Published online Sep 27, 2024. doi: 10.4240/wjgs.v16.i9.3032
Revised: July 17, 2024
Accepted: July 22, 2024
Published online: September 27, 2024
Processing time: 205 Days and 6.5 Hours
Through continuous improvement in transplantation medicine, a wider range of solid organ transplant (SOT) recipients is considered suitable for complex procedures. Despite advances in modern transplantation practice, transpiring invasive fungal infections pose a substantial threat for SOT recipients. To our knowledge, cryptococcal infection confined amidst sole pancreas SOT recipients has not been described to date. Enforcement of a multidisciplinary transplant team approach in the management of pancreas SOT recipients presenting with complex cryptococcal complications is fundamental in improving patient outcomes.
We present the case of a female pancreas transplant recipient, with confirmed meningeal cryptococcosis, referred to our institution for further evaluation and treatment from the Regional Center for Infectious Diseases. On admission, the patient was weaned from the protocolized immunosuppression therapy for two consecutive weeks, in addition to tapering systemic corticosteroid remedial treatment. Our novel multidisciplinary transplant team approach embodied exhaustive discussions of possible complex and diverse multiple organ system physiologic and pathologic challenges associated with distinct management strategies in pancreas transplant recipients. Owing to the potentially devastating impact of invasive cryptococcosis in terms of morbidity and mortality, a definitive surgical intervention of pancreas transplant grafectomy was reinforced, as a pathway towards secure access to early meaningful expertise care. The patient was discharged to the Regional Center for Infectious Diseases 2 mo after the admittance further advancing to a clinical improvement.
The precision transplantation approach by tailoring complex medical interventions to individual needs proved indispensable in improving our patient’s outcomes.
Core Tip: The morbidity and mortality arising from invasive cryptococcosis in solid organ transplant recipients are an incentive to establish the lowest possible level of immunosuppression necessary to maintain stable graft function. There is a paucity of data on optimum management strategies and the absence of standard transplantation protocols for the complex subgroup of pancreas organ transplant recipients with invasive cryptococcosis. This short report demonstrates that the intervention of pancreas transplant grafectomy might be an effective surgical treatment option for pancreas transplant recipients with meningeal cryptococcosis, as a path towards securing good outcomes.
- Citation: Lulic I, Fingler G, Lulic D, Pavicic Saric J, Mikulic D, Filipec Kanizaj T, Goluza E. Meningeal cryptococcosis in a pancreas transplant recipient requiring grafectomy: A case report. World J Gastrointest Surg 2024; 16(9): 3032-3040
- URL: https://www.wjgnet.com/1948-9366/full/v16/i9/3032.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i9.3032
Invasive fungal infections pose considerable challenges in solid organ transplant (SOT) recipients, with up to 8% of them arising as a result of cryptococcosis[1]. Temporal trends in modern transplantation medicine diagnostic and therapeutic option tools have significantly improved SOT recipients’ outcomes after invasive cryptococcosis[2,3]. Additionally, the furtherance of fungal pathogenesis understanding and anticipation of disparate host immune mechanisms coupled with pharmacological advancement in antifungal compounds enabled transplantation teams to tailor complex medical decisions, practices, and interventions to individual SOT recipients’ needs[4].
The most prevalent sites of cryptococcal infection are the lung and central nervous system (CNS) alongside multiple organ infection dissemination in about two-thirds of SOT recipients[5]. Although the majority of cryptococcosis cases occur up to 5 years post-transplantation, owing to transplantation medicine advancement we are witnessing an inclining shift in infection rates later in the post-transplantation period[5].
Within this context, meningeal cryptococcal infection confined amidst sole pancreas SOT recipients has not been described to date, with gaps in standard transplantation protocols and data conditioning on optimum management strategies for this complex subgroup of SOT recipients[6]. Amid contemporary anti-cryptococcosis therapeutic modalities, a cautious reduction of immunosuppression in SOT recipients is of paramount importance, in some instances triggering a detrimental sequence of allograft damage and/or loss[7]. We present the first case of pancreas transplant grafectomy as an effective surgical treatment option for pancreas transplant recipients with meningeal cryptococcosis, in the act of securing good outcomes.
A 61-year-old Caucasian female pancreas transplant recipient with a body mass index of 14.4 kg/m2 and confirmed meningeal cryptococcosis was referred to our institution for further evaluation and treatment from the Regional Center for Infectious Diseases.
During the preceding hospitalization, a computed tomography (CT) scan of the brain described a larger lacunar lesion in the basal ganglia on the right, a small punctiform lesion in the right frontal white matter, and a few smaller hypodensity areas in the right frontal and left temporal white matter. Further, evaluation of the cerebrospinal fluid (CSF) culture showed signs of cryptococcal infection (number of cells 29/mm3, percentage of polynuclear cells 1%, percentage of mononuclear cells 99%, number of erythrocytes 48/mm3, number of fungal elements 160/mm3, morphologically resem
The past medical history revealed arterial hypertension, chronic kidney disease (stage 3b), anemia of chronic disease, malabsorption syndrome, and osteopenia.
Two months before admittance, the patient was treated for bilateral interstitial lung disease within the realm of severe acute respiratory syndrome coronavirus 2 infection. A timeline of the patient's precedent surgical procedures is depicted in Table 1.
| Year | Procedure |
| 2008 | Pancreaticoduodenectomy due to solid pseudopapillary pancreas neoplasm |
| 2009 | Distal pancreatectomy and splenectomy due to insulinoma |
| 2010 | Pancreas transplantation for optimization of glycemic control |
| 2012 | Ventral hernia repair |
| 2017 | Excision of the basal cell carcinoma on the nose |
On initial evaluation, the patient was alert, with a chief complaint of mild headache, hemodynamically stable, afebrile, and having a good urinary output. The Glasgow Coma Scale score was 15 (E4, V5, M6), alongside equal pupils normally reacting to light, and unremarkable brainstem reflexes. Lung auscultation disclosed diminished breath sounds bilaterally. On heart auscultation, a whooshing rasping precordial heart sound was noted. The skin and mucous membranes were pale, and the surrounding painless skin hematoma of the left forehead and hands was validated. No motor or sensory deficits, apart from level one immobility, were prominent during the comprehensive neurological examination.
The extended laboratory findings at admission are presented in Table 2.
| Cell count | |
| White blood count | 14230/mL |
| Platelet count | 14400/mL |
| Hemoglobin | 87 g/L |
| Hematopoietic cell transplant | 0.241 L/L |
| Electrolites | |
| Sodium | 138 mmol/L |
| Potassium | 3.1 mmol/L |
| Chemistry | |
| Urea | 14.7 mmol/L |
| Creatinine | 139 umol/L |
| C-reactive protein | 2.3 mg/L |
| Enzymes | |
| Aspartate aminotransferase | 9 U/L |
| Alanine aminotransferase | 7 U/L |
| Gamma-glutamyl transferase | 22 U/L |
| Alkaline phosphatase | 30 U/L |
| Coagulation profile | |
| Prothrombin time ratio | 0.94 s |
| Activated partial thromboplastin time | 20 s |
| Fasting glucose | 2.3 g/L |
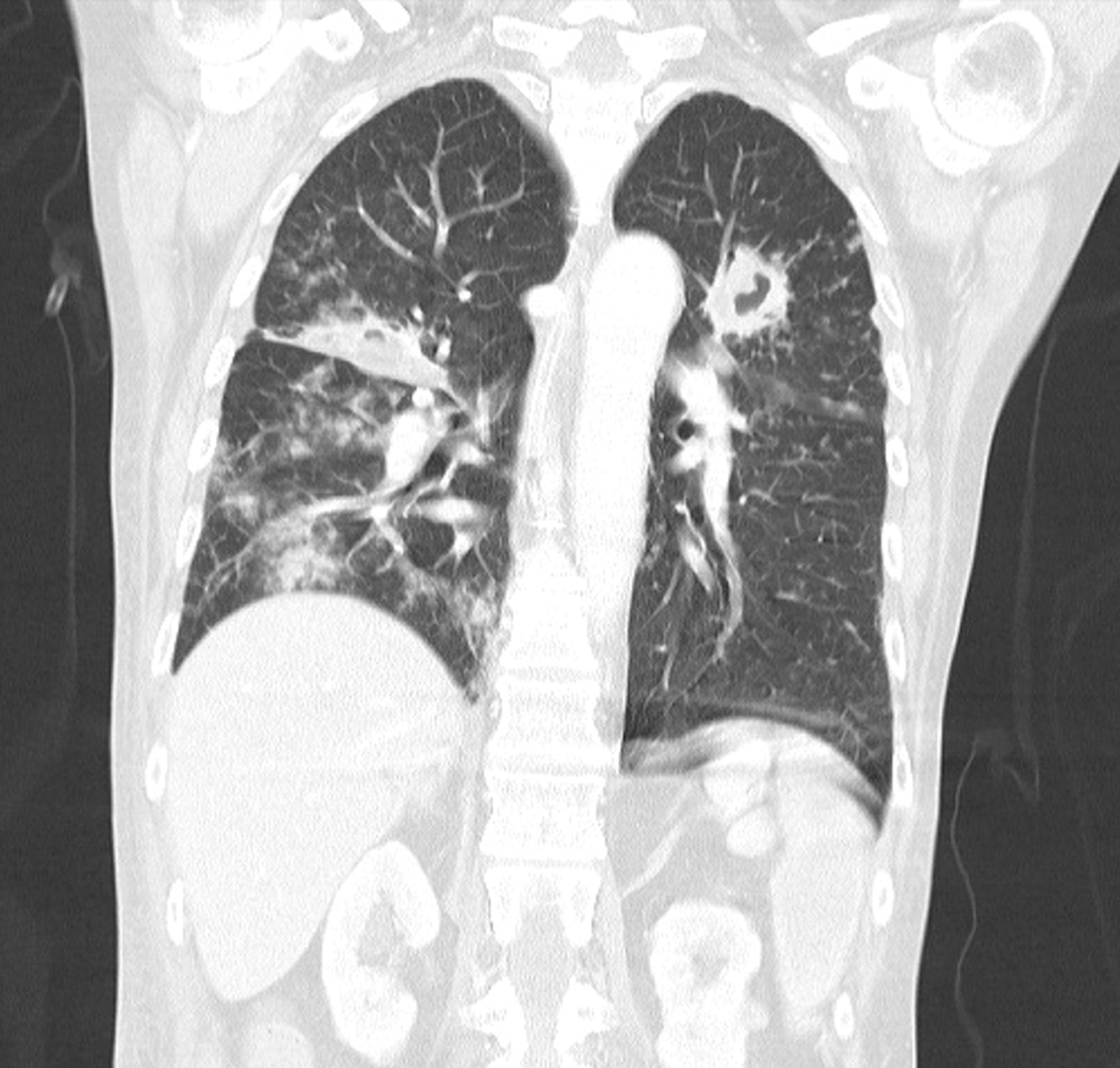
A subsequent CT scan of the chest revealed the progression of the previously described lung infiltrate in the left upper lung field, coupled with a newly formed infiltrate in the projection of the middle right-sided lung field, and bilateral abscesses (Figure 1). The patient refused the recommended bronchoscopy.
Owing to the potentially devastating impact of invasive cryptococcosis in terms of morbidity and mortality, our multidisciplinary transplant team enforced a well-defined surgical plan of pancreas transplant grafectomy.
We enforced a multidisciplinary transplant team approach in the management of our pancreas transplant recipient. The careful consideration of possible complex and diverse multiple organ system physiologic and pathologic challenges, as well as a high probability of immune reconstitution syndrome development (IRIS) because of protocolized immunosuppression therapy withdrawal, were taken into account before the decision on a definitive management strategy.
Five days after the admission, an exhaustive pre-anesthesia evaluation was conducted to assess the pancreas transplant recipient's present medical status, peri-operative risks, and readiness for the planned procedure, to create an anesthetic plan according to the Solid Transplant Unit (STU) SOT anesthetic protocol. The next day, as a premedication the patient was given midazolam (0.25 mg/kg orally) 30 min before coming to the operating room.
Before anesthesia induction, two large-bore peripheral intravenous catheters were inserted. Rapid sequence induction anesthesia protocol was installed with intravenous sufentanyl (slowly titrated up to 5 μg/kg), etomidate (2 mg/kg), and rocuronium (1.2 mg/kg). After endotracheal intubation, an arterial line with invasive arterial monitoring and a central venous line were introduced. Balanced anesthesia was maintained with a mixture of oxygen, air, and sevoflurane, rocuronium for muscle relaxation, and sufentanyl for analgesia. The ventilation was adjusted to maintain normocapnia and the patient remained normotensive during the intra-operative period. Volume replacement included balanced crystalloid solutions and human albumin, with adequate urine output.
The operative procedure of pancreas transplant grafectomy was conducted 6 d after the admission, with the patient positioned supine. The abdominal cavity was accessed through an old median laparotomy mark. Partial omental and inter-intestinal adhesions were separated from the surrounding structures, with vascular structure ligation, to section the transplanted pancreas. Further, the part of the small intestine, to which the anastomosis of the duodenum was sewn, was resected followed by T-T anastomosis formation. After adequate hemostasis, two drains were placed in the abdominal cavity. The pancreas transplant grafectomy procedure was performed smoothly with no damage to the surrounding structures and significant bleeding.
The patient was transferred to the STU intensive care unit (ICU) and was eventually weaned off over the next 2 h. Recommended antifungal therapy (amphotericin B 250 mg/d orally and 5-fluorocytosine 3000 mg/d orally) and antiviral therapy (valganciclovir 450 mg/d orally on alternate days), together with gastroprotection (pantoprazole 40 mg/d intravenously and metoclopramide 10 mg/d intravenously), thromboprophylaxis (enoxaparin sodium 30 mg/d subcutaneously), patient-controlled analgesia (non-steroidal anti-inflammatory drugs and opioid analgesics), and antimicrobial therapy (meropenem 3 g/d), were continued throughout the patient’s ICU stay. Furthermore, systemic synthetic corticosteroid therapy with methylprednisolone (12 mg/d orally) alone was sustained. On the fourth postoperative day, the patient was discharged to the surgical ward.
On the eighth postoperative day, the patient underwent a surgical exploration due to hematoperitoneum, during which no active bleeding site was established, except diffuse bleeding at the pancreas transplant grafectomy site. Following surgical exploration, no postoperative complications were noted and the patient was conscious, orientated, cardiorespiratorily compensated, hemodynamically stable, and afebrile throughout the admission. Further treatment regimen consisted of 7 wk of induction therapy with amphotericin B 250 mg/d orally and 8 wk of consolidation therapy with fluconazole 400 mg/d intravenously. Repeated CSF culture was sterile, and cryptococcal antigen significantly dropped in serum (1:1024) and CSF (1:256). Additionally, CT scans of the brain and chest revealed no significant dynamic changes. The corresponding extended laboratory findings at discharge are presented in Table 3. The patient was discharged to the Regional Center for Infectious Diseases 2 mo after the admittance further advancing to a clinical improvement, without complications or related sequelae at the time of writing. Afterward, the recommended fungal treatment (fluconazole 200 mg/d orally) was continued for one year, with complete suppression of customized immunosuppression therapy.
| Cell count | |
| White blood count | 10000/mL |
| Platelet count | 44600/mL |
| Hemoglobin | 93 g/L |
| Hematopoietic cell transplant | 0.277 l/L |
| Electrolites | |
| Sodium | 136 mmol/L |
| Potassium | 4.3 mmol/L |
| Chemistry | |
| Urea | 8.1 mmol/L |
| Creatinine | 94 μmol/L |
| C-reactive protein | 19.2 mg/L |
| Enzymes | |
| Aspartate aminotransferase | 17 U/L |
| Alanine aminotransferase | 9 U/L |
| Gamma-glutamyl transferase | 49 U/L |
| Alkaline phosphatase | 149 U/L |
| Coagulation profile | |
| Prothrombin time ratio | 1.27 s |
| Activated partial thromboplastin time | 21.3 s |
| Fasting glucose | 3.2 g/L |
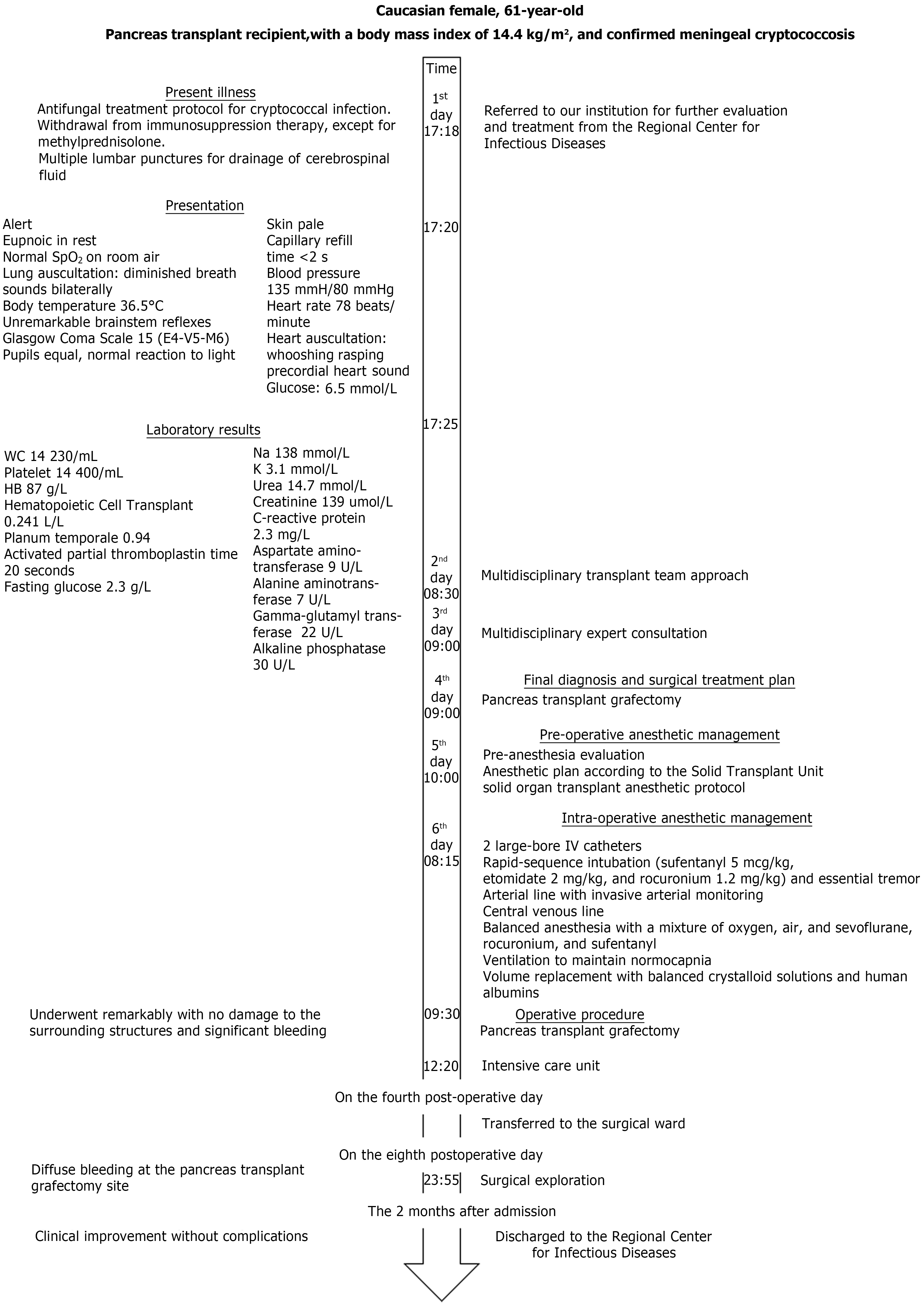
The timeline of diagnoses, laboratory and imaging results, and treatment of the pancreas transplant recipient is described in detail in Figure 2.
The late complication in the pancreas transplant recipient, meningeal cryptococcosis, requiring grafectomy and advancing to a clinical improvement was successfully managed by our institution's multidisciplinary transplant team.
Cryptococcosis is the third most prevalent cause of fungal infections in SOT recipients, mostly presenting in kidney, and to a lesser extent liver, lung, and heart transplant recipients, with overall survival rates of approximately 80%[8,9]. The most common cryptococcal habitats are the lung and CNS, consistent with observed CNS involvement in nearly 50% of SOT recipients[10].
Trends in SOT recipient diversity concerning age, ethnicity, and comorbidities refined our understanding of the impact of cryptococcal pathogenesis on host immune mechanisms and provided new avenues for utilization of disparate prevention tones, as well as breakthroughs in optimal treatment regimens. Over the past years, there has been a renaissance in evidence-based points of view regarding the most appropriate treatment modalities for complex cryptococcal complications among the SOT population[11]. One of the foremost aspects of anti-cryptococcosis therapy in SOT recipients is an adjustment in the immunosuppression therapy regimen.
In current literature, cryptococcal infection confined amidst the sole pancreas SOT population has not been described to date[6]. At the time of admission, our pancreas transplant recipient was cautiously withdrawn from the protocolized enhanced immunosuppression therapy, in addition to tapering systemic synthetic corticosteroid remedial treatment, to elude an IRIS, reportedly manifesting in up to 10% of SOT patients and resulting in higher rates of allograft loss[12,13].
Infectious complications represent the primary cause of morbidity and mortality in pancreas transplant recipients, predominantly bacterial infections, followed by viral and fungal infections. Late diagnosis, regardless of the type of infection, could result in pertinent complications and graft rejection[14]. Expeditious elimination of immunosuppression, as well as reversal of cryptococcal-induced immunosuppression in SOT recipients receiving antifungal therapy, may lead to IRIS and result in allograft dysfunction[15]. Criteria for diagnosis of IRIS in patients with cryptococcosis include new onset or worsening of any of the following clinical manifestations: Clinical or radiographic manifestations of inflammatory process in the CNS, CSF pleocytosis (> 5 white blood cells or increased intracranial pressure), and pulmonary lesions[16]. A significant proportion of SOT recipients with cryptococcosis progress to allograft loss either due to reduced immunosuppression therapy or infection, thus our institution's multidisciplinary transplant team determined pancreas transplant grafectomy as the definitive management strategy, therefore improving the prognosis and survival rate of our patient.
Attributable to the potential brunt of invasive cryptococcosis on the SOT recipient outcomes, it is now standard practice to enforce a multidisciplinary transplant team approach in the management of patients presenting with complex cryptococcal complications.
The key strength in our novel multidisciplinary transplant team approach stands in the interplay between the urgency of decisive surgical intervention of pancreas transplant grafectomy and the potential peri-procedural surgical, anesthetic, and critical care peculiarities, which were tackled by exhaustive analysis and anticipation of possible problems that may resign during the disparate procedural actions. A harmonized precision medical approach by customizing complex medical proceedings to our pancreas transplant recipient’s individual needs, as a passage towards early vital proficiency avenue, proved to be indispensable in improving our pancreas transplant recipient’s outcomes. This was followed by comprehensive treatment-related recommendations, as well as verbal and written instructions on the patient's follow-up.
Future research should focus on ways to improve the sensitivity and specificity of late pancreas transplant recipient complications recognition and management to optimize patient care and ensure appropriate and timely resource utilization in SOT practice.
The multidisciplinary transplant approach to the potentially devastating impact of invasive cryptococcosis in SOT recipients demonstrated that pancreas transplant grafectomy might be an effective surgical treatment option.
| 1. | Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1114] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 2. | Perfect JR, Bicanic T. Cryptococcosis diagnosis and treatment: What do we know now. Fungal Genet Biol. 2015;78:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Singh N, Lortholary O, Alexander BD, Gupta KL, John GT, Pursell KJ, Muñoz P, Klintmalm GB, Stosor V, Del Busto R, Limaye AP, Somani J, Lyon M, Houston S, House AA, Pruett TL, Orloff S, Humar A, Dowdy LA, Garcia-Diaz J, Kalil AC, Fisher RA, Heitman J, Husain S. Antifungal management practices and evolution of infection in organ transplant recipients with cryptococcus neoformans infection. Transplantation. 2005;80:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Baddley JW, Forrest GN; AST Infectious Diseases Community of Practice. Cryptococcosis in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Davis JA, Horn DL, Marr KA, Fishman JA. Central nervous system involvement in cryptococcal infection in individuals after solid organ transplantation or with AIDS. Transpl Infect Dis. 2009;11:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kontoyiannis DP, Lewis RE, Alexander BD, Lortholary O, Dromer F, Gupta KL, John GT, Del Busto R, Klintmalm GB, Somani J, Lyon GM, Pursell K, Stosor V, Munoz P, Limaye AP, Kalil AC, Pruett TL, Garcia-Diaz J, Humar A, Houston S, House AA, Wray D, Orloff S, Dowdy LA, Fisher RA, Heitman J, Albert ND, Wagener MM, Singh N. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob Agents Chemother. 2008;52:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Vilchez RA, Fung J, Kusne S. Cryptococcosis in organ transplant recipients: an overview. Am J Transplant. 2002;2:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | George IA, Santos CAQ, Olsen MA, Powderly WG. Epidemiology of Cryptococcosis and Cryptococcal Meningitis in a Large Retrospective Cohort of Patients After Solid Organ Transplantation. Open Forum Infect Dis. 2017;4:ofx004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Singh N, Lortholary O, Dromer F, Alexander BD, Gupta KL, John GT, del Busto R, Klintmalm GB, Somani J, Lyon GM, Pursell K, Stosor V, Munoz P, Limaye AP, Kalil AC, Pruett TL, Garcia-Diaz J, Humar A, Houston S, House AA, Wray D, Orloff S, Dowdy LA, Fisher RA, Heitman J, Wagener MM, Husain S; Cryptococcal Collaborative Transplant Study Group. Central nervous system cryptococcosis in solid organ transplant recipients: clinical relevance of abnormal neuroimaging findings. Transplantation. 2008;86:647-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Singh N. How I treat cryptococcosis in organ transplant recipients. Transplantation. 2012;93:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, Janoff EN, Bohjanen PR. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202:962-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Sun HY, Alexander BD, Huprikar S, Forrest GN, Bruno D, Lyon GM, Wray D, Johnson LB, Sifri CD, Razonable RR, Morris MI, Stosor V, Wagener MM, Singh N. Predictors of immune reconstitution syndrome in organ transplant recipients with cryptococcosis: implications for the management of immunosuppression. Clin Infect Dis. 2015;60:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Meirelles Júnior RF, Salvalaggio P, Pacheco-Silva A. Pancreas transplantation: review. Einstein (Sao Paulo). 2015;13:305-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Gushiken AC, Saharia KK, Baddley JW. Cryptococcosis. Infect Dis Clin North Am. 2021;35:493-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Baddley JW, Forrest GN; AST Infectious Diseases Community of Practice. Cryptococcosis in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/