Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.109700
Revised: July 30, 2025
Accepted: August 21, 2025
Published online: October 27, 2025
Processing time: 121 Days and 24 Hours
Gastric cancer is a malignant tumor with high morbidity and mortality world
To identify and analyze the predictive factors associated with achieving pCR after NAC in gastric cancer patients, thereby providing evidence-based guidance for clinical decision-making.
A retrospective analysis was performed on 215 patients from Shandong Cancer Hospital and Tai’an Central Hospital with locally advanced gastric cancer who underwent NAC followed by radical surgery at our hospital between January 2015 and December 2023. Comprehensive clinical and pathological data were collected, including age, gender, tumor location, Lauren classification, clinical staging, chemotherapy regimens, number of chemotherapy cycles, and baseline hematological indicators. The baseline hematological indicators included neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio, albumin level, carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9. Univariate and multivariate logistic regression analyses were employed to determine the independent predictive factors for pCR.
Among 215 gastric cancer patients, 41 (19.1%) achieved pCR after NAC. Multivariate analysis identified five independent predictive factors for pCR: Lauren intestinal type [odds ratio (OR) = 3.28], lower clinical T stage (OR = 2.75), CEA decrease ≥ 70% after NAC (OR = 3.42), pre-treatment NLR < 2.5 (OR = 2.13), and ≥ 4 chemotherapy cycles (OR = 2.87). The fluorouracil, leucovorin, oxaliplatin, docetaxel regimen achieved the highest pCR rate (27.5%), and oxaliplatin-containing regimens were superior to cisplatin-containing regimens (22.3% vs 12.7%, P = 0.034). Patients with both low NLR and platelet-to-lymphocyte ratio had the highest pCR rate (33.8%), while those with both high inflammatory markers had the lowest rate (10.7%). Earlier clinical stage disease (cT3N+ vs cT4N+: 28.6% vs 13.0%) and lower lymph node burden were associated with higher pCR rates.
The achievement of pCR after NAC in gastric cancer patients is closely associated with Lauren intestinal type, lower clinical T stage, a significant decrease in CEA after chemotherapy, low pre-treatment NLR, and an adequate number of chemotherapy cycles.
Core Tip: This study included patients with pathologically confirmed gastric adenocarcinoma who received neoadjuvant chemotherapy and completed radical gastrectomy + D2 dissection. Unified tumor regression grade assessment defines pathological complete response as the absence of residual primary tumor and lymph node metastasis. The system collected demographic data, comorbidities, tumor location and clinical stage, Lauren classification, human epidermal growth factor receptor 2/microsatellite instability/programmed death-ligand 1, baseline and dynamic laboratory indicators imaging and endoscopic responses, chemotherapy regimens/cycles/dose intensities and adverse reactions. With pathological complete responses the primary outcome, univariate and multivariate logistic regression were used to screen independent predictors.
- Citation: Bi B, Liu C, Chai J, Duan YM. Retrospective analysis of predictive factors for pathological complete response after neoadjuvant chemotherapy in gastric cancer. World J Gastrointest Surg 2025; 17(10): 109700
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/109700.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.109700
Gastric cancer is one of the malignant tumors with high incidence and mortality rates worldwide, particularly with a high disease burden in East Asia. Despite continuous advances in diagnostic and treatment technologies, the overall prognosis of gastric cancer remains unfavorable, especially for patients with locally advanced disease. Surgery remains the cornerstone of gastric cancer treatment; however, surgery alone has limited efficacy for locally advanced patients, with high risks of recurrence and metastasis[1-3].
Over the past two decades, multimodal treatment strategies have gradually become the mainstream approach for locally advanced gastric cancer. Among these, neoadjuvant chemotherapy (NAC) has demonstrated significant advantages in multiple clinical studies. NAC can not only downstage tumors and improve radical resection rates but also potentially eliminate micrometastases and improve overall patient survival. Key clinical trials such as MAGIC, FNCLCC/FFCD, and FLOT4-AIO have confirmed the survival benefits of NAC in patients with locally advanced gastric cancer[4-6].
Pathological complete response (pCR) refers to the absence of viable tumor cells in the surgical specimen after neoadjuvant treatment. Multiple studies have shown that patients achieving pCR typically have longer disease-free survival and overall survival, making it an important indicator of treatment success and favorable prognosis. However, the incidence of pCR after NAC in gastric cancer patients is relatively low, usually between 10%-30%, and the factors influencing pCR achievement are complex and diverse, not yet fully clarified[7-9].
Previous studies have indicated that tumor biological characteristics (such as histological type, differentiation degree, Lauren classification), clinical stage, pre-treatment inflammatory indicators, tumor marker levels, as well as chemothe
Furthermore, with the advent of precision medicine, searching for biomarkers that can predict treatment response has become a research hotspot. NAC has significant toxic side effects, and some patients may respond poorly to specific regimens, which not only delays the optimal treatment timing but may also increase patient suffering and economic burden. Therefore, accurately identifying patient populations likely to benefit from NAC before treatment is important for clinical decision-making and optimizing resource allocation. Based on this, domestic and international scholars are actively exploring various predictive tools including imaging features, hematological indicators, and molecular biological markers, hoping to establish more precise prediction models to guide clinical practice[14-16].
In recent years, potential value has been shown in emerging biomarkers such as changes in tumor markers after NAC, dynamic changes in inflammatory indicators before and after treatment, and immune microenvironment status in predicting treatment response. In particular, the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), as indicators reflecting systemic inflammation and immune status, have shown the ability to predict treatment response and prognosis in various tumors. However, the predictive value of these indicators in gastric cancer NAC still needs further validation through more large-sample, prospective studies. This study aims to establish a prediction model integrating multi-dimensional information by analyzing the relationship between various clinicopathological factors and pCR, providing more reliable decision-making tools for clinical practice, and ultimately achieving individualized and precise treatment for gastric cancer patients.
This study employed a retrospective cohort study design, collecting clinical data from locally advanced gastric cancer patients who received NAC followed by radical surgery at Shandong Cancer Hospital and Tai’an Central Hospital from January 2015 to December 2023. The study subjects were patients aged 18-75 years, with gastric adenocarcinoma confirmed by gastroscopic biopsy pathology, with preoperative clinical staging of cT3-4N + M0 or cTanyN + M0 (according to the American Joint Committee on Cancer 8th edition tumor-node-metastasis staging system), who received at least 2 cycles of NAC followed by radical gastrectomy (with D2 Lymph node dissection), and had complete clinical data including pre- and post-chemotherapy imaging evaluations, hematological examinations, and pathology reports. Patients were excluded if they had previously received gastric surgery or radiotherapy, had a history of other primary malignant tumors, had severe cardiac, hepatic, or renal dysfunction or other severe systemic diseases, experienced severe adverse reactions during NAC leading to treatment interruption, had incomplete surgical specimen pathology reports, died within 30 days after surgery, or had missing follow-up data. Based on postoperative pathological results, patients were divided into a pCR group and a non-pCR group. pCR was defined as the absence of viable tumor cells in both the primary tumor site and all resected lymph nodes (ypT0N0M0), assessed based on complete pathological examination following standardized D2 Lymph node dissection. All patients underwent D2 Lymph node dissection as per standard surgical protocol, and pathologists performed comprehensive histological examination of all resected lymph nodes to determine the presence of viable tumor cells. The non-pCR group included patients with viable tumor cells found in the primary site and/or any of the resected lymph nodes after surgery. This standardized approach to pCR assessment, involving systematic examination of all harvested lymph nodes rather than selective sampling, ensures the reliability and consistency of pCR classification across all study participants.
All patients received platinum-based (oxaliplatin or cisplatin) NAC regimens, mainly including fluorouracil, leucovorin, oxaliplatin, docetaxel (FLOT), S-1, oxaliplatin (SOX), capecitabine, oxaliplatin (XELOX), or docetaxel, cisplatin, fluorouracil (DCF). The number of chemotherapy cycles was determined based on the patient's clinical response and tolerance, typically ranging from 2 cycles to 6 cycles. After completing NAC, patients underwent reassessment and received radical gastrectomy within 2-4 weeks after chemotherapy completion.
This study collected patients’ demographic data [age, gender, body mass index (BMI)], tumor characteristics [primary site location, Lauren classification, histological differentiation degree, clinical staging (clinical T stage, N stage), pre-treatment hematological indicators NLR, PLR, albumin level, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9)], and other baseline characteristics; it also recorded treatment-related indicators such as NAC regimen, number of chemotherapy cycles, post-chemotherapy imaging response evaluation (RECIST 1.1 criteria), and changes in tumor marker levels before and after chemotherapy; pathological assessment included yp tumor-node-metastasis staging (American Joint Committee on Cancer 8th edition), pCR status (defined as no viable tumor cells found in the primary site and all resected lymph nodes), lymph node metastasis, lymphovascular invasion, and perineural invasion.
SPSS 25.0 and R software version 4.0.5 were used for statistical analysis. Continuous variables were expressed as mean ± SD or median (interquartile range) and compared between groups using t-test or Mann-Whitney U test; categorical variables were expressed as frequency (percentage) and compared using χ2 test or Fisher’s exact test. Univariate and multivariate logistic regression analyses were used to determine independent predictive factors for pCR, with results expressed as odds ratio (OR) and 95% confidence interval (CI). For continuous variables, optimal cutoff values were determined through receiver operating characteristic curve analysis. Based on the independent predictive factors identified by multivariate analysis. All statistical tests were two-sided, with P < 0.05 considered statistically significant.
From January 2015 to December 2023, a total of 215 Locally advanced gastric cancer patients who received NAC followed by radical surgery were included in the study. Among them, 41 patients (19.1%) achieved pCR, while 174 patients (80.9%) did not achieve pCR. There were no significant differences between the two groups in demographic characteristics such as age (median age: 61.3 years in the pCR group vs 60.8 years in the non-pCR group), gender ratio (male: 63.4% in the pCR group vs 65.5% in the non-pCR group), and BMI (mean BMI: 22.4 kg/m2 in the pCR group vs 22.1 kg/m2 in the non-pCR group) (P > 0.05). Regarding tumor characteristics, the proportion of Lauren intestinal type was significantly higher in the pCR group than in the non-pCR group (63.4% vs 31.6%, P < 0.001), while diffuse type (24.4% in the pCR group vs 46.6% in the non-pCR group) and mixed type (12.2% in the pCR group vs 21.8% in the non-pCR group) were more prevalent in the non-pCR group. Tumor location distribution showed that antral tumors were slightly more common in the pCR group (43.9% vs 38.5%), but the difference was not statistically significant (P = 0.218). Patients in the pCR group had significantly lower clinical T and N stages than those in the non-pCR group, with cT3 stage patients accounting for 53.7% in the pCR group compared to 34.5% in the non-pCR group (P = 0.022); cN1 stage patients accounted for 48.8% in the pCR group compared to 31.0% in the non-pCR group (P = 0.038, Table 1).
| Characteristics | pCR group (n = 41) | Non-pCR group (n = 174) | P value |
| Demographic characteristics | |||
| Age (years), median | 61.3 | 60.8 | 0.742 |
| Gender | 0.796 | ||
| Male | 26 (63.4) | 114 (65.5) | |
| Female | 15 (36.6) | 60 (34.5) | |
| BMI (kg/m2), mean | 22.4 | 22.1 | 0.631 |
| Tumor characteristics | |||
| Lauren classification | < 0.001 | ||
| Intestinal | 26 (63.4) | 55 (31.6) | |
| Diffuse | 10 (24.4) | 81 (46.6) | |
| Mixed | 5 (12.2) | 38 (21.8) | |
| Tumor location | 0.218 | ||
| Cardia | 10 (24.4) | 46 (26.4) | |
| Body | 12 (29.3) | 57 (32.8) | |
| Antrum | 18 (43.9) | 67 (38.5) | |
| Diffuse | 1 (2.4) | 4 (2.3) | |
| Clinical staging | |||
| Clinical T stage | 0.022 | ||
| cT3 | 22 (53.7) | 60 (34.5) | |
| cT4 | 19 (46.3) | 114 (65.5) | |
| Clinical N stage | 0.038 | ||
| cN1 | 20 (48.8) | 54 (31.0) | |
| cN2 | 14 (34.1) | 70 (40.2) | |
| cN3 | 7 (17.1) | 50 (28.8) | |
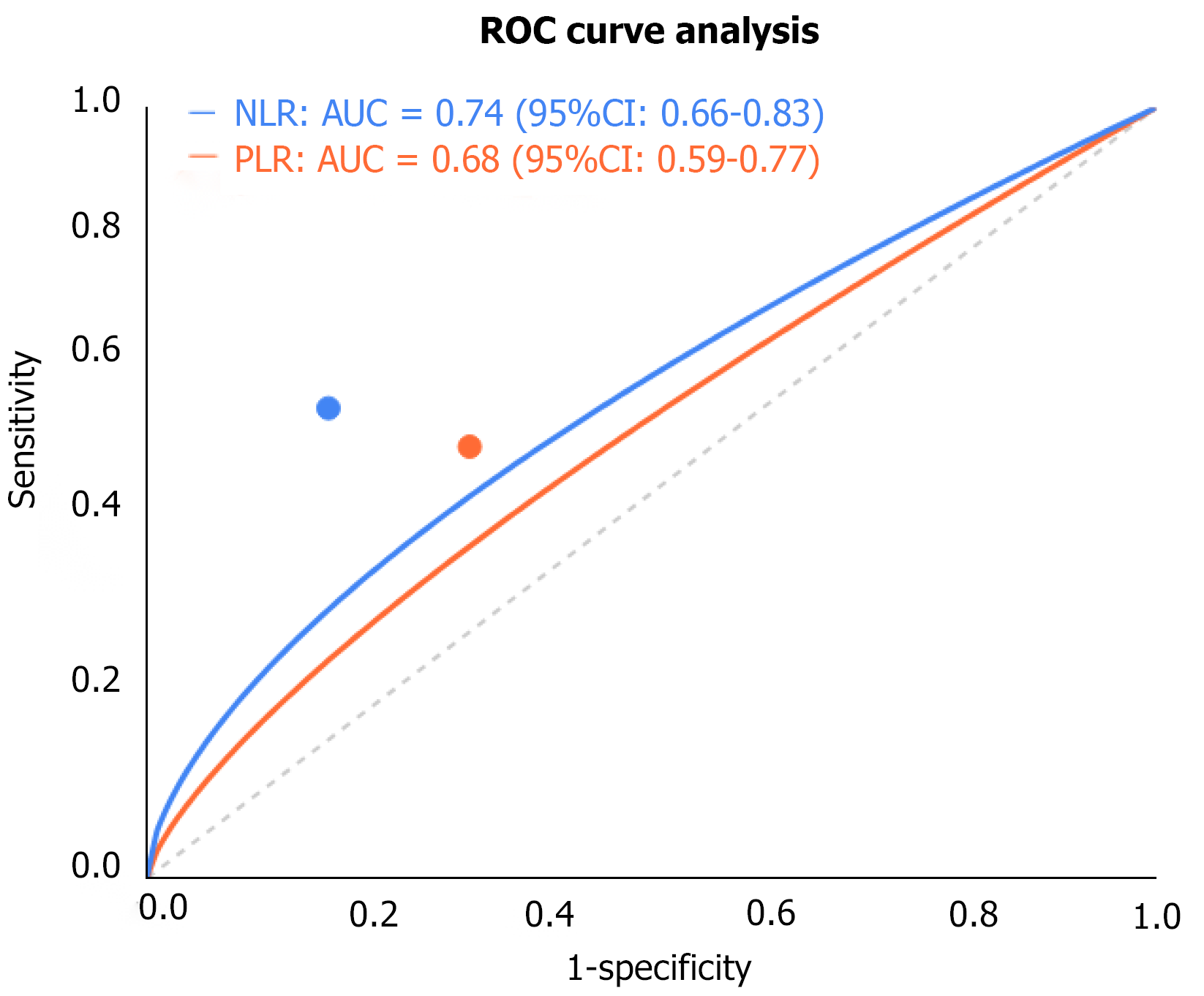
Patients in the pCR group had significantly lower pre-treatment NLR and PLR compared to those in the non-pCR group, with median values of 2.1 vs 3.4 (P = 0.002) and 117.6 vs 156.3 (P = 0.008), respectively. Receiver operating characteristic curve analysis determined the optimal cutoff values for NLR and PLR to be 2.5 and 135, with areas under the curve of 0.74 (95%CI: 0.65-0.83) and 0.68 (95%CI: 0.59-0.77), respectively. Multivariate analysis revealed that NLR < 2.5 (OR = 2.86, 95%CI: 1.54-5.32, P = 0.001) and PLR < 135 (OR = 2.41, 95%CI: 1.28-4.53, P = 0.006) were independent factors predicting pCR. Pre-treatment albumin levels did not differ significantly between the two groups (P = 0.137), but patients with albumin < 35 g/L had significantly lower pCR rates (15.2% vs 32.7%, P = 0.024). Pre-treatment CEA and CA19-9 Levels also showed no significant differences between the two groups, but patients in the pCR group had markedly greater decreases in tumor markers after chemotherapy compared to the non-pCR group (CEA decrease rate: 78.4% vs 48.2%, P = 0.003; CA19-9 decrease rate: 82.1% vs 53.6%, P = 0.001), and tumor marker decrease ≥ 50% was significantly associated with higher pCR rates (OR = 3.14, 95%CI: 1.72-5.76, P < 0.001, Figure 1).
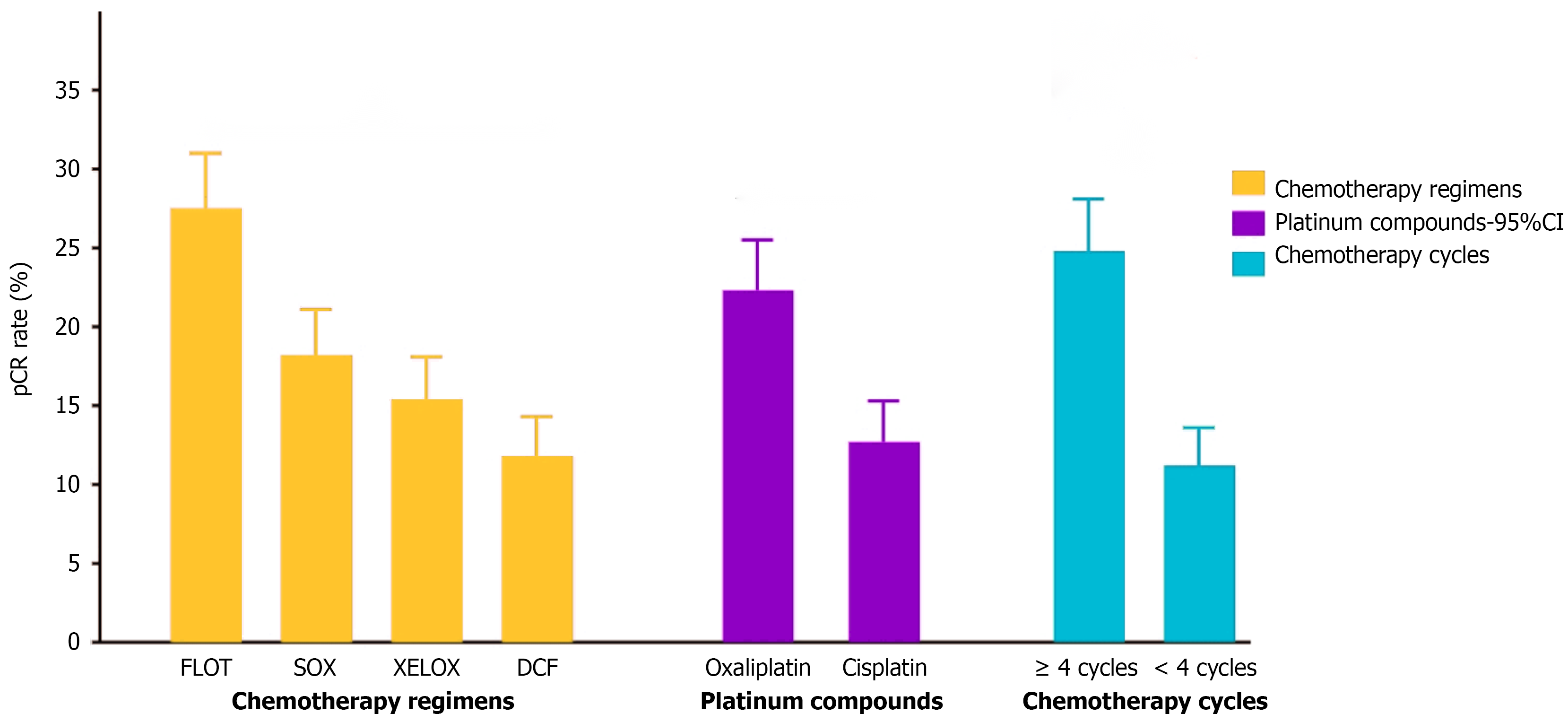
Analysis showed significant differences in pCR rates among different chemotherapy regimens (P = 0.023), with the FLOT regimen showing the best efficacy (27.5%), followed by SOX (18.2%) and XELOX (15.4%) regimens, while the DCF regimen had the lowest pCR rate (11.8%). Comparison of drug combinations revealed that oxaliplatin-containing regimens had significantly higher pCR rates than cisplatin-containing regimens (22.3% vs 12.7%, P = 0.034). The number of chemotherapy cycles was also an important factor affecting pCR, with patients receiving ≥ 4 cycles of chemotherapy showing markedly better pCR rates than those receiving < 4 cycles (24.8% vs 11.2%, P = 0.006). These results suggest that FLOT regimen, oxaliplatin-containing drug combinations, and chemotherapy cycles (≥ 4 cycles) may be key elements for optimizing NAC regimens for gastric cancer (Figure 2).
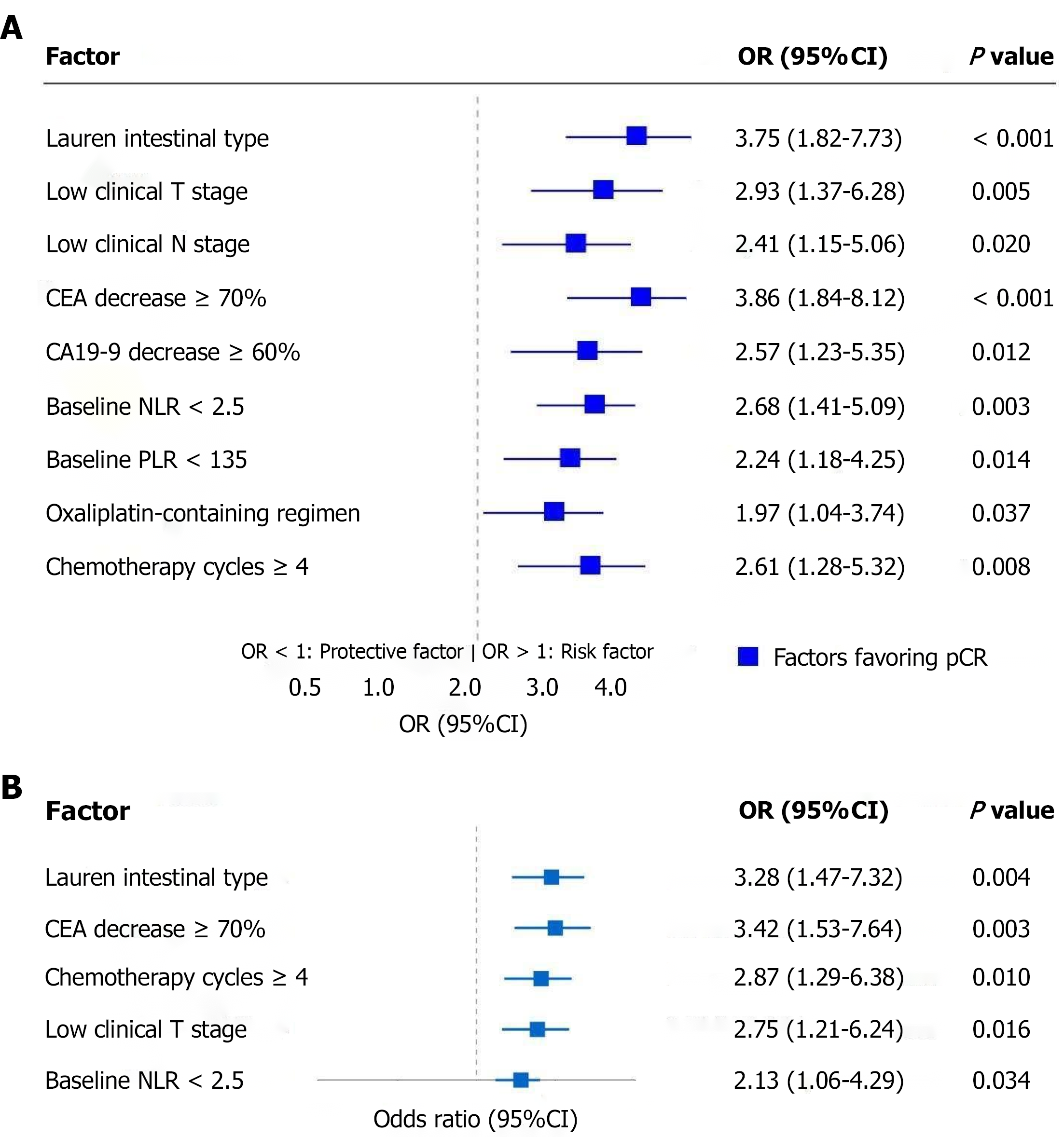
Univariate analysis showed multiple factors significantly associated with pCR. Regarding tumor characteristics, Lauren intestinal type showed the strongest correlation (OR = 3.75, 95%CI: 1.82-7.73, P < 0.001); in terms of clinical staging, low clinical T stage (OR = 2.93, 95%CI: 1.37-6.28, P = 0.005) and low clinical N stage (OR = 2.41, 95%CI: 1.15-5.06, P = 0.020) were both favorable factors for achieving pCR; tumor marker changes showed that CEA decrease ≥ 70% after NAC (OR = 3.86, 95%CI: 1.84-8.12, P < 0.001) and CA19-9 decrease ≥ 60% (OR = 2.57, 95%CI: 1.23-5.35, P = 0.012) both suggested higher pCR probability; among baseline inflammatory indicators, pre-treatment NLR < 2.5 (OR = 2.68, 95%CI: 1.41-5.09, P = 0.003) and PLR < 135 (OR = 2.24, 95%CI: 1.18-4.25, P = 0.014) also showed significant positive correlations with pCR; additionally, treatment regimen characteristics including oxaliplatin-containing chemotherapy regimens (OR = 1.97, 95%CI: 1.04-3.74, P = 0.037) and chemotherapy cycles ≥ 4 (OR = 2.61, 95%CI: 1.28-5.32, P = 0.008) similarly contributed to increased pCR rates (Figure 3A).
Multivariate logistic regression analysis included all variables with P < 0.1 in the univariate analysis, resulting in five factors being identified as independent predictors of achieving pCR. Lauren intestinal type (OR = 3.28, 95%CI: 1.47-7.32, P = 0.004) and CEA decrease ≥ 70% after NAC (OR = 3.42, 95%CI: 1.53-7.64, P = 0.003) demonstrated the strongest predictive power, followed by chemotherapy cycles ≥ 4 (OR = 2.87, 95%CI: 1.29-6.38, P = 0.010) and low clinical T stage (OR = 2.75, 95%CI: 1.21-6.24, P = 0.016), while pre-treatment NLR < 2.5 (OR = 2.13, 95%CI: 1.06-4.29, P = 0.034) was statistically significant but had relatively weaker predictive strength. These findings provide reliable predictive tools for clinical assessment of gastric cancer patients' response to NAC (Figure 3B).
Pre-treatment inflammatory indicator levels showed significant correlation with pCR achievement after NAC in gastric cancer. Research analysis demonstrated that NLR and PLR were both important predictive markers. Specifically, patients with NLR < 2.5 had a pCR rate of 29.6%, significantly higher than patients with NLR ≥ 2.5 (13.4%) (P = 0.003); patients with PLR < 135 had a pCR rate of 26.1%, higher than patients with PLR ≥ 135 (13.9%) (P = 0.017). The interaction between the two inflammatory indicators was even more pronounced, with patients having both low NLR (< 2.5) and low PLR (< 135) showing the highest pCR rate (33.8%), while those with both high NLR (≥ 2.5) and high PLR (≥ 135) showing the lowest pCR rate (10.7%) (P < 0.001), presenting a clear gradient trend; groups with abnormal single indicators showed intermediate pCR rates (low NLR/high PLR group: 22.4%, high NLR/Low PLR group: 16.9%, Table 2).
| Group | NLR | PLR | pCR rate (%) | P value | Clinical significance |
| Individual NLR analysis | |||||
| Low NLR group | < 2.5 | - | 29.6 | 0.003 | Significantly higher pCR |
| High NLR group | ≥ 2.5 | - | 13.4 | - | Lower pCR rate |
| Individual PLR analysis | |||||
| Low PLR group | - | < 135 | 26.1 | 0.017 | Significantly higher pCR |
| High PLR group | - | ≥ 135 | 13.9 | - | Lower pCR rate |
| Combined analysis | |||||
| Optimal group | < 2.5 | < 135 | 33.8 | < 0.001 | Highest pCR rate |
| Single low NLR | < 2.5 | ≥ 135 | 22.4 | - | Intermediate pCR rate |
| Single low PLR | ≥ 2.5 | < 135 | 16.9 | - | Intermediate pCR rate |
| Poor prognosis group | ≥ 2.5 | ≥ 135 | 10.7 | - | Lowest pCR rate |
The choice of NAC regimen and the number of chemotherapy cycles significantly influenced pCR rates in gastric cancer patients. Analysis indicated that oxaliplatin-containing chemotherapy regimens (FLOT, SOX, XELOX) generally performed better than cisplatin-containing regimens (DCF), with pCR rates of 22.3% and 12.7%, respectively (P = 0.034). Among these, the FLOT regimen demonstrated the best efficacy across various cycle numbers, with a pCR rate of 27.5%, while SOX (18.2%) and XELOX (15.4%) regimens were next, and the DCF regimen showed the lowest efficacy (11.8%). The number of chemotherapy cycles was also a key factor affecting pCR achievement, with patients receiving ≥ 4 cycles of chemotherapy having significantly higher pCR rates (24.8%) than those receiving < 4 cycles (11.2%) (P = 0.006), and showing a clear dose-response relationship: The pCR rate was 11.2% in the 2-3 cycle group, rose to 21.6% in the 4-5 cycle group, reached 28.3% in the 6-cycle group, and was 27.9% in the > 6 cycle group, indicating that pCR rates increased with the number of chemotherapy cycles but plateaued after exceeding 6 cycles, suggesting that a reasonable number of NAC cycles should be 4-6 cycles, considering the balance between efficacy and toxicity (Table 3).
| Factor | Category | pCR rate (%) | P value |
| Chemotherapy regimen | Platinum compound type | ||
| Oxaliplatin-containing (FLOT, SOX, XELOX) | 22.3% | 0.034 | |
| Cisplatin-containing (DCF) | 12.7% | - | |
| Specific regimen | 0.023 | ||
| FLOT | 27.5% | ||
| SOX | 18.2% | ||
| XELOX | 15.4% | ||
| DCF | 11.8% | ||
| Chemotherapy cycles | General comparison | ||
| ≥ 4 cycles | 24.8% | 0.006 | |
| < 4 cycles | 11.2% | - | |
| Detailed cycle ranges | - | N/A | |
| 2-3 cycles | 11.2% | ||
| 4-5 cycles | 21.6% | ||
| 6 cycles | 28.3% | ||
| > 6 cycles | 27.9% | ||
Gastric cancer, as one of the most common malignant tumors globally, has a particularly high disease burden in East Asia. Despite advances in diagnostic and treatment technologies, the prognosis for patients with locally advanced gastric cancer remains unfavorable. NAC, as an important component of multimodal treatment strategies, has become a key measure for improving the prognosis of patients with locally advanced gastric cancer by downstaging tumors, increasing radical resection rates, and eliminating micrometastases. pCR is considered the “gold standard” for evaluating the effectiveness of neoadjuvant treatment, and multiple studies have confirmed that patients achieving pCR have significantly longer disease-free survival and overall survival. However, the pCR rate after NAC for gastric cancer is relatively low and influenced by complex factors, posing challenges for clinical decision-making[17-19].
Tumor biological characteristics have important effects on chemosensitivity. Lauren classification, as an important basis for gastric cancer pathological classification, reflects different growth patterns and biological behaviors of tumors. Intestinal-type gastric cancer typically has more regular glandular structures with tighter intercellular adhesion, while diffuse-type presents as infiltration of poorly differentiated individual tumor cells with reduced expression of cell adhesion molecules. These differences may lead to variations in the penetration and sensitivity of chemotherapy drugs between the two types of tumors. Intestinal-type gastric cancer cells usually proliferate more rapidly, while most chemotherapy drugs primarily target rapidly dividing cells, which may explain the greater sensitivity of intestinal-type gastric cancer to chemotherapy[20-22].
Inflammatory responses play complex roles in tumor occurrence, development, and treatment response. NLR and PLR, as markers of systemic inflammation, can reflect the status of the tumor microenvironment. High NLR and PLR may indicate the predominance of pro-tumor inflammatory environments, including increased tumor-associated macro
Tumor burden is a key factor influencing treatment response. Patients with low clinical T and N stages have a lower total number of tumor cells, presenting less resistance to overcome with treatment; additionally, early-stage tumors typically have better blood supply, allowing better penetration of chemotherapy drugs. As tumors progress, hypoxic regions and heterogeneous clones may develop within the tumor, with cells in these regions often being insensitive to chemotherapy or even developing resistance. Furthermore, advanced tumors often have more cancer stem cells, which have intrinsic resistance to conventional chemotherapy[26-28].
Tumor markers have unique value in monitoring treatment response. CEA, as a commonly used serum marker for gastrointestinal tumors, can partially reflect changes in tumor burden through its level changes. Significant decreases in CEA after chemotherapy suggest effective tumor cell killing, which is closely related to pathological response. Dynamic changes in markers have more clinical significance than single measurements, providing early information about tumor response to treatment and helping to adjust treatment strategies in a timely manner[29-31].
The choice of chemotherapy regimen and dosing intensity are equally crucial. This study found that oxaliplatin-containing regimens (especially FLOT) were superior to cisplatin-containing regimens, which may be related to the different mechanisms of action and pharmacokinetic properties of oxaliplatin and cisplatin, with the former having stronger cytotoxic effects and relatively more manageable adverse reactions. The number of chemotherapy cycles re
This retrospective study has several limitations including inherent selection bias, relatively small sample size (n = 215), and potential confounding factors that limit causal inference. The analysis lacks modern molecular biomarkers (microsatellite instability, programmed death-ligand 1, human epidermal growth factor receptor 2) and inadequately controls for important clinical variables such as performance status and comorbidities. The single-country cohort and extended study period (2015-2023) may limit generalizability due to population differences and evolving treatment protocols. Future prospective, multi-center studies with larger cohorts, comprehensive biomarker analysis, and long-term survival data are needed to validate these predictive factors.
These findings help identify patient populations most likely to benefit from NAC, providing a basis for clinical decision-making. For example, NAC can be actively recommended for patients with multiple favorable factors; while for patients expected to benefit little, direct surgery or adjusted treatment strategies can be considered.
| 1. | Cann C, Ciombor KK. Systemic therapy for gastric cancer: Perioperative strategies and beyond. J Surg Oncol. 2022;125:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Cosma LS, Schlosser S, Tews HC, Müller M, Kandulski A. Hereditary Diffuse Gastric Cancer: Molecular Genetics, Biological Mechanisms and Current Therapeutic Approaches. Int J Mol Sci. 2022;23:7821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 3. | Huang RJ, Hwang JH. Improving the Early Diagnosis of Gastric Cancer. Gastrointest Endosc Clin N Am. 2021;31:503-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Li GZ, Doherty GM, Wang J. Surgical Management of Gastric Cancer: A Review. JAMA Surg. 2022;157:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 196] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 239] [Reference Citation Analysis (3)] |
| 6. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 916] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 7. | André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, Jary M, Tournigand C, Aparicio T, Desrame J, Lièvre A, Garcia-Larnicol ML, Pudlarz T, Cohen R, Memmi S, Vernerey D, Henriques J, Lefevre JH, Svrcek M. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol. 2023;41:255-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 294] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 8. | Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, Barnes JA, O'Brien SM, Chávez JC, Duell J, Rosenwald A, Crombie JL, Ufkin M, Li J, Zhu M, Ambati SR, Chaudhry A, Lowy I, Topp MS. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9:e327-e339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 9. | Chen YH, Xiao J, Chen XJ, Wang HS, Liu D, Xiang J, Peng JS. Nomogram for predicting pathological complete response to neoadjuvant chemotherapy in patients with advanced gastric cancer. World J Gastroenterol. 2020;26:2427-2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Fonseca T, Coimbra M, Barbosa E, Barbosa J. Gastric cancer: histological response of tumor and metastatic lymph nodes for perioperative chemotherapy. Cir Cir. 2022;90:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Hunzeker ZE, Bhakta P, Gudipally SR, Kavuri SB, Venkatesan R, Nwanze C. Complete Response of High Microsatellite Instability Gastric Cancer and Synchronous Microsatellite Stability Rectal Cancer. Cureus. 2022;14:e25820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 522] [Article Influence: 104.4] [Reference Citation Analysis (1)] |
| 13. | Sakaue M, Sugimura K, Masuzawa T, Takeno A, Katsuyama S, Shinnke G, Ikeshima R, Kawai K, Hiraki M, Katsura Y, Ohmura Y, Hata T, Takeda Y, Murata K. Long-term survival of HER2 positive gastric cancer patient with multiple liver metastases who obtained pathological complete response after systemic chemotherapy: A case report. Int J Surg Case Rep. 2022;94:107097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Tang X, Li M, Wu X, Guo T, Zhang L, Tang L, Jia F, Hu Y, Zhang Y, Xing X, Shan F, Gao X, Li Z. Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer. Oncoimmunology. 2022;11:2135819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 15. | Wada T, Yoshikawa T, Sekine S, Kamiya A, Hayashi T, Otsuki S, Yamagata Y, Katai H. Pathological complete response at the para-aortic nodes as a possible surrogate endpoint in gastric cancer surgery with para-aortic node dissection after neoadjuvant chemotherapy. Eur J Surg Oncol. 2022;48:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Wang ZL, Li YL, Li XT, Tang L, Li ZY, Sun YS. Role of CT in the prediction of pathological complete response in gastric cancer after neoadjuvant chemotherapy. Abdom Radiol (NY). 2021;46:3011-3018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Zhang SX, Liu W, Ai B, Sun LL, Chen ZS, Lin LZ. Current Advances and Outlook in Gastric Cancer Chemoresistance: A Review. Recent Pat Anticancer Drug Discov. 2022;17:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol. 2022;86:566-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (1)] |
| 19. | Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. J Surg Oncol. 2022;125:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 20. | Bayram E, Kidi MM, Camadan YA, Biter S, Yaslikaya S, Toyran T, Mete B, Kara IO, Sahin B. Can the Pathological Response in Patients with Locally Advanced Gastric Cancer Receiving Neoadjuvant Treatment Be Predicted by the CEA/Albumin and CRP/Albumin Ratios? J Clin Med. 2024;13:2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Biondi A, Lorenzon L, Santoro G, Agnes A, Laurino A, Persiani R, D'Ugo D. Profiling complete regression after pre-operative therapy in gastric cancer patients using clinical and pathological data. Eur J Surg Oncol. 2023;49:106969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Botta GP, Chao J, Ma H, Hahn M, Sierra G, Jia J, Hendrix AY, Nolte Fong JV, Ween A, Vu P, Miller A, Choi M, Heyman B, Daniels GA, Kaufman D, Jamieson C, Li Z, Cohen E. Metastatic gastric cancer target lesion complete response with Claudin18.2-CAR T cells. J Immunother Cancer. 2024;12:e007927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 23. | Chen Y, He J, Zheng J, Lin Y, Wang H, Lian L, Peng J. Impact of pathological complete response on survival in gastric cancer after neoadjuvant chemotherapy: a propensity score matching analysis. BMC Gastroenterol. 2025;25:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Chen Y, Liu D, Wei K, Lin Y, Wang Z, Sun Q, Wang H, Peng J, Lian L. Carcinoembryonic antigen trajectory predicts pathological complete response in advanced gastric cancer after neoadjuvant chemotherapy. Front Oncol. 2025;15:1525324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Guo F, Xiang X, Huang Y, Chen A, Ma L, Zhu X, Abdulla Z, Jiang W, Li J, Li G. Long-term survival outcome of locally advanced gastric cancer patients who achieved a pathological complete response to neoadjuvant chemotherapy. Int J Clin Oncol. 2023;28:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Jun C, Yamauchi S, Yube Y, Egawa H, Yoshimoto Y, Kubota A, Tsuda K, Kaji S, Orita H, Oka S, Mine S, Fukunaga T. Pathological complete response with nivolumab for recurrence of liver metastasis after gastrectomy of gastric cancer. Surg Case Rep. 2023;9:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Katsumata K, Morimoto Y, Aoyama J, Yamada T, Katsuki Y, Nishiyama R, Egawa T. Conversion surgery for gastric remnant cancer with liver metastasis after nivolumab combination chemotherapy achieving pathological complete response: a case report and literature review. Surg Case Rep. 2024;10:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Leong T, Smithers BM, Michael M, Haustermans K, Wong R, Gebski V, O'Connell RL, Zalcberg J, Boussioutas A, Findlay M, Willis D, Moore A, Murray WK, Lordick F, O'Callaghan C, Swallow C, Darling G, Miller D, Strickland A, Liberman M, Mineur L, Simes J; Australasian Gastro-Intestinal Trials Group, National Health and Medical Research Council Clinical Trials Centre, Trans-Tasman Radiation Oncology Group, European Organisation for Research and Treatment of Cancer, and Canadian Cancer Trials Group. Preoperative Chemoradiotherapy for Resectable Gastric Cancer. N Engl J Med. 2024;391:1810-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 29. | Li C, Tian Y, Zheng Y, Yuan F, Shi Z, Yang L, Chen H, Jiang L, Wang X, Zhao P, Zhang B, Wang Z, Zhao Q, Dong J, Lian C, Xu S, Zhang A, Zheng Z, Wang K, Dang C, Wu D, Chen J, Xue Y, Liang B, Cheng X, Wang Q, Chen L, Xia T, Liu H, Xu D, Zhuang J, Wu T, Zhao X, Wu W, Wang H, Peng J, Hou Z, Zheng R, Chen Y, Yin K, Zhu Z. Pathologic Response of Phase III Study: Perioperative Camrelizumab Plus Rivoceranib and Chemotherapy Versus Chemotherapy for Locally Advanced Gastric Cancer (DRAGON IV/CAP 05). J Clin Oncol. 2025;43:464-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 30. | Li L, Chen G, Chen EY, Strickland MR, Zhao W, Zhang J, Li Z. Development and validation of a nomogram to predict pathological complete response in patients with locally advanced gastric adenocarcinoma treated with neoadjuvant chemotherapy in combination with PD-1 antibodies. J Gastrointest Oncol. 2023;14:2373-2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, Wei M, Zhou W, Wang J, Zhao Z, Dai X, Xu Q, Zhang X, Huang M, Huang K, Wang J, Li J, Sheng L, Liu L. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (1)] |
| 32. | Saltalamacchia G, Bernardo A, Quaquarini E. Prognostic Role of Pathological Complete Response in Early Stage Epithelial Solid Tumors. Cancer Control. 2023;30:10732748231161466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Sandø AD, Fougner R, Røyset ES, Dai HY, Grønbech JE, Bringeland EA. Response Evaluation after Neoadjuvant Chemotherapy for Resectable Gastric Cancer. Cancers (Basel). 2023;15:2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/