Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.109920
Revised: June 29, 2025
Accepted: September 4, 2025
Published online: October 27, 2025
Processing time: 151 Days and 23.9 Hours
Anastomotic leakage (AL) is a serious and challenging complication following low anterior resection (LAR) for low rectal cancer. This case series presents the successful management of AL in three patients using a combined approach of transanal opening of the intersphincteric space (TROPIS) surgery and adjuvant Kangfuxin liquid enema therapy.
Three male patients underwent laparoscopic LAR with diverting ileostomy for low rectal cancer. Case 1: A 39-year-old, presented with fever and abdominal distension 2 weeks after discharge. A digital rectal examination revealed partial anastomotic separation. Case 2: A 74-year-old, developed abdominal pain and fever on postoperative day 5, with fecal discharge through the pelvic drain, and computed tomography scan confirmed AL. Case 3: A 51-year-old, was asymp
The combination of TROPIS and Kangfuxin enema appears to be a safe and effective approach for managing AL following LAR. This minimally invasive strategy offers a promising alternative to conventional surgical inter
Core Tip: This case series presents a novel, minimally invasive strategy for managing anastomotic leakage following low anterior resection for rectal cancer. By combining the transanal opening of the intersphincteric space technique with Kangfuxin liquid enemas, all three patients achieved complete healing with preserved anal function. This approach specifically targets leaks confined to the intersphincteric space and may serve as an effective adjunct to conventional protective measures, potentially enhancing patient outcomes and accelerating recovery.
- Citation: Li H, Huang HB, Xiang T, Yang L, Furnée EJB, Sun G, Chen WB. Transanal intersphincteric approach combined with Kangfuxin enema for treating anastomotic leakage after low anterior resection: Three case reports. World J Gastrointest Surg 2025; 17(10): 109920
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/109920.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.109920
Rectal cancer is a common malignancy worldwide, with its incidence continuing to rise. In Asia, it accounts for more than 50% of all colorectal cancer cases, with low rectal cancer comprising approximately 70%-80% of these cases[1]. Sphincter-preserving resection has become the preferred surgical approach for patients with low rectal cancer. However, despite the emphasis on standardized and meticulous surgical techniques, postoperative complications related to intestinal anastomosis, such as anastomotic leakage (AL), bleeding, and stenosis, remain unavoidable.
The reported incidence of AL after rectal cancer surgery ranges from 2.4% to 15.9%[2-4]. Although advances in surgical instruments and techniques have improved overall outcomes, the rate of AL following low anterior resection (LAR) for mid and low rectal cancer has not significantly declined. This underscores the ongoing need to prioritize anastomotic safety during surgery.
AL may result in serious complications, including peritonitis and sepsis, and adversely affect patient quality of life by prolonging hospitalization, impairing postoperative anal function, and ultimately worsening treatment outcomes[5,6].
Many strategies have been proposed for managing AL, but their overall effectiveness remains limited[4,7,8]. Tra
In recent years, there has been a growing shift toward less invasive, anastomosis-preserving strategies for AL ma
The transanal opening of the intersphincteric space (TROPIS) procedure is a recently developed surgical technique that may fulfill the need for a sphincter-preserving solution to AL. Originally described by Garg[9] in 2017 for the treatment of high, complex anal fistulas, TROPIS involves making a transanal intersphincteric incision to lay open the fistula tract or abscess cavity, facilitating drainage and promoting healing by secondary intention. Importantly, this technique preserves the external anal sphincter, aiming to resolve sepsis while minimizing the risk of incontinence.
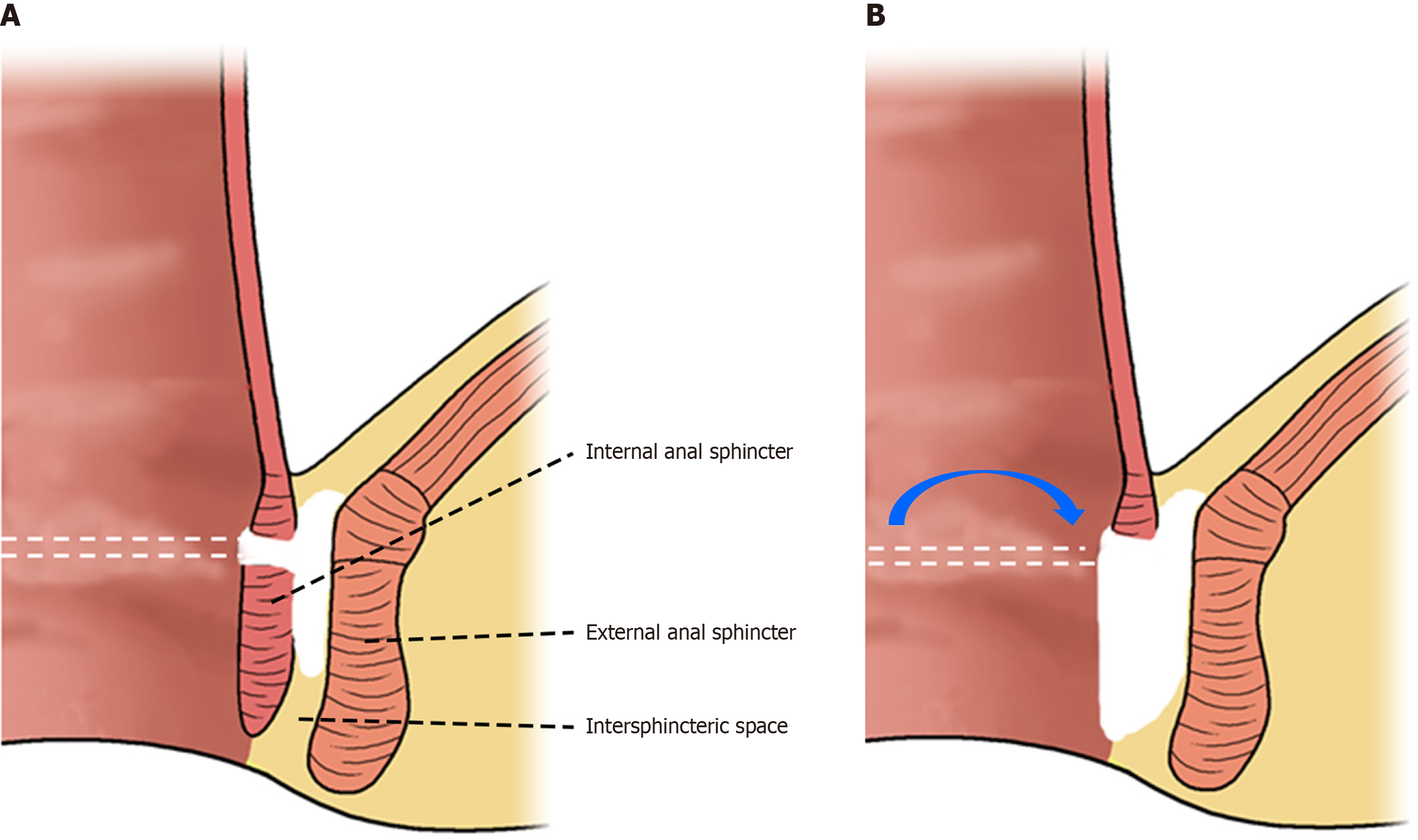
In the setting of complex fistula-in-ano, TROPIS has demonstrated success rates of approximately 80%-90%, with favorable continence outcomes. Based on these features, we hypothesized that a similar transanal, sphincter-sparing approach could be applied to AL of the rectum - essentially unroofing the leak cavity from below to allow effective drainage and healing (Figure 1). When combined with a protective diverting stoma, this strategy may enable anastomosis salvage without the need for major resection[9].
Another innovative component of our approach is the use of Kangfuxin liquid (KFL) enema as adjunctive therapy. KFL is a traditional Chinese medicine extract derived from the Periplaneta americana insect, and is widely recognized for its wound-healing and anti-inflammatory properties[10-12]. In gastrointestinal practice, KFL has been employed topically to promote mucosal repair - for instance, as a retention enema in the management of radiation-induced proctitis and in the healing of peptic ulcers[12-14]. Pharmacological studies suggest that KFL can accelerate tissue repair and regeneration[12-14]. We hypothesized that applying KFL locally to the anastomotic leak cavity, after surgical debridement and drainage via TROPIS, could further promote healing and facilitate closure of the defect.
To date, there is limited literature on combining a surgical intersphincteric approach with pharmacotherapeutic enema therapy for the management of AL. The novel concept behind this strategy is that TROPIS converts a contained anastomotic leak into an open, clean wound, while KFL promotes healing from the inside out. This combined approach may provide an effective treatment for AL that avoids permanent stoma formation and preserves anorectal function.
In this case series, we report the outcomes of 3 patients with AL following LAR for low rectal cancer who were treated with a combination of TROPIS surgery and postoperative KFL retention enema. We describe their clinical courses and healing processes, emphasizing the feasibility, safety, and potential benefits of this novel combined approach for managing rectal AL.
Case 1: The patient, a 39-year-old male, presented with fever and abdominal distension 2 weeks after hospital discharge.
Case 2: On postoperative day 5, the patient, a 74-year-old male, developed abdominal pain and high-grade fever (temperature up to 39.4 °C), accompanied by fecal fluid drainage from the pelvic drain.
Case 3: The patient, a 51-year-old male with low rectal cancer, presented 1 week after discharge at the outpatient clinic, and digital rectal examination revealed that the anastomosis was located 2.5 cm from the anal verge, with a 0.5 cm defect in the anterior wall. The patient was asymptomatic at the time and was managed conservatively. However, after 1 year of follow-up, the anastomotic defect remained unhealed.
Case 1: A 39-year-old male was diagnosed with low rectal cancer and underwent laparoscopic LAR with the creation of a diverting ileostomy. His preoperative body mass index (BMI) was 26.8, and his nutritional status was classified as Nutri-Score level B (score 1). Histopathological examination of the resected specimen revealed a stage IIA tumor [pT3N0M0 according to the 8th edition of American Joint Committee on Cancer (AJCC)] with negative resection margins.
Case 2: A 74-year-old male was diagnosed with low rectal cancer and underwent laparoscopic LAR with a diverting ileostomy. His preoperative BMI was 22.4 and his nutritional score was 2 (Nutri-Score level B). Pathological examination revealed a moderately differentiated adenocarcinoma (AJCC 8th edition: PT3N0M0, stage IIA).
Case 3: A 51-year-old male with low rectal cancer underwent laparoscopic LAR with a diverting ileostomy. His preoperative BMI was 29.4, and his nutritional score was 1 (Nutri-Score level B). He received neoadjuvant radiotherapy prior to surgery (25 fractions, total of 50 Gy). Pathological analysis revealed tumor stage IIA (PT3N0M0, AJCC 8th edition), with negative resection margins.
The three patients had no previous medical history.
Case 1: The patient had a medical history of hypertension, type 2 diabetes mellitus, gout, and hyperlipidemia.
Case 2: The patient had a medical history of hypertension, dilated cardiomyopathy, and chronic heart failure.
Case 3: The patient had no personal and family history.
Case 1: Digital rectal examination at the outpatient clinic revealed the anastomosis located 2 cm from the anal verge, with a 0.5 cm detachment in the left-posterior wall.
Case 2: Digital rectal examination at the outpatient clinic revealed a localized detachment on the left rectal wall.
Case 3: One week after discharge, digital rectal examination at the outpatient clinic revealed that the anastomosis was located 2.5 cm from the anal verge, with a 0.5 cm defect in the anterior rectal wall.
Case 1: Laboratory blood tests demonstrated signs of mild inflammation (white blood cell: 14000/μL; C-reactive protein: 13.75 mg/dL).
Case 2: Laboratory tests showed significantly increased C-reactive protein (108.46 mg/dL).
Case 3: All findings were within normal range.
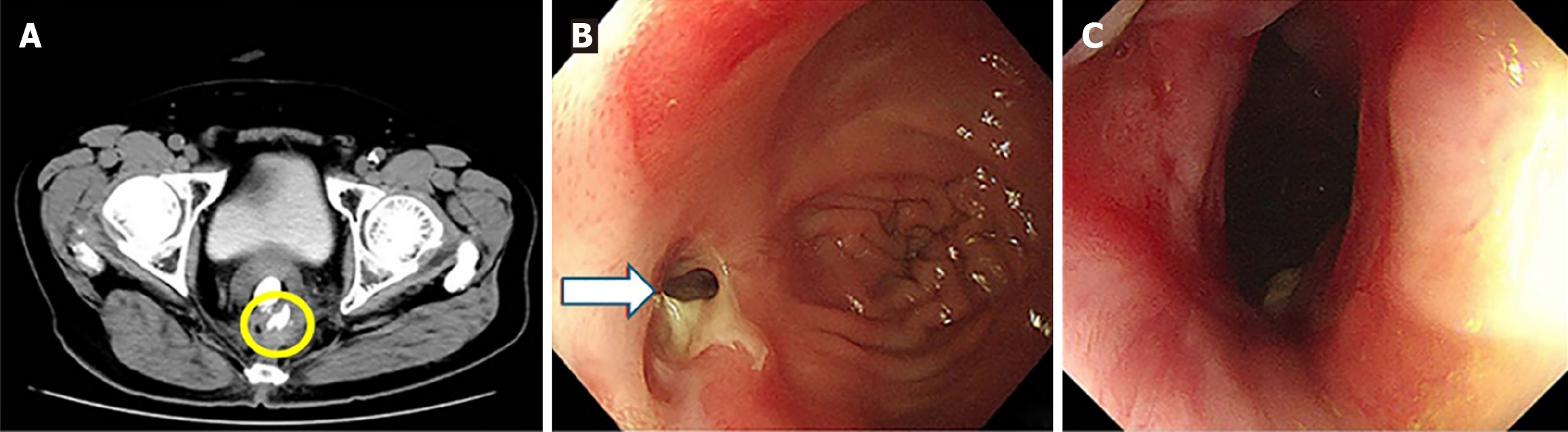
Case 1: Computed tomography (CT) scan revealed extraluminal contrast leakage outside the rectum (Figure 2A). Colonoscopy subsequently revealed a pin-hole defect in the rectal wall at the anastomotic site, confirming the presence of an anastomotic leak (Figure 2B). Following treatment, colonoscopic re-examination revealed an intact anastomosis exhibiting localized scar hyperplasia, with no evidence of mucosal depression or defect (Figure 2C).
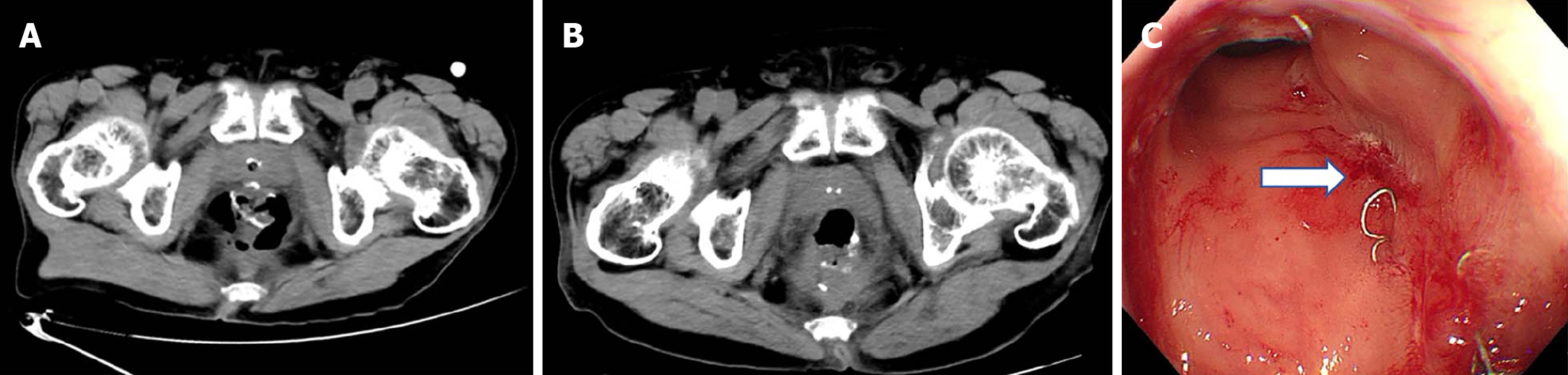
Case 2: Abdominal CT scan revealed a discontinuous anastomosis on the left rectal wall (Figure 3A). A follow-up CT scan post-treatment demonstrated a continuous anastomosis with no evident gap (Figure 3B). Subsequently, colonoscopy confirmed that the fistula had substantially healed (Figure 3C).
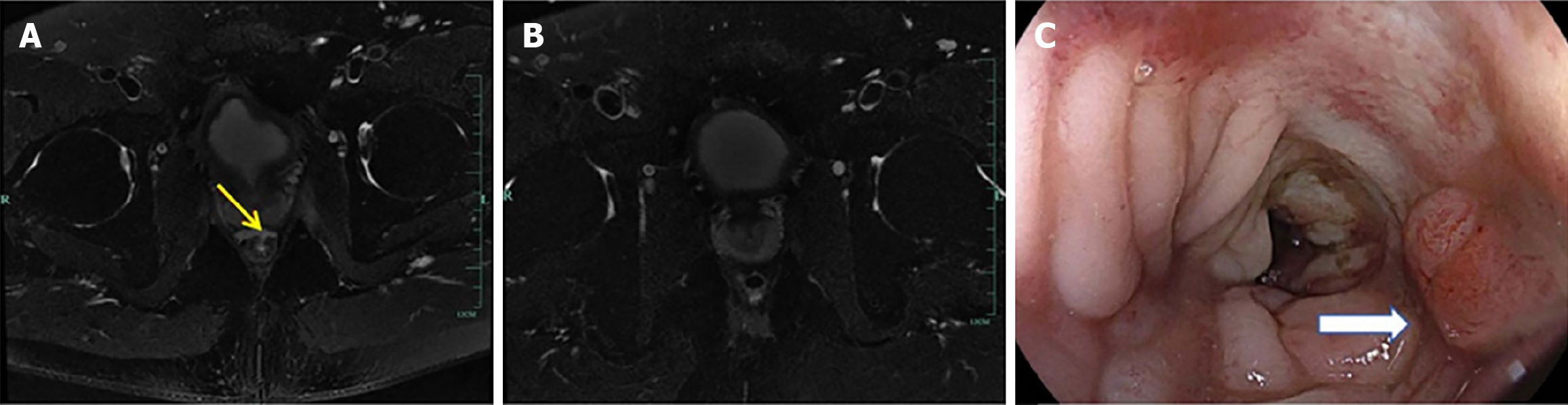
Case 3: After 1 year of follow-up, the anastomotic defect remained unhealed. Pelvic magnetic resonance imaging con
All three patients were diagnosed with AL.
The TROPIS technique is based on the concept of treating infections within the intersphincteric space. Through a transanal approach (Figure 1), the intersphincteric portion of the anal canal is opened from the internal orifice of the anal fistula into the intersphincteric space, creating a large saucer-shaped wound within the anal canal. This facilitates continuous and adequate drainage from the intersphincteric space into the anal canal. Originally developed for fistula surgery, the TROPIS technique aims to open the internal fistula orifice and effectively drain the intersphincteric space transanally (Figure 1).
These procedures were performed at The First Affiliated Hospital of Zhejiang University between January 2022 and December 2024. Written informed consent was obtained from each patient prior to surgery. The TROPIS procedure was performed using neuraxial anesthesia, which was administered either as spinal or epidural. Patients were placed in a prone position with appropriate padding and support to ensure both stability and comfort. The standard surgical instrumentation consisted of an anal speculum, anal dilator, and perianal retractors. Wound closure was achieved with absorbable sutures (3-0 Vicryl). The operative duration typically ranged from 10 minutes to 30 minutes. Postoperative management emphasized a multimodal approach to pain control in order to optimize patient well-being. Open drainage was employed for wound management, and dressings were routinely changed to maintain a clean and dry surgical environment. Early resumption of oral intake and ambulation was encouraged to promote recovery. Patients were instructed to initiate warm saline sitz baths within 24 hours postoperatively, particularly after bowel movements, to improve hygiene and enhance comfort. In addition, stool softeners were recommended to facilitate regular and pain-free bowel movements.
We applied KFL to patients with AL following rectal cancer surgery and achieved favorable outcomes both internally and externally. KFL was administered via rectal retention enemas, leveraging the rich blood supply and loose submucosal tissue of the rectal area. The solution dissolves in rectal secretions and is absorbed through the mucosa, increasing its local bioavailability. Through this mechanism, KFL can promote the healing of AL by suppressing the inflammatory response, increasing antioxidant activity, and stimulating the production of growth factors that support tissue regeneration. A standardized enema protocol was implemented for the administration of KFL as a local adjuvant therapy. Specifically, KFL was diluted with normal saline at a ratio ranging from 1:1 to 1:3 before administration. Each enema delivered 30-100 mL of the diluted solution, and patients were instructed to retain the enema for a minimum of 30 minutes to ensure adequate contact between the therapeutic agent and the target tissue. The treatment schedule involved one to two enemas per day, with the total duration of therapy ranging from 2 weeks to 4 weeks, subject to minor adjustments based on individual wound healing progress. Throughout the treatment period, no absolute contraindications to KFL were identified. The only potential adverse effect observed was mild local irritation, manifested as transient burning, stinging, or urgency to defecate, all of which resolved spontaneously without requiring discontinuation of treatment.
The patient initially received conservative management, including oral antibiotics and once daily KFL enemas; however, the anastomotic defect did not heal. Seven months after LAR, the patient underwent TROPIS surgery, followed by twice-daily KFL enemas postoperatively.
The patient was initially managed with fasting, antibiotics, nutritional support, and pelvic cavity drainage from a pelvic drain. Infection was controlled, and the patient was discharged on postoperative day 19 with the drainage tube in place. Despite this, the anastomotic defect persisted during follow-up. Approximately 3 months after LAR, the patient underwent TROPIS surgery, followed by twice-daily KFL enemas postoperatively.
The patient underwent the TROPIS procedure, followed by twice-daily postoperative KFL liquid enemas.
Two months post-TROPIS, digital rectal examination revealed an intact anastomosis. The patient was asymptomatic, reporting no anal pain, swelling, or discomfort. Colonoscopic reevaluation demonstrated an intact anastomosis with a localized scar but no mucosal depression or defect (Figure 2C). Subsequently, ileostomy reversal was performed to restore bowel continuity.
Approximately two months after surgery, digital rectal examination at the outpatient clinic revealed a healed anastomosis with local scar formation. The patient reported no postoperative anal symptoms. Five months after TROPIS, abdominal CT scan with rectal contrast showed a continuous anastomosis without any evident defects (Figure 3B). Colonoscopy confirmed complete healing of the anastomosis (Figure 3C), and ileostomy reversal was performed. After recovery of anal function, the patient’s Wexner score was 5, indicating mild fecal incontinence.
Follow-up visits showed progressive healing of the anastomotic defect. Digital rectal examination approximately 3 months after TROPIS confirmed an intact anastomosis with localized scar formation. Contrast-enhanced magnetic resonance imaging demonstrated no evidence of leakage (Figure 4B), and colonoscopy revealed a smooth and intact anastomosis (Figure 4C). Ileostomy reversal was subsequently performed to restore bowel continuity.
AL remains a significant complication following mid- to low-rectal cancer surgery. Even with strict adherence to established surgical principles - such as ensuring a tension-free anastomosis, preserving adequate blood supply, and performing thorough bowel preparation - AL may still occur unpredictably, often in the absence of clear risk factors. The onset of AL markedly prolongs hospitalization and places a substantial physiological and psychological burdens on patients[15]. As noted by Xu et al[16] in their 2025 review, AL should be considered a spectrum, ranging from asy
In this study, we report a novel therapeutic approach that combines the TROPIS technique with KFL enemas. This combination treatment was used to manage AL in three patients following rectal cancer surgery. All cases achieved complete healing with preservation of anorectal function, suggesting a promising role for this approach in selected clinical scenarios.
The TROPIS technique allows for effective drainage and debridement of the intersphincteric space without com
KFL, an extract derived from Periplaneta americana, has demonstrated anti-inflammatory and anti-edematous properties in preclinical and clinical studies. Xiao et al[19] reported that KFL reduces tissue swelling, inflammatory exudation, and may also provide analgesic effects. These anti-inflammatory actions are particularly relevant in the context of AL, where an exaggerated inflammatory response can worsen both local tissue damage and systemic morbidity. Preclinical studies have shown of gastrointestinal anastomoses, including in esophageal surgery, where it has been associated with reduced systemic inflammation and enhanced tissue regeneration[4]. Additionally, KFL has demonstrated efficacy in improving gastric mucosal healing and decreasing recurrence of peptic ulcers, likely through mechanisms involving neovascularization, growth factor expression, and suppression of local inflammation[13,14,20].
However, this combined approach should be viewed as complementary to, rather than a replacement for, established management strategies. In our case series, all three patients had a protective diverting ileostomy at the time of leakage. The presence of a stoma likely mitigated the severity of clinical symptoms and created a therapeutic window in which the TROPIS procedure could be safely implemented. In contrast, for patients without a protective stoma, particularly those with severe or generalized leaks, the formation of a diverting loop ileostomy remains the standard of care. As demon
Anatomical factors - especially anastomotic height - play a pivotal role in AL risk. Lower anastomoses, particularly those created after total mesorectal excision, are associated with a substantially higher incidence of leakage, as confirmed by McDermott et al[22] in a comprehensive systematic review. In our practice, protective stomas are routinely constructed for anastomoses ≤ 5 cm from the anal verge. Consistent with this, all three patients in our series experienced low anastomotic leaks confined to the intersphincteric space, a location where the TROPIS technique is both technically feasible and effective.
This study is limited by its small sample size, absence of a control group, and short follow-up period. Although the transanal intersphincteric incision (TROPIS) procedure combined with Kangfuxin solution enema shows therapeutic potential for managing AL following LAR, its successful application depends on several critical factors: Contraindications, appropriate patient selection, cost-effectiveness, and surgical expertise. Three primary contraindications have been identified. First, the technique is not suitable for patients with anastomotic sites located more than 6 cm above the anal verge, due to the anatomical limitations of transanal access. Second, patients with systemic instability (e.g., septic shock) or coagulopathy should be excluded to minimize procedural risks. Third, individuals with a documented history of hypersensitivity to Kangfuxin solution must avoid this regimen to prevent potential local or systemic allergic reactions.
Compared to conventional conservative management, which typically involves prolonged hospitalization (often 4-6 weeks), high costs associated with extended antibiotic use, and a 30%-40% reoperation rate, the TROPIS-Kangfuxin protocol has demonstrated significant cost-saving potential. When effectively implemented, this integrated approach can reduce hospital stays to 2-3 weeks, eliminate the need for reoperation in responsive cases, and decrease antibiotic consumption - thereby lowering overall healthcare expenditures. Given that general surgeons often lack extensive experience with the anatomical structures of the intersphincteric plane, the adoption of the TROPIS procedure typically requires approximately 20 supervised cases to achieve technical proficiency. Proficiency is defined as consistent operative durations under 30 minutes and a procedural success rate of at least 90% in achieving effective drainage and anastomotic reinforcement. These outcomes align with findings reported in other studies on transanal minimally invasive surgery, underscoring the importance of structured training for safe and widespread clinical adoption.
Additionally, the effect of this approach on associated complications - such as organ-space surgical site infections or the need for further interventions - requires validation in larger cohorts. Future prospective studies, ideally with stratification based on anastomotic level and clinical severity, will be essential to determine the optimal indications, timing, and long-term outcomes of combination TROPIS plus KFL therapy in the management of AL.
This case series highlights a novel therapeutic approach combining the TROPIS technique with KFL enemas for managing AL following LAR in rectal cancer patients. The favorable outcomes observed - complete healing and preservation of anal function - suggest that this minimally invasive strategy may be a promising adjunct in selected cases. By facilitating effective drainage and reducing inflammation, the combined method offers a potential alternative to more invasive surgical interventions, with implications for improved recovery and quality of life. Further prospective studies are necessary to validate these preliminary results and to develop standardized protocols for broader clinical application.
| 1. | Yang Y, Wang HY, Chen YK, Chen JJ, Song C, Gu J. Current status of surgical treatment of rectal cancer in China. Chin Med J (Engl). 2020;133:2703-2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Yamamoto S, Fujita S, Akasu T, Inada R, Moriya Y, Yamamoto S. Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech. 2012;22:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Kawada K, Hasegawa S, Hida K, Hirai K, Okoshi K, Nomura A, Kawamura J, Nagayama S, Sakai Y. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28:2988-2995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 348] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 5. | Arron MNN, Custers JAE, van Goor H, van Duijnhoven FJB, Kampman E, Kouwenhoven EA, de Wilt JHW, Kok DE. The association between anastomotic leakage and health-related quality of life after colorectal cancer surgery. Colorectal Dis. 2023;25:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 6. | Jutesten H, Buchwald PL, Angenete E, Rutegård M, Lydrup ML. High Risk of Low Anterior Resection Syndrome in Long-term Follow-up After Anastomotic Leakage in Anterior Resection for Rectal Cancer. Dis Colon Rectum. 2022;65:1264-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Sun G, Zhang H, Furnee E, Liu Q, Gong H, Sun P, Zhang W. Endoscopic closure of a postoperative rectal anastomotic leakage with hemoclips: A case report. Int J Surg Case Rep. 2021;80:105525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Talboom K, van Kesteren J, Sonneveld DJA, Tanis PJ, Bemelman WA, Hompes R. Early transanal closure after vacuum-assisted drainage for anastomotic leakage in rectal cancer surgery - a video vignette. Colorectal Dis. 2020;22:973-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Garg P. Transanal opening of intersphincteric space (TROPIS) - A new procedure to treat high complex anal fistula. Int J Surg. 2017;40:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Cao RQ. [Pharmacological effects and clinical applications of Kangfuxin liquid]. Linchuang Yixue Shijian. 2022;31:682-684. [DOI] [Full Text] |
| 11. | Li CG, Liu XX, Dai LM, Liu XJ, Ma J, Li ZH, Wei YH, Gu F, Chen ZH, Jiang YJ. [The effect of traditional Chinese medicine Neibu Huangqi Decoction combined with Kangfuxin Liquid on wound healing after hemorrhoid and fistula surgery]. Guoji Zhongyi Zhongyao Zazhi. 2023;45:973-976. [DOI] [Full Text] |
| 12. | Guo YH. [Preventive effect of Kangfuxin liquid on perineal skin and mucosa injury in patients with cervical cancer after radiotherapy]. Zhongguo Xiandai Yixue Zazhi. 2017;19:70-71. [DOI] [Full Text] |
| 13. | Zou JB, Zhang XF, Shi YJ, Tai J, Wang Y, Liang YL, Wang F, Cheng JX, Wang J, Guo DY. Therapeutic Efficacy of Kangfuxin Liquid Combined with PPIs in Gastric Ulcer. Evid Based Complement Alternat Med. 2019;2019:1324969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Tian M, Dong J, Wang Z, Lu S, Geng F. The effects and mechanism of Kangfuxin on improving healing quality and preventing recurrence of gastric ulcer. Biomed Pharmacother. 2021;138:111513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Gooszen JAH, Goense L, Gisbertz SS, Ruurda JP, van Hillegersberg R, van Berge Henegouwen MI. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg. 2018;105:552-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Xu V, La K, Ma R, Solis-Pazmino P, Smiley A, Barnajian M, Ellenhorn J, Bergamaschi R, Nasseri Y. Short-term outcomes of low anterior resection with and without ileostomy for low, mid and upper rectal cancers. Updates Surg. 2025;77:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Zhou ZY, Hu B, Liu DC, Peng H, Xie SK, Su D, Ren DL. Clinical Significance of 2 Deep Posterior Perianal Spaces to Complex Cryptoglandular Fistulas. Dis Colon Rectum. 2016;59:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Sugrue J, Nordenstam J, Abcarian H, Bartholomew A, Schwartz JL, Mellgren A, Tozer PJ. Pathogenesis and persistence of cryptoglandular anal fistula: a systematic review. Tech Coloproctol. 2017;21:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Xiao XQ, Wang SP, Xu SR, Liu XQ, Luo C, Wu SJ, Zeng SH. [Experimental study on the anti-inflammatory and analgesic effects of Periplaneta Americana extract]. Zhongguo Bingyuan Shengwuxue Zazhi. 2007;2:140-143. [DOI] [Full Text] |
| 20. | Wang J, Zhong L, Bo Y, Luo N, Hao P. Pharmaceutical preparations of Periplaneta americana (KangFuXin liquid) in the treatment of pressure ulcer: A meta-analysis. Int Wound J. 2023;20:2855-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 629] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/