Published online Nov 27, 2024. doi: 10.4240/wjgs.v16.i11.3499
Revised: September 3, 2024
Accepted: September 19, 2024
Published online: November 27, 2024
Processing time: 83 Days and 6.5 Hours
Laparoscopic hernia repair is a minimally invasive surgery, but patients may experience emergence agitation (EA) during the post-anesthesia recovery period, which can increase pain and lead to complications such as wound reopening and bleeding. There is limited research on the risk factors for this agitation, and few effective tools exist to predict it. Therefore, by integrating clinical data, we have developed nomograms and random forest predictive models to help clinicians predict and potentially prevent EA.
To establish a risk nomogram prediction model for EA in patients undergoing laparoscopic hernia surgery under total inhalation combined with sacral block anesthesia.
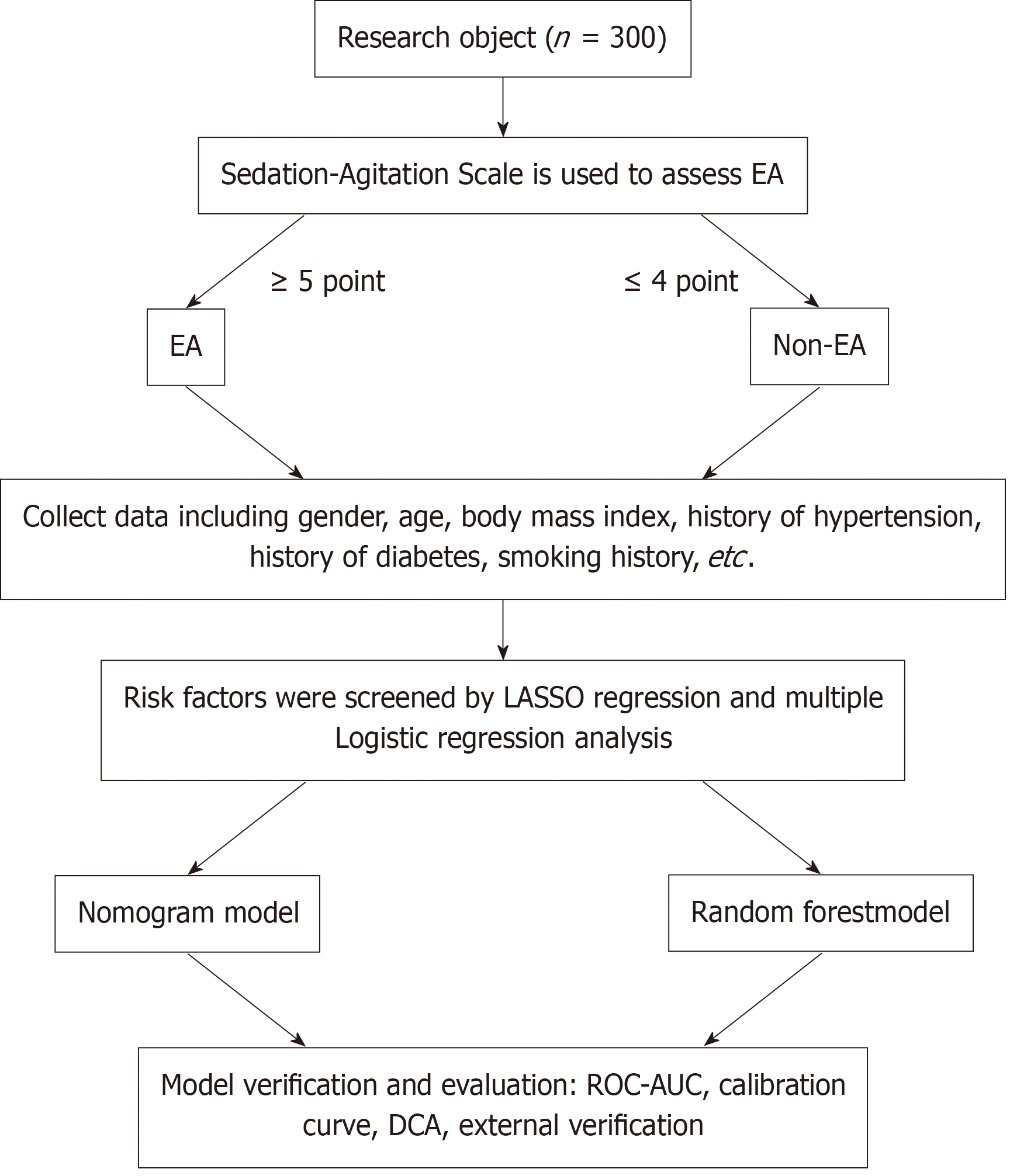
Based on the clinical information of 300 patients who underwent laparoscopic hernia surgery in the Nanning Tenth People’s Hospital, Guangxi, from January 2020 to June 2023, the patients were divided into two groups according to their sedation-agitation scale score, i.e., the EA group (≥ 5 points) and the non-EA group (≤ 4 points), during anesthesia recovery. Least absolute shrinkage and selection operator regression was used to select the key features that predict EA, and incorporating them into logistic regression analysis to obtain potential pre
Out of the 300 patients, 72 had agitation during anesthesia recovery, with an incidence of 24.0%. American Society of Anesthesiologists classification, preoperative anxiety, solid food fasting time, clear liquid fasting time, indwelling catheter, and pain level upon awakening are key predictors of EA in patients undergoing laparoscopic hernia surgery with total intravenous anesthesia and caudal block anesthesia. The nomogram predicts EA with an area under the receiver operating characteristic curve (AUC) of 0.947, a sensi
Independent predictors of EA in patients undergoing laparoscopic hernia repair with total intravenous anesthesia combined with caudal block include American Society of Anesthesiologists classification, preoperative anxiety, duration of solid food fasting, duration of clear liquid fasting, presence of an indwelling catheter, and pain level upon waking. The nomogram and random forest models based on these factors can help tailor clinical decisions in the future.
Core Tip: This study identified American Society of Anesthesiologists classification, preoperative anxiety, fasting duration, catheterization, and pain level during emergence as major risk factors for emergence agitation. It constructed nomograms and a random forest prediction model with high accuracy and clinical utility, aiding physicians assess and predict emergence agitation and guide personalized medical interventions, improving patient safety and recovery after surgery.
- Citation: Zhu YF, Yi FY, Qin MH, Lu J, Liang H, Yang S, Wei YZ. Factors influencing agitation during anesthesia recovery after laparoscopic hernia repair under total inhalation combined with caudal block anesthesia. World J Gastrointest Surg 2024; 16(11): 3499-3510
- URL: https://www.wjgnet.com/1948-9366/full/v16/i11/3499.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i11.3499
A groin hernia, commonly referred to as “hernia”, is a common disease in the groin area. Usually, it can be pushed back into the abdomen, but if it becomes trapped and cannot be reduced, immediate treatment is required. If a strangulated hernia is not treated promptly, it can cut off blood supply to the intestinal tract, potentially leading to necrosis of the intestines[1]. Laparoscopic hernia repair is favored by both doctors and patients due to its shorter operating time, faster postoperative recovery, and lower recurrence rates[2,3]. However, it requires expert handling of pneumoperitoneum, necessitating proper depth of general anesthesia to tolerate laparoscopy, imposing significant demands on anesthesiologists in terms of intraoperative sedation and pain management, postoperative safety, rapid recovery, and awakening.
Inhalation anesthesia has the advantages of rapid absorption and elimination, good controllability, and rapid awa
Predictive models are commonly used in clinical practice to predict the adverse events and prognosis of survival, etc.[9]. They help medical teams identify potential causes and risk factors for EA, improving anesthesia and postoperative care. There is currently no research that clearly identifies risk factors for EA in patients undergoing laparoscopic hernia surgery under general inhalation combined with caudal block anesthesia, and there is a lack of related predictive models. Therefore, we conducted a retrospective analysis to identify factors affecting EA in patients undergoing laparoscopic hernia surgery under general inhalation combined with caudal block anesthesia, and constructed a risk prediction model to help take early, targeted measures to reduce EA incidence.
This was a retrospective study involving 300 patients who underwent laparoscopic hernia repair surgery at the Nanning Tenth People’s Hospital, Guangxi, from January 2020 to June 2023. Inclusion criteria were as follows: (1) Patients who had combined general inhalation anesthesia and sacral epidural block, and were transferred to the post-anesthesia care unit after laparoscopic hernia surgery; (2) Patients with normal intelligence, clear consciousness, and the ability to commu
The main results of this study involve creating a predictive model for EA using logistic regression analysis. According to the events per variable method[11], which requires 10 events per independent variable, we need 60 EA cases for a model with 6 variables. Since EA incidence in adults ranges from 4.7% to 22.2%[12], this translates to needing a sample size of at least 271 to 1277 cases. Considering the actual situation of our hospital, 300 patients were finally included in the study.
Methods of anesthesia: Once in the operating room, all patients underwent routine electrocardiogram monitoring and intravenous access was established, with their heart rate, blood pressure, pulse oximetry, and electrocardiogram being monitored: (1) General inhalation anesthesia: Inhalation anesthesia was administered at a rate of 3 mL/minute with sevoflurane (Shanghai Hengrui Pharmaceutical Co., Ltd., China, drug approval number H20070172, specification, 250 mL), along with 0.1 mg/kg atracurium besylate and 1.0 μg/kg fentanyl citrate for induction; and (2) Sacral epidural block anesthesia: After general anesthesia, the patient was positioned in the lateral decubitus position with hips and knees flexed at 90° to locate the sacral hiatus. A 6-gauge needle was inserted subcutaneously, followed by a 25-gauge needle angled at 25° to the coronal plane, passing through the sacrococcygeal ligament until a loss of resistance was felt, with no cerebrospinal fluid or blood return upon aspiration. Then, 12 mL of normal saline was injected, followed by 1.0 mL/kg of 0.25% ropivacaine (produced by Ruiyang Pharmaceutical Co., Ltd., China drug approval number H20183152). Once the baseline anesthesia took effect, sevoflurane was continued at a concentration of 2% to 3%, at a rate of 4 L/minute. The anesthesia effect was monitored, and 0.5 μg/kg of fentanyl citrate was given if needed.
Methods of laparoscopic hernia operation: The patient is in a supine position to allow access to the groin area. The doctor makes small incisions, typically about 1 cm below the navel, and injects carbon dioxide gas into the abdomen to create space for the laparoscopic surgery. Through the small incision, the doctor inserts a laparoscope to observe the groin area and guide the operation of surgical tools. The laparoscope allows the doctor to accurately locate the hernia sac. Once located, tension-free mesh is inserted through the incision to cover the hernia, reinforce the abdominal wall, and prevent recurrence. After the surgery, the laparoscope and surgical instruments are removed and the incision is closed with sutures or adhesive tape.
The sedation-agitation scale (SAS)[13], developed by Riker et al[13], helps identify and quantify agitation and sedation levels. We used the SAS to assess agitation during the postoperative recovery. The scale ranges from 1 to 7, with a score of ≤ 4 indicating no agitation, and a score of ≥ 5 indicating agitation, where 5 points is mild agitation, 6 points is moderate agitation, and 7 points is severe agitation (Table 1).
| Classification | Point | Description |
| Unable to wake up | 1 | The patient has little or no response to harmful stimuli, does not communicate, or does not follow instructions |
| Very calm | 2 | Patients respond to physical stimuli but cannot communicate or follow commands and may move spontaneously |
| Calm | 3 | The patient is awakened by verbal stimulation or slight shaking, but falls asleep again; follow simple instructions |
| Cool fit | 4 | The patient is calm, wakes easily, and follows instructions |
| EA | 5 | The patient is anxious or mildly agitated and tries to sit up and calm down on verbal instructions |
| Moderate EA | 6 | The patient is not calm, despite the doctor’s frequent verbal reminders of limitations; the patient needs to be physically restrained and bite the tracheal catheter |
| Severe EA | 7 | Patients pulled tracheal tubes, tried to remove them, climbed over bed bars, hit staff, convulsed back to back |
General information on all patients was gathered, including gender, age, body mass index, history of hypertension, his
Statistical analysis was performed using SPSS 23.0 and R Studio software. The normality of continuous variables was tested using the Kolmogorov-Smirnov method. Skewed continuous variables were expressed as median (P25, P75) values, and categorical variables were presented as frequencies and percentages. Univariate analysis was conducted using the χ² test and Mann-Whitney U test. Least absolute shrinkage and selection operator (LASSO) regression selected the best predictive features among risk factors, which were then analyzed with logistic regression to identify all potential predictors. A predictive model for EA risk was constructed using the R software. A calibration curve was used to assess the model’s accuracy. The bootstrap method validated the model internally and measured the discriminatory ability of the model. Furthermore, predictive model’s efficiency was evaluated using the receiver operating characteristic curve. A clinical decision curve assessed the clinical utility of the model. A difference was considered statistically significant when P < 0.05.
We conducted this study following the process outlined in Figure 1.
Patients were divided into the EA group (≥ 5 points) and the non-EA group (≤ 4 points) based on their SAS scores. Significant differences between the two groups were observed in gender, ASA classification, preoperative anxiety, pre
| EA (n = 72) | Non-EA (n = 228) | χ2/Z | P value | |
| Gender | ||||
| Male/female | 45/27 | 89/139 | 12.190 | < 0.001 |
| Age (year) | 38 (36, 40) | 39 (34, 43) | -1.413 | 0.158 |
| Body mass index (kg/m2) | 23.43 (22.01, 25.03) | 23.36 (21.84, 25.19) | -0.372 | 0.710 |
| Hypertension | 11 (15.28) | 54 (23.68) | 2.278 | 0.131 |
| Diabetes | 13 (18.06) | 58 (25.44) | 1.651 | 0.199 |
| Smoking | 27 (37.50) | 60 (26.32) | 3.324 | 0.068 |
| ASA classification | ||||
| I | 11 (15.28) | 71 (31.14) | -3.068 | 0.002 |
| II | 52 (72.22) | 145 (63.60) | ||
| III | 9 (12.50) | 12 (5.26) | ||
| Preoperative anxiety | 59 (81.94) | 81 (35.53) | 47.371 | < 0.001 |
| Preoperative fasting duration (hours) | ||||
| Solid food | 7.45 (6.95, 7.80) | 6.90 (6.60, 7.20) | -7.045 | < 0.001 |
| Clear liquid food | 3.30 (2.93, 3.50) | 2.85 (2.60, 3.20) | -5.709 | < 0.001 |
| Intraoperative hypothermia | 35 (48.61) | 63 (27.63) | 10.950 | 0.001 |
| Intraoperative blood loss (mL) | 11 (10, 12) | 11 (9, 12) | -0.128 | 0.898 |
| Operation time (minutes) | 41.00 (37.00, 44.00) | 40.00 (36.00, 44.75) | -0.525 | 0.600 |
| Indwelling catheter | 44 (61.11) | 82 (35.96) | 14.204 | < 0.001 |
| Pain degree during recovery | 6 (6, 7) | 5 (4, 6) | -7.901 | < 0.001 |
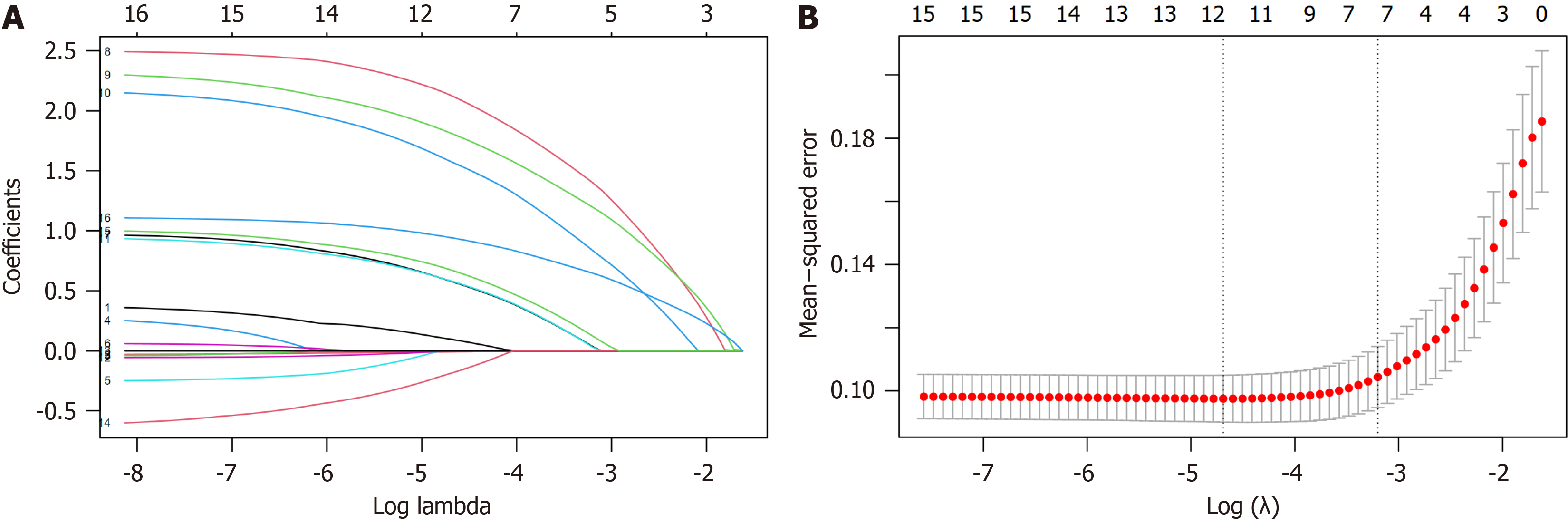
LASSO regression was used to identify the most influential factors among demographic and perioperative indicators. As lambda increased, the coefficients of the initial 16 variables were compressed, leading to unimportant variables being shrunk to 0 (Figure 2A). The lambda value that resulted in the minimum mean squared error within one standard error of the cross-validated error was selected as the optimal value for the model (lambda.1se), which corresponded to 7 selected variables indicated by the right dashed line. These variables, ranked in descending order, include preoperative anxiety, duration of solid food fasting, duration of clear fluid fasting, pain degree during recovery, indwelling catheter, intraoperative hypothermia, and ASA classification (Figure 2B).
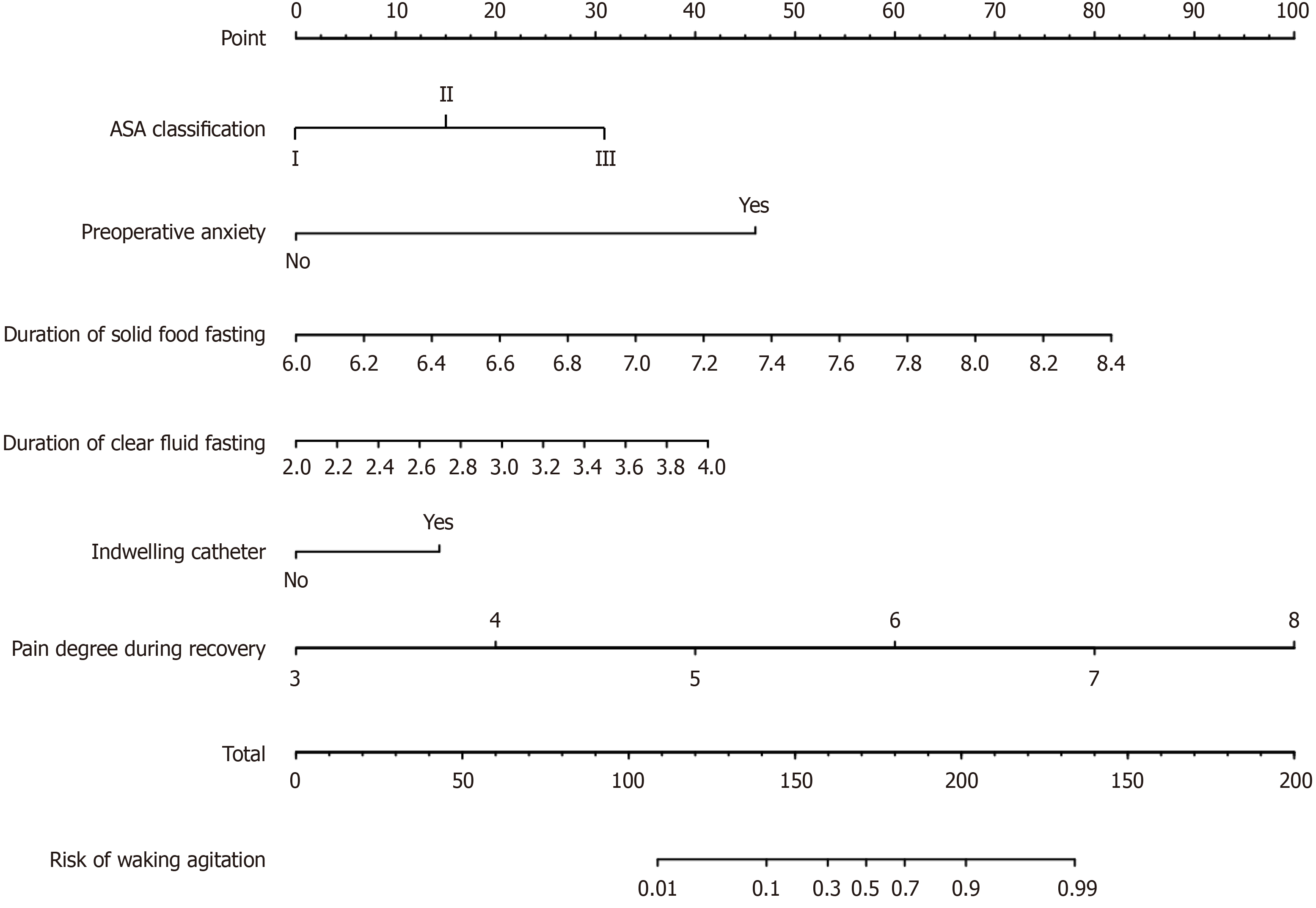
The variables selected by LASSO regression were included in the logistic regression model. The results identified ASA classification, preoperative anxiety, duration of solid food fasting, duration of clear fluid fasting, indwelling catheter, and pain degree during recovery as risk factors for EA in patients undergoing laparoscopic hernia repair under combined inhalation and epidural anesthesia (Table 3).
| Variable | β | SE | P value | OR | 95%CI |
| ASA classification | 0.935 | 0.405 | 0.021 | 6.487 | 1.328-31.671 |
| Preoperative anxiety | 2.591 | 0.508 | < 0.001 | 13.340 | 4.929-36.101 |
| Duration of solid food fasting | 2.259 | 0.490 | < 0.001 | 4.860 | 2.482-9.515 |
| Duration of clear fluid fasting | 2.119 | 0.564 | < 0.001 | 4.408 | 2.035-9.551 |
| Indwelling catheter | 0.968 | 0.444 | 0.029 | 2.633 | 1.103-6.287 |
| Pain degree during recovery | 1.172 | 0.228 | < 0.001 | 10.430 | 4.268-25.488 |
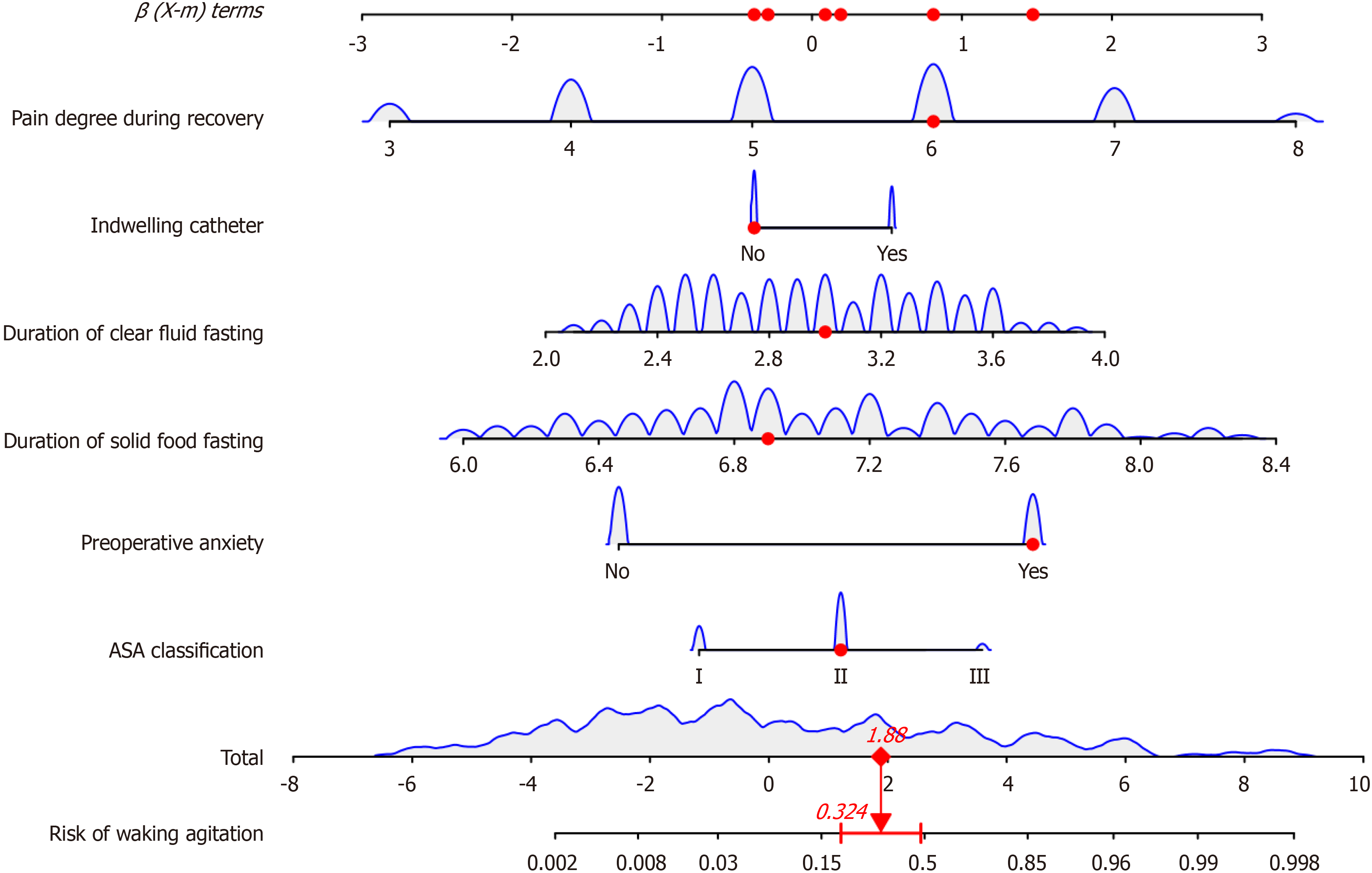
Based on 6 independent risk factors (ASA classification, preoperative anxiety, duration of solid food fasting, duration of clear fluid fasting, indwelling catheter, and pain degree during recovery), a nomogram predictive model was constructed (Figure 3). For example, if a patient has a recovery pain score of 6, a clear fluid fasting time of 3 hours, a solid food fasting time of 6.9 hours, preoperative anxiety, and ASA classification of grade II, the estimated risk of EA is 32.4% (Figure 4).
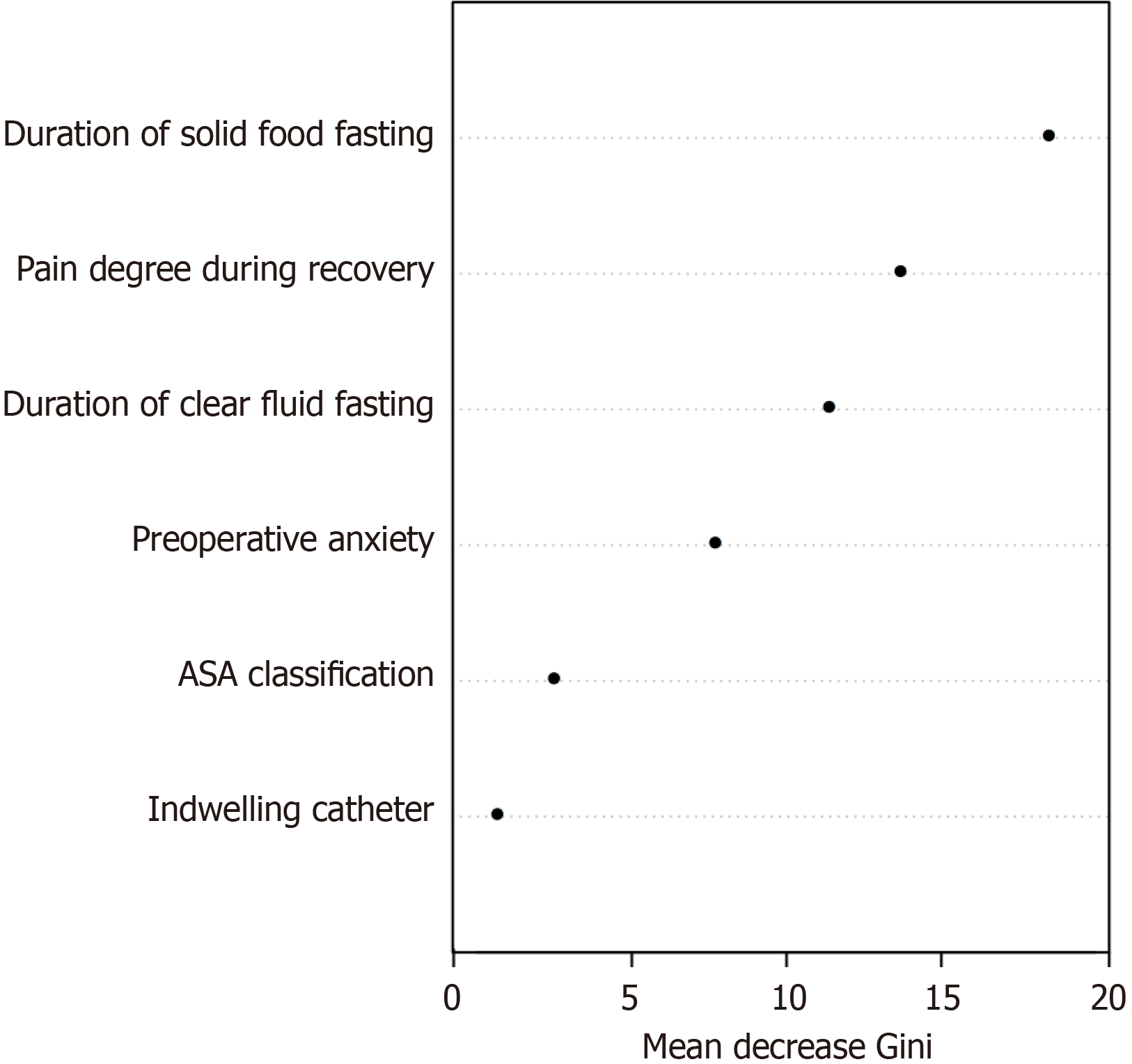
A random forest prediction model based on multifactor logistic regression analysis was built. The out-of-bag error of the random forest model is 8.96%, with the importance ranking of 6 most important variables as duration of solid food fasting, pain degree during recovery, duration of clear fluid fasting, preoperative anxiety, ASA classification, and ind
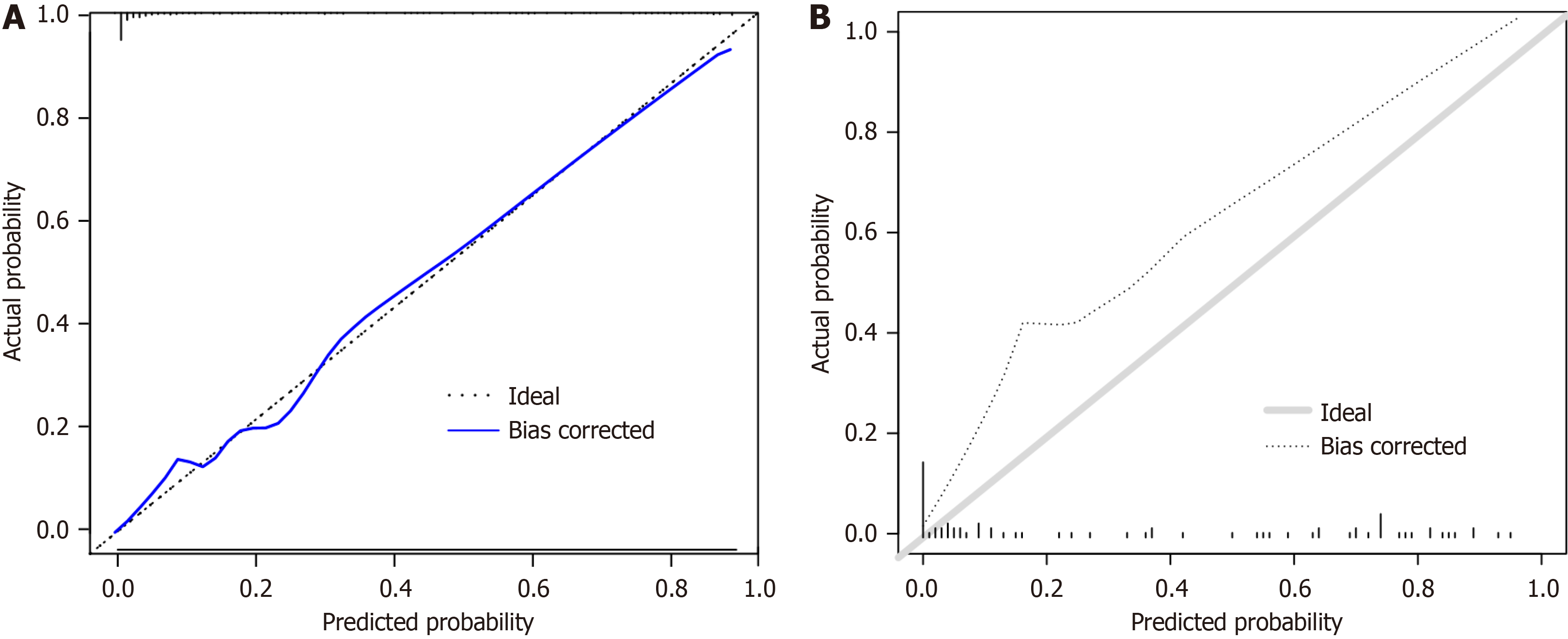
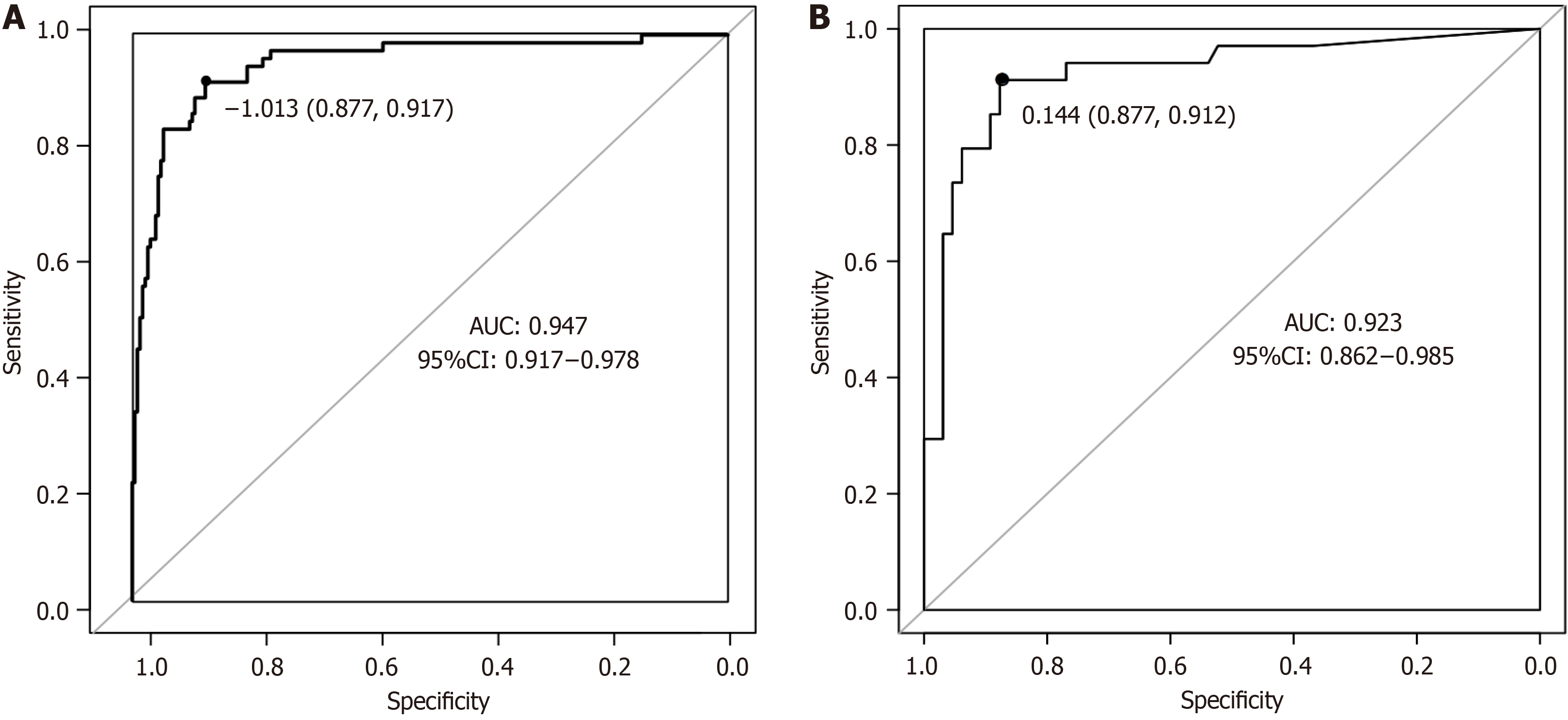
The prediction model for postoperative EA occurrence and the actual occurrence relationship through the calibration curve were evaluated, and it is found that the calibration curve is close to the reference line, indicating that the model’s calibration is good (Figure 6). The receiver operating characteristic curve analysis shows that the nomogram model has an area under the receiver operating characteristic curve (AUC) of 0.947 (95% confidence interval: 0.917-0.978), with a sen
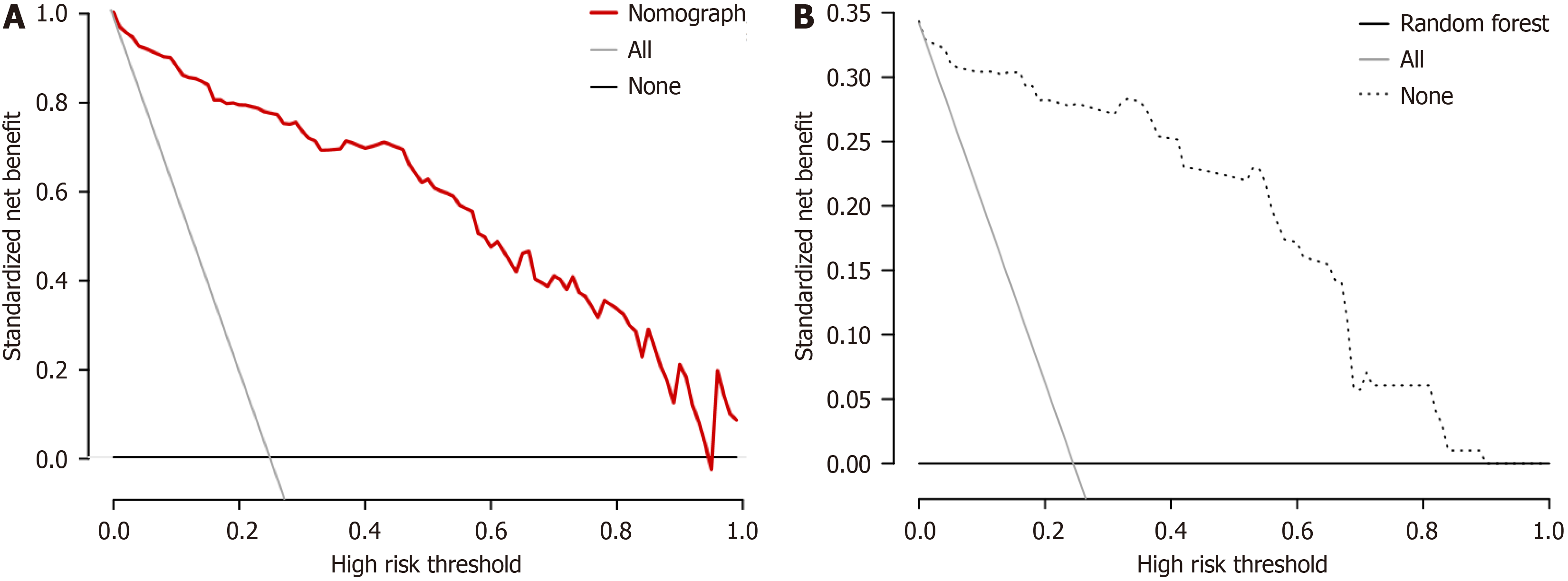
The decision curve shows that the nomogram model provides good net benefits for predicting EA within a threshold range of 0.02 to 0.96. The random forest model also offers good net benefits within a threshold range of 0.03 to 0.90 (Figure 8).
From November 2023 to April 2024, we tested the models on 50 patients from our hospital. The nomogram model predicted EA with a sensitivity of 83.33% (10/12), a specificity of 86.84% (33/38), and an accuracy of 86.00%. The random forest model also predicted EA with a sensitivity of 75.00% (9/12), a specificity of 78.95% (30/38), and an accuracy of 78.00% (Table 4).
| Models | Reality | Prediction | Total | |
| EA | Non-EA | |||
| Nomogram | EA | 10 | 2 | 12 |
| Non-EA | 5 | 33 | 38 | |
| Total | 15 | 35 | 50 | |
| Random forest | EA | 9 | 3 | 12 |
| Non-EA | 6 | 30 | 38 | |
| Total | 17 | 33 | 50 | |
EA can lead to self-harm, harm to healthcare providers, catheter removal, airway spasm, displacement, rupture, bleeding, and other adverse outcomes, prolonging hospital stay, increasing healthcare burden, and mortality rates[8,9]. Therefore, it is necessary to identify risk factors for EA early, reduce its incidence, and provide appropriate treatment. Developing a predictive model helps healthcare professionals provide precise medical care and develop personalized and effective treatment plans. The nomogram is a reliable and practical predictive tool that integrates different prognostic and de
How to screen important features from multidimensional and complex medical data, deeply explore influencing factors, and make subsequent intervention measures more targeted is a question worth pondering. In this study, the incidence of EA was 24%. LASSO regression and multivariate logistic regression determined ASA classification, pre
A meta-analysis of 21 studies on postoperative agitation in non-cardiac surgery patients found that a high ASA classification is the main risk factor for EA[19]. The ASA classification system assesses the patient’s condition and surgery risk before an operation. The higher the ASA classification, the worse the patient’s condition and tolerance to anesthetic drugs, and the more likely there will be fluctuations in respiration, circulation, and internal environment during anes
Previous studies have linked preoperative anxiety to EA[24]. The results of this study also confirm that preoperative anxiety is a risk factor. On the one hand, laparoscopic surgery is a traumatic event that may trigger stress; on the other hand, preoperative anxiety and tension can affect sleep and dietary habits and emotional reactions before surgery, and procedural stimuli during surgery can activate the patient’s sympathetic nervous system, prolonging arousal and in
Preoperative fasting is required for laparoscopic hernia surgery, but there is limited research on its impact on EA. The ASA and other organizations recommend fasting from solid foods for 6 hours and clear fluids for 2 hours before surgery[26]. In this study, the agitation group had significantly longer fasting times than the non-agitation group, exceeding re
Indwelling catheters are commonly used in the perioperative period to collect urine for measuring urine output and assessing blood volume. However, indwelling catheters may cause bladder discomfort, irritation and burning sensations, pain, urgency, and frequency during the postoperative awakening period[29], which increases the risk of EA. Although laparoscopic surgery is minimally invasive and efficient, the manipulation of muscle traction can still cause postoperative pain. This pain may lead to reflex resistance during the awakening period, triggering complications such as tachycardia and hypoxemia[30], and may also lead to complex neurobehavioral responses[31], resulting in EA. A systematic study reported that inadequate postoperative analgesia is a risk factor for EA[32]. Based on the aforementioned study, it is recommended to use multimodal postoperative analgesia, particularly preventive analgesia, such as local nerve blocks before the surgery ends. This can control pain early in the recovery phase and reduce pain level. Effective pain manage
In summary, ASA classification, preoperative anxiety, duration of solid food fasting, duration of clear fluid fasting, indwelling catheter, and pain degree during recovery are key risk factors for postoperative agitation in patients under
| 1. | Mansoor A, Shaukat R. Inguinal hernia leading to omental torsion: Role of CT in differentiating from other clinical mimics - a case report and literature review. J Radiol Case Rep. 2023;17:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Xu LS, Li Q, Wang Y, Wang JW, Wang S, Wu CW, Cao TT, Xia YB, Huang XX, Xu L. Current status and progress of laparoscopic inguinal hernia repair: A review. Medicine (Baltimore). 2023;102:e34554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Xu TQ, Higgins RM. The Minimally Invasive Inguinal Hernia: Current Trends and Considerations. Surg Clin North Am. 2023;103:875-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Pehlivan B, Akçay M, Atlas A, Erol MK, Duran E, Karahan MA, Binici O, Büyükfırat E, Altay N. Comparison of General Anesthesia (Sevoflurane) and Spinal Anesthesia (Levobupivacaine) Methods on QT Dispersion in Inguinal Hernia Operations. Cureus. 2020;12:e9079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Wang LZ, Hu XX, Zhang YF, Chang XY. A randomized comparison of caudal block by sacral hiatus injection under ultrasound guidance with traditional sacral canal injection in children. Paediatr Anaesth. 2013;23:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Benka AU, Pandurov M, Galambos IF, Rakić G, Vrsajkov V, Drašković B. [Effects of caudal block in pediatric surgical patients: a randomized clinical trial]. Braz J Anesthesiol. 2020;70:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Tolly B, Waly A, Peterson G, Erbes CR, Prielipp RC, Apostolidou I. Adult Emergence Agitation: A Veteran-Focused Narrative Review. Anesth Analg. 2021;132:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73:471-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Record SM, Chanenchuk T, Parrish KM, Kaplan SJ, Kimmick G, Plichta JK. Prognostic Tools for Older Women with Breast Cancer: A Systematic Review. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Hocevar LA, Fitzgerald BM. American Society of Anesthesiologists Staging. 2023 Jan 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 11. | van Smeden M, Moons KG, de Groot JA, Collins GS, Altman DG, Eijkemans MJ, Reitsma JB. Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat Methods Med Res. 2019;28:2455-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 439] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 12. | Munk L, Andersen G, Møller AM. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol Scand. 2016;60:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 625] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 14. | Dunstan DA, Scott N. Norms for Zung's Self-rating Anxiety Scale. BMC Psychiatry. 2020;20:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 374] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 15. | He S, Renne A, Argandykov D, Convissar D, Lee J. Comparison of an Emoji-Based Visual Analog Scale With a Numeric Rating Scale for Pain Assessment. JAMA. 2022;328:208-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | König C, Grensemann J, Czorlich P, Schlemm E, Kluge S, Wicha SG. A dosing nomograph for cerebrospinal fluid penetration of meropenem applied by continuous infusion in patients with nosocomial ventriculitis. Clin Microbiol Infect. 2022;28:1022.e9-1022.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Yu S, Yuan J, Lin H, Xu B, Liu C, Shen Y. A predictive model based on random forest for shoulder-hand syndrome. Front Neurosci. 2023;17:1124329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Shi G, Liu G, Gao Q, Zhang S, Wang Q, Wu L, He P, Yu Q. A random forest algorithm-based prediction model for moderate to severe acute postoperative pain after orthopedic surgery under general anesthesia. BMC Anesthesiol. 2023;23:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 19. | Sadeghirad B, Dodsworth BT, Schmutz Gelsomino N, Goettel N, Spence J, Buchan TA, Crandon HN, Baneshi MR, Pol RA, Brattinga B, Park UJ, Terashima M, Banning LBD, Van Leeuwen BL, Neerland BE, Chuan A, Martinez FT, Van Vugt JLA, Rampersaud YR, Hatakeyama S, Di Stasio E, Milisen K, Van Grootven B, van der Laan L, Thomson Mangnall L, Goodlin SJ, Lungeanu D, Denhaerynck K, Dhakharia V, Sampson EL, Zywiel MG, Falco L, Nguyen AV, Moss SJ, Krewulak KD, Jaworska N, Plotnikoff K, Kotteduwa-Jayawarden S, Sandarage R, Busse JW, Mbuagbaw L. Perioperative Factors Associated With Postoperative Delirium in Patients Undergoing Noncardiac Surgery: An Individual Patient Data Meta-Analysis. JAMA Netw Open. 2023;6:e2337239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Wongtangman K, Aasman B, Garg S, Witt AS, Harandi AA, Azimaraghi O, Mirhaji P, Soby S, Anand P, Himes CP, Smith RV, Santer P, Freda J, Eikermann M, Ramaswamy P. Development and validation of a machine learning ASA-score to identify candidates for comprehensive preoperative screening and risk stratification. J Clin Anesth. 2023;87:111103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 21. | Katz JA, Sehlhorst CS, Thompson GA, Denson DD, Coyle D, Bridenbaugh PO. Pharmacokinetics of intravenous and epidural ropivacaine in the rhesus monkey. Biopharm Drug Dispos. 1993;14:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Castillo P, Forestier J, Wiegele M, Finnbogasson T, Lönnqvist PA. Primary spread of caudal blockade in children: the possible limiting role of the lumbar spinal cord enlargement (tumenescence) in combination with the cerebrospinal fluid rebound mechanism. Paediatr Anaesth. 2021;31:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Omidvar S, Ebrahimi F, Amini N, Modir H, Kia MK, Rahmaty B, Zarei A. Comparing the Effect of Ketamine and Lidocaine on Agitation and Pain in Rhinoplasty: A Randomized Clinical Trial. J Cutan Aesthet Surg. 2023;16:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Yu H, Sun X, Li P, Deng X. Prevalence and risk factors of emergence agitation among pediatric patients undergo ophthalmic and ENT Surgery: a cross-sectional study. BMC Pediatr. 2023;23:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | Zheng L, Zhao J, Zheng L, Jing S, Wang X. Effect of Dexmedetomidine on Perioperative Stress Response and Immune Function in Patients With Tumors. Technol Cancer Res Treat. 2020;19:1533033820977542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 520] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 27. | Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 28. | Li Y, Lu Q, Wang B, Tang W, Fan L, Li D. Preoperative Fasting Times for Patients Undergoing Elective Surgery at a Pediatric Hospital in Shanghai: The Big Evidence-Practice Gap. J Perianesth Nurs. 2021;36:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Bach H, Kaasby K, Sørensen A, Løfqvist S, Laursen BS. Incidence and Severity of Catheter-Related Bladder Discomfort Among Nonurological Adult Patients in a Postanesthesia Care Unit. J Perianesth Nurs. 2020;35:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Lee YJ, Kim BY, Park JH, Kim SY, Park HY, Do SH. The Effect of Intraoperative Magnesium Sulphate Infusion on Emergence Agitation after Ambulatory Ophthalmic Surgery in Children. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Menser C, Smith H. Emergence Agitation and Delirium: Considerations for Epidemiology and Routine Monitoring in Pediatric Patients. Local Reg Anesth. 2020;13:73-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Wei B, Feng Y, Chen W, Ren D, Xiao D, Chen B. Risk factors for emergence agitation in adults after general anesthesia: A systematic review and meta-analysis. Acta Anaesthesiol Scand. 2021;65:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/