Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.357
Peer-review started: November 3, 2023
First decision: December 6, 2023
Revised: December 16, 2023
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: February 27, 2024
Processing time: 113 Days and 21.9 Hours
Gastric cancer (GC) is prevalent and aggressive, especially when patients have distant lung metastases, which often places patients into advanced stages. By identifying prognostic variables for lung metastasis in GC patients, it may be po
To investigate the predictors of GC with lung metastasis (GCLM) to produce nomograms for OS and generate CIP by using cancer-specific survival (CSS) data.
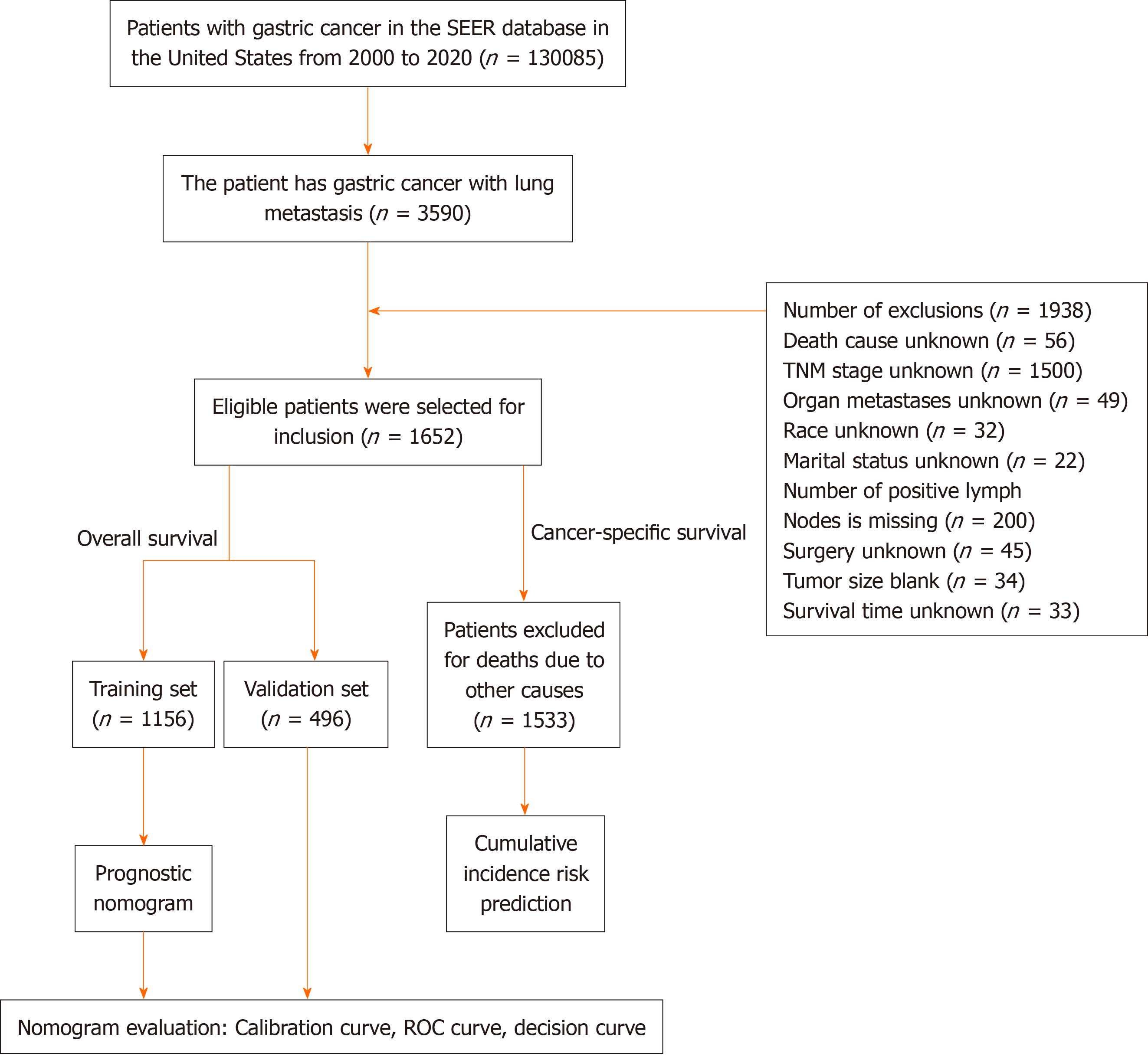
Data from January 2000 to December 2020 involving 1652 patients with GCLM were obtained from the Surveillance, epidemiology, and end results program database. The major observational endpoint was OS; hence, patients were se
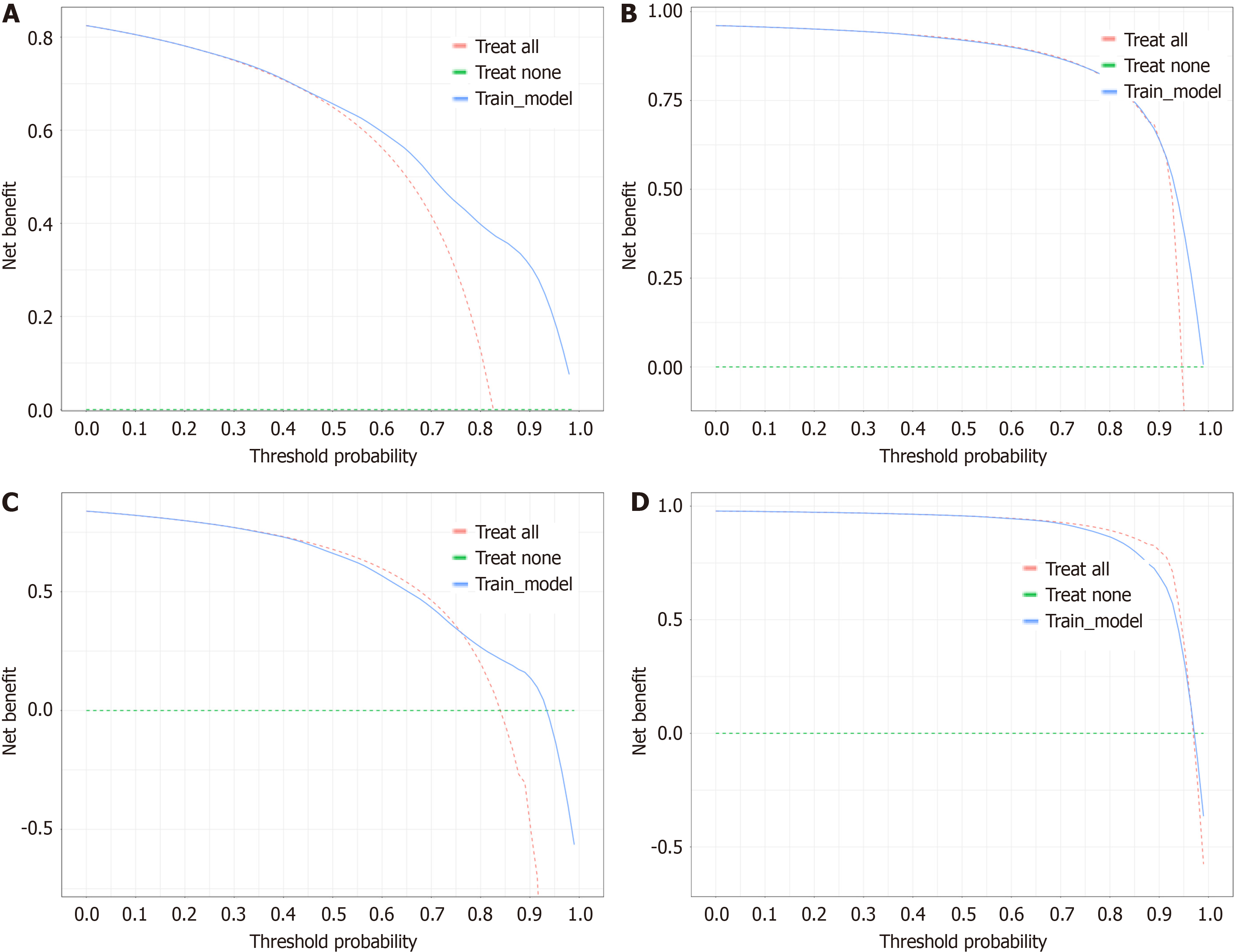
For the purpose of creating the OS nomogram, a CIP plot based on CSS was generated. Cox multivariate regression analysis identified eleven significant prognostic factors (P < 0.05) related to liver metastasis, bone metastasis, primary site, surgery, regional surgery, treatment sequence, chemotherapy, radiotherapy, positive lymph node count, N staging, and time from diagnosis to treatment. It was clear from the DCA (net benefit > 0), time-de
The OS nomogram for GCLM successfully predicts 1- and 3-year OS. Moreover, this approach can help to ap
Core Tip: From the viewpoints of overall survival and cancer-specific survival, this study investigated the survival probability based on independent prognostic indicators in gastric cancer with lung metastasis (GCLM) patients and generated a nomogram; the cumulative incidence of disease initiation was predicted. A risk score is assigned to each patient, and vital assistance is offered for individualized treatment plans in GCLM. Moreover, this groundbreaking study provides a model for the prognosis and prevention of various malignancies.
- Citation: Chen ZR, Yang MF, Xie ZY, Wang PA, Zhang L, Huang ZH, Luo Y. Risk stratification in gastric cancer lung metastasis: Utilizing an overall survival nomogram and comparing it with previous staging. World J Gastrointest Surg 2024; 16(2): 357-381
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/357.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.357
As the third most common cause of cancer-related deaths worldwide, gastric cancer (GC) continues to pose a serious threat to public health[1]. Clinical care for stomach cancer is complicated by its metastatic course. Although the liver has been the subject of most studies on GC metastases[2], lung metastases are also gradually gaining increased attention. The clinical circumstances involving the intersection of GC and lung metastasis (GCLM) are multifaceted and require accurate methods for diagnosis, treatment, and prognosis. The incidence and number of cases of lung metastasis from stomach cancer have increased over the past several decades. Clinical studies have shown that regional differences in the incidence rates of lung metastasis from stomach cancer have an effect on overall survival (OS)[3].
Early detection is crucial for improving the prognoses of GCLM patients. Imaging technology advancements, including computed tomography (CT), magnetic resonance imaging, and positron emission tomography, have improved the de
Treatment strategies for GCLM have developed over time and include a variety of surgical, chemotherapeutic, and targeted therapeutic methods. The incorporation of immunotherapy and diverse treatment techniques heralds the start of a new era in customized medicine[6]. Even with these improvements, many GC patients still experience late recurrence, and it is difficult to predict OS and disease cumulative incidence[7]. However, the prognosis of GCLM patients remains unknown, thus emphasizing the importance of continued research into novel treatment options and prognostic models. Multiple factors influence the prognosis of GCLM, including the severity of the disease, the occurrence of metastases elsewhere in the body, and the patient's overall health. An understanding of clinical prediction markers and the use of prognostic models are critical for directing treatment decisions and giving patients a realistic picture of their illness trajectory[8].
Due to their simplicity and accessibility of testing, nomograms have become standard tools for prognostic prediction in patients with various malignancies. These findings can assist clinicians in making more informed treatment decisions and provide personalized survival predictions for patients[9,10]. This study created a large GCLM dataset based on the Surveillance, epidemiology, and end results program (SEER) database, with the goal of developing an accurate predictive nomogram for GCLM patients.
This study used SEER-stat software version 8.4.2 to obtain the data. The SEER database, which is funded by the National Cancer Institute, is a decentralized registry spanning multiple centres and populations. It operates independently of medical ethics review processes and does not mandate the acquisition of informed consent[11]. This study used data from the 2010–2020 SEER Research Plus database, which met strict inclusion and exclusion criteria. The inclusion criteria for the study were multifaceted. First, GC patients with International Classification of Diseases-O-3/the World health Or
Patient data were divided into training and validation sets by using the R "caret" package's CreateDataPartition function. The random seed was 2345, therefore, the training set (1156 samples) was 7:3, and the validation set included 496 sam
All of the statistical analyses were performed in the RStudio environment by using R software, version 4.1.3. Categorical data are presented as frequencies and percentages for the creation of a three-line table and descriptive statistics of cli
In this investigation describing the OS of patients with GC, 1652 patients were included. There were 529 females (32.23%) and 1123 males (67.98%) among the participants, the overwhelming majority of whom were white (76.45%). The participants were randomly assigned to the training (n = 1156) or validation (n = 499) sets at a 7:3 ratio. The majority of patients with GC (62.89%) had malignant gastric adenocarcinomas, and 69.43% of these patients were older than 60 years. Nearly half of the primary tumours (43.16%) originated in the cardia, whereas a small percentage also originated in the larger curvature of the stomach (2.91%). Significantly, 95.58% of patients did not undergo surgery at the primary site, 94.9% did not undergo surgery on regional lymph nodes, and only 4.66% underwent other noncancer-related operations. The vast majority (97.82%) of patients in this study did not receive direct radiation or cancer-related surgery, as indicated by radiation therapy sequence data. Only 18.64% of patients received radiation therapy, whereas 55.21% received che
| Variable | Total (n = 1652) | Train set (n = 1156) | Valid set (n = 496) | Statistic | P value |
| Months from diagnosis to treatment, M (Q1, Q3) | 1.00 (1.00 - 1.00) | 1.00 (1.00 - 1.00) | 1.00 (1.00 - 1.00) | Z = 1.394 | 0.252 |
| Tumor size | χ2 = 1.983 | 0.371 | |||
| ≤ 5 | 421 (25.48) | 302 (26.12) | 119 (23.99) | ||
| 5-10 | 1159 (70.16) | 800 (69.20) | 359 (72.38) | ||
| ≥ 10 | 72 (4.36) | 54 (4.67) | 18 (3.63) | ||
| Age | χ2 = 0.077 | 0.782 | |||
| < 60 | 505 (30.57) | 351 (30.36) | 154 (31.05) | ||
| ≥ 60 | 1147 (69.43) | 805 (69.64) | 342 (68.95) | ||
| Race | χ2 = 1.121 | 0.571 | |||
| Black | 190 (11.5) | 127 (10.99) | 63 (12.70) | ||
| Other | 199 (12.05) | 138 (11.94) | 61 (12.30) | ||
| White | 1263 (76.45) | 891 (77.08) | 372 (75.00) | ||
| Sex | χ2 = 1.552 | 0.213 | |||
| Female | 529 (32.02) | 381 (32.96) | 148 (29.84) | ||
| Male | 1123 (67.98) | 775 (67.04) | 348 (70.16) | ||
| Primary site | χ2 = 3.797 | 0.803 | |||
| Antrum | 165 (9.99) | 119 (10.29) | 46 (9.27) | ||
| Body | 116 (7.02) | 78 (6.75) | 38 (7.66) | ||
| Cardia | 713 (43.16) | 494 (42.73) | 219 (44.15) | ||
| Fundus | 91 (5.51) | 62 (5.36) | 29 (5.85) | ||
| Greater curvature | 48 (2.91) | 34 (2.94) | 14 (2.82) | ||
| Lesser curvature | 75 (4.54) | 48 (4.15) | 27 (5.44) | ||
| Others | 426 (25.79) | 309 (26.73) | 117 (23.59) | ||
| Pylorus | 18 (1.09) | 12 (1.04) | 6 (1.21) | ||
| Histologic | χ2 = 6.574 | 0.037a | |||
| Adenocarcinoma | 1039 (62.89) | 704 (60.90) | 335 (67.54) | ||
| Others | 372 (22.52) | 275 (23.79) | 97 (19.56) | ||
| Signet ring cell carcinoma | 241 (14.59) | 177 (15.31) | 64 (12.90) | ||
| Surgery | χ2 = 1.650 | 0.199 | |||
| No | 1579 (95.58) | 1100 (95.16) | 479 (96.57) | ||
| Yes | 73 (4.42) | 56 (4.84) | 17 (3.43) | ||
| Regional lymph node surgery | χ2 = 1.063 | 0.302 | |||
| No | 1568 (94.92) | 1093 (94.55) | 475 (95.77) | ||
| Yes | 84 (5.08) | 63 (5.45) | 21 (4.23) | ||
| Surgery other regional/distant | χ2 = 0.001 | 0.976 | |||
| No | 1575 (95.34) | 1102 (95.33) | 473 (95.36) | ||
| Yes | 77 (4.66) | 54 (4.67) | 23 (4.64) | ||
| Treatment sequence | - | 0.057 | |||
| No radiation and/or cancer-directed surgery | 1616 (97.82) | 1126 (97.40) | 490 (98.79) | ||
| Radiation after surgery | 24 (1.45) | 22 (1.90) | 2 (0.40) | ||
| Radiation before and after surgery | 1 (0.06) | 1 (0.09) | 0 (0.00) | ||
| Radiation prior to surgery | 11 (0.67) | 7 (0.61) | 4 (0.81) | ||
| Radiation | χ2 = 0.380 | 0.537 | |||
| No | 1344 (81.36) | 936 (80.97) | 408 (82.26) | ||
| Yes | 308 (18.64) | 220 (19.03) | 88 (17.74) | ||
| Chemotherapy | χ2 = 0.601 | 0.438 | |||
| No | 740 (44.79) | 525 (45.42) | 215 (43.35) | ||
| Yes | 912 (55.21) | 631 (54.58) | 281 (56.65) | ||
| Nodes positive | χ2 = 5.288 | 0.021 | |||
| < 4 | 60 (3.63) | 50 (4.33) | 10 (2.02) | ||
| ≥ 4 | 1592 (96.37) | 1106 (95.67) | 486 (97.98) | ||
| Metastases at bone | χ2 = 2.578 | 0.108 | |||
| No | 1305 (79) | 901 (77.94) | 404 (81.45) | ||
| Yes | 347 (21) | 255 (22.06) | 92 (18.55) | ||
| Metastases at brain | χ2 = 3.751 | 0.053 | |||
| No | 1581 (95.7) | 1099 (95.07) | 482 (97.18) | ||
| Yes | 71 (4.3) | 57 (4.93) | 14 (2.82) | ||
| Metastases at liver | χ2 = 2.773 | 0.096 | |||
| No | 781 (47.28) | 562 (48.62) | 219 (44.15) | ||
| Yes | 871 (52.72) | 594 (51.38) | 277 (55.85) | ||
| Marital status at diagnosis | χ2 = 0.216 | 0.642 | |||
| 0 | 990 (59.93) | 697 (60.29) | 293 (59.07) | ||
| 1 | 662 (40.07) | 459 (39.71) | 203 (40.93) | ||
| T stage | χ2 = 3.078 | 0.688 | |||
| T0 | 10 (0.61) | 5 (0.43) | 5 (1.01) | ||
| T1 | 327 (19.79) | 227 (19.64) | 100 (20.16) | ||
| T2 | 62 (3.75) | 42 (3.63) | 20 (4.03) | ||
| T3 | 180 (10.9) | 132 (11.42) | 48 (9.68) | ||
| T4 | 285 (17.25) | 200 (17.30) | 85 (17.14) | ||
| TX | 788 (47.7) | 550 (47.58) | 238 (47.98) | ||
| N stage | χ2 = 0.708 | 0.950 | |||
| N0 | 588 (35.59) | 408 (35.29) | 180 (36.29) | ||
| N1 | 626 (37.89) | 440 (38.06) | 186 (37.50) | ||
| N2 | 59 (3.57) | 43 (3.72) | 16 (3.23) | ||
| N3 | 63 (3.81) | 46 (3.98) | 17 (3.43) | ||
| NX | 316 (19.13) | 219 (18.94) | 97 (19.56) |
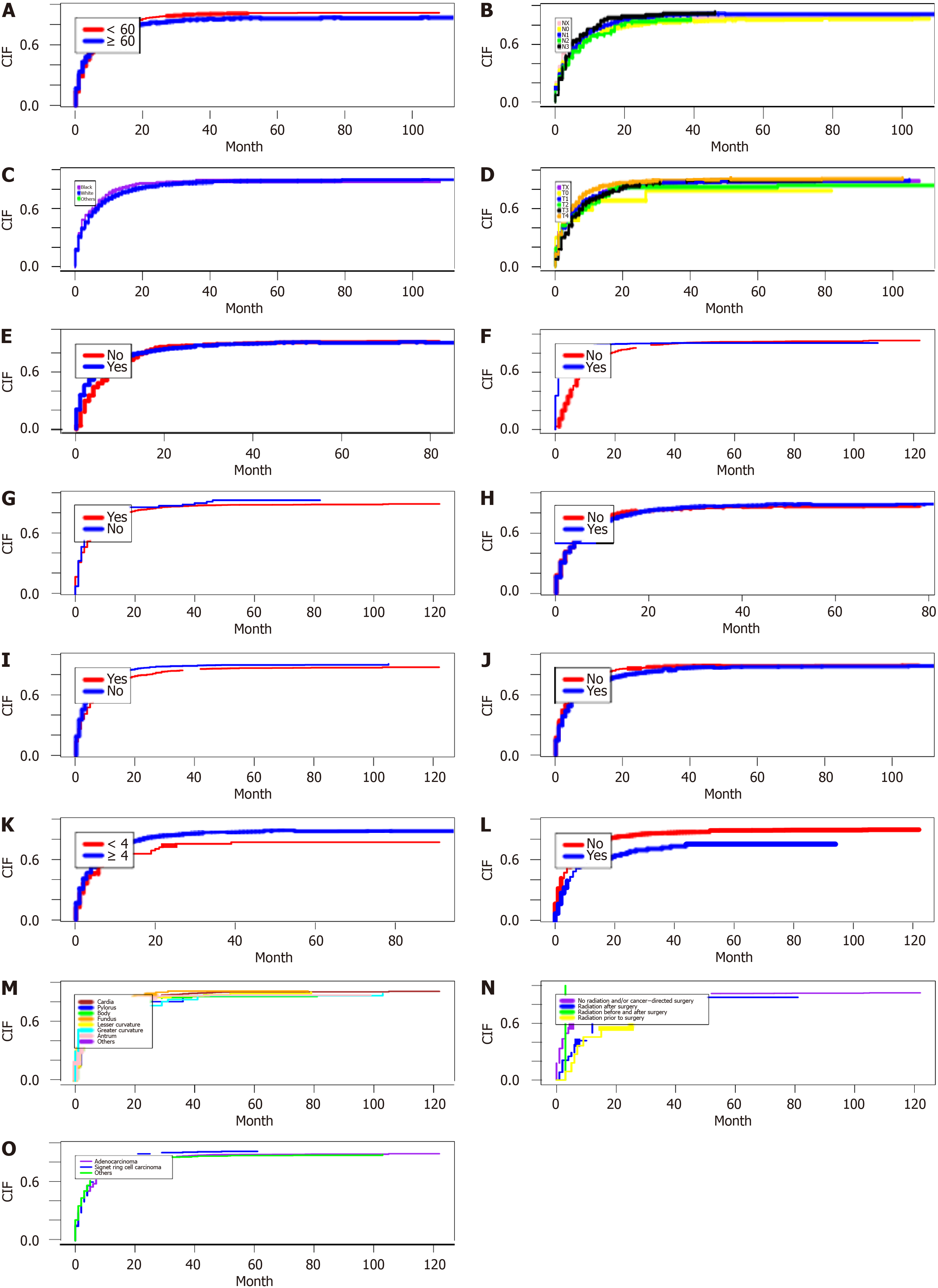
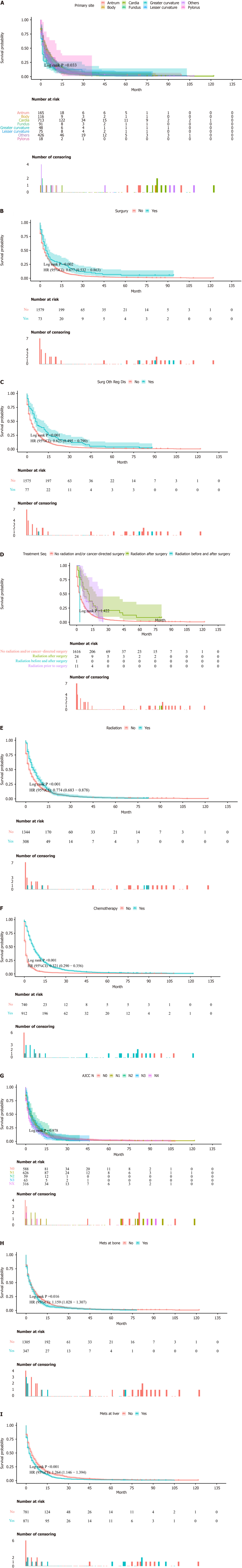
According to the analysis of CSS data, the competitive risk model demonstrated the following trends in cumulative incidence rates. As the follow-up period increased, there was a marked increase in the cumulative incidence among patients with GC lung metastases, particularly in individuals older than 60 years (Figure 2A), those with stage N2 tu
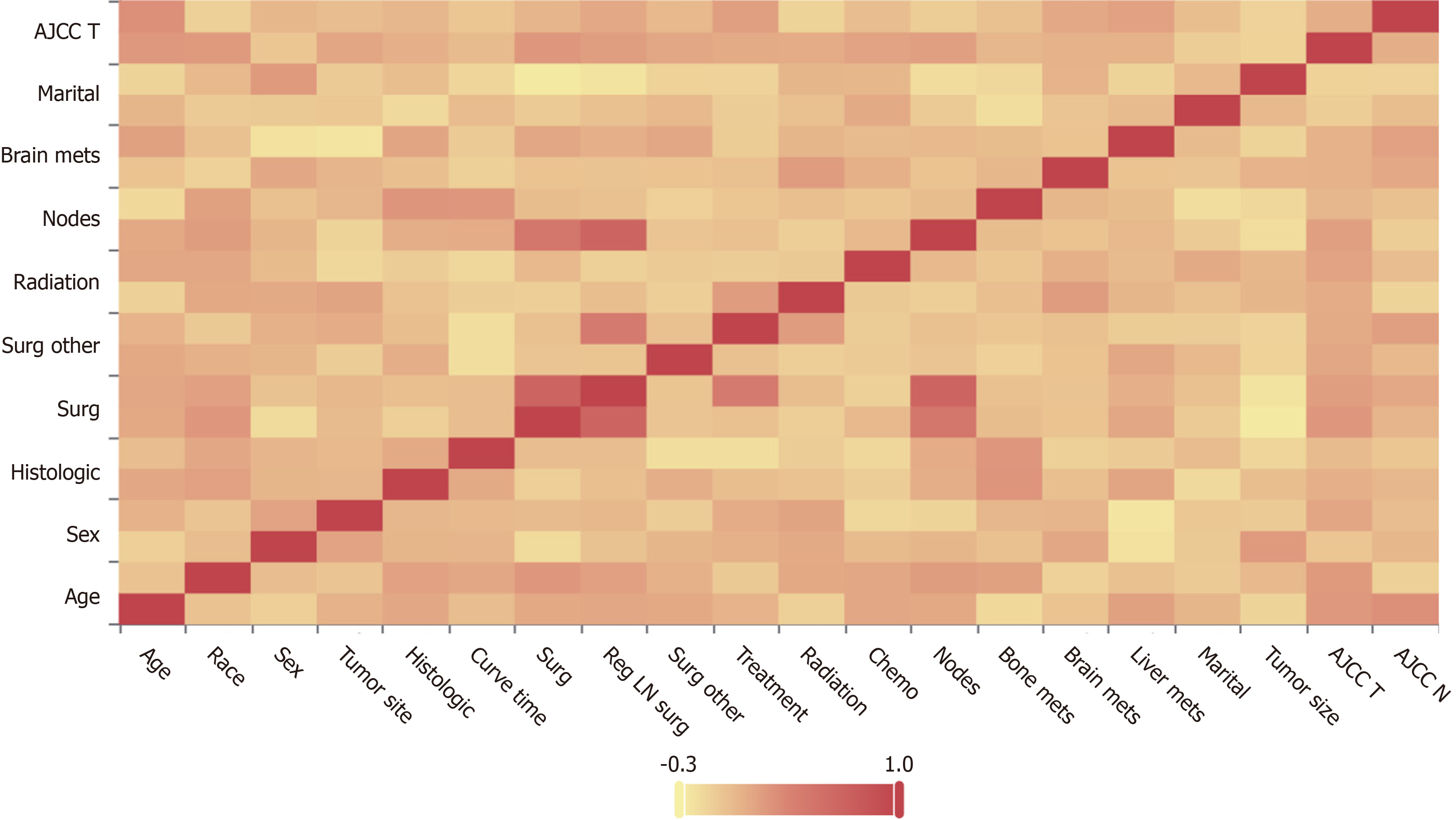
Before conducting the Cox regression analysis, to ensure that there was no collinearity among the variables, we utilized Spearman correlation analysis. The results of the correlation analysis can be found in Figure 3.
The results of the univariate Cox regression analysis indicated that a total of 11 variables, including months from diagnosis to treatment, primary site, surgery, treatment sequence, surgery regarding other regional distance, chemo
| Variables | Beta | SE | Z | P value | HR (95%CI) | m_Beta | m_SE | m_Z | P value | HR (95%CI) |
| Months from diagnosis to treatment | -0.15 | 0.03 | -4.90 | < 0.001a | 0.86 (0.81-0.92) | -0.15 | 0.04 | -4.11 | < 0.001b | 0.86 (0.80-0.93) |
| Tumor size | ||||||||||
| ≤ 5 | Ref. | |||||||||
| 5-10 | 0.13 | 0.07 | 1.96 | 0.05a | 1.14 (1.01-1.31) | |||||
| ≥ 10 | 0.08 | 0.15 | 0.53 | 0.593 | 1.08 (0.81-1.45) | |||||
| Age | ||||||||||
| 2 | Ref. | |||||||||
| 1 | -0.10 | 0.07 | -1.52 | 0.127 | 0.91 (0.80-1.03) | |||||
| Race | ||||||||||
| White | Ref. | |||||||||
| Other | 0.19 | 0.09 | 1.99 | 0.047a | 1.20 (1.01-1.45) | |||||
| Black | 0.14 | 0.10 | 1.42 | 0.154 | 1.15 (0.95-1.39) | |||||
| Sex | ||||||||||
| Female | Ref. | |||||||||
| Male | 0.03 | 0.06 | 0.42 | 0.677 | 1.03 (0.91-1.16) | |||||
| Primary site | ||||||||||
| Antrum | Ref. | Ref. | ||||||||
| Others | 0.19 | 0.11 | 1.72 | 0.085a | 1.21 (0.97-1.50) | 0.24 | 0.11 | 2.10 | 0.036b | 1.26 (1.02-1.58) |
| Cardia | -0.06 | 0.10 | -0.54 | 0.591 | 0.95 (0.77-1.16) | 0.13 | 0.11 | 1.22 | 0.222 | 1.14 (0.92-1.41) |
| Body | 0.14 | 0.15 | 0.97 | 0.331 | 1.16 (0.86-1.55) | 0.41 | 0.15 | 2.73 | 0.006b | 1.51 (1.12-2.04) |
| Pylorus | 0.03 | 0.30 | 0.09 | 0.928 | 1.03 (0.57-1.86) | 0.44 | 0.31 | 1.42 | 0.154 | 1.55 (0.85-2.85) |
| Fundus | 0.19 | 0.16 | 1.19 | 0.234 | 1.21 (0.88-1.65) | 0.12 | 0.16 | 0.77 | 0.442 | 1.13 (0.83-1.55) |
| Lesser curvature | 0.10 | 0.17 | 0.57 | 0.571 | 1.10 (0.79-1.55) | 0.28 | 0.18 | 1.61 | 0.107 | 1.33 (0.94-1.88) |
| Greater curvature | 0.50 | 0.20 | 2.57 | 0.010a | 1.66 (1.13-2.43) | 0.72 | 0.20 | 3.61 | < 0.001b | 2.05 (1.39-3.02) |
| Histologic | ||||||||||
| Signet ring cell carcinoma | Ref. | |||||||||
| Adenocarcinoma | -0.14 | 0.09 | -1.62 | 0.106 | 0.87 (0.74-1.03) | |||||
| Others | -0.11 | 0.10 | -1.15 | 0.250 | 0.89 (0.74-1.08) | |||||
| Surgery | ||||||||||
| No | Ref. | Ref. | ||||||||
| Yes | -0.50 | 0.14 | -3.54 | < 0.001a | 0.60 (0.46-0.80) | -0.83 | 0.17 | -4.77 | < 0.001b | 0.43 (0.31-0.61) |
| Regional lymph node surgery | ||||||||||
| No | Ref. | |||||||||
| Yes | -0.44 | 0.13 | -3.31 | < 0.001a | 0.64 (0.50-0.84) | |||||
| Treatment sequence | ||||||||||
| No radiation and/or cancer-directed surgery | Ref. | Ref. | ||||||||
| Radiation prior to surgery | -0.44 | 0.38 | -1.17 | 0.243 | 0.64 (0.31-1.35) | 0.89 | 0.44 | 2.00 | 0.045b | 2.43 (1.02-5.78) |
| Radiation after surgery | -0.78 | 0.23 | -3.44 | < 0.001a | 0.46 (0.29-0.72) | -0.21 | 0.25 | -0.84 | 0.400 | 0.81 (0.49-1.33) |
| Radiation before and after surgery | 0.33 | 1.00 | 0.33 | 0.742 | 1.39 (0.20-9.89) | 1.60 | 1.02 | 1.58 | 0.115 | 4.98 (0.68-36.63) |
| Surgery other regional distance | ||||||||||
| No | Ref. | Ref. | ||||||||
| Yes | -0.53 | 0.14 | -3.74 | < 0.001a | 0.59 (0.44-0.78) | -0.32 | 0.16 | -1.95 | 0.051 | 0.73 (0.53-1.00) |
| Chemotherapy | ||||||||||
| Yes | Ref. | Ref. | ||||||||
| No | 1.21 | 0.06 | 19.12 | < 0.001a | 3.35 (2.96-3.79) | 1.29 | 0.07 | 19.02 | < 0.001b | 3.63 (3.18-4.14) |
| Radiation | ||||||||||
| No | Ref. | Ref. | ||||||||
| Yes | -0.27 | 0.08 | -3.59 | < 0.001a | 0.76 (0.66-0.88) | -0.17 | 0.09 | -1.99 | 0.046b | 0.84 (0.71-0.99) |
| Nodes positive | ||||||||||
| ≥ 4 | Ref. | Ref. | ||||||||
| < 4 | -0.38 | 0.15 | -2.56 | 0.011a | 0.68 (0.51-0.91) | 0.28 | 0.18 | 1.54 | 0.125 | 1.32 (0.93-1.89) |
| Metastases at brain | ||||||||||
| No | Ref. | |||||||||
| Yes | -0.04 | 0.14 | -0.28 | 0.777 | 0.96 (0.73-1.26) | |||||
| Metastases at bone | ||||||||||
| No | Ref. | Ref. | ||||||||
| Yes | 0.17 | 0.07 | 2.40 | 0.017a | 1.19 (1.03-1.37) | 0.28 | 0.07 | 3.71 | < 0.001b | 1.32 (1.14-1.53) |
| Metastases at liver | ||||||||||
| No | Ref. | Ref. | ||||||||
| Yes | 0.22 | 0.06 | 3.60 | < 0.001a | 1.24 (1.10-1.39) | 0.26 | 0.06 | 4.14 | < 0.001b | 1.30 (1.15-1.47) |
| Marital status at diagnosis | ||||||||||
| 1 | Ref. | |||||||||
| 0 | -0.20 | 0.06 | -3.19 | 0.001a | 0.82 (0.73-0.93) | |||||
| T stage | ||||||||||
| TX | Ref. | |||||||||
| T3 | -0.24 | 0.10 | -2.40 | 0.016a | 0.79 (0.65-0.96) | |||||
| T4 | 0.02 | 0.08 | 0.24 | 0.808 | 1.02 (0.87-1.20) | |||||
| T2 | -0.20 | 0.16 | -1.21 | 0.228 | 0.82 (0.59-1.13) | |||||
| T1 | -0.11 | 0.08 | -1.35 | 0.177 | 0.90 (0.77-1.05) | |||||
| T0 | -0.47 | 0.50 | -0.94 | 0.347 | 0.62 (0.23-1.67) | |||||
| N stage | ||||||||||
| N1 | Ref. | Ref. | ||||||||
| NX | 0.18 | 0.08 | 2.09 | 0.037a | 1.19 (1.01-1.40) | 0.06 | 0.09 | 0.73 | 0.463 | 1.07 (0.90-1.26) |
| N2 | 0.00 | 0.16 | 0.03 | 0.979 | 1.00 (0.73-1.37) | 0.30 | 0.17 | 1.79 | 0.073 | 1.35 (0.97-1.86) |
| N0 | 0.05 | 0.07 | 0.67 | 0.506 | 1.05 (0.91-1.20) | -0.03 | 0.07 | -0.38 | 0.703 | 0.97 (0.84-1.12) |
| N3 | 0.14 | 0.16 | 0.91 | 0.361 | 1.15 (0.85-1.56) | 0.37 | 0.16 | 2.36 | 0.018b | 1.45 (1.07-1.98) |
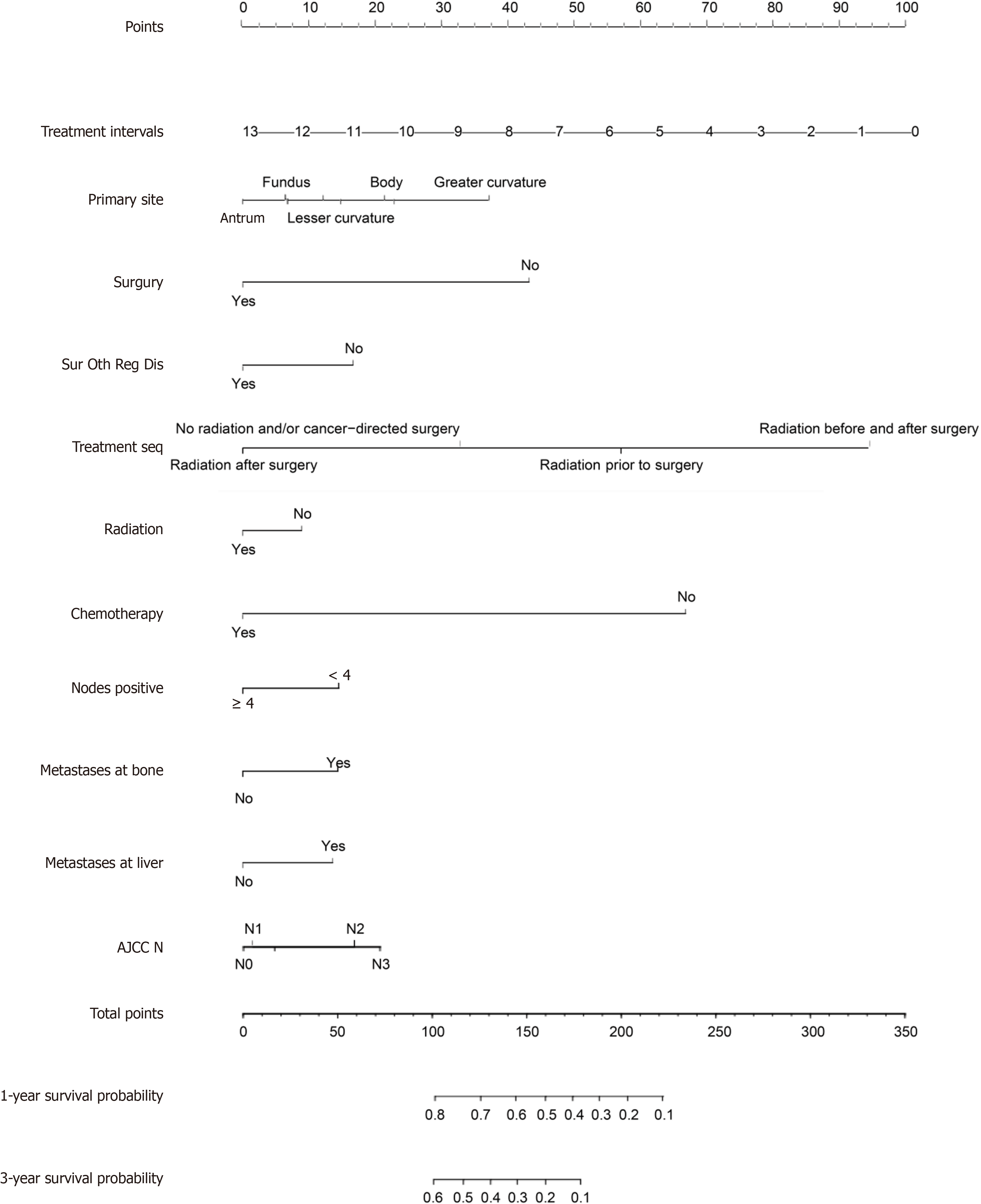
Univariate Cox regression analysis was also conducted for all of the patients, with a significance level of P < 0.1. Covariates that were found to be significant were subsequently included in the multivariate Cox regression analysis, with a significance level of P < 0.05. Figure 4 shows the independent predictive variables for individuals diagnosed with GCLM. The study examined a total of ten variables, encompassing primary tumour location, surgical intervention, other regional surgeries, treatment sequence, radiation therapy, chemotherapy, the number of positive lymph nodes, the presence of bone metastases, liver metastases, and tumour N staging. These variables were utilized to predict the 1-year and 3-year survival rates for patients diagnosed with GCLM. The patients were assigned scores for each risk factor by mapping them upwards on a scale based on the classification of each risk factor. There was a negative correlation bet
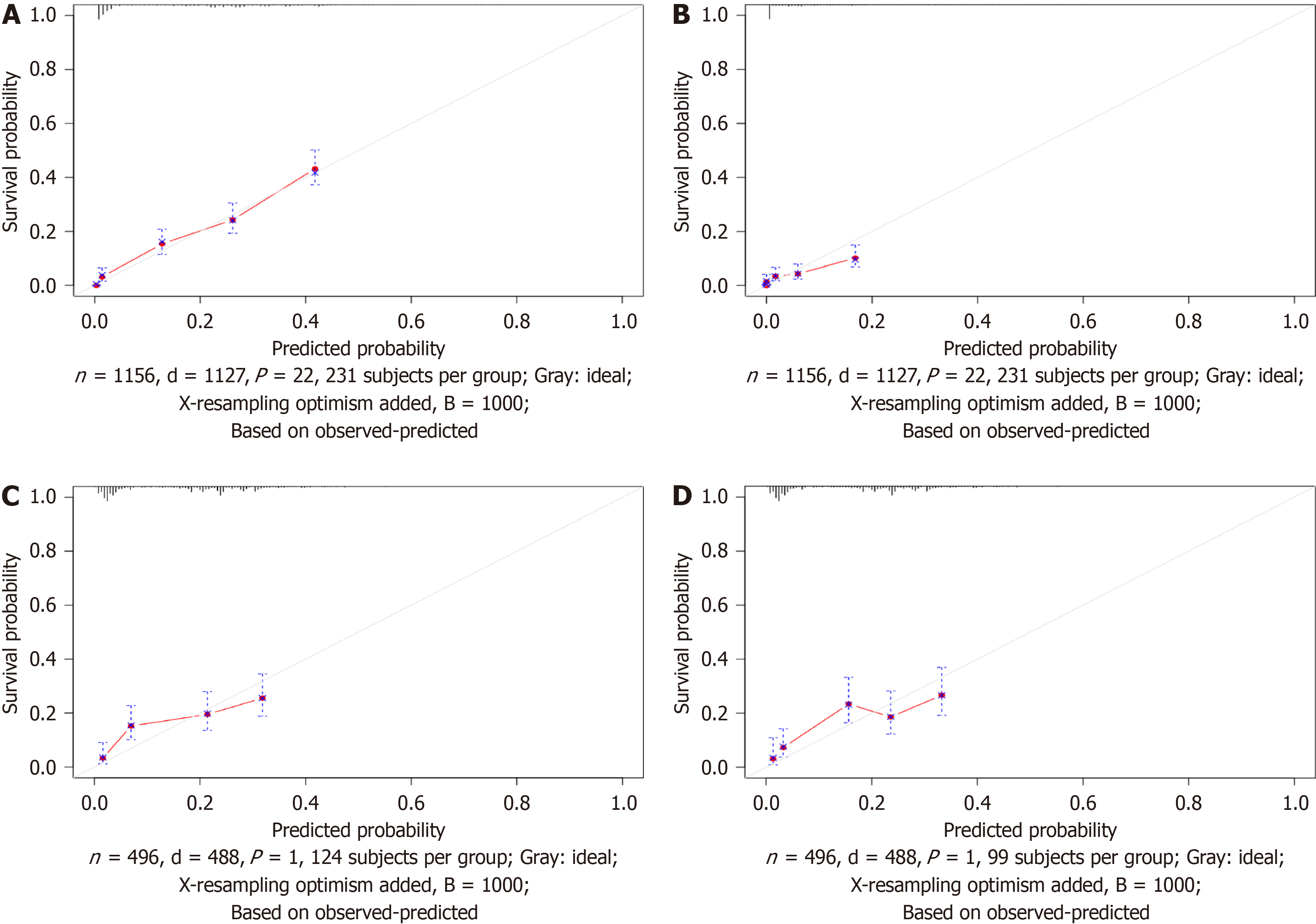
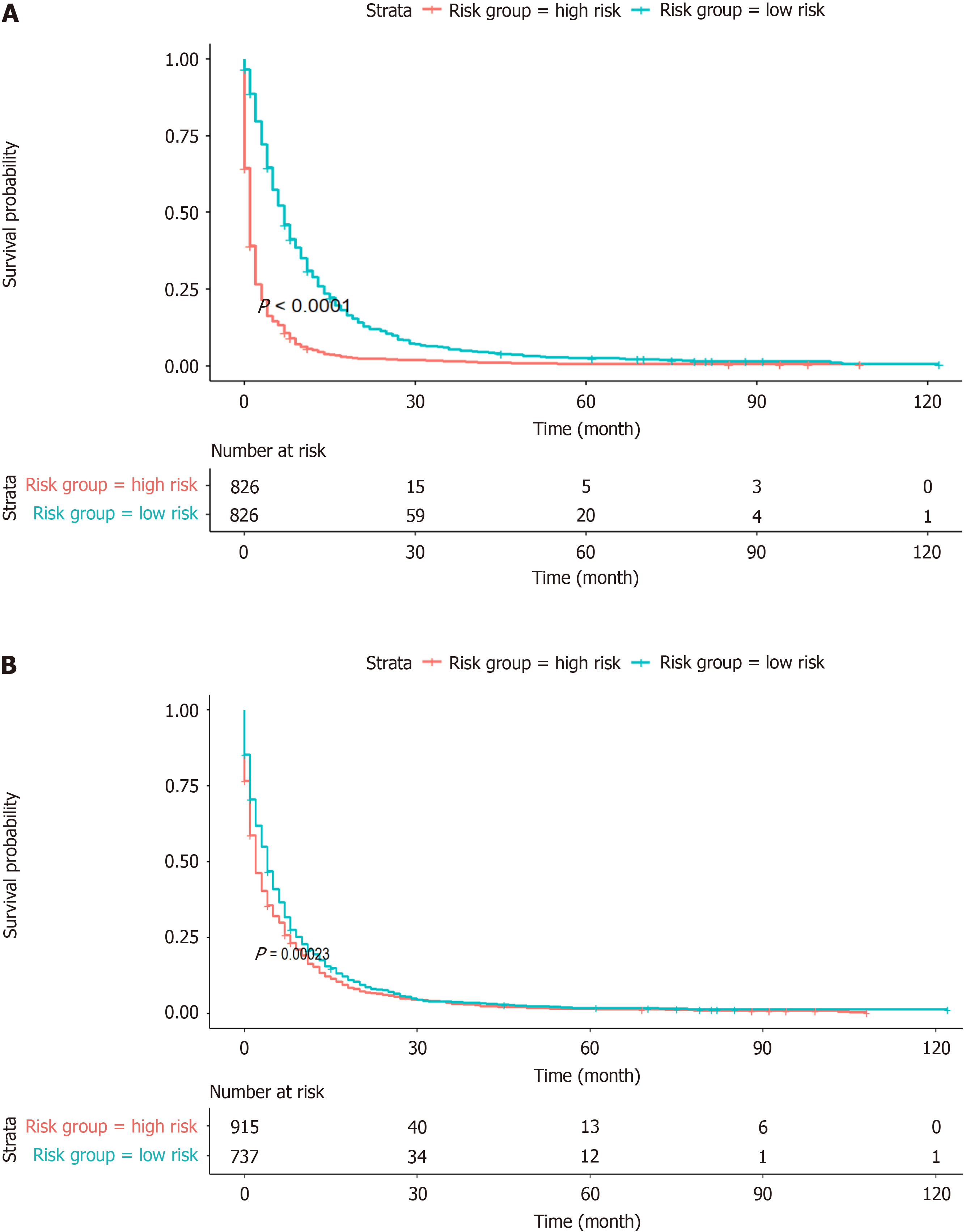
In this study, to address various independent prognostic factors, we utilized the 'survival' package in the R language, in addition to the ggsurvplot function[14], to illustrate the Kaplan‒Meier (KM) survival curves and to perform risk stratification for both the OS model and the AJCC model. By utilizing the OS nomogram, we generated a thorough survival score for the patients using the OS nomogram. Patients were divided into two main cohorts (the high-risk group and the low-risk group) based on the median risk score (Figure 9). According to the KM survival analysis, the OS of patients in the low-risk subgroup was greater than that of patients in the high-risk subgroup (Figure 9A). Although the AJCC staging system showed low discriminative power in risk stratification, the OS risk stratification showed substantial discriminative ability (Figure 9B).
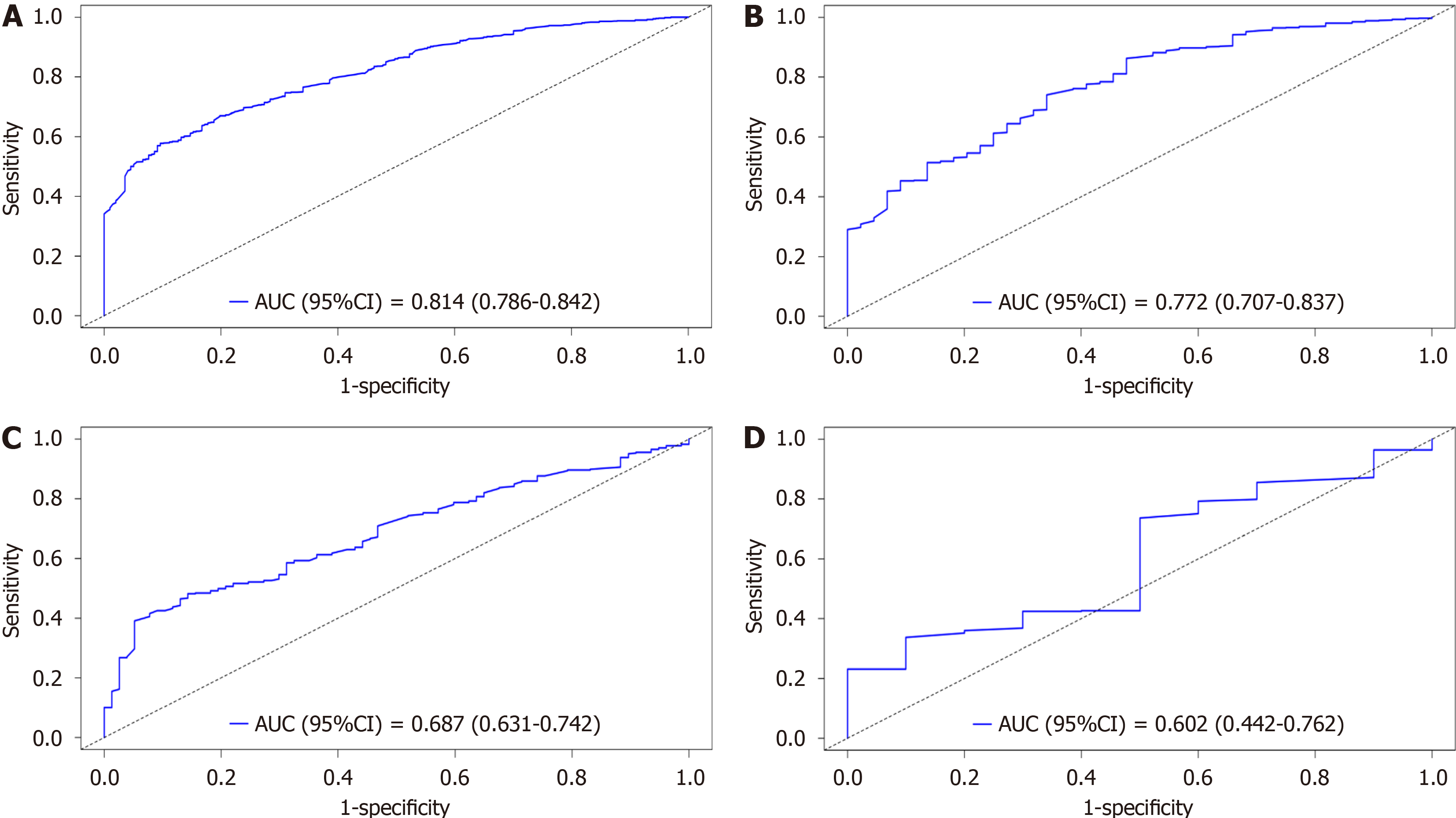
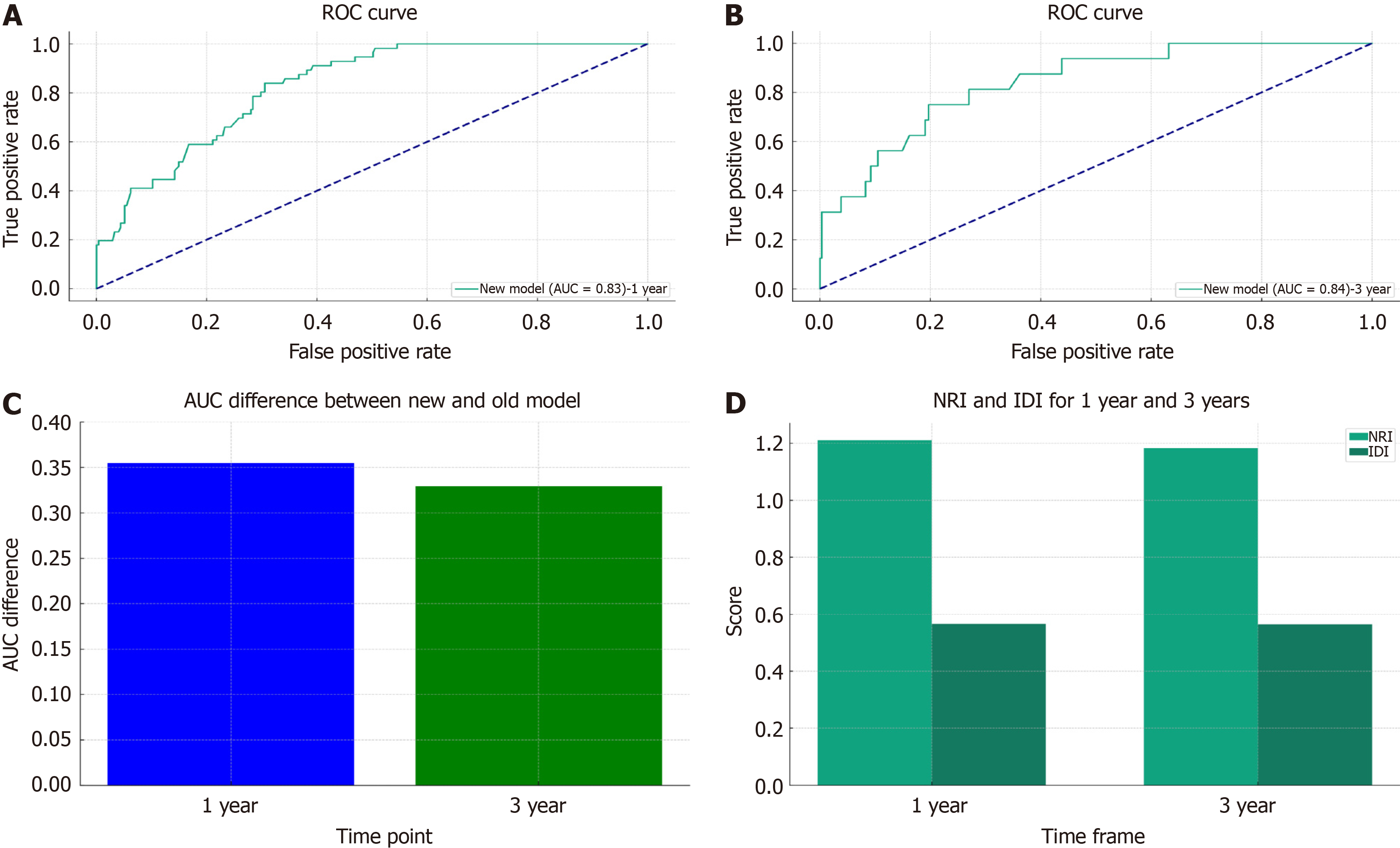
Individualized prediction has become the cornerstone of such research due to the notable prognostic variations in GC patients with lung metastases. Previously, the prognosis was based on the 7th edition of the AJCC staging method. However, the sole use of the TNM staging system within the AJCC system is not enough to ensure complete and accurate prognostic evaluations. Therefore, it is crucial to combine variables with additional clinical factors. To assess the accuracy and capacity for improvement of the recently constructed model, the nomogram that was developed in this study was contrasted with the previous AJCC staging method. The Cox-AJCC older model architecture, which was based on the Cox nomogram model, considered only age, race, sex, T stage, and N stage. By considering improvements at particular cut-off points, the NRI is used to compare the prediction skills of the old and new models. IDI is used to observe the model's capacity for overall improvement[15]. The ROC curves of the participants at 1 year (Figure 10A) and 3 years (Figure 10B) IDI of the new model indicated favourable performance (AUC > 0.8), and Figure 10C shows a bar graph demonstrating the difference in the AUC between the two models. The bar graph clearly shows that the new model has a higher AUC than the previous model for both 1-year and 3-year forecasts. This shows that the new model outperforms the previous model in terms of prediction. Furthermore, the NRI and IDI values for the first and third years were greater than 0. As shown in Figure 10D, the new model outperforms the AJCC prediction model in terms of accuracy and total improvement.
As a common digestive tract cancer, GC frequently faces difficulties such as poor identification, limited surgical resection options, and a high likelihood of recurrence[16]. However, stomach cancer is actually a preventable and treatable type of cancer if it is found early and combined with proactive therapeutic methods. Such tactics can significantly reduce the incidence and fatality rates of this disease[17]. Our work, which prognostically examined the 1-year and 3-year survival times of patients with GC pulmonary metastasis using the extensive sample database SEER, provided insights that surpassed the conventional thoroughness and accuracy of the AJCC staging system[18]. The established nomogram model exhibited good discriminatory capacity and calibration. Additionally, risk stratification significantly divided GCLM patients into high- and low-risk groups. The OS rates of these patients at both the 1-year and 3-year intervals showed a distinct decreasing trend as the follow-up period progressed.
The most frequent liver metastases in individuals with recurrences and metastases from GC were those that were accompanied by distant lymph node metastases. The majority of research on GC has primarily focused on liver meta
Oh et al[20] reported that the development of GC and its doubling time significantly decreased as the stage progressed after confirmation of GC without the need for any therapeutic interventions and by merely observing tumour progression via CT and endoscopy; moreover, the survival rate also decreased during follow-up. For T1 stomach cancer, the doubling time was 11.8 months; however, for T4, it was 6.2 months. It took an average of 34 months for early-stage stomach cancer to develop into an advanced stage[20]. The urgency from diagnosis to treatment introduction is further highlighted by this rate of advancement. The risk to the patient was increased by more than 14% in comparison to timely treatment for every month of treatment delay following diagnosis according to multivariate Cox proportional hazards regression analysis (HR = 0.86, CI = 0.80-0.93; P = 0.001). These findings demonstrate the critical importance of early diagnosis and therapy for individuals with stomach cancer and other malignancies in terms of their prognosis. Neoadjuvant therapy for GC should be administered no later than 4 wk after surgery, according to studies on this form of treatment by Ahn et al[21]. If this delay is delayed past this point, the patient's chance of survival may suffer. Patients with locally progressed and late-stage GC who underwent surgery within 3-5 wk of receiving neoadjuvant therapy experienced the greatest improvements in survival according to studies by Wang et al[22]. However, our research showed that different treatment modalities and approaches can lead to differences in patient prognosis. In particular, patients who receive radiotherapy prior to surgery at the primary site have a 2.43-fold increased risk of poor survival (P = 0.045, CI = 1.02–5.78) compared to patients who did not receive radiation or surgery. This result deviates from prior research[23].
Patients with original tumours on the greater curvature of the stomach had considerably greater OS rates than did those with tumours in the antrum, cardia, or lesser curvature according to Korivi et al's retrospective analysis[24]. In contrast to original tumours in the gastric antrum, predictive risk variables for lung metastasis in individuals with GC included those originating from the cardia, pylorus, stomach body, stomach fundus, greater curvature, and lesser cur
Through Cox regression analysis, Dong et al[15] reported that surgery was an independent prognostic factor impacting patient survival. When regarding the surgical management of patients with far-reaching metastases, debate has persisted in recent years[15]. In addition, the single-factor and multifactor analyses of this study's data regarding surgical therapy at the primary site showed that patients who underwent surgery at the primary lesion had a 57% lower risk than did those who did not (P = 0.001, HR = 0.43, CI = 0.31–0.61). Additionally, surgical intervention in these metastatic areas may be able to stop the spread of the disease. Lung metastasis from GC may also occur, as may lymph node and distant organ metastases in other regions. This study provided concrete evidence that these surgical procedures improved patients' chances of survival. Some Japanese specialists have recommended against performing palliative surgery to remove the main lesion for patients with incurable distant metastases. However, resection of the metastatic location may be an option for cancers that have limited metastatic spread[25].
For advanced stomach cancer, radiation and chemotherapy are considered essential supplementary treatment stra
In this study, patients with liver and bone metastases had a 1.30- and 1.32-fold greater chance of dying from GC than did those without liver and bone metastases, respectively, at a very significant level (P < 0.001). Liang et al[27] conducted a retrospective investigation by using Cox survival analysis and public databases. Early bone metastasis in GC is rare, and synchronous bone metastasis is even rarer. Those individuals with GC who had surgery at the main site and who had bone metastases had a longer median survival time (9.0 months) than did those who did not have surgery (3.0 months). Furthermore, the median survival time for patients with GCLM was 7 months among those who did not have any skeletal-related disorders at the time of bone metastasis diagnosis. Additionally, treatment may improve a patient's relative survival in patients with stomach cancer that metastasizes to the bone[27,28]. CT and retrospective analysis allowed Hori et al[29] to categorize liver metastasis patients into H1, H2, and H3 grades. According to their study, the size and number of tumours that had spread to the liver were positively correlated with the prognosis of liver metastasis in patients with GC[29].
Younger patient populations were found to be independent risk variables for underestimating lymph node metastases and clinical N staging in Kim et al's study[30]. Younger patients with GC had an increased risk of lymph node metastases, as well as an inclination to underestimate staging[30]. In this study, the risk of a poor prognosis increased with increasing stage; this difference was most noticeable at the N3 stage (P = 0.018, HR = 1.45, CI = 1.07-1.98), when the cumulative incidence was highest.
This study used the SEER database to evaluate the long-term prognostic outcomes of patients with GC pulmonary metastasis and presented a unique model. The study utilized extensive sample data from GC patients and clinicians worldwide. The accuracy of prognostic predictions for GCLM improved when using this new approach, which differs significantly from the traditional AJCC model in that it incorporates variables such as clinical auxiliary treatment elements and demographic variables that were not present in earlier models. Precision medicine is becoming a reality with rapid progress in medical technology. Rapid advancements in fields such as radiomics, metabolomics, and genomics have led to the wider use of multiomics analytic techniques. Doctors carefully collect all of the relevant information during patient visits and perform in-depth evaluations. Thorough case studies have shown that the prognosis of patients with GC is significantly influenced by the extent of tumour resection, the depth of tumour invasion, and the extent of lymph node metastasis[31-33]. Researchers are also paying attention to the relationship between prognosis and the following three important parameters: The number of negative lymph nodes, the rate of lymph node metastases, and the presence of free tumour cells in the peritoneal cavity[34]. These discoveries have the potential to improve the survival rates of stomach cancer patients by enabling the development of more customized treatment plans. In the future, prognostic prediction models that make use of large amounts of medical data and sophisticated algorithms are expected to be fundamental to customized care. These developments are expected to improve treatment outcomes for patients with stomach cancer, thereby prolonging their survival.
There are undoubtedly certain aspects of this study that need to be further refined. In the present study, we analysed data from 1652 patients with GCLM, of which 67.98% were male, and 32.02% were female. Although this distribution reflects the sample available in the SEER database, we acknowledge that the unequal sex ratio may introduce certain biases in our findings. This disproportionate sex distribution could affect the generalizability of our results. Future studies should aim to include a more balanced male-to-female patient ratio to increase the applicability of the findings across genders. Additionally, the investigation of any sex-specific differences in the prognosis of GCLM could offer more nuanced insights into disease progression and management. Other limitations include the retrospective design of the study and the lack of additional validation through randomized controlled trials. Crucially, the study focused mainly on the ability of lung metastasis to predict the survival of patients with GC, and the clinical research characteristics in the database were limited to patients with metastases to the liver, bone, or brain. Other metastatic sites (such as the ab
A risk nomogram for the OS of patients with lung metastases from stomach cancer was effectively established in this study. The suggested nomogram uses CSS to determine the cumulative incidence of patient prognosis and efficiently separates prognostic groupings. This nomogram showed constant reliability and clinical application after validation. One of the most important innovations in our study is the use of extremely extensive and precise clinicopathological variables. These characteristics are expected to substantially improve the predictive power for OS and CSS. This approach will help surgeons in creating more individualized therapeutic and prognostic strategies for these patients. Subsequent studies will require additional external verification to prospectively evaluate the model.
Gastric cancer (GC) is a globally prevalent malignancy known for its aggressive behaviour and poor survival outcomes, especially when metastasis occurs. Recent research has focused on identifying more precise prognostic factors to tailor individual treatment strategies. By developing a nomogram using e Surveillance, epidemiology, and end results program (SEER) database, this study addresses a critical gap in understanding GC lung metastasis (GCLM). This approach goes beyond traditional American Joint Committee on Cancer staging, thus offering a more accurate predictive model for overall survival (OS) and risk categorization in GCLM patients. This contribution is significant because it can inform better clinical decision-making and potentially improve outcomes in this patient population.
This study was motivated by the need to improve prognostic predictions for GCLM, which is a condition associated with notably poor survival outcomes. The aim was to address key problems in current prognostic models, such as their limited ability to accurately predict OS and cumulative incidence prediction (CIP) in GCLM patients. The significance of solving these problems lies in providing clinicians with a more effective tool for risk stratification, which can guide personalized treatment plans and potentially improve patient outcomes. By developing a more accurate and comprehensive nomogram using data from the SEER database, this research contributes to the advancement of precision medicine in GC care, particularly for those with lung metastases.
The primary objective of this study was to develop an accurate prognostic nomogram for patients with GCLM by using data from the SEER database. This nomogram aims to predict OS and CIP more effectively than existing models. The study successfully identified significant prognostic factors related to GCLM, thus integrating these factors into a model that offers more precise survival predictions and risk stratification. These objectives are significant for future research, as they will enhance the understanding of GCLM and aid in the advancement of personalized treatment strategies, thus potentially improving patient outcomes in this challenging cancer subtype.
This research utilized a retrospective analysis of data from the SEER database comprising patients with GCLM from January 2000 to December 2020. The methods included univariate and multivariate Cox regression analyses to identify independent prognostic factors, and a nomogram was developed for predicting OS. This nomogram was validated by using the time-dependent area under the curve and calibration curves. Additionally, decision curve analysis was used to assess the clinical usefulness of the model. The novelty of this research lies in the comprehensive approach combining various clinical and demographic variables that have not been previously integrated in traditional models, thereby enhancing the prognostic accuracy for GCLM patients.
This research established a novel prognostic nomogram for predicting OS in patients with GCLM that included factors such as age, sex, race, tumour size, and treatment modality. This model demonstrated superior predictive accuracy compared to traditional staging systems, thereby significantly contributing to personalized treatment planning and risk assessment in GCLM patients. However, challenges remain in validating the nomogram across diverse patient po
The research concluded with the successful development of a prognostic nomogram for predicting the OS of patients with lung metastases from GC based on an extensive and precise collection of clinicopathological variables. Moreover, this approach helps to appropriately classify patients into high-risk and low-risk groups, thereby guiding treatment. This model, which was validated for its reliability and clinical application, represents a significant innovation in personalized treatment and prognosis strategies for GC.
Future efforts will focus on additional external validation and prospective evaluations to further establish the model's efficacy and applicability in clinical settings.
I would like to express my sincere gratitude to the Surveillance, epidemiology, and end results program database for providing invaluable data resources for this study. I also wish to extend my deepest appreciation to my research team, whose expertise, dedication, and support made this research possible. A special thanks to Wei-Wei Chen for her invaluable insights and feedback; her contributions were pivotal in enhancing the quality of this research.
| 1. | Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. 2022;28:1187-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 239] [Cited by in RCA: 292] [Article Influence: 73.0] [Reference Citation Analysis (23)] |
| 2. | Luo Z, Rong Z, Huang C. Surgery Strategies for Gastric Cancer With Liver Metastasis. Front Oncol. 2019;9:1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Gu J, Chu X, Huo Y, Liu C, Chen Q, Hu S, Pei Y, Ding P, Pang S, Wang M. Gastric cancer-derived exosomes facilitate pulmonary metastasis by activating ERK-mediated immunosuppressive macrophage polarization. J Cell Biochem. 2023;124:557-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 4. | Yashima K, Onoyama T, Kurumi H, Takeda Y, Yoshida A, Kawaguchi K, Yamaguchi N, Isomoto H. Current status and future perspective of linked color imaging for gastric cancer screening: a literature review. J Gastroenterol. 2023;58:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Röcken C. Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol. 2023;149:467-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 116] [Reference Citation Analysis (5)] |
| 6. | Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Yago A, Haruta S, Ueno M, Hamada Y, Ogawa Y, Ohkura Y, Urabe M, Udagawa H. Adequate period of surveillance in each stage for curatively resected gastric cancer: analyzing the time and rates of recurrence. Gastric Cancer. 2021;24:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Liu S, Chen L, Zeng J, Chen Y. A prognostic model based on the COL1A1-network in gastric cancer. Am J Transl Res. 2023;15:1640-1653. [PubMed] |
| 9. | Kong J, Zheng J, Cai J, Wu S, Diao X, Xie W, Chen X, Liao C, Yu H, Fan X, Huang C, Liu Z, Chen W, Lv Q, Qin H, Huang J, Lin T. A nomogram for individualized estimation of survival among adult patients with adrenocortical carcinoma after surgery: a retrospective analysis and multicenter validation study. Cancer Commun (Lond). 2019;39:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2577] [Article Influence: 234.3] [Reference Citation Analysis (0)] |
| 11. | Sampson JH, Lad SP, Herndon JE 2nd, Starke RM, Kondziolka D. SEER insights. J Neurosurg. 2014;120:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G; STROCSS Group. STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1232] [Article Influence: 176.0] [Reference Citation Analysis (0)] |
| 13. | Avalos-Pacheco A, Ventz S, Arfè A, Alexander BM, Rahman R, Wen PY, Trippa L. Validation of Predictive Analyses for Interim Decisions in Clinical Trials. JCO Precis Oncol. 2023;7:e2200606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sonabend R, Király FJ, Bender A, Bischl B, Lang M. mlr3proba: an R package for machine learning in survival analysis. Bioinformatics. 2021;37:2789-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Dong Z, Zhang Y, Geng H, Ni B, Xia X, Zhu C, Liu J, Zhang Z. Development and validation of two nomograms for predicting overall survival and cancer-specific survival in gastric cancer patients with liver metastases: A retrospective cohort study from SEER database. Transl Oncol. 2022;24:101480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Zhou L, Li SH, Wu Y, Xin L. Establishment of a prognostic model of four genes in gastric cancer based on multiple data sets. Cancer Med. 2021;10:3309-3322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Norwood DA, Montalvan-Sanchez E, Dominguez RL, Morgan DR. Gastric Cancer: Emerging Trends in Prevention, Diagnosis, and Treatment. Gastroenterol Clin North Am. 2022;51:501-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | RuiYang W, ZhiMing Y, Jiao F, Liang Z, Gang Z. Evaluation and Recommendation of the 8th Edition of American Joint Committee on Cancer (AJCC) Staging System for Intrahepatic Cholangiocarcinoma (ICC) in 820 Patients from the Surveillance, Epidemiology, and End Results (SEER) Database. J Gastrointest Surg. 2021;25:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Muroi T, Okui M, Sasaki A. [Surgical Resection of Pulmonary Metastases from Gastric Cancer;Report of a Case]. Kyobu Geka. 2020;73:1045-1047. [PubMed] |
| 20. | Oh SY, Lee JH, Lee HJ, Kim TH, Huh YJ, Ahn HS, Suh YS, Kong SH, Kim GH, Ahn SJ, Kim SH, Choi Y, Yang HK. Natural History of Gastric Cancer: Observational Study of Gastric Cancer Patients Not Treated During Follow-Up. Ann Surg Oncol. 2019;26:2905-2911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Ahn GT, Baek SK, Han JJ, Kim HJ, Jeong SJ, Maeng CH. Optimal time interval from surgery to adjuvant chemotherapy in gastric cancer. Oncol Lett. 2020;20:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Liu Z, Shan F, Ying X, Zhang Y, Li S, Jia Y, Li Z, Ji J. Optimal Timing to Surgery After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer. Front Oncol. 2020;10:613988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Chen J, Guo Y, Fang M, Yuan Y, Zhu Y, Xin Y, Zhang L. Neoadjuvant chemoradiotherapy for resectable gastric cancer: A meta-analysis. Front Oncol. 2022;12:927119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Korivi BR, Faria S, Aly A, Sun J, Patnana M, Jensen CT, Wagner-Bartak N, Bhosale PR. Intestinal and diffuse gastric cancer: a retrospective study comparing primary sites. Clin Imaging. 2019;56:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1415] [Article Influence: 283.0] [Reference Citation Analysis (2)] |
| 26. | Sato Y, Okamoto K, Kida Y, Mitsui Y, Kawano Y, Sogabe M, Miyamoto H, Takayama T. Overview of Chemotherapy for Gastric Cancer. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 27. | Liang C, Chen H, Yang Z, Han C, Ren C. Risk factors and prognosis of bone metastases in newly diagnosed gastric cancer. Future Oncol. 2020;16:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Imura Y, Tateiwa D, Sugimoto N, Inoue A, Wakamatsu T, Outani H, Tanaka T, Tamiya H, Yagi T, Naka N, Okawa S, Tabuchi T, Takenaka S. Prognostic factors and skeletal-related events in patients with bone metastasis from gastric cancer. Mol Clin Oncol. 2020;13:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Hori S, Honda M, Kobayashi H, Kawamura H, Takiguchi K, Muto A, Yamazaki S, Teranishi Y, Shiraso S, Kono K, Kamiga T, Iwao T, Yamashita N. A grading system for predicting the prognosis of gastric cancer with liver metastasis. Jpn J Clin Oncol. 2021;51:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Kim W, Youn S, Won Y, Min S, Park YS, Ahn SH, Park DJ, Kim HH. Clinicopathologic Characteristics of Young Gastric Cancer Patients: Diagnostic Staging Accuracy and Survival. J Minim Invasive Surg. 2020;23:163-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Li X, Yang J. Association of tumor deposits with tumor-infiltrating lymphocytes and prognosis in gastric cancer. World J Surg Oncol. 2022;20:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Woo Y, Behrendt CE, Yang A, Hahn M, Goel A, Li H, Yuan YC, Fong Y. Tumor Epigenetic Signature and Survival in Resected Gastric Cancer Patients. J Am Coll Surg. 2021;232:483-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Wang X, Chen Y, Gao Y, Zhang H, Guan Z, Dong Z, Zheng Y, Jiang J, Yang H, Wang L, Huang X, Ai L, Yu W, Li H, Dong C, Zhou Z, Liu X, Yu G. Predicting gastric cancer outcome from resected lymph node histopathology images using deep learning. Nat Commun. 2021;12:1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 34. | Chen ZD, Zhang PF, Xi HQ, Wei B, Chen L, Tang Y. Recent Advances in the Diagnosis, Staging, Treatment, and Prognosis of Advanced Gastric Cancer: A Literature Review. Front Med (Lausanne). 2021;8:744839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nittari G, Italy S-Editor: Li L L-Editor: A P-Editor: Xu ZH