by severe bowel obstruction, and patients with severe infection due to colon injury[2,3,9].
In general, the placement of a balloon obstruction and diversion of the enteric contents was effective in preventing AL. This method proved to be an effective alternative to colostomy, which is associated with considerable morbidity and mortality. In the clinical setting, the balloon and diverting drain can easily be placed in the proximal intestine using an open surgery or laparoscopy and can effectively help prevent AL.
This study demonstrated that total enteric flow diversion using a modified ileostomy tube with a balloon in pigs effectively prevented colonic AL. Compared with the control group, the diversion group presented no peritonitis or abscess, with successful enteric content diversion at a pressure of 20 kPa. The technique avoids defunctioning stomas, offering a feasible approach to eliminating AL after colorectal surgery.
We sincerely thank Dr. Hong-Liang Yao (The Second Xiangya Hospital) for his surgical expertise and clinical supervision of the study. Additionally, we extend our gratitude to the staff of the experimental animal husbandry room at the Second Xiangya Hospital, Central South University for their significant contributions to the conduct of this research.
| 1. | Wada T, Kawada K, Hirai K, Toda K, Iwamoto M, Hasegawa S, Sakai Y. Enhanced anastomotic healing by Daikenchuto (TJ-100) in rats. Sci Rep. 2018;8:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Messias BA, Botelho RV, Saad SS, Mocchetti ER, Turke KC, Waisberg J. Serum C-reactive protein is a useful marker to exclude anastomotic leakage after colorectal surgery. Sci Rep. 2020;10:1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Rodríguez-Padilla Á, Morales-Martín G, Pérez-Quintero R, Rada-Morgades R, Gómez-Salgado J, Ruiz-Frutos C. Diversion Colitis and Probiotic Stimulation: Effects of Bowel Stimulation Prior to Ileostomy Closure. Front Med (Lausanne). 2021;8:654573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Kimura K, Watanabe J, Suwa Y, Kotake M, Noura S, Suwa H, Tei M, Takano Y, Munakata K, Matoba S, Yamagishi S, Yasui M, Kato T, Ozawa M, Shiozawa M, Ishii Y, Yabuno T, Nitta T, Saito S, Nagata N, Ichikawa D, Hasegawa S, Katsuno G, Takahashi H, Kawai K, Furuhata T, Tonooka T, Kanazawa A, Kuriu Y, Sakamoto K, Kinjo T, Otsuka H, Uemura M, Watanabe T, Ueda K, Ikeda M, Takemasa I; On the behalf of EssentiAL Trial Group. Impact of Low Ligation on Bowel Perfusion and Anastomotic Leakage in Minimally Invasive Rectal Cancer Surgery: A Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum. 2025;68:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Harada T, Numata M, Atsumi Y, Fukuda T, Izukawa S, Suwa Y, Watanabe J, Sato T, Saito A. Risk factors for anastomotic leakage in rectal cancer surgery reflecting current practices. Surg Today. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Hiraki M, Tanaka T, Ikeda O, Sadashima E, Kimura N, Nakamura S, Nakamura H, Yamada K, Okuyama K, Yamaji K, Manabe T, Miyoshi A, Kitahara K, Sato S, Noshiro H. Retrospective Risk Analysis for Anastomotic Leakage Following Laparoscopic Rectal Cancer Surgery in a Single Institute. J Gastrointest Cancer. 2020;51:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 823] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 8. | Yang J, Chen Q, Jindou L, Cheng Y. The influence of anastomotic leakage for rectal cancer oncologic outcome: A systematic review and meta-analysis. J Surg Oncol. 2020;121:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009;96:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Kverneng Hultberg D, Svensson J, Jutesten H, Rutegård J, Matthiessen P, Lydrup ML, Rutegård M. The Impact of Anastomotic Leakage on Long-term Function After Anterior Resection for Rectal Cancer. Dis Colon Rectum. 2020;63:619-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Gastinger I, Marusch F, Steinert R, Wolff S, Koeckerling F, Lippert H; Working Group 'Colon/Rectum Carcinoma'. Protective defunctioning stoma in low anterior resection for rectal carcinoma. Br J Surg. 2005;92:1137-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Biondo S, Kreisler E, Millan M, Fraccalvieri D, Golda T, Martí Ragué J, Salazar R. Differences in patient postoperative and long-term outcomes between obstructive and perforated colonic cancer. Am J Surg. 2008;195:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 13. | Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, Strong SA, Oakley JR. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 1997;185:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 347] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Wang XT, Li L, Kong FB, Zhong XG, Mai W. Surgical-related risk factors associated with anastomotic leakage after resection for rectal cancer: a meta-analysis. Jpn J Clin Oncol. 2020;50:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Biondo S, Parés D, Kreisler E, Ragué JM, Fraccalvieri D, Ruiz AG, Jaurrieta E. Anastomotic dehiscence after resection and primary anastomosis in left-sided colonic emergencies. Dis Colon Rectum. 2005;48:2272-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Slooter MD, Talboom K, Sharabiany S, van Helsdingen CPM, van Dieren S, Ponsioen CY, Nio CY, Consten ECJ, Wijsman JH, Boermeester MA, Derikx JPM, Musters GD, Bemelman WA, Tanis PJ, Hompes R; IMARI-study group. IMARI: multi-Interventional program for prevention and early Management of Anastomotic leakage after low anterior resection in Rectal cancer patIents: rationale and study protocol. BMC Surg. 2020;20:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Bennis M, Parc Y, Lefevre JH, Chafai N, Attal E, Tiret E. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg. 2012;255:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 19. | Park W, Park WC, Kim KY, Lee SY. Efficacy and Safety of Laparoscopic Hartmann Colostomy Reversal. Ann Coloproctol. 2018;34:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Biondo S, Kreisler E, Millan M, Fraccalvieri D, Golda T, Frago R, Miguel B. Impact of surgical specialization on emergency colorectal surgery outcomes. Arch Surg. 2010;145:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Ertürer Oruç E, Albayrak D. Impact of Gentamicin-Impregnated Collagen on the Intra-Abdominal Adhesions and Integrity of Colonic Anastomosis: An Experimental Study. Med Sci Monit. 2021;27:e931959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Edwards DP, Leppington-Clarke A, Sexton R, Heald RJ, Moran BJ. Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. Br J Surg. 2001;88:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Hoeppner J, Crnogorac V, Hopt UT, Weiser HF. The pig as an experimental model for colonic healing study of leakage and ischemia in colonic anastomosis. J Invest Surg. 2009;22:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/