Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.345
Peer-review started: December 4, 2023
First decision: December 17, 2023
Revised: January 1, 2024
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 27, 2024
Processing time: 83 Days and 1 Hours
Although accurately evaluating the overall survival (OS) of gastric cancer patients remains difficult, radiomics is considered an important option for studying pro
To develop a robust and unbiased biomarker for predicting OS using machine learning and computed tomography (CT) image radiomics.
This study included 181 stage II/III gastric cancer patients, 141 from Lichuan People's Hospital, and 40 from the Cancer Imaging Archive (TCIA). Primary tumors in the preoperative unenhanced CT images were outlined as regions of interest (ROI), and approximately 1700 radiomics features were extracted from each ROI. The skeletal muscle index (SMI) and skeletal muscle density (SMD) were measured using CT images from the lower margin of the third lumbar vertebra. Using the least absolute shrinkage and selection operator regression with 5-fold cross-validation, 36 radiomics features were identified as important predictors, and the OS-associated CT image radiomics score (OACRS) was cal
Patients with a high OACRS had a poorer prognosis than those with a low OACRS score (P < 0.05) and those in the TCIA cohort. Univariate and multivariate analyses revealed that OACRS was a risk factor [RR = 3.023 (1.896-4.365), P < 0.001] independent of SMI, SMD, and pathological features. Moreover, OACRS outperformed SMI and SMD and could improve OS prediction (P < 0.05).
A novel biomarker based on machine learning and radiomics was developed that exhibited exceptional OS discrimination potential.
Core Tip: We investigated 141 patients with locally advanced gastric cancer and developed the overall survival-associated computed tomography image radiomics score (OACRS) using machine learning and radiomics. The data revealed that OACRS was associated with overall survival (OS) in these patients, in addition to OS in the Cancer Imaging Archive cohort. Importantly, OACRS could improve the predicted accuracy of OS.
- Citation: Xiang YH, Mou H, Qu B, Sun HR. Machine learning-based radiomics score improves prognostic prediction accuracy of stage II/III gastric cancer: A multi-cohort study. World J Gastrointest Surg 2024; 16(2): 345-356
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/345.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.345
Gastric cancer is the fourth most common malignancy, and the application of multidisciplinary approaches in recent years has significantly improved its prognosis. However, the 5-year overall survival (OS) rate for locally advanced gastric cancer is less than 60%[1,2]. Tumor, node, and metastasis (TNM) staging is the cornerstone for guiding OS; however, the outcomes of gastric cancer patients who undergo radical resection with the same TNM stage can vary significantly[3]. Current research indicates that the prognosis of malignant tumors not only depends on immutable tumor-specific factors, such as histology and pathology but is also closely related to postoperative adjuvant therapy and the patient's own nutri
Previous studies have revealed an association between preoperative computed tomography (CT) image data and the prognosis of malignant tumors[5], such as the skeletal muscle index (SMI) and skeletal muscle density (SMD)[6]. However, accurately evaluating OS remains challenging, as evidenced by the fact that approximately 50% of patients with locally advanced gastric cancer undergoing curative resection develop distant metastases in subsequent years. Radiomic progression has provided methods for extracting and analyzing thousands of image-related features to aid in diagnosis and treatment[7,8]. With applications beyond clinical decision-making, such as predicting peritoneal recurrence, disease-free survival, non-invasive tumor microenvironment evaluation, and treatment response, radiomics has shown great potential in personalized medicine, as it can improve OS prediction[9-11]. Recent studies have revealed that machine learning algorithms are highly flexible and powerful tools for modeling[12,13]. Radiomics provides an opportunity for machine learning as it is more suitable for data with multiple variables. However, the efficacy of a machine learning algorithm model using radiomics for evaluating OS and determining whether it outperforms manual indicators such as SMI and SMD in stage II/III gastric cancer remains unclear.
This study hypothesized that machine learning model-based CT-derived radiomics could be a predictive biomarker to assess OS for stage II/III gastric cancer and outperform SMI and SMD.
Clinical data from 186 patients who underwent radical resection for gastric cancer at Lichuan People’s Hospital between 2013 and 2019 were collected, and all data were accessed between June 2023 and September 2023. The inclusion criteria for this study were as follows: Age of 18-80 years and primary stage II/III gastric adenocarcinoma. Forty-five patients were excluded due to tumor perforation and acute bleeding (n = 2), R1 or R2 resection (n = 8), missing preoperative CT images (n = 6), number of harvested lymph nodes < 12 (n = 10), and missing follow-up (n = 19), and 141 patients were included. This study was approved by the medical ethics committee of Lichuan People's Hospital, and the authors had access to information that could identify individual participants during or after data collection. The clinical variables included in this study were as follows: Gender, age, height, American Association of Anesthesiologists (ASA) score, preoperative carcinoembryonic antigen (CEA), type of gastrectomy, tumor differentiation, tumor size, T, N, and TNM stages, nerve or vascular invasion, tumor deposition (TD), and postoperative chemotherapy. All patients were restaged according to the 8th AJCC staging system. Gastric cancer patients were followed up every three months for the first two years, every six months during postoperative 3-5 years, and every 12 months after five years at our center. The primary outcome of the current study was OS, defined as the time at which a patient died postoperatively from any cause. Another cohort of 40 patients was included in the Cancer Imaging Archive (TCIA cohort; https://www.cancerimagingarchive.net/).
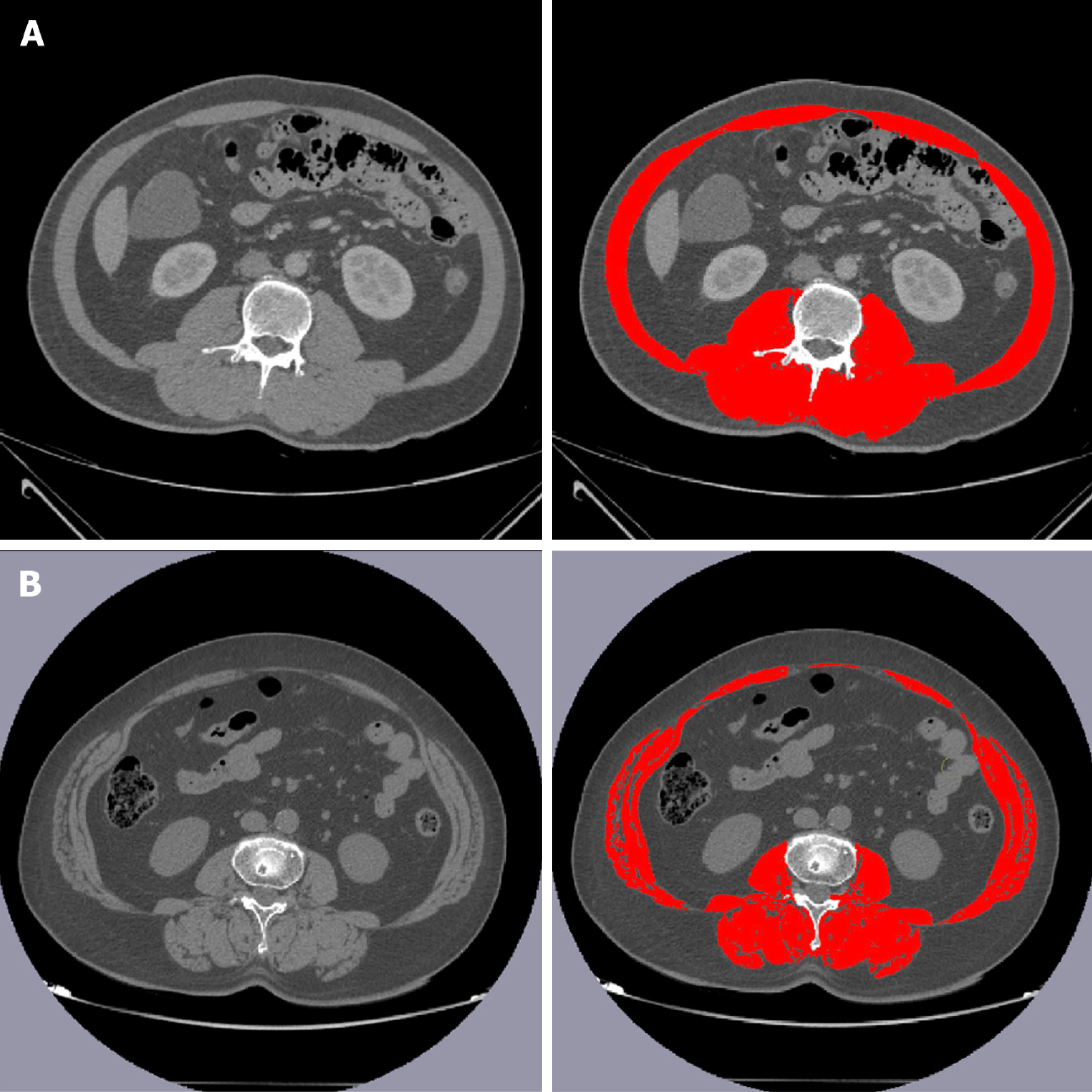
Martin et al[14] proposed a classical method for calculating SMD. All CT examinations were performed within one week before surgery, and unenhanced images from the lower margin of the third lumbar vertebra were analyzed. First, soft tissue was visualized using Hounsfield Units (HU) [-150, 180]. Next, the skeletal muscle tissue area (SMA) was delineated using 29 to 150 HU, and the average value of HU was defined as SMD[14]. Moreover, SMI was calculated using SMA/height2[15,16]. In this study, SMD and SMI were calculated using the Slice-O-Matic software (Tomovision, Montreal, Canada, version 5.0). A schematic diagram for calculating SMD and SMI is shown in Figure 1.
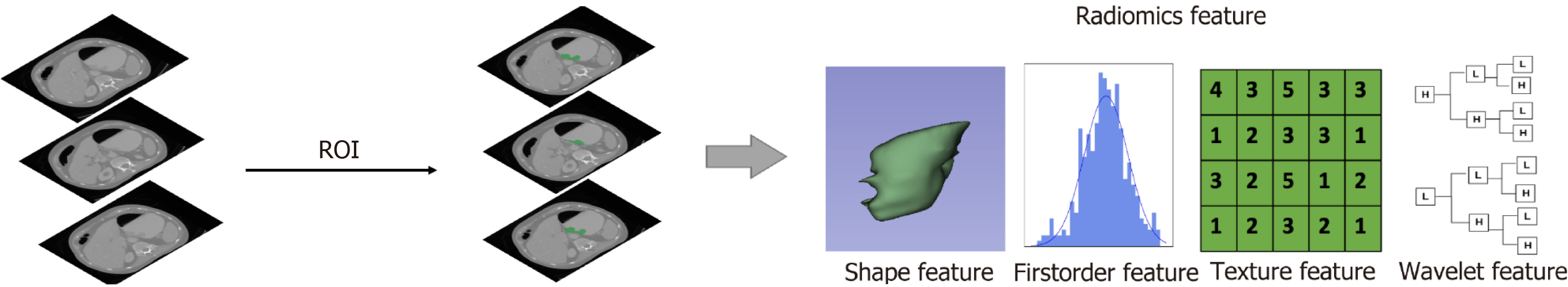
CT image radiomics was extracted using 3D-Slicer (5.4.0), a widely used freeware for medical image data. To evaluate gastric cancer, the ‘Segmentation’ module was used to delineate the primary tumor as a region of interest (ROI) from unenhanced CT images by two surgeons with at least eight years of clinical experience (Sun HR and Qu B). Next, the 'Radiomics' module was used to calculate the CT image radiomics of ROIs, such as ‘shape’, ‘first-order’, ’glcm’, ’gldm’, ’glrlm’, ‘glszm’ and ‘ngtdm’ with their derived features. Finally, approximately 1700 CT image radiomics features were extracted. Figure 2 depicts the radiomics extraction procedure.
Excessive features could increase the model complexity and make clinical applications inconvenient. A machine learning algorithm, the least absolute shrinkage and selection operator (LASSO) regression, is widely used for feature selection and modeling, which can filter unimportant features and discriminate significantly important features for predictions. Cox LASSO regression with 5-fold cross-validation was used to select the most useful predictors associated with OS to select radiomics features for modeling. The radiomics score calculated using the Cox LASSO regression model was defined as the OS-associated CT radiomics score (OACRS) and was used for subsequent machine learning modeling. Moreover, radiomics of the patients from TCIA cohort were extracted, and OACRSs were calculated using the 36 predictors. The predictive model was developed using random survival forest (RSF), a popular machine learning algorithm. Patients from our center were included in the training cohort and were classified as the discovery cohort. The high-performance model was trained using a 5-fold cross-validation and hyperparameter-adjustment approach.
Categorical variables are represented as the number of cases (percentage), while continuous variables are represented as mean ± SD. The χ2 test was used to compare categorical variables, whereas the t-test was used for continuous variables. The ‘Survminer’, ’glmnet’, and ‘randomForstSRC’ packages in R (version 0.4.9) were used to determine the best cut-off value, select features, and develop a predictive model. Independent risk factors associated with OS were analyzed using univariate and multivariate proportional risk regression models (Cox). The Kaplan-Meier method and log-rank test were used to compare survival rates. All P values represented the correlation by a two-tailed test, and P < 0.05 indicated statistically significant differences. All analyses were performed using IBM SPSS 25.0 (SPSS for Windows, IBM Corporation, Armonk, NY, United States) or R (4.3.0).
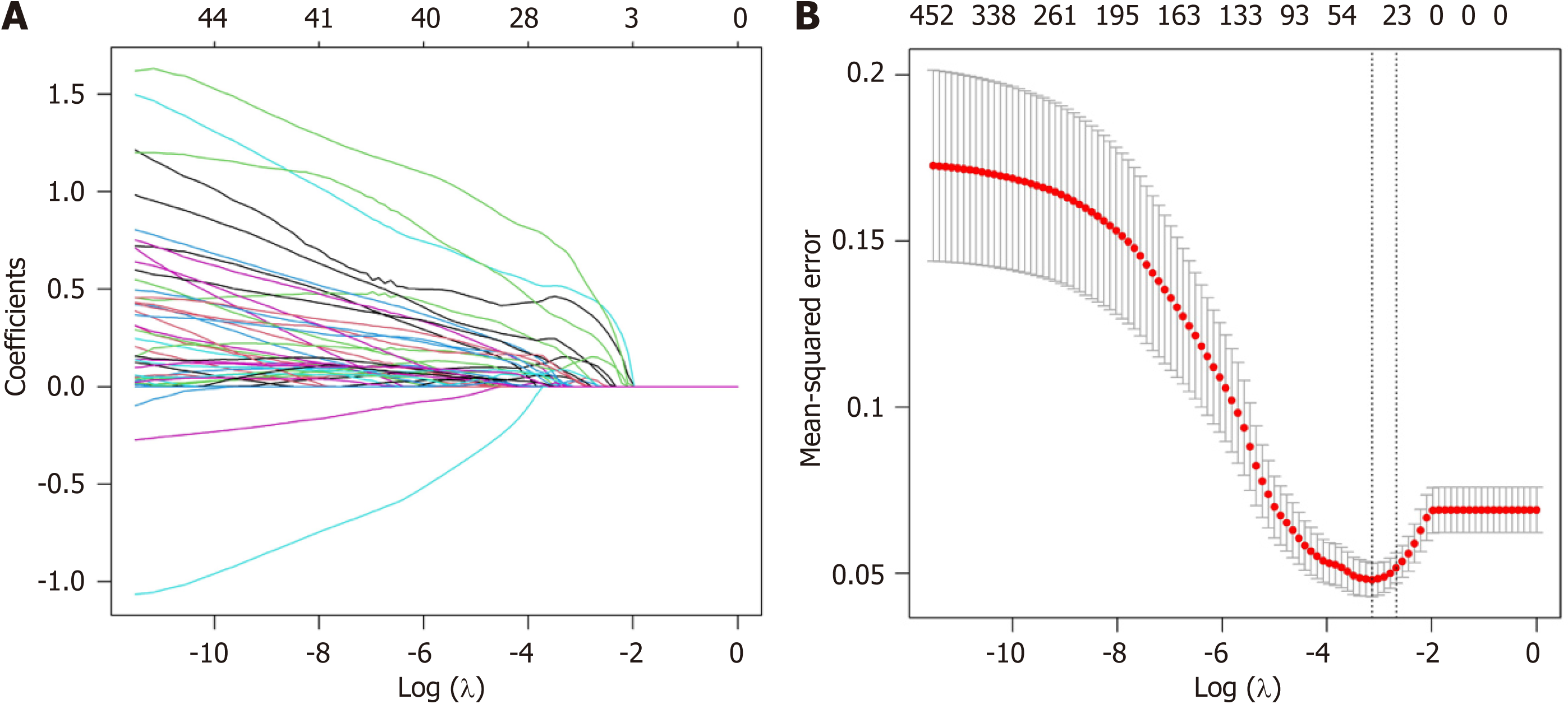
OS-related features were selected from the 1689 radiomics features initially extracted from CT images using LASSO regression with 5-fold cross-validation. A coefficient profile plot was produced according to the log (λ) sequence (–10, 0) (Figure 3A). The binomial deviance curve indicated that when the log (λ) value was 4.33 × 10–2, there was a minimum mean-squared error, and 36 features were selected as important (Figure 3B).
In this study, 141 patients were included in the discovery cohort, and 40 were included in the TCIA cohort. To elucidate the relationship between radiomics features and OS, the OACRS of all patients was calculated using the LASSO regression model. The mean values of OACRS, SMI, and SMD were 0.48, 46.8, and 33.2 for discovery cohort patients and 0.53, 44.6, and 32.6 for TCIA cohort patients (P < 0.05), respectively. Compared with the discovery cohort, males and elders were predominant in the TCIA cohort (P < 0.05). Patients in the TCIA cohort exhibited later N and TNM stages (P < 0.05). This may be due to the early screening for cancer in recent years (Table 1). In addition, patients in the discovery cohort received fewer proximal gastrectomies (12.8% vs 42.5%), which may be linked to the severe decline in the quality of life for proximal gastric surgery.
| Discovery cohort | TCIA cohort | P value | |
| (n = 141) | (n = 40) | ||
| Gender | 0.002 | ||
| Male | 79 (56.0) | 33 (82.5) | |
| Female | 62 (44.0) | 7 (17.5) | |
| Age (yr) | 56.3 (12.1) | 65.0 (9.6) | < 0.001 |
| ASA | NA | ||
| 1 | 70 (49.6) | Not reported | |
| 2 | 40 (28.4) | ||
| 3 | 31 (22.0) | ||
| CEA (ng/mL) | NA | ||
| ≤ 5 | 109 (77.3) | Not reported | |
| > 5 | 32 (22.7) | ||
| Operation | < 0.001 | ||
| Proximal | 18 (12.8) | 17 (42.5) | |
| Distal | 84 (59.6) | 11 (27.5) | |
| Total | 39 (27.7) | 12 (30.0) | |
| Tumor size | 4.3 (1.2) | Not reported | NA |
| Nerve or vascular invasion | NA | ||
| Yes | 54 (38.3) | Not reported | |
| No | 87 (61.7) | ||
| SMI | 46.8 (14.5) | 44.8 (13.4) | 0.009 |
| SMD | 33.2 (6.8) | 34.6 (7.2) | 0.032 |
| Differentiation | NA | ||
| Good | 13 (9.2) | Not reported | |
| Moderate | 63 (44.7) | ||
| Poor | 65 (46.1) | ||
| T stage | 0.097 | ||
| 2-3 | 66 (46.8) | 23 (57.5) | |
| 4 | 75 (53.2) | 17 (42.5) | |
| N stage | < 0.001 | ||
| 0-1 | 33 (23.4) | 16 (40.0) | |
| > 1 | 108 (76.6) | 24 (60.0) | |
| TD | NA | ||
| Yes | 29 (20.6) | Not reported | |
| No | 112 (79.4) | ||
| TNM stage | |||
| II | 65 (46.1) | 12 (30.0) | < 0.001 |
| III | 76 (53.9) | 28 (70.0) | |
| Chemotherapy | NA | ||
| Yes | 90 (63.8) | Not reported | |
| No | 51 (36.2) |
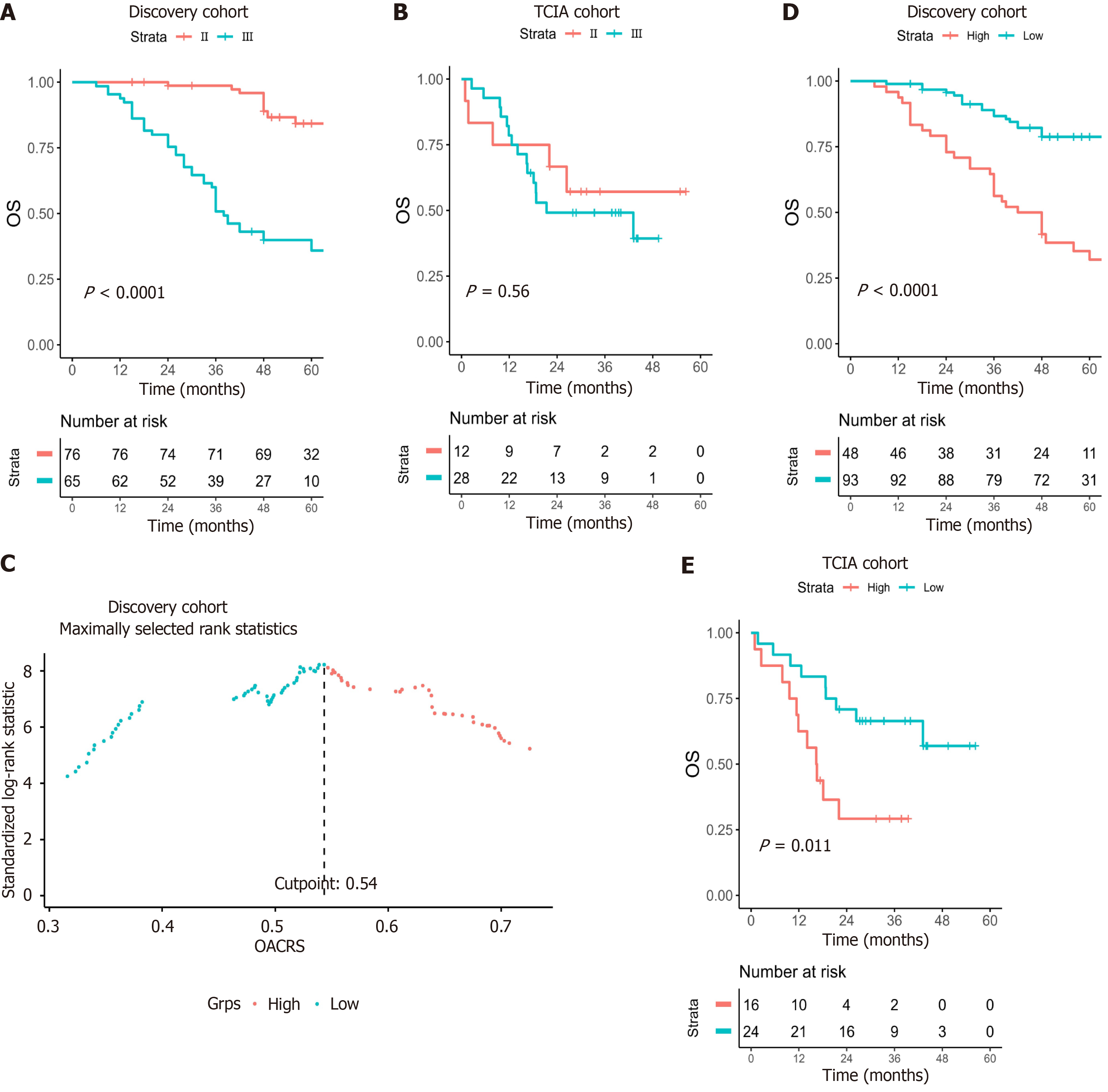
TNM staging is widely recognized as a useful prognostic indicator. Patients with stage Ⅱ had a better OS than that of stage Ⅲ in the discovery cohort (P < 0.001; Figure 4A) but not in the TCIA cohort (P = 0.56) (Figure 4B), possibly due to an insufficient number of cases. The receiver operating characteristic curve (ROC) is the most popular method for calculating the optimal cut-off value. However, ROC is applicable only to binary diagnostic tests and not to survival analysis. Con
Collinearity may occur because SMI, SMD, and OACRS were calculated from unenhanced CT images. Consequently, univariate and multivariate Cox regression were used to analyze the role of OACRS in OS. Univariate analysis revealed that SMI, SMD, OACRS, CEA, nerve or vascular invasion, TD, N stage, TNM stage, and postoperative chemotherapy were significantly associated with OS (P < 0.05). Furthermore, multivariate analysis indicated that SMI, SMD, CEA, OACRS, TNM stage, and postoperative chemotherapy were independent risk factors linked to OS (P < 0.05; Table 2). These results indicate that OACRS is an OS predictor independent of SMI, SMD, and pathological features.
| Univariate | Multivariate | |||
| RR (95%CI) | P value | RR (95%CI) | P value | |
| Gender (female) | 0.885 (0.523-1.498) | 0.649 | ||
| Age (yr) | 1.002 (0.981-1.024) | 0.843 | ||
| ASA | ||||
| 1 | Ref | |||
| 2 | 1.338 (0.669-2.675) | 0.411 | ||
| 3 | 0.979 (0.496-1.934) | 0.952 | ||
| SMI | 0.958 (0.930-0.986) | 0.004 | 0.968 (0.932-0.991) | 0.009 |
| SMD | 0.907 (0.872-0.943) | < 0.001 | 0.936 (0.889-0.975) | 0.002 |
| OACRS | 3.158 (1.378-5.247) | < 0.001 | 3.023 (1.896-4.365) | < 0.001 |
| Operation | ||||
| Proximal | Ref | |||
| Distal | 1.499 (0.632-3.553) | 0.369 | ||
| Total | 1.018 (0.390-2.652) | 0.972 | ||
| CEA (< 5 ng/mL) | 2.236 (1.275-3.921) | 0.005 | 3.174 (1.635-6.035) | 0.003 |
| Tumor size | 1.035 (0.917-1.168) | 0.58 | ||
| Nerve or vascular invasion | 1.700 (1.006-2.871) | 0.047 | ||
| TD (No) | 2.167 (1.233-3.810) | 0.007 | ||
| Differentiation | ||||
| Good | Ref | |||
| Moderate | 1.808 (0.814-4.016) | 0.146 | ||
| Poor | 0.996 (0.569-1.744) | 0.989 | ||
| T stage (2/3) | 1.472 (0.867-2.499) | 0.153 | ||
| N stage (0-1) | 4.501 (1.793-11.299) | 0.001 | ||
| TNM stage (II) | 6.325 (3.425-11.680) | < 0.001 | 3.698 (2.478-7.635) | < 0.001 |
| Chemotherapy (No) | 0.410 (0.241-0.697) | 0.001 | 0.496 (0.268-0.768) | 0.002 |
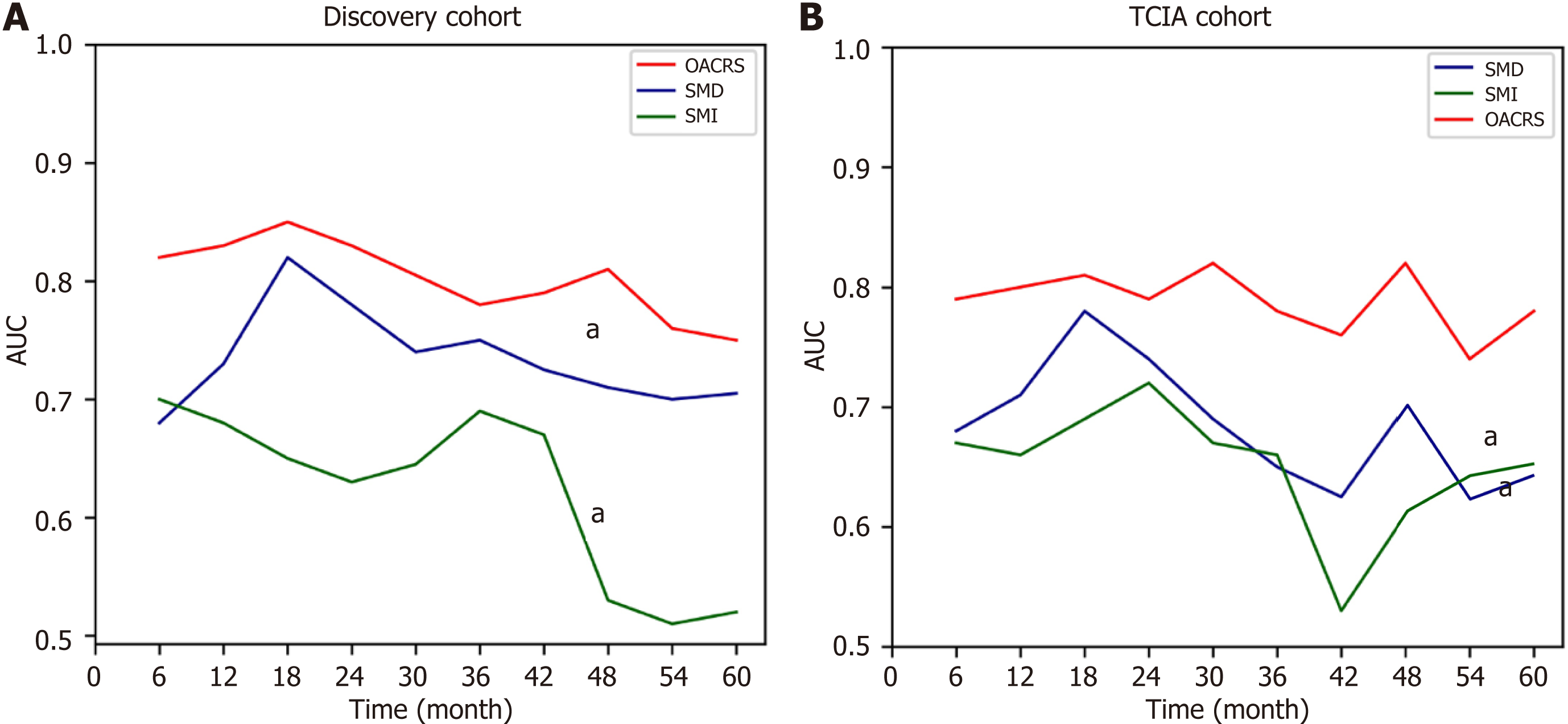
Previous studies have demonstrated that SMI and SMD are independent risk factors for OS in gastric cancer, consistent with the present data. Moreover, the current study revealed that OACRS is an independent risk factor for OS in stage II/III gastric cancer, which has rarely been reported. OACRS might be a more robust predictor (P < 0.001) than SMI (P = 0.009) and SMD (P = 0.002). To compare the OS prediction performance of SMI, SMD, and OACRS, the time-dependent area under the curve (TAUC) was plotted, a novel method that can calculate the area under ROC of multiple time points. The TAUCs of SMI, SMD, and OACRS indicated that OACRS could predict OS more accurately (aP < 0.05) in the discovery and TCIA cohorts (Figure 5). These findings revealed that OACRS outperformed SMI and SMD for OS pre
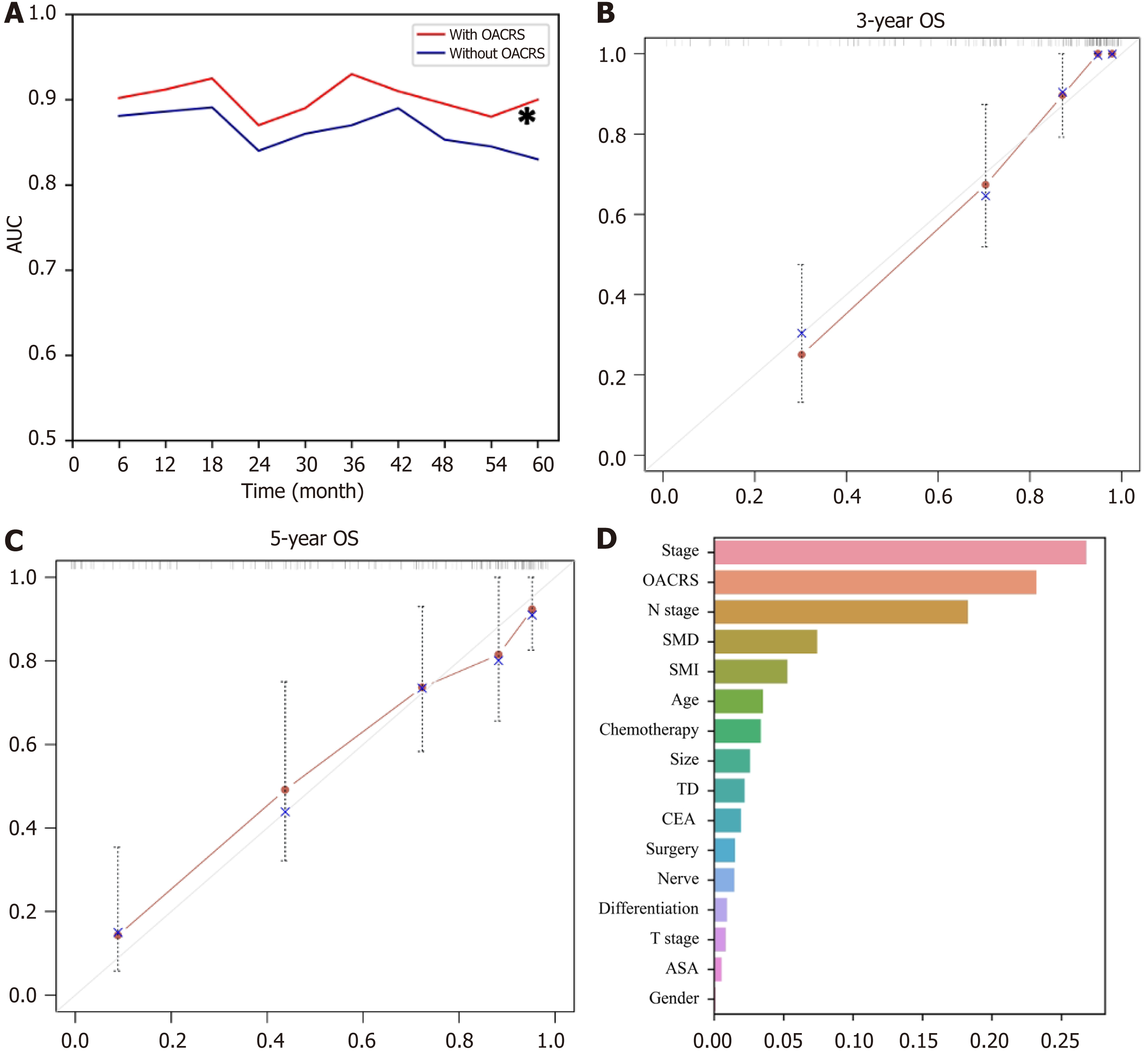
RSF, a prevalent, flexible, and capable algorithm, was applied to develop a prediction model using the discovery cohort to meet clinical practice requirements. Two RSF models were developed to determine whether OACRS could improve predictive accuracy: one with and one without OACRS. The AUCs of the RSF model demonstrating good discrimination of OS are displayed in Figure 6A. The model with OACRS outperformed that without OACRS (aP < 0.05). These findings revealed that OACRS could improve the predictive accuracy of OS. Furthermore, the C-index values for 3- and 5-year OS were 0.835 and 0.806, respectively, indicating favorable performance. The calibration curves of the 3- and 5-year OS also demonstrated good concordance between the predictions and the ground truth (Figure 6B and C). In addition, decision curve analysis indicated that the model, including OACRS, outperformed the models, including SMI or SMD (Supp
Radiomics assessment is a novel approach for evaluating tumor prognosis. SMI and SMD have been identified as bio
The OS of locally advanced gastric cancer remains very poor and patients undergo radical surgery and extended nodal dissection. Gastric cancer is heterogeneous, necessitating accurate prognostic prediction for the selection of appropriate treatment or long-term management. Compared to expensive or invasive assessments, noninvasive and inexpensive biomarkers are more easily accepted by patients and clinicians. Imaging data provides some opportunities to overcome these challenges, and accumulating studies have been reported to predict the outcome of gastric cancer using pre
Gastric cancer is a highly heterogeneous disease, and a reliable and comprehensive evaluation of gastric cancer may provide new insights into improving OS. Pathology and genomics are two typical approaches for predicting OS; however, the need for high-quality tissue and intratumoral spatial heterogeneity is limited in clinical practice. Radiomics provides some unique advantages that allow the evaluation of tumors in a wide-ranging and non-invasive manner, and growing evidence has revealed that radiomics is associated with the response to chemoradiotherapy, immunotherapy, and the heterogeneity of tumor cells[23-26]. Moreover, several previous studies have revealed the potential association between radiomics and the tumor microenvironment, such as the number of tumor-infiltrating lymphocytes, macro
In summary, this study demonstrated that the independent predictive value of radiomics for stage Ⅱ/Ⅲ gastric cancer outperformed SMI and SMD. Moreover, a prediction model incorporating OACRS was developed to meet the clinical application requirements, which exhibited exceptional discrimination potential. This study had several limitations. First, a selection bias was unavoidable as this was a retrospective study. Second, the types and timing of anticancer drugs and radiotherapy applications were excluded, which may have increased the uncertainty in the results. Third, other factors associated with body composition, such as glucocorticoid usage and athlete status, were excluded, which may have affected the accuracy of the results. Finally, the RSF model requires external validation. Consequently, prospective studies with large sample sizes are recommended to further validate the correlation between radiomics and stage II/III gastric cancer OS.
Accurately evaluating the overall survival (OS) of gastric cancer patients remains difficult. Compelling evidence showed that radiomics was related to tumor stroma, heterogeneity, antitumor immunity and tumor microenvironment.
To develop an OS-associated computed tomography image radiomics score (OACRS) based on 141 patients from two cohorts using machine learning and radiomics.
To investigate the association between radiomics and OS of gastric cancer to develop a robust and non-invasive bio
A retrospective multi-cohort study was conducted. Approximately 1700 radiomics features were extracted from primary tumor and 36 important features were selected as predictors to calculated OACRS.
OACRS was a risk factor and was independent of skeletal muscle index (SMI), skeletal muscle density (SMD), and pathological features. Importantly, OACRS outperformed SMI and SMD and could improve OS prediction.
A novel biomarker based on machine learning and radiomics was developed that exhibited exceptional OS discrimination potential. Gastric cancer patients who have a higher OACSR might have a poor OS.
Considering the nature of retrospective studies, prospective studies with large sample sizes are recommended to further validate the correlation between radiomics and stage II/III gastric cancer OS.
| 1. | Carlisle JW, Steuer CE, Owonikoko TK, Saba NF. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin. 2020;70:505-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 774] [Article Influence: 96.8] [Reference Citation Analysis (1)] |
| 3. | Li X, Zhai Z, Ding W, Chen L, Zhao Y, Xiong W, Zhang Y, Lin D, Chen Z, Wang W, Gao Y, Cai S, Yu J, Zhang X, Liu H, Li G, Chen T. An artificial intelligence model to predict survival and chemotherapy benefits for gastric cancer patients after gastrectomy development and validation in international multicenter cohorts. Int J Surg. 2022;105:106889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Sexton RE, Hallak MNA, Uddin MH, Diab M, Azmi AS. Gastric Cancer Heterogeneity and Clinical Outcomes. Technol Cancer Res Treat. 2020;19:1533033820935477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Chen Q, Zhang L, Liu S, You J, Chen L, Jin Z, Zhang S, Zhang B. Radiomics in precision medicine for gastric cancer: opportunities and challenges. Eur Radiol. 2022;32:5852-5868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography-defined low skeletal muscle index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle. 2021;12:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3948] [Article Influence: 438.7] [Reference Citation Analysis (0)] |
| 8. | Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022;19:132-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 544] [Article Influence: 136.0] [Reference Citation Analysis (1)] |
| 9. | Jiang Y, Zhou K, Sun Z, Wang H, Xie J, Zhang T, Sang S, Islam MT, Wang JY, Chen C, Yuan Q, Xi S, Li T, Xu Y, Xiong W, Wang W, Li G, Li R. Non-invasive tumor microenvironment evaluation and treatment response prediction in gastric cancer using deep learning radiomics. Cell Rep Med. 2023;4:101146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 10. | Jiang Y, Zhang Z, Yuan Q, Wang W, Wang H, Li T, Huang W, Xie J, Chen C, Sun Z, Yu J, Xu Y, Poultsides GA, Xing L, Zhou Z, Li G, Li R. Predicting peritoneal recurrence and disease-free survival from CT images in gastric cancer with multitask deep learning: a retrospective study. Lancet Digit Health. 2022;4:e340-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Specogna AV, Sinicrope FA. Defining colon cancer biomarkers by using deep learning. Lancet. 2020;395:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Deo RC. Machine Learning in Medicine. Circulation. 2015;132:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 2155] [Article Influence: 215.5] [Reference Citation Analysis (7)] |
| 13. | Fleuren LM, Klausch TLT, Zwager CL, Schoonmade LJ, Guo T, Roggeveen LF, Swart EL, Girbes ARJ, Thoral P, Ercole A, Hoogendoorn M, Elbers PWG. Machine learning for the prediction of sepsis: a systematic review and meta-analysis of diagnostic test accuracy. Intensive Care Med. 2020;46:383-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 14. | Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1512] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 15. | Jin Y, Ma X, Yang Z, Zhang N. Low L3 skeletal muscle index associated with the clinicopathological characteristics and prognosis of ovarian cancer: a meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:697-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 16. | Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210:489-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 560] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 17. | Chen S, Nie RC, OuYang LY, Li YF, Xiang J, Zhou ZW, Chen Y, Peng J. Body mass index (BMI) may be a prognostic factor for gastric cancer with peritoneal dissemination. World J Surg Oncol. 2017;15:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Ding P, Yang P, Yang L, Sun C, Chen S, Li M, Lowe S, Guo H, Tian Y, Liu Y, Zhao Q. Impact of skeletal muscle loss during conversion therapy on clinical outcomes in lavage cytology positive patients with gastric cancer. Front Oncol. 2022;12:949511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Bir Yucel K, Karabork Kilic AC, Sutcuoglu O, Yazıcı O, Aydos U, Kilic K, Özdemir N. Effects of Sarcopenia, Myosteatosis, and the Prognostic Nutritional Index on Survival in Stage 2 and 3 Gastric Cancer Patients. Nutr Cancer. 2023;75:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Ma LX, Taylor K, Espin-Garcia O, Anconina R, Suzuki C, Allen MJ, Honorio M, Bach Y, Allison F, Chen EX, Brar S, Swallow CJ, Yeung J, Darling GE, Wong R, Kalimuthu SN, Jang RW, Veit-Haibach P, Elimova E. Prognostic significance of nutritional markers in metastatic gastric and esophageal adenocarcinoma. Cancer Med. 2021;10:199-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Surov A, Benkert F, Pönisch W, Meyer HJ. CT-defined body composition as a prognostic factor in multiple myeloma. Hematology. 2023;28:2191075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Li LQ, Zhao WD, Su TS, Wang YD, Meng WW, Liang SX. Effect of Body Composition on Outcomes in Patients with Hepatocellular Carcinoma Undergoing Radiotherapy: A Retrospective Study. Nutr Cancer. 2022;74:3302-3311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Porcu M, Solinas C, Mannelli L, Micheletti G, Lambertini M, Willard-Gallo K, Neri E, Flanders AE, Saba L. Radiomics and "radi-…omics" in cancer immunotherapy: a guide for clinicians. Crit Rev Oncol Hematol. 2020;154:103068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Shin J, Seo N, Baek SE, Son NH, Lim JS, Kim NK, Koom WS, Kim S. MRI Radiomics Model Predicts Pathologic Complete Response of Rectal Cancer Following Chemoradiotherapy. Radiology. 2022;303:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 25. | Zhao H, Gao J, Bai B, Wang R, Yu J, Lu H, Cheng M, Liang P. Development and external validation of a non-invasive imaging biomarker to estimate the microsatellite instability status of gastric cancer and its prognostic value: The combination of clinical and quantitative CT-imaging features. Eur J Radiol. 2023;162:110719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 26. | Wang X, Xie T, Luo J, Zhou Z, Yu X, Guo X. Radiomics predicts the prognosis of patients with locally advanced breast cancer by reflecting the heterogeneity of tumor cells and the tumor microenvironment. Breast Cancer Res. 2022;24:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 27. | Li G, Li L, Li Y, Qian Z, Wu F, He Y, Jiang H, Li R, Wang D, Zhai Y, Wang Z, Jiang T, Zhang J, Zhang W. An MRI radiomics approach to predict survival and tumour-infiltrating macrophages in gliomas. Brain. 2022;145:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 28. | Yu Y, He Z, Ouyang J, Tan Y, Chen Y, Gu Y, Mao L, Ren W, Wang J, Lin L, Wu Z, Liu J, Ou Q, Hu Q, Li A, Chen K, Li C, Lu N, Li X, Su F, Liu Q, Xie C, Yao H. Magnetic resonance imaging radiomics predicts preoperative axillary lymph node metastasis to support surgical decisions and is associated with tumor microenvironment in invasive breast cancer: A machine learning, multicenter study. EBioMedicine. 2021;69:103460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yin J, China S-Editor: Yan JP L-Editor: Webster JR P-Editor: ZhangYL